Abstract
The medial thalamic parafascicular nucleus (PF) and the rostral anterior cingulate cortex (rACC) are implicated in the processing and suppression of the affective dimension of pain. The present study evaluated the functional interaction between PF and rACC in mediating the suppression of pain affect in rats following administration of morphine or carbachol (acetylcholine agonist) into PF. Vocalizations that occur following a brief noxious tailshock (vocalization afterdischarges) are a validated rodent model of pain affect, and were preferentially suppressed by injection of morphine or carbachol into PF. Vocalizations that occur during tailshock were suppressed to a lesser degree, whereas, spinal motor reflexes (tail flick and hindlimb movements) were only slightly suppressed by injection of carbachol into PF and unaffected by injection of morphine into PF. Blocking glutamate receptors in rACC (NMDA and non-NMDA) by injecting D-2-amino-5-phosphonovalerate (AP-5) or 6-Cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) produced dose-dependent antagonism of morphine-induced increases in vocalization thresholds. Carbachol-induced increases in vocalization thresholds were not affected by injection of either glutamate receptor antagonist into rACC. The results demonstrate that glutamate receptors in the rACC contribute to the suppression of pain affect produced by injection of morphine into PF, but not to the suppression of pain affect generated by intra-PF injection of carbachol.
Keywords: parafascicular nucleus, pain, morphine, carbachol, glutamate, rat
Introduction
Four decades of neurosurgical observations demonstrate that the anterior cingulate cortex (ACC) contributes to the affective dimension of pain. Ablation of large portions of the ACC or its underlying white matter (cingulum bundle) alleviates the emotional suffering of patients in chronic pain, but fails to impair their ability to judge the location or intensity of their pain (Foltz and White, 1962; Ballantine et al., 1967; Hassenbusch et al., 1990; Yen et al., 2005). However, recent studies indicate that regional differences exist within the ACC in the processing versus modulation of pain affect.
Neuronal activation in the caudal ACC (cACC) elicited by noxious stimulation is related to the processing of pain affect in humans and animals (Bentley et al., 2003; Porro et al., 2003; Vogt et al., 2003; Kulkarni et al., 2007). Conversely, the rostral ACC (rACC) contributes to inhibitory modulation of nociceptive processing. Electrical stimulation of the rACC in rats increased response latencies on the hot-plate and tail-flick tests (Hardy, 1985), and reduced the aversive quality of noxious tactile stimulation in nerve-injured rats (LaBuda and Fuchs, 2005). Human neuroimaging studies demonstrated preferential activation of rACC during a variety of interventions reported to reduce the perception of pain unpleasantness, and these increases in rACC activation were accompanied by a reduction of noxious-evoked activity in cACC (Garcia-Larrea et al., 1997; Garcia-Larrea et al., 1999; Casey et al., 2000; Faymonville et al., 2000; Bantick et al., 2002; Petrovic et al., 2002; Willoch et al., 2003; Petrovic et al., 2005; Zubieta et al., 2005).
The medial thalamic parafascicular nucleus (PF) is also implicated in the processing and modulation of pain affect, and has extensive reciprocal connections throughout the ACC (Vogt et al., 1979; Deschenes et al., 1996; Vercelli et al., 2003; Weigel and Krauss, 2004). The PF receives nociceptive afferents (Stevens et al., 1989; Krout and Loewy, 2000) and noxious peripheral stimulation evokes neural activity in PF (Casey et al., 1974; Yen et al., 1989; Bullitt, 1990). Ablation of PF relieves the emotional suffering associated with chronic pain in humans (Mark et al., 1961; Whittle and Jenkinson, 1995) and reduces affective responses of animals to noxious stimulation (Kaelber et al., 1975; Saade et al., 2007). Escape and avoidance conditioning in rats supported by noxious peripheral stimulation or stimulation of nucleus reticularis gigantocellularis (i.e., medullarly link of the spino-retuculo-thalamic pathway) are also attenuated following lesion of PF (Mitchell and Kaelber, 1967; Delacour, 1971; Roberts, 1991). Recent reports that failed to observe deficits in avoidance conditioning in rats with medial thalamic lesions did not include PF in their analysis (Wang et al., 2007; Wilson et al., 2008). Alternately, high frequency stimulation of PF results in reports of intense pain and unpleasantness in humans (Thoden et al., 1979;Velasco et al., 1998), and aversive pain-like reactions in animals (Kaelber and Mitchell, 1967; Rosenfeld and Holzman, 1978).
Nociceptive-reactive neurons within the PF are the primary relay of nociceptive input to the ACC. Stimulation of nociceptive-reactive neurons in the PF produces excitatory responses within the ACC (Hsu and Shyu, 1997; Hsu et al., 2000), and suppression of neural activity in the PF or lesion of PF eliminated noxious-evoked neural activity in ACC (Sikes and Vogt, 1993; Yang et al., 2006). Projections from PF to ACC appear to be glutamatergic. PF synapses in the ACC are characterized by anatomical indices indicative of excitatory output, namely asymmetrical junctions and round synaptic vesicles (Marini et al., 1996). Further, histological and autoradiographic analyses identified postsynaptic glutamate receptors throughout the ACC (Unnerstall and Wamsley, 1983; Ohishi et al., 1995; Ohishi et al., 1998). Intracellular recordings demonstrated that glutamate, acting at both NMDA and AMPA/kainate receptors, mediates excitatory neurotransmission within the ACC (Sah and Nicoll, 1991; Liauw et al., 2003), and neural activity in ACC evoked by stimulation of PF is reduced by injection of NMDA (AP-5) and AMPA/kainate (CNQX) receptor antagonists into ACC (Yang et al., 2006; Lee et al., 2007).
The PF contains opioid and acetylcholine receptors (Spencer et al., 1986; Mansour et al., 1987), and opioids and acetylcholine modulate neuronal activity within PF (Dafny and Gildenberg, 1984; Capozzo et al., 2003). We reported that microinjection of morphine or carbachol (broad spectrum acetylcholine agonist) into PF preferentially reduced rats’ affective response to noxious stimulation through their action at μ-opioid and muscarinic receptors, respectively (Harte et al., 2000; Harte et al., 2004). The present study evaluated the contribution of glutamate receptors in the rACC to the suppression of pain affect generated by injection of morphine or carbachol into PF.
Experimental Procedures
Animals
Forty-two, male Long-Evans rats (Charles River, Portage, MI) between 100 and 150 days old at the time of surgery were used. Rats were housed as pairs in polycarbonate cages in a climate-controlled vivarium (lights on 0700 h to 1900 h), and given ad libitum access to food and water. Testing occurred during the light portion of the cycle. Rats were handled daily for one week prior to surgery to minimize the effects of stress from human contact. All procedures were approved by the IACUC of Wayne State University.
Anatomical Definition of rostral ACC
There is no common scheme to differentiate rostral and caudal regions of the rodent ACC. For purposes of this study, ACC was divided following anatomical landmarks indicated in the rat brain atlas of Paxinos and Watson (1998). The rACC (or perigenual ACC) was identified as the area encompassing both the precallosal cingulate gyrus that lies immediately anterior and ventral to the genu of the corpus callosum (bregma 4.20 mm to 1.70 mm) and the supracollosal cingulate gyrus at the level of the genu (bregma 1.60 mm to 0.70 mm). The cACC (or midcingulate cortex) was defined as the cingulate gyrus superior to the body of the corpus callosum extending caudally from the genu to the splenium (bregma 0.48 mm to −1.40 mm). This delineation is in general agreement with the regional segregation of the rodent ACC used elsewhere (Johansen et al., 2001; Vogt et al., 2004).
There is also considerable variation in the nomenclature used to identify equivalent areas of the rodent ACC (Uylings et al., 2003; Jones et al., 2005). The nomenclature used here follows that adopted by Zilles and Wree (1995) in which the rACC is divided into dorsal and ventral cingulate areas, identified as Cg1 and Cg2, respectively, that correspond to Brodmann area (BA) 24in primates (Vogt et al., 2004). The rACC also comprises the prelimbic (BA 32) and infralimbic (BA 25) cortices.
Stereotaxic Surgery and Histology
Under aseptic conditions, rats were anesthetized with sodium pentobarbital (60 mg/kg, ip) following pretreatment with atropine sulfate (1 mg/kg, ip). Stereotaxic coordinates (Paxinos and Watson, 1998) were measured relative to the bregma suture and the top of the level skull. Stainless steel 26-gauge double-cannulae (Plastics One, Roanoke, VA) were implanted above the rACC (AP: + 2.70 mm, L: ± 0.6 mm, DV: −1.2 mm) and PF (AP: −4.3 mm, Lat: ± 1.2 mm, DV: − 4.0mm). Cannulae were affixed to the skull with bone screws and cranioplastic dental cement, and fitted with an obturator that extended the length of the cannulae to maintain patency. Rats were given 7–10 days to recover before testing.
At the conclusion of testing, rats were sacrificed by carbon dioxide asphyxiation. Injection sites were marked by safrin-O dye (0.25 µl) and brains were extracted and placed in a 20% (w/v) sucrose formalin solution for 48–72 hours. Brains were sectioned at 40 µm on a freezing microtome, and injection sites were localized using the rat brain atlas of Paxinos and Watson (1998). Histological analysis was done without knowledge of the behavioral results.
Assessment of Pain Affect
Research in this laboratory validated vocalization afterdischarges (VADs) as a rodent model of pain affect. These vocalizations occur immediately following application of noxious tailshock, are organized within the forebrain, and have distinct spectrographic characteristics compared to vocalizations that occur during shock (VDSs; Carroll and Lim, 1960; Hoffmeister, 1968; Borszcz, 1995b; Borszcz and Leaton, 2003; Borszcz, 2006). Systemically administered drug treatments that preferentially suppress the affective reaction of humans to pain (Gracely et al., 1978; Price et al., 1985) also preferentially suppress production of VADs (Borszcz et al., 1994). Generation of VADs is suppressed by damage of or drug treatments into forebrain sites known to contribute to production of the affective response of humans to clinical and experimental pain (Mark et al., 1961; Hoffmeister, 1968; Sweet, 1980; Borszcz, 1999; Harte et al., 2000; Zubieta et al., 2001; Borszcz and Leaton, 2003; Nandigama and Borszcz, 2003; Harte et al., 2004). Additionally, the capacity of noxious tailshock to support fear conditioning is directly related to its production of VADs (Borszcz, 1993; Borszcz, 1995b; Borszcz and Leaton, 2003; Borszcz, 2006). In the present study, the effects of experimental treatments on VAD threshold were compared with their effects on the thresholds of other tail shock– elicited responses that are organized at medullary (VDS = Vocalizations During Shock) and spinal (SMR = Spinal Motor Reflexes) levels of the neuraxis (Carroll and Lim, 1960; Borszcz et al., 1992).
Testing Apparatus
Testing was controlled by custom computer programs via a multifunction interface board (DT-2801, Data Translation, Marlboro, MA) installed in a PC. Rats were placed into custom made Velcro body suits and restrained on a Plexiglas pedestal using Velcro strapping that passes through loops located on the underside of the suits. This design maintains the rat in a crouching posture throughout testing, permits normal respiration and vocalizing, and allows unobstructed access to the head for intracerebral injections (see photograph in Borszcz, 1995b). Testing was conducted within a sound attenuating, lighted, and ventilated chamber equipped with a small window that enabled visual monitoring of rats during testing.
Tailshock (20 ms pulses at 25 Hz for 1,000 ms) was delivered by a computer controlled constant current shocker (STIMTEK, Arlington, MA) through electrodes (0-gauge stainless steel insect pins) placed intracutaneously on opposite sides of the tail, 7.0 cm (cathode) and 8.5 cm (anode) from the base. The intensity, duration, and timing of tailshocks were controlled by the computer. Current intensity was monitored by an A-to-D converter that digitized (500 Hz sampling rate) an output voltage of the shocker that was proportional to the current delivered.
Spinal motor reflexes (SMRs) were measured with a semi-isotonic displacement transducer (Lafayette Instruments Model 76614, Lafayette, IN) attached to the rat’s tail with cotton thread. The arm of the transducer was positioned behind and perpendicular to the tail such that the thread extended in a straight line directly behind the rat. The output voltage of the transducer was amplified (x50) and then digitized (500 Hz sampling rate) by an analog-to-digital converter of the interface board. SMR was defined as movement of the transducer arm by at least 1.0 mm following shock onset. The computer recorded the latency (ms), peak amplitude (mm), and magnitude (cm × ms) of tail movement on each trial. Displacements up to 100 mm can be detected, and latencies in 2 ms increments can be measured.
Vocalizations were measured by a pressure-zone microphone (Realistic model 33–1090, Tandy, Ft. Worth, TX) located on the wall of the testing chamber 15 cm from the rat’s head. The microphone was connected to an audio amplifier (Technics model SA-160, Tandy, Ft. Worth, TX) and a 10-band frequency equalizer adjusted to selectively amplify frequencies above 1500 Hz. The filtering of low frequencies prevented extraneous noise (i.e., rats’ respiration and movement artifacts) from contaminating vocalization records. The output of the amplifier was integrated by a Coulbourn Instruments (Allentown, PA) contour following integrator (2 ms time base) and digitized (500 Hz sampling rate) by a separate analog-to-digital converter of the interface board. The peak intensity (in decibels: SPL, B scale), latency (ms), and duration (ms) of vocalizations during the shock epoch (VDS) and for the 2,000 ms interval following shock termination (VAD), were recorded by the computer.
Pain Testing
On 3 consecutive days prior to testing, rats were adapted to the testing apparatus for 20–25 min/day to minimize the effects of restraint stress. Test sessions consisted of 20 randomly presented trials. On 16 trials tailshocks between 0.02 mA and 2.50 mA were delivered, and on 4 trials no current was delivered so as to assess false alarm rates. Trials were presented with a minimum intertrial interval of 30 seconds, and each test session concluded within 20 minutes. These procedures caused no observable damage to the tail. Following each test session, the testing apparatus was cleaned with 5% ammonia hydroxide to eliminate stress odors (Fanselow, 1985).
Experimental Design
Two groups of rats (morphine group, n = 14; carbachol group, n = 15) were implanted with bilateral cannulae directed toward PF and rACC. Morphine group rats received the following bilateral microinjections prior to pain testing: rACC/saline + PF/saline (baseline condition); rACC/saline + PF/morphine (5 µg/side); rACC/AP-5 (2 or 4 µg/side) + PF/morphine (5 µg/side); rACC/CNQX (1 or 2 µg/side) + PF/morphine (5 µg/side); rACC/ AP-5 (2 or 4 µg/side) + PF/saline; rACC/CNQX (1 or 2 µg/side) + PF/saline. The carbachol group rats received the following bilateral microinjections prior to pain testing: rACC/saline + PF/saline (baseline condition); rACC/sal + PF/carbachol (2 µg/side); rACC/AP-5 (2 or 4 µg/side) + PF/carbachol (2 µg/side); rACC/CNQX (1 or 2 µg/side) + PF/carbachol (2 µg/side); rACC/ AP-5 (2 or 4 µg/side) + PF/saline; rACC/CNQX (1 or 2 µg/side) + PF/saline. Therefore, every animal in both groups received each antagonist in rACC, but the different doses of each antagonist were evaluated in separate subgroups. One subgroup received the high dose of AP-5 and CNQX, whereas the other subgroup received the low dose of AP-5 and CNQX. Injections into PF and rACC were separated by 10 min, and the PF injection was always administered second. Pain testing began 7–10 min following completion of PF injections.
The total of six treatment conditions were presented on a quasi-Latin square schedule that maintained the rACC/saline + PF/saline and rACC/saline + PF/agonist treatments at either the beginning or the end of each testing sequence. Comparison of the rACC/saline + PF/saline treatment at these times permitted evaluation of multiple test sessions on baseline thresholds. Comparison of the rACC/saline + PF/agonist treatment at these times permitted evaluation of the effects of multiple test sessions on morphine- or carbachol-induced increases in response thresholds. Test sessions were separated by 5–7 days, and the rACC/antagonist + PF/saline treatment conditions were always tested between treatment conditions in which an agonist was administered into the PF. Thus, injections of agonist into the PF were separated by a minimum of 10 days. This time interval reduced the likelihood that tolerance would develop following repeated drug injections (Yaksh et al., 1976).
Following the completion of the testing sequence, a subset of rats (n = 7) underwent an additional pain test following administration of NMDA (1 µg/side) into rACC. Animals were taken from both the morphine and carbachol groups, and were selected on the basis of health and cannulae condition. This subset of animals tested the direct effect of glutamatergic receptor activation of the rACC on pain response thresholds.
Anatomical specificity of rACC antagonist administration was evaluated in a separate group of rats (n = 4) by microinjecting morphine into PF, and AP-5 and CNQX into sites ventral or lateral to the Cg1 region of the rACC (extra-rACC). Each animal received bilateral injections of antagonist at multiple D/V coordinates using a series of different length injectors, starting at the most dorsal position and moving ventrally in increments of 0.25–0.5 mm. In total, AP-5 and CNQX were injected into 15 unique extra-rACC locations.
Drug Injections
Intracerebral injections were administered in a constant volume of 0.25 µl via 33-guage injectors that extended 1.2 mm (rACC) and 3 mm (PF) beyond the cannula tip. Injections were made at a constant rate over 1 min via an infusion pump (Harvard Model PHD 2000), and injectors were left in place for 2 min after the completion of injections to aid the diffusion of drugs into the tissue. Morphine, carbachol, CNQX (6-cyano-7-nitroquinoxaline-2,3-dione), and NMDA (N-methyl-d-aspartic acid) were purchased from Sigma-Aldrich (St. Louis, MO); AP-5 (2-amino-5-phosphonovaleric acid) was purchased from Tocris (Ellisville, MO). CNQX was dissolved in distilled water; all other drugs were dissolved in sterile isotonic saline.
Data Analysis
Following each test session, data were reorganized in ascending order according to tailshock intensity. SMR, VDS, and VAD thresholds were calculated as the lesser current intensity from a string of at least two consecutive intensities that generated the response. Response thresholds following rACC/saline + PF/saline and rACC/saline + PF/agonist treatments were collapsed across antagonist subgroups, and directly compared using MANOVA. A significant omnibus MANOVA was followed by within-subject contrasts of response thresholds, and one-way ANOVA across individual treatment levels. The effects of agonist on individual responses were compared using Student’s t-test for independent samples. Planned comparisons that assessed the capacity of intra-rACC antagonist treatment to reduce increases in response thresholds produced by intra-PF morphine or carbachol were conducted using Student’s t-test for independent samples. The alpha level was 0.05 for all analyses. Rats with injections outside of the PF and/or rACC were separately grouped and analyzed.
Response thresholds deviating two standard deviations or more from the mean threshold in any treatment condition were considered outliers and rejected from analysis. Five threshold outliers were removed in the morphine group and two threshold outliers were removed from the carbachol group. One additional response threshold from the morphine group and two from the carbachol group were removed from analysis due to experimental error (e.g., injection failure, corrupted/lost data file).
Results
Response Profile
As demonstrated by Carroll and Lim (1960), SMR, VDS, and VAD reflect nociceptive processing at progressively higher levels of the neuraxis. Their analysis of rats that received transections of the neuraxis revealed that SMRs are organized at the spinal level (also see Borszcz et al., 1992), VDSs within the medulla below the pontomedullary border, and VADs within the forebrain. Consistent with our previous reports, rostrally organized responses were rarely generated without those integrated more caudally within the CNS. VAD generation, without concomitant generation of VDS or SMR, occurred on 0.68% of trials. Similarly, VDS was generated without SMR on 0.40% of trials in which VDS was the most rostrally elicited response. False alarm rates for each response were low: SMR = 3.74%, VDS = 2.84%, VAD = 2.85%. A low incidence of false alarms indicates that responses were not induced by drug administration, were not occurring spontaneously, and were not conditioned responses to the context, but instead were generated by tailshock.
Response Performance
The effects of morphine and carbachol on the performance of SMR, VDS and VAD were evaluated. Performance variables at threshold following vehicle (rACC/saline + PF/saline) treatment were compared to performance variables at threshold following rACC/saline + PF/agonist treatment. The latency, amplitude, and magnitude of SMRs, and latency, amplitude, and duration of VDSs and VADs did not differ following vehicle and morphine treatments, ts < 2.05, ps > 0.05. On the other hand, carbachol treatment resulted in selective decrements in SMR and VDS performance. Compared to SMR latency following vehicle treatment, the SMR latency at threshold following PF-carbachol administration was significantly increased (228.0 ms vs. 347.2 ms), t(22) = 2.49, p < 0.05. PF-carbachol treatment also significantly reduced VDS duration compared to baseline performance (660.4 ms vs. 437.8 ms), t(22) = 3.35, p < 0.01. SMR amplitude and magnitude, VDS latency and amplitude, and VAD latency, amplitude, and duration were not altered by PF-carbachol treatment, ts(22) < 1.99, ps > 0.05.
Intra-PF Morphine vs. Intra-rACC AP-5 and CNQX
The effects of intra-PF administered morphine (5 µg/side) on SMR, VDS, and VAD thresholds are shown in Figure 1. Comparison of response thresholds following rACC/saline + PF/saline treatment revealed no differences in baseline responding, F(2, 29) = 2.22, p > 0.05. However, response thresholds were differentially affected by rACC/saline + PF/morphine treatment. Comparison of response thresholds following rACC/saline + PF/saline and rACC/saline + PF/morphine treatments (repeated measures MANOVA, Hotelling’s Trace) revealed significant main effects of treatment, F(2, 29) = 57.32, p < 0.001, and response, F(2, 40) = 26.40, p < 0.001, and a significant Treatment × Response interaction, F(2, 40) = 24.47, p < 0.001. This interaction reflects the finding that morphine preferentially increased VAD threshold. Pair-wise contrasts of VAD threshold with VDS and SMR thresholds yielded significant main effects of response, Fs > 24.01, ps < 0.001, and significant Treatment × Response interactions, Fs > 21.63, ps < 0.001. Comparison of baseline thresholds with those following the administration of rACC/saline + PF/morphine revealed significant threshold increases for VAD and VDS [ts(20) > 5.02, ps < 0.001], but not for SMR, t(20) = 1.39, p > 0.05. Direct comparison of VAD and VDS thresholds following rACC/saline + PF/morphine treatment revealed that VAD threshold was significantly elevated above VDS threshold, t(22) = 5.30, p < 0.001.
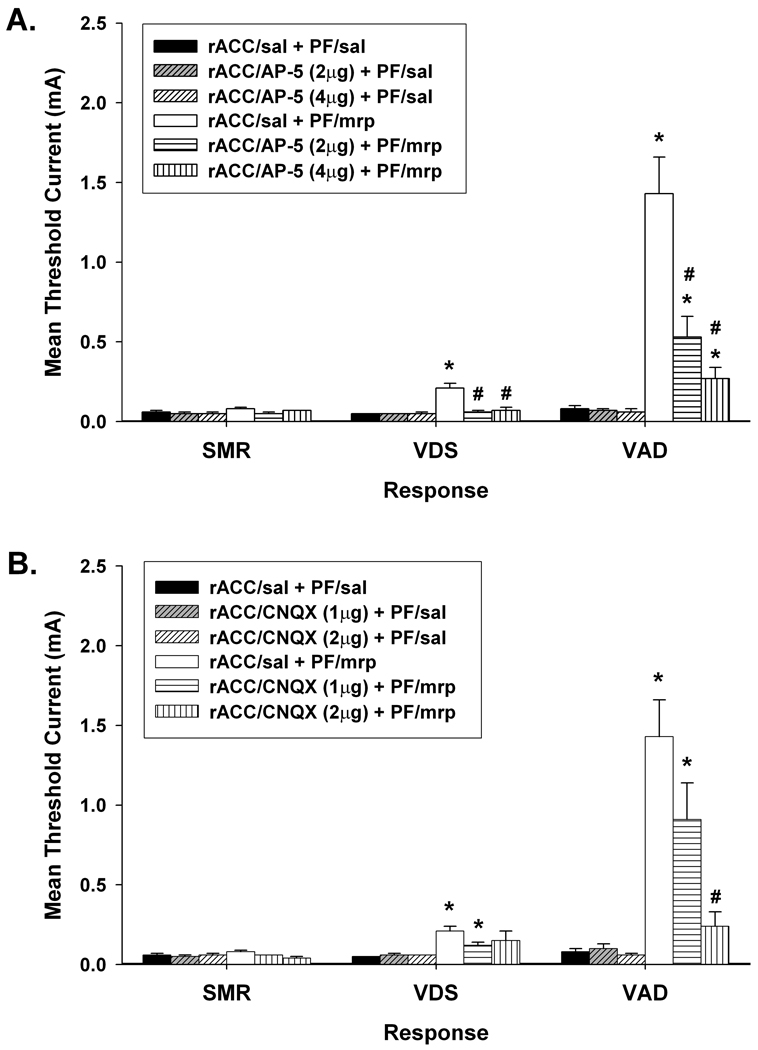
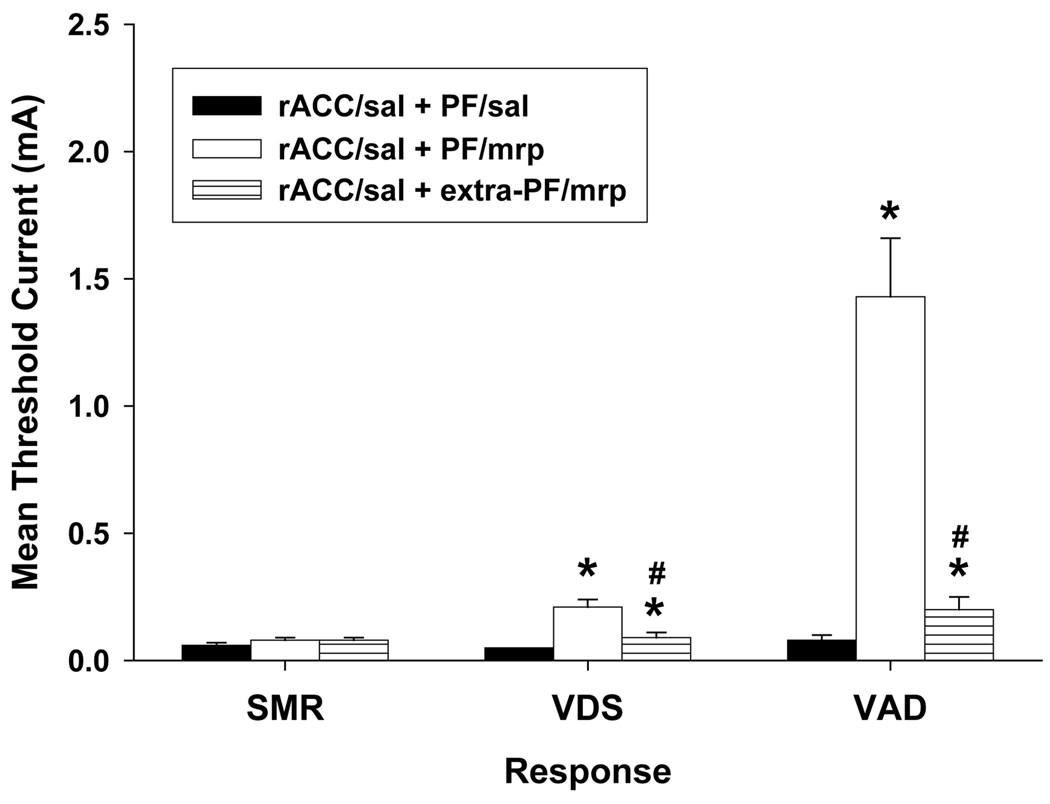
Figure 1.
Effects of bilateral administration of (A) AP-5 and (B) CNQX into the rostral anterior cingulate cortex (rACC) on increases in response thresholds produced by 5µg/side morphine (mrp) administered into the parafascicular nucleus (PF). Data are plotted as the mean (+/− S.E.M.) threshold of spinal motor reflexes (SMRs), vocalizations during shock (VDSs), and vocalization afterdischarges (VADs). Asterisk (*) indicates thresholds significantly elevated above rACC/saline (sal) + PF/sal treatment (p < 0.05). Pound sign (#) indicates thresholds significantly reduced compared to rACC/sal + PF/mrp treatment (p < 0.05).
Intra-rACC administration of AP-5 resulted in the dose-dependent antagonism of morphine-induced increases in VDS and VAD thresholds (Figure 1A). Planned comparisons revealed that the 2 and 4 µg/side doses of AP-5 significantly reduced morphine-induced increases in VDS threshold, ts > 3.91, ps < 0.01. Following injection of either dose of AP-5, VDS threshold was restored to its baseline level, ts < 1.20, ps > 0.05. Morphine-induced increases in VAD threshold were also significantly reduced by the administration of 2 and 4 µg/side AP-5, ts > 2.22, ps ≤ 0.05, but it remained elevated compared to baseline following both doses of AP-5, ts > 2.65, ps < 0.05. Furthermore, AP-5 had no effect on baseline responding. Administration of either dose of AP-5 into rACC (rACC/AP-5 + PF/saline) failed to alter baseline thresholds of any of the responses, ts < 1.64, ps > 0.05.
Intra-rACC administration of CNQX also resulted in the dose-dependent antagonism of morphine-induced increases in vocalization thresholds (Figure 1B). Administration of 2 µg/side CNQX significantly reduced VAD threshold [t(10) = 3.46, p < 0.01], and restored VAD and VDS thresholds to baseline levels, ts < 2.02, ps > 0.05. VDS and VAD thresholds remained elevated compared to baseline following administration of 1 µg/side CNQX, ts > 3.22, ps < 0.05. Similar to AP-5 treatment, neither dose of CNQX altered baseline thresholds, ts < 1.
PF-Carbachol vs. Intra-rACC AP-5 and CNQX
The effects of intra-PF administered carbachol (2 µg/side) on SMR, VDS, and VAD thresholds are shown in Figure 2. Similar to the results observed following PF-morphine treatment, baseline thresholds of SMR, VDS, and VAD did not differ, F < 1, but the intra-PF administration of carbachol differentially affected response thresholds. Comparison of response thresholds following rACC/saline + PF/saline and rACC/saline + PF/carbachol treatments (repeated measures MANOVA, Hotelling’s Trace) revealed significant main effects of treatment, F(1, 26) = 96.84, p < 0.001 and response, F(2, 52) = 46.20, p < 0.001, and a significant Treatment × Response interaction, F(2, 52) = 44.41, p < 0.001. This interaction reflects the finding that carbachol also preferentially increased VAD threshold. Pair-wise comparisons of VAD threshold with VDS and SMR thresholds yielded significant main effects of response, Fs(1, 26) > 35.29, ps < 0.001, and significant Treatment × Response interactions, Fs(1, 26) > 33.74, ps < 0.001. Compared to baseline, VAD and VDS thresholds were significantly elevated following rACC/saline + PF/carbachol treatment, ts(26) > 4.82, ps < 0.001. In addition, PF-administered carbachol produced a slight, yet significant, increase in SMR threshold over baseline, t(26) = 3.06, p < 0.01. Direct comparison of response thresholds following rACC/saline + PF/carbachol treatment revealed that both VAD and VDS thresholds were significantly elevated above SMR threshold [ts(24) > 4.14, ps < 0.01], and VAD threshold was significantly elevated over VDS threshold, t(24) = 4.37, p < 0.001.
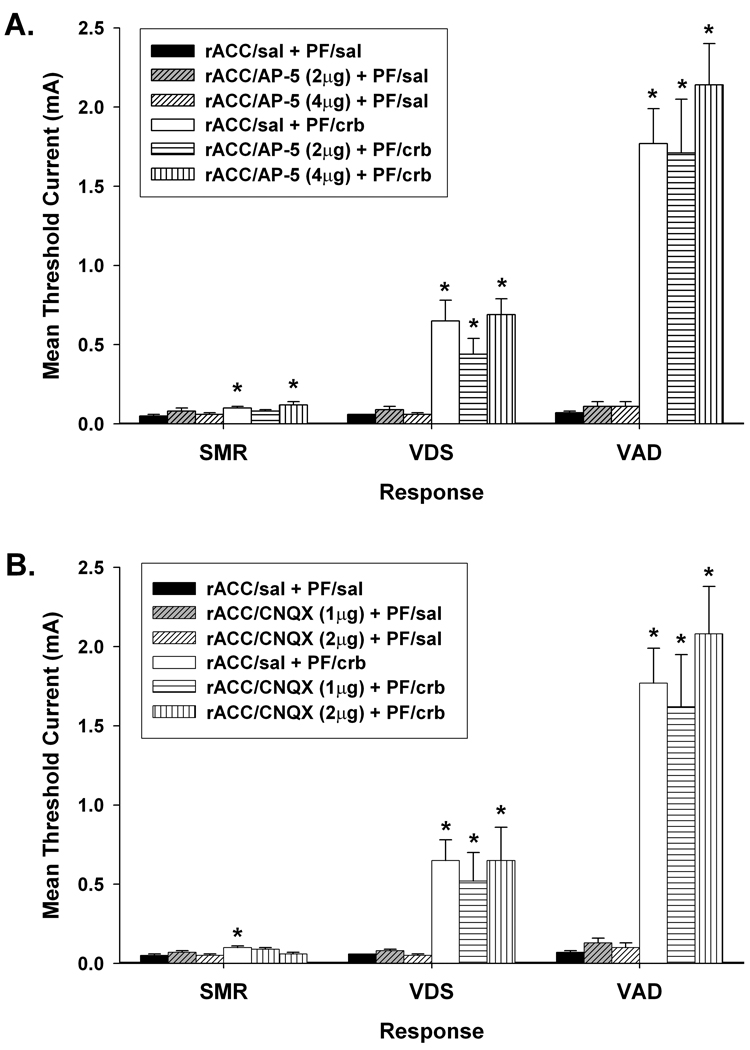
Figure 2.
Effects of bilateral administration of (A) AP-5 and (B) CNQX into the rostral anterior cingulate cortex (rACC) on increases in response thresholds produced by 2 µg/side carbachol (crb) administered into the parafascicular nucleus (PF). Data are plotted as the mean (+/− S.E.M.) threshold of spinal motor reflexes (SMRs), vocalizations during shock (VDSs), and vocalization afterdischarges (VADs). Asterisk (*) indicates thresholds significantly elevated above rACC/saline (sal) + PF/sal treatment (p < 0.05).
Intra-rACC administered AP-5 or CNQX failed to reduce threshold increases in vocalizations thresholds produced by PF-carbachol treatment. Compared to rACC/saline + PF/carbachol treatment, no differences were observed in VDS or VAD thresholds following administration of 2 or 4 µg/side AP-5 (Figure 2A), or 1 or 2 µg/side CNQX (Fig. 2B) into rACC, ts < 1.21, ps > 0.05. VDS and VAD thresholds remained elevated compared to baseline following both doses of AP-5 (ts > 3.75, ps < 0.001) or CNQX (ts > 2.53, ps < 0.05). Intra-rACC administration of either dose of AP-5 or CNQX failed to alter baseline thresholds, ts < 2.11, ps > 0.05.
Histological Analysis & Anatomical Specificity
Schematics depicting representative dual-injection sites (PF and rACC) from the morphine group are shown in Figure 3. Figure 3A shows the bilateral distribution of AP-5 and CNQX injection sites within the rACC. The majority of microinjections were localized bilaterally within the Cg1 region of the rACC; a small number of injections were also localized along the dorsal border of Cg1 within the secondary motor cortex. No systematic differences were observed in the distribution of sites that received antagonist treatment. All injection sites within Cg1 were effective in reducing the increases in VDS and VAD thresholds produced by PF-morphine administration.
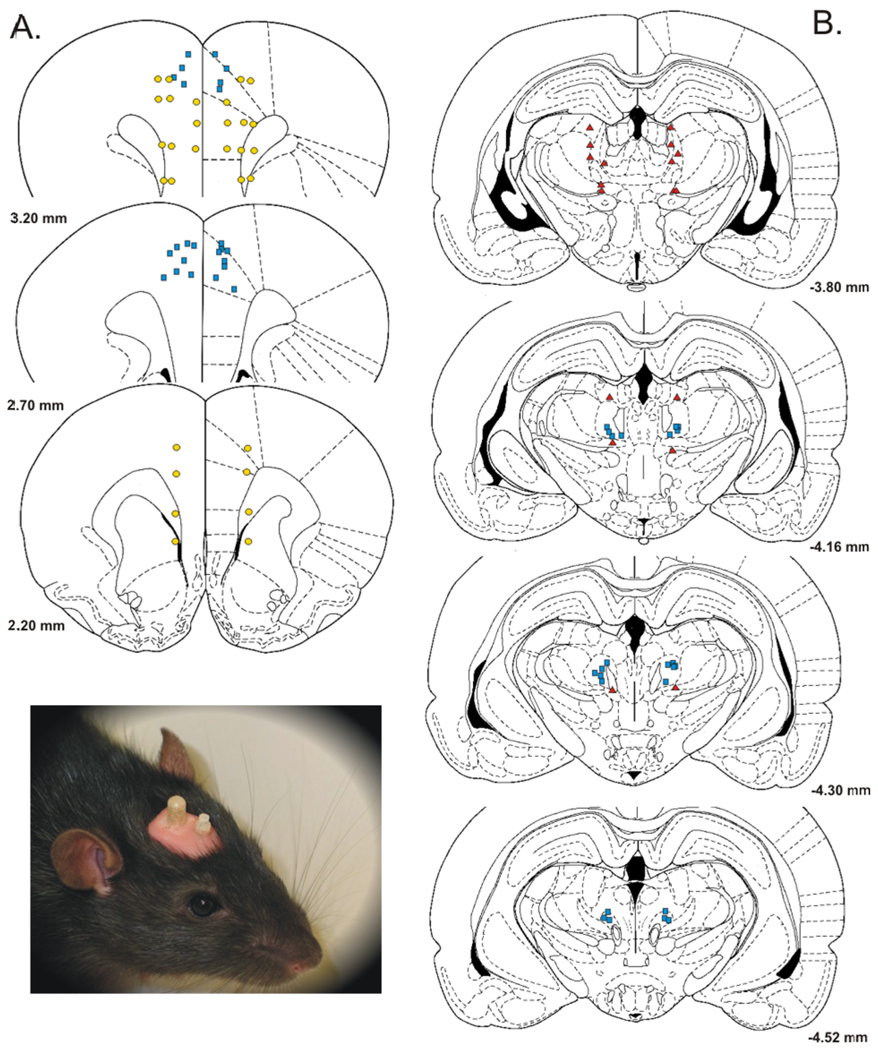
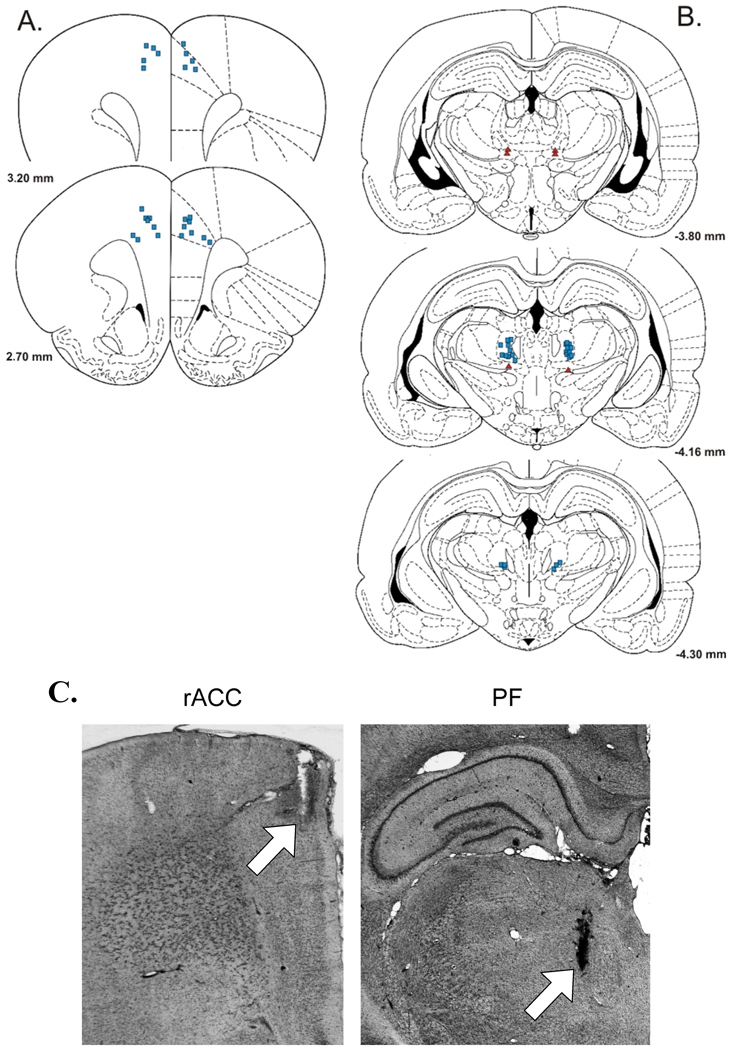
Figure 3.
Selected injection sites in and around (A) rostral anterior cingulate cortex (rACC) and (B) parafascicular nucleus (PF). Blue squares = bilateral morphine injection sites in PF that were effective in elevating response thresholds, and corresponding bilateral injection sites of AP-5 and CNQX in rACC that were effective in reducing increases in response thresholds produced by PF-morphine treatment. Red triangles = morphine injections into areas dorsal or ventral to PF (extra-PF). Yellow circles = injection sites of AP-5 and CNQX located lateral or ventral to the Cg1 region of the rACC (extra-rACC). Coordinates are in millimeters from bregma. Schematics were derived from the rat brain atlas of Paxinos and Watson (1998). Inset: Photograph of a rat with two bilateral cannulae targeting the rACC and PF.
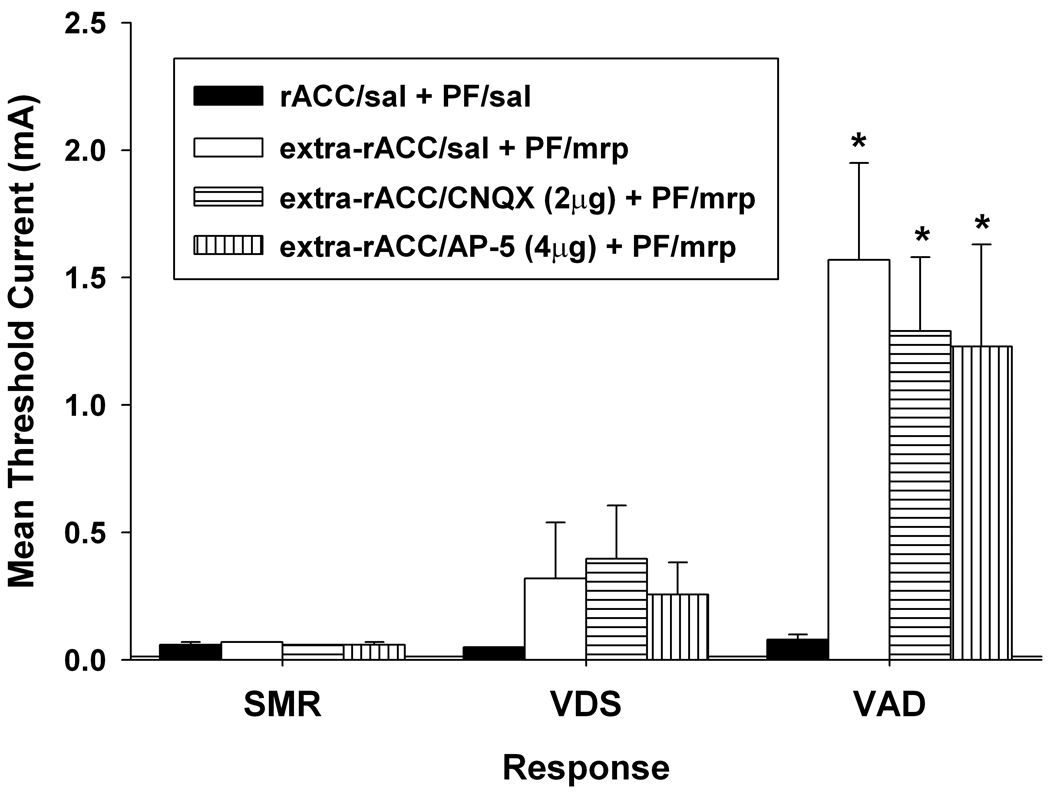
Anatomical specificity of rACC antagonist administration to reduce threshold increases produced by PF-administered morphine was evaluated in a separate group of rats by microinjecting AP-5 and CNQX into sites ventral or lateral to the Cg1 region of the rACC (extra-rACC). As shown in Figure 3A, extra-rACC injections were localized in one of following locations: secondary motor cortex, prelimbic cortex (BA 32), infralimbic cortex (BA 25), corpus collosum, and caudate putamen. Rats found to have unilateral Cg1 placements during postmortem histological examination were also collapsed into the extra-rACC group. Extra-rACC administered AP-5 or CNQX failed to reduce threshold increases produced by PF-administered morphine (Figure 4). Compared to extra-rACC/saline + PF/morphine treatment, no differences were observed in VDS or VAD thresholds following administration of 2 µg/side CNQX or 4 µg/side AP-5 into extra-rACC sites, ts < 0.95, ps > 0.05. Compared to rACC/saline + PF/saline treatment, increases in VAD threshold following PF-morphine administration remained elevated following extra-ACC administration of AP-5 (t = 2.83, p < 0.05) or CNQX (t = 3.29, p < 0.01). Thus, the capacity of AP-5 and CNQX to attenuate elevations in vocalization thresholds produced by PF-morphine treatment appears limited to a bilateral action within the Cg1 region of the rACC. SMR and VDS thresholds were not significantly elevated compared to baseline in this group of animals following intra-PF morphine administration (ts < 1.74, ps > 0.05).
Figure 4.
Response thresholds following morphine (5 µg/side) administered into the parafascicular nucleus (PF) and antagonist administered into sites outside the anatomical boundaries of the Cg1 region of the rostral anterior cingulate cortex (extra-rACC). Thresholds following morphine (mrp) administration into PF were compared to those obtained following AP-5 or CNQX administration into extra-rACC sites. Data are plotted as the mean (+/− S.E.M.) threshold of spinal motor reflexes (SMRs), vocalizations during shock (VDSs), and vocalization afterdischarges (VADs). Asterisk (*) indicates thresholds significantly elevated above extra-rACC/saline (sal) + PF/sal treatment (p < 0.05).
The distribution of morphine injection sites within PF (Figure 3B) is similar to those we previously reported (Harte et al., 2000), and consists of bilateral injections localized to the portion of PF lateral to fasciculus retroflexus. No systematic differences were observed in the distribution of sites within PF that received morphine injections. All injection sites within PF were effective in generating morphine-induced increases in vocalization thresholds.
As shown in Figure 3B, several injection sites of morphine were also identified dorsal and ventral to PF (extra-PF). These injections provided control data for the possibility that the effects of morphine on response thresholds resulted from its spread into other sites near PF. Administration of morphine into extra-PF sites (rACC/saline + extra-PF/morphine, n = 9) produced significant increases in VDS and VAD thresholds over baseline thresholds as compared to rats that received injections of saline into PF and rACC, ts > 2.46, ps < 0.05 (Figure 5). However, these increases in vocalization thresholds were much smaller compared to those observed following the intra-PF injection of morphine and intra-rACC injection of saline, ts > 3.14, ps < .001. Therefore, the increase in vocalization thresholds observed following injection of morphine into PF is likely the result of the action of morphine within PF. SMR threshold was not affected by extra-PF morphine, t(17) = 1.02, p > 0.05.
Figure 5.
Response thresholds following bilateral morphine (5 µg/side) administered into sites outside the anatomical boundaries of the parafascicular nucleus (extra-PF). Thresholds following morphine (mrp) administration were compared to those attained following saline (sal) administration into the rostral anterior cingulate cortex (rACC) and PF. Data are plotted as the mean (+/− S.E.M.) threshold of spinal motor reflexes (SMRs), vocalizations during shock (VDSs), and vocalization afterdischarges (VADs). Asterisk (*) indicates thresholds significantly elevated above rACC/sal + PF/sal treatment (p < 0.05). Pound sign (#) indicates thresholds significantly lower compared to rACC/sal + PF/mrp treatment (p < 0.05).
Schematics depicting representative dual-injection sites from the carbachol group are shown in Figure 6. Figure 6A shows the bilateral distribution of injection sites of AP-5 and CNQX into the rACC. The distribution of injection sites was similar to that found in the morphine group, and no systematic differences were observed in the distribution of sites within rACC that received antagonist treatment. All injections of antagonists into the rACC failed to reduce increases in vocalization thresholds produced by intra-PF carbachol treatment. VAD and VDS thresholds following rACC/antagonist + PF/carbachol treatments were not significantly different from those following the rACC/sal + PF/carbachol treatment, ts < 1.21, ps > 0.05. In addition, two injection sites were located ventral to rACC in the prelimbic cortex (not shown). Injections of AP-5 and CNQX into this area were also ineffective in reducing carbachol-induced threshold increases.
Figure 6.
Selected injection sites in and around (A) rostral anterior cingulate cortex (rACC) and (B) parafascicular nucleus (PF). Blue squares = carbachol injection sites in PF that were effective in elevating response thresholds, and corresponding injection sites of AP-5 and CNQX in rACC that failed to reduce increases in response thresholds produced by PF-carbachol treatment. Red triangles = injections of carbachol into areas outside the boundaries of PF (extra-PF). Coordinates are in millimeters from bregma. Schematics were derived from the rat brain atlas of Paxinos and Watson (1998). (C) Photomicrographs of representative microinjections (arrows) into rACC and PF. Both sites received bilateral injections.
The distribution of carbachol injection sites within PF (Figure 6B) is similar to that which we previously reported (Harte et al., 2004). Similar to the morphine group, the carbachol injection sites were bilateral and localized to the lateral portion of PF. Figure 6B also depicts three extra-PF injections of carbachol. Extra-PF injections of carbachol did not appear to increase the threshold of any response, although the small number of injections into these areas precluded statistical analysis of this subset of data. We previously reported that the administration of carbachol in the immediate vicinity of PF was ineffective in elevating response thresholds (Harte et al., 2004).
Effects of Intra-rACC NMDA on Response Thresholds
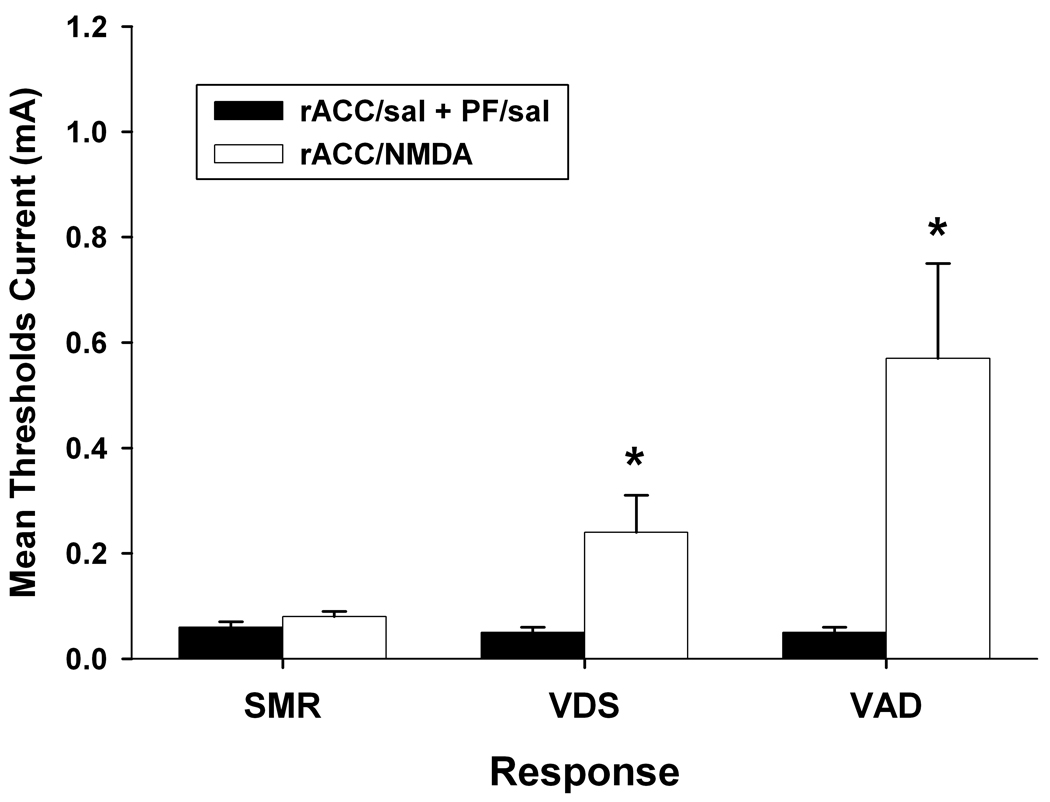
Microinjection of NMDA into the rACC following completion of their normal testing sequence. As shown in Figure 7, intra-rACC NMDA (1 µg/side) elevated VDS and VAD thresholds above baseline levels, ts > 2.57, ps < 0.05. SMR threshold was not affected by administration of NMDA into the rACC, t(11) = 1.66, p > 0.05.
Figure 7.
Effects of bilateral administration of NMDA (1 µg/side) into the rostral anterior cingulate cortex (rACC). Thresholds following intra-rACC NMDA administration were compared to those attained following saline (sal) administered into rACC and nucleus parafascicularis (PF). Data are plotted as the mean (+/− S.E.M.) threshold of spinal motor reflexes (SMRs), vocalizations during shock (VDSs), and vocalization afterdischarges (VADs). Asterisk (*) indicates thresholds significantly elevated above rACC/sal + PF/sal treatment (p < 0.05).
Analysis of Testing Effects and Tolerance
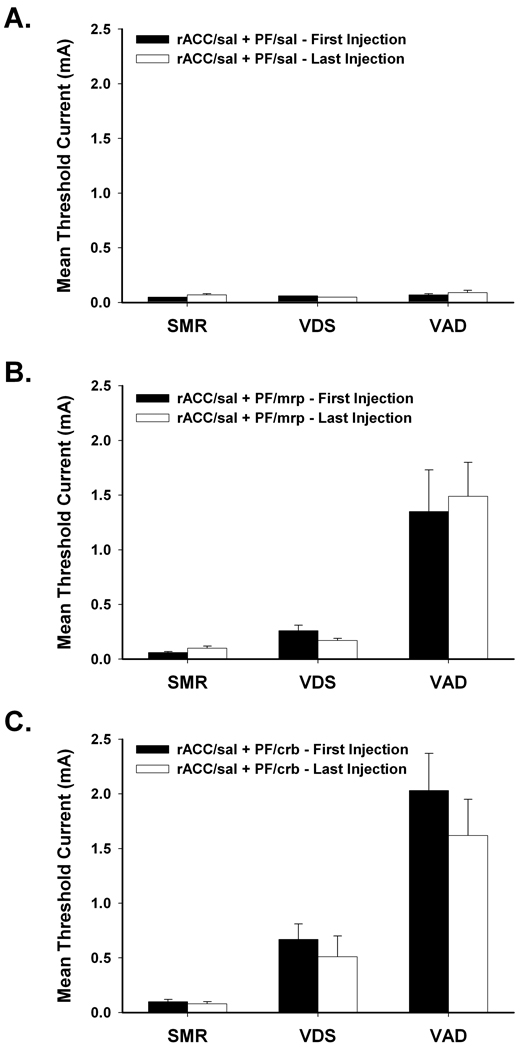
The effects of multiple test sessions on response thresholds are shown in Figure 8. Multiple testing sessions did not alter baseline responding (Figure 8A). Comparison of thresholds following vehicle treatment (rACC/saline + PF/saline) from the beginning (First Injection) and the end (Last Injection) of the testing sequence revealed no differences in baseline responding, ts < 1.69, ps > 0.05. Increases in response thresholds produced by agonist treatments (rACC/saline + PF/morphine; rACC/saline + PF/carbachol) also were not affected by multiple test sessions. Response thresholds following morphine (Figure 8B) and carbachol (Figure 8C) administration attained at the beginning of the testing sequence did not differ from those following agonist administration at the end of the testing sequence, ts < 1.69, ps > 0.05.
Figure 8.
Effects of multiple test sessions on response thresholds. Response thresholds from the beginning (First Injection) and the end (Last Injection) of the testing sequence were compared following administration of (A) saline (sal), (B) 5 µg/side morphine (mrp), and (C) 2 µg/side carbachol (crb) into the rostral anterior cingulate cortex (rACC) and parafascicular nucleus (PF). Data are plotted as the mean (+/− S.E.M.) threshold of spinal motor reflexes (SMRs), vocalizations during shock (VDSs), and vocalization afterdischarges (VADs).
Therefore, elevations in response thresholds do not reflect the deleterious effects possible with multiple test sessions and microinjections, including the development of stress-induced analgesia/hyperalgesia and excessive neuronal damage. Furthermore, the finding that morphine- and carbachol-induced increases in response thresholds did not differ when assessed at the beginning and end of the testing sequence demonstrates that tolerance did not develop following repeated PF microinjections of morphine or carbachol.
Discussion
The present study provides the first behavioral evidence of a functional interaction between the medial thalamus and rACC in the modulation of pain affect. As described earlier, VADs are a validated rodent model of pain affect. Consistent with our earlier reports, administration of morphine or carbachol into PF preferentially elevated VAD threshold (Harte et al., 2000; Harte et al., 2004). Increases in VAD and VDS thresholds produced by PF-morphine treatment were blocked in a dose-dependent manner by administration of AP-5 or CNQX into the Cg1 region of the rACC. Therefore, glutamatergic neurotransmission in rACC is critical for the expression of antinociception produced by PF-morphine administration. The observation that direct stimulation of rACC by NMDA also preferentially increased VAD threshold supports this conclusion. Alternately, increases in vocalization thresholds generated by administration of carbachol into PF were unaffected by injection of AP-5 or CNQX into rACC.
Our earlier work demonstrated that elevations in vocalization thresholds reflect the action of morphine and carbachol at μ-opioid and muscarinic acetylcholine receptors within PF, respectively (Harte et al., 2000; Harte et al., 2004). In the present study, morphine and carbachol injections outside the PF produced significantly smaller increases in vocalization thresholds. This finding is also consistent with our previous reports and demonstrates that increases in vocalization thresholds reflect the action of morphine and carbachol within PF.
Also consistent with our previous report, administration of morphine into PF failed to elevate SMR threshold. However, the slight increase in SMR threshold following carbachol administration into PF is inconsistent with our earlier findings. This increase in SMR threshold may reflect induction of a motor deficit as reflected in the increased latency of SMRs at threshold. The capacity of the monitored performance variables to detect drug-induced motor deficits that confound threshold measurement was previously demonstrated (Borszcz et al., 1994). In our earlier report, no increase in SMR threshold was observed following administration of the same dose of carbachol into PF and no change in SMR performance was observed. The failure to increase SMR threshold does not reflect the resistance of this response to antinociceptive treatments as we previously demonstrated significant increases in SMR threshold following administration of morphine into other sites within the neuraxis (Borszcz, 1995a; Borszcz et al., 1996). Similarly, motor deficits may contribute to carbachol-induced increase in VDS threshold as VDS duration at threshold was reduced. However, we previously observed that injection of the same dose of carbachol into PF increased VDS threshold without altering performance. We therefore conclude that administration of morphine and carbachol into PF preferentially suppresses nociceptive processing at supraspinal levels of the neuraxis.
Administration of morphine into PF suppresses the response of nociceptive neurons to noxious stimulation and activates non-nociceptive neurons in PF (Dafny and Gildenberg, 1984; Prieto-Gomez et al., 1989). We hypothesize that the latter set of PF neurons are glutamatergic and project preferentially to the rACC thereby activating antinociceptive projections from the rACC. Findings that systemically administered morphine facilitate glutamatergic transmission from PF to rACC supports this hypothesis (Yang et al., 2006). Furthermore, nociceptive responsive neurons in PF are hypothesized to project preferentially to the cACC and thereby contribute to the processing of pain affect. Therefore, morphine in PF suppresses pain transmission by activating antinociceptive projections from the rACC and blocking the throughput of nociceptive transmission to the cACC.
The preferential increase in VAD threshold over VDS threshold following injection of morphine into PF or NMDA into rACC is postulated to reflect activation of antinociceptive projections from the ventrolateral periaqueductal gray (vPAG). Activation of the rACC results in the release of met-enkephalin from interneurons in vPAG. Hardy and Haigler (1985) reported that vPAG neurons were modulated by rACC stimulation, and these effects were mimicked by the microiontophoretic application of the μ-opioid receptor agonist met-enkephalin into vPAG. These investigators posited that stimulation of the rACC either directly activates antinociceptive projections from the vPAG or indirectly activates these vPAG projections via inhibition of tonically active intrinsic inhibitory interneurons (Behbehani, 1995). Consistent with this hypothesis, human neuroimaging studies revealed that opioid analgesia and placebo analgesia covary with activation of the rACC and PAG (Petrovic et al., 2002; Bingel et al., 2006), and a recent functional connectivity MRI study revealed an intrinsic connection between rACC and vPAG (Kong et al., 2010). Conversely, vPAG lesions block the suppression of pain affect in rats produced by rACC stimulation (LaBuda and Fuchs, 2005).
In a series of studies, we demonstrated that increasing the level of μ-opioid receptor activation in vPAG, via microinjection of increasing doses of morphine into vPAG, results in progressive increases in VAD, VDS, and SMR thresholds that are mediated by successive recruitment of antinociceptive projections from vPAG to the limbic forebrain, medulla and spinal dorsal horn (Borszcz et al., 1996; Borszcz, 1999; Borszcz and Streltsov, 2000). In the present study, administration of morphine into PF or NMDA into rACC presumably evokes the release of met-enkephalin within vPAG that activates antinociceptive projections to the forebrain and medulla. These antinociceptive projections suppress nociceptive processing in limbic forebrain sites (cACC, PF, amygdaloid central nucleus) that contribute to the production of VADs, and to medullary sites (nucleus reticularis gigantocellularis) that contribute to generation of VDSs (Borszcz, 1999). Increases in VAD threshold also reflect the engagement of descending projections of the vPAG to the medulla that suppresses the throughput of nociceptive transmission to forebrain sites responsible for generating VADs. The dual inhibition of nociceptive processing at medullary and forebrain levels could account for the greater effect of PF administered morphine and rACC administered NMDA on VAD versus VDS thresholds (Borszcz, 1999). Consistent with this interpretation is our recent observation that increases in vocalization thresholds generated by morphine administered into PF is blocked by inactivation of the vPAG (Munn et al., 2009).
It is well established that opiate activation of vPAG engages spinopetal projections that suppress pain transmission at the level of the spinal dorsal horn, and inhibit spinally organized withdrawal reflexes (see review Milan, 2002). If PF administered morphine produces antinociception through activation of rACC projections to vPAG, then it might be expected that noxious evoked withdrawal reflexes would be suppressed. Although we did not observe an increase in SMR threshold following PF administered morphine, Hardy (1985) reported that electrical stimulation of rACC elevated tail flick latencies from a heated water bath. Additionally, electrical stimulation of rACC was shown to inhibit noxious-evoked neural activity in the spinal dorsal horn (Senapati et al., 2005). Our failure to observe elevation of SMR threshold following administration of morphine into PF or NMDA into rACC may reflect insufficient activation of the vPAG. As noted above, we previously demonstrated that a greater level of opiate-mediated activation of vPAG is required to engage spinopetal antinociceptive projections compared to antinociceptive projections that inhibit nociceptive processing at medullary and forebrain levels (Borszcz et al., 1996; Borszcz, 1999; Borszcz and Streltsov, 2000).
In addition to glutamate’s involvement in engaging antinociceptive mechanisms in the rACC that suppress pain affect, it may also contribute to the processing of pain affect by the cACC. Administration of the NMDA receptor antagonist MK-801 into cACC reduced autotomy generated by sciatic nerve denervation (López-Avila et al., 2004), and allodynia generated by sciatic nerve injury is accompanied by elevated efflux of glutamate in cACC (Niikura et al., 2010). These results are consistent with our finding that administration of AP-5 into the cACC produces preferential elevation of VAD threshold similar to that observed in the present study with administration of NMDA into rACC (Greer et al., 2006). Therefore, regional differences may exist within the ACC regarding the involvement of NMDA receptors in antinociceptive versus pronociceptive processes.
The rACC may contribute to an endogenous antinociceptive system that is activated by noxious stimulation and functions to modulate subsequent nociceptive processing. The rACC is highly interconnected with the cACC (Vogt, 2005), and there is evidence that nociceptive input to the cACC from PF is relayed to rACC. Cho et al. (2003) used differential regression analysis coupled with fMRI to investigate the dynamic responses of the medial thalamus and ACC during peripheral painful thermal stimulation in humans. The medial thalamus was activated first, the cACC was activated shortly thereafter, and eventually the rACC was activated. Activation of the rACC through cACC ➔ rACC projections may only occur if noxious input reaches a certain threshold. rACC activation required higher temperatures compared to cACC activation, and only occurred when thermal stimulation produced “stressful pain”. Activation of the rACC by the cACC may reflect engagement of endogenous antinociceptive mechanisms that provide a compensatory action following the detection of a noxious stimulus that elicits stress in the individual. The cACC contributes to processing the affective dimension of nociceptive input and determines the elicited level of stress. When the level of cACC activation reaches a particular threshold (i.e., noxious stimulation becomes stressful) it engages the rACC and its antinociceptive projections.
Recently, reduced activation of the rACC and its related brainstem structures in response to noxious peripheral stimulation was correlated with increased pain perception in fibromyalgia patients (Jensen et al., 2009). Placebo analgesia also engages the rACC-mediated antinociceptive circuit. Human neuroimaging studies revealed that placebo-induced increases opioid neurotransmission in rACC and its interconnected subcortical sites (PAG, nucleus accumbens, amygdala, hypothalamus), were correlated with reductions of pain-elicited activation of the cACC (Petrovic et al., 2002; Eippert et al., 2009; Zubieta and Stohler, 2009).
Unlike the results produced by morphine, threshold elevations produced by PF-carbachol treatment were not blocked by administration of AP-5 or CNQX into the rACC. Therefore, the antinociceptive effects generated by PF-carbachol treatment do not rely on glutamate receptors in rACC. Given that noxious-evoked neural activity in PF is attenuated following the intraventricular administration of acetylcholine (Zhao et al., 1988), it is likely that intra-PF administered carbachol suppresses noxious-evoked activity in PF. Thereby, intra-PF administered carbachol, like intra-PF administered morphine, may suppress transmission of noxious stimulation to cACC and inhibit processing of pain affect and production of VADs. Carbachol may also activate non-nociceptive neurons in PF that project to neurons in vPAG (Sakata et al., 1988) that provide projections to the medulla (Sakata et al., 1989). These descending projections may inhibit processing by medullary neurons that underlie production of VDSs and suppress nociceptive throughput to forebrain sites responsible for production of VADs. As a result, intra-PF carbachol would also elevate VAD threshold greater than VDS threshold, but these increases in thresholds would not involve glutamate activation of rACC.
The present results add to an accumulating literature that supports the existence of mesolimbic circuits that preferentially suppress pain affect by inhibiting nociceptive transmission principally within the brain. We demonstrated that increases in VAD threshold produced by injection of morphine into vPAG relies on the interaction between PF and amygdaloid central nucleus (Borszcz and Streltsov, 2000). The present study demonstrates that the increase in VAD threshold generated by injection of morphine into PF is mediated by glutamatergic activation of rACC neurons that presumably engage antinociceptive projections of the vPAG. Antinociception elicited from other limbic forebrain sites (habenula, amygdala, nucleus accumbens) are also mediated via projections to vPAG (Yu and Han, 1990; Ma et al., 1992a; Pavlovic et al., 1996), and reciprocal interactions between vPAG and forebrain sites are critical for the antinociception elicited from the vPAG (Ma et al., 1992b; Borszcz, 1999; Borszcz and Streltsov, 2000). Further understanding of brain circuits that contribute to suppression of nociceptive processing principally at supraspinal levels will provide insight into mechanisms that suppress affective responding to pain. As the affective response to pain underlies the suffering and disability associated with pain and contributes to development of secondary emotional disturbances (depression, anxiety), an understanding of these brain antinociceptive circuits is of clinical importance and warrants additional study.
Acknowledgements
Grant R01 NS045720 from the National Institute of Neurological Disorders and Stroke supported this research. This research was conducted in partial fulfillment of the requirements for a doctorate of philosophy in Psychology from Wayne State University by S.E.H.
Abbreviations
- PF
(nucleus parafascicularis thalami)
- rACC
(rostral anterior cingulate cortex)
- cACC
(caudal anterior cingulate cortex)
- VAD
(vocalization afterdischarge)
- VDS
(vocalizations during shock)
- SMR
(spinal motor reflex)
- AP-5
(D-2-amino-5-phosphonovalerate)
- CNQX
(6-Cyano-7-nitroquinoxaline-2,3-dione disodium)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballantine HT, Jr, Cassidy WL, Flanagan NB, Marino R., Jr Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26:488–495. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Behbehani M. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Bentley DE, Derbyshire SW, Youell PD, Jones AK. Caudal cingulate cortex involvement in pain processing: an inter-individual laser evoked potential source localisation study using realistic head models. Pain. 2003;102:265–271. doi: 10.1016/S0304-3959(02)00405-0. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. The capacity of motor reflex and vocalization thresholds to support avoidance conditioning in the rat. Behav Neurosci. 1993;107:678–693. doi: 10.1037//0735-7044.107.4.678. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. Increases in vocalization and motor reflex thresholds are influenced by the site of morphine microinjection: Comparisons following administration into the periaqueductal gray, ventral medulla, and spinal subarachnoid space. Behav Neurosci. 1995a;109:502–522. doi: 10.1037//0735-7044.109.3.502. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. Pavlovian conditional vocalizations of the rat: A model system for analyzing the fear of pain. BehavNeurosci. 1995b;109:648–662. doi: 10.1037//0735-7044.109.4.648. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. Differential contributions of medullary, thalamic, and amygdaloid serotonin to the antinociceptive action of morphine administered into the periaqueductal gray: A model of morphine analgesia. Behav Neurosci. 1999;113:612–631. doi: 10.1037//0735-7044.113.3.612. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. Contribution of the ventromedial hypothalamus to generation of the affective dimension of pain. Pain. 2006;123:155–168. doi: 10.1016/j.pain.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borszcz GS, Johnson CP, Anderson ME, Young BJ. Characterization of tailshock elicited withdrawal reflexes in intact and spinal rats. Physiol Behav. 1992;52:1055–1062. doi: 10.1016/0031-9384(92)90459-f. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Johnson CP, Fahey KA. Comparison of motor reflex and vocalization thresholds following systemically administered morphine, fentanyl, and diazepam in the rat: Assessment of sensory and performance variables. Pharmacol Biochem Behav. 1994;49:827–834. doi: 10.1016/0091-3057(94)90230-5. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Johnson CP, Thorp MV. The differential contribution of spinopetal projections to increases in vocalization and motor reflex thresholds generated by the microinjection of morphine into the periaqueductal gray. Behav Neurosci. 1996;110:368–388. doi: 10.1037//0735-7044.110.2.368. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Leaton RN. The effect of amygdala lesions on conditional and unconditional vocalizations in rats. Neurobiol Learn Mem. 2003;79:212–225. doi: 10.1016/s1074-7427(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Streltsov NG. Amygdaloid-thalamic interactions mediate the antinociceptive action of morphine microinjected into the periaqueductal gray. Behav Neurosci. 2000;114:574–584. doi: 10.1037//0735-7044.114.3.574. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Capozzo A, Florio T, Cellini R, Moriconi U, Scarnati E. The pedunculopontine nucleus projection to the parafascicular nucleus of the thalamus: an electrophysiological investigation in the rat. J Neural Transm. 2003;110:733–747. doi: 10.1007/s00702-003-0820-1. [DOI] [PubMed] [Google Scholar]
- Carroll MN, Lim KS. Observations on the neuropharmacology of morphine and morphinelike analgesia. Arch Int Pharmacodyn Ther. 1960;125:383–403. [PubMed] [Google Scholar]
- Casey KL, Keene JJ, Morrow T. Bulboreticular and medial thalamic unit activity in relation to aversive behavior and pain. Adv Neurol. 1974;4:197–205. [Google Scholar]
- Casey KL, Svensson P, Morrow TJ, Raz J, Jone C, Minoshima S. Selective opiate modulation of nociceptive processing in the human brain. J Neurophysiol. 2000;84:525–533. doi: 10.1152/jn.2000.84.1.525. [DOI] [PubMed] [Google Scholar]
- Cho ZH, Son YD, Kang CK, Han JY, Wong EK, Bai SJ. Pain dynamics observed by functional magnetic resonance imaging: Differential regression analysis technique. J Magn Reson Imaging. 2003;18:273–283. doi: 10.1002/jmri.10368. [DOI] [PubMed] [Google Scholar]
- Dafny N, Gildenberg P. Morphine effects on spontaneous, nociceptive, antinociceptive and sensory evoked responses of parafasciculus thalami units in morphine naive and morphine dependent rats. Brain Res. 1984;323:11–20. doi: 10.1016/0006-8993(84)90260-9. [DOI] [PubMed] [Google Scholar]
- Delacour J. Effects of medial thalamic lesions in the rat. A review and an interpretation. Neuropsychologia. 1971;9:157–174. doi: 10.1016/0028-3932(71)90040-6. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Odors released by stressed rats produce opioid analgesia in unstressed rats. BehavNeurosci. 1985;99:589–592. doi: 10.1037//0735-7044.99.3.589. [DOI] [PubMed] [Google Scholar]
- Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Lamy M, Maquet P. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 2000;92:1257–1267. doi: 10.1097/00000542-200005000-00013. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE., Jr Pain "relief" by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Bonnefoi F, Mauguiere F, Laurent B, Sindou M. Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neurosurg. 1997;68:141–148. doi: 10.1159/000099915. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguiere F, Sindou M, Laurent B. Electrical stimulation of motor cortex for pain control: A combined PET-scan and electrophysiological study. Pain. 1999;83:259–273. doi: 10.1016/s0304-3959(99)00114-1. [DOI] [PubMed] [Google Scholar]
- Gracely RH, McGrath P, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1978;5:19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- Greer CA, Wronkowicz R, Harte S, Borszcz G. Program No 141.8 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2006. Regional differences within the anterior cingulate cortex in antinociception elicited by NMDA agonists and antagonists. [Google Scholar]
- Hardy SG. Analgesia elicited by prefrontal stimulation. Brain Res. 1985;339:281–284. doi: 10.1016/0006-8993(85)90093-9. [DOI] [PubMed] [Google Scholar]
- Hardy SG, Haigler HJ. Prefrontal influences upon the midbrain: a possible route for pain modulation. Brain Res. 1985;339:285–293. doi: 10.1016/0006-8993(85)90094-0. [DOI] [PubMed] [Google Scholar]
- Harte SE, Hoot MR, Borszcz GS. Involvement of the intralaminar parafascicular nucleus in muscarinic-induced antinociception in rats. Brain Res. 2004;1019:152–161. doi: 10.1016/j.brainres.2004.05.096. [DOI] [PubMed] [Google Scholar]
- Harte SE, Lagman AL, Borszcz GS. Antinociceptive effects of morphine injected into the nucleus parafascicularis thalami of the rat. Brain Res. 2000;874:78–86. doi: 10.1016/s0006-8993(00)02583-x. [DOI] [PubMed] [Google Scholar]
- Hassenbusch SJ, Pillay PK, Barnett GH. Radiofrequency cingulotomy for intractable cancer pain using stereotaxis guided by magnetic resonance imaging. Neurosurgery. 1990;27:220–223. doi: 10.1097/00006123-199008000-00008. [DOI] [PubMed] [Google Scholar]
- Hoffmeister F. Effects of psychotropic drugs on pain. In: Soulariarc A, et al., editors. Pain. New York: Academic Press; 1968. pp. 309–319. [Google Scholar]
- Hsu MM, Kung JC, Shyu BC. Evoked responses of the anterior cingulate cortex to stimulation of the medial thalamus. Chin J Physiol. 2000;43:81–89. [PubMed] [Google Scholar]
- Hsu MM, Shyu BC. Electrophysiological study of the connection between medial thalamus and anterior cingulate cortex in the rat. Neuroreport. 1997;8:2701–2707. doi: 10.1097/00001756-199708180-00013. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144:95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience. 2005;133:193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Kaelber WW, Mitchell CL. The centrum medianum - central tegmental fasciculus complex. A stimulation, lesion and degeneration study in the cat. Brain. 1967;90:83–100. doi: 10.1093/brain/90.1.83. [DOI] [PubMed] [Google Scholar]
- Kaelber WW, Mitchell CL, Yarmat AJ, Afifi AK, Lorens SA. Centrum medianum-parafascicularis lesions and reactivity to noxious and non-noxious stimuli. Exp Neurol. 1975;46:282–290. doi: 10.1016/0014-4886(75)90135-1. [DOI] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krout KE, Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2000;428:475–494. doi: 10.1002/1096-9861(20001218)428:3<475::aid-cne6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El-Deredy W, Jones AK. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–1354. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. Attenuation of negative pain affect produced by unilateral spinal nerve injury in the rat following anterior cingulate cortex activation. Neuroscience. 2005;136:311–322. doi: 10.1016/j.neuroscience.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lee CM, Chang WC, Chang KB, Shyu BC. Synaptic organization and input-specific short-term plasticity in anterior cingulate cortical neurons with intact thalamic inputs. Eur J Neurosci. 2007;25:2847–2861. doi: 10.1111/j.1460-9568.2007.05485.x. [DOI] [PubMed] [Google Scholar]
- Liauw J, Wang GD, Zhuo M. NMDA receptors contribute to synaptic transmission in anterior cingulate cortex of adult mice. Sheng Li Xue Bao. 2003;55:373–380. [PubMed] [Google Scholar]
- Lopez-Avila A, Coffeen U, Ortega-Legaspi JM, del Angel R, Pellicer F. Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex. Pain. 2004;111:136–143. doi: 10.1016/j.pain.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Ma Q-P, Shi Y-S, Han J-S. Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res. 1992a;583:292–295. doi: 10.1016/s0006-8993(10)80036-8. [DOI] [PubMed] [Google Scholar]
- Ma QP, Shi YS, Han JS. Naloxone blocks opioid peptide release in nucleus accumbens and amygdala elicited by morphine injected into periaqueductal gray. Brain Res Bull. 1992b;28:351–354. doi: 10.1016/0361-9230(92)90202-9. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Marini G, Pianca L, Tredici G. Thalamocortical projection from the parafascicular nucleus to layer V pyramidal cells in frontal and cingulate areas of the rat. Neurosci Lett. 1996;203:81–84. doi: 10.1016/0304-3940(95)12266-4. [DOI] [PubMed] [Google Scholar]
- Mark VH, Ervin FR, Yakovlev PI. Correlation of pain relief, sensory loss, and anatomical lesion sites in pain patients treated with stereotactic thalamotomy. Trans Am Neurol Assoc. 1961;86:86–90. [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mitchell CL, Kaelber WW. Unilateral vs. bilateral medial thalamic lesions and reactivity to noxious stimuli. Arch Neurol. 1967;17:653–660. doi: 10.1001/archneur.1967.00470300095016. [DOI] [PubMed] [Google Scholar]
- Munn EM, Harte SE, Lagman A, Borszcz GS. Contribution of the periaqueductal gray to the suppression of pain affect produced by administration of morphine into the intralaminar thalamus of rat. J Pain. 2009;10:426–435. doi: 10.1016/j.jpain.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandigama P, Borszcz GS. Affective analgesia following the administration of morphine into the amygdala of rats. Brain Res. 2003;959:343–354. doi: 10.1016/s0006-8993(02)03884-2. [DOI] [PubMed] [Google Scholar]
- Niikura K, Furuya M, Narita M, Torigoe K, Kobayashi Y, Takemura Y, Yamazaki M, Horiuchi H, Enomoto T, Iseki M, Kinoshita H, Tomiyasu S, Imai S, Kuzumaki N, Suzuki T. Enhancement of glutamatergic transmission in the cingulate cortex in response to mild noxious stimuli under a neuropathic pain-like state. Synapse. Epub ahead of print. 2010 doi: 10.1002/syn.20859. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: An immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30:65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- Pavlovic ZW, Cooper ML, Bodnar RJ. Opioid antagonists in the periaqueductal gray inhibit morphine and beta- endorphin analgesia elicited from the amygdala of rats. Brain Res. 1996;741:13–26. doi: 10.1016/s0006-8993(96)00880-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam: Academic Press; 1998. [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M, Lui F, Giuliani D, Pellegrini M, Baraldi P. Independent time courses of supraspinal nociceptive activity and spinally mediated behavior during tonic pain. Pain. 2003;104:291–301. doi: 10.1016/s0304-3959(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- Prieto-Gomez B, Dafny N, Reyes-Vazquez C. Dorsal raphe stimulation, 5-HT and morphine microiontophoresis effects on noxious and nonnoxious identified neurons in the medial thalamus of the rat. Brain Res Bull. 1989;22:937–943. doi: 10.1016/0361-9230(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Roberts VJ. NGC-evoked nociceptive behaviors: II. Effect of midbrain and thalamus lesions. Physiol Behav. 1991;51:73–80. doi: 10.1016/0031-9384(92)90205-g. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JP, Holzman BS. Effects of morphine on medial thalamic and medial bulboreticular aversive stimulation thresholds. Brain Res. 1978;150:436–440. doi: 10.1016/0006-8993(78)90297-4. [DOI] [PubMed] [Google Scholar]
- Saade NE, Al Amin H, Abdel Baki S, Chalouhi S, Jabbur SJ, Atweh SF. Reversible attenuation of neuropathic-like manifestations in rats by lesions or local blocks of the intralaminar or the medial thalamic nuclei. Exp Neurol. 2007;204:205–219. doi: 10.1016/j.expneurol.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Sah P, Nicoll RA. Mechanisms underlying potentiation of synaptic transmission in rat anterior cingulate cortex in vitro. J Physiol. 1991;433:615–630. doi: 10.1113/jphysiol.1991.sp018446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Shima F, Kato M, Fukui M. Effects of thalamic parafascicular stimulation on the periaqueductal gray and adjacent reticular formation neurons: A possible contribution to pain control mechanisms. Brain Res. 1988;451:85–96. doi: 10.1016/0006-8993(88)90752-4. [DOI] [PubMed] [Google Scholar]
- Sakata S, Shima F, Kato M, Fukui M. Dissociated mesencephalic responses to medial and ventral thalamic nuclei stimulation in rats. Relationship to analgesic mechanisms. J Neurosurg. 1989;70:446–453. doi: 10.3171/jns.1989.70.3.0446. [DOI] [PubMed] [Google Scholar]
- Senapati AK, Lagraize SC, Huntington PJ, Wilson HD, Fuchs PN, Peng YB. Electrical stimulation of the anterior cingulate cortex reduces responses of rat dorsal horn neurons to mechanical stimuli. J Neurophysiol. 2005;94:845–851. doi: 10.1152/jn.00040.2005. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1993;68:1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Jr, Horvath E, Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res. 1986;380:59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- Stevens RT, Hodge CJ, Jr, Apkarian AV. Medial, intralaminar, and lateral terminations of lumbar spinothalamic tract neurons: a fluorescent double-label study. Somatosens Mot Res. 1989;6:285–308. doi: 10.3109/08990228909144678. [DOI] [PubMed] [Google Scholar]
- Sweet WH. Central mechanisms of chronic pain (neuralgias and certain other neurogenic pain) In: Bonica JJ, editor. Pain. New York: Raven Press; 1980. pp. 287–303. [PubMed] [Google Scholar]
- Thoden U, Doerr M, Dieckmann G, Krainick JU. Medial thalamic permanent electrodes for pain control in man: An electrophysiological and clinical study. Electroencephalogr Clin Neurophysiol. 1979;47:582–591. doi: 10.1016/0013-4694(79)90259-1. [DOI] [PubMed] [Google Scholar]
- Unnerstall JR, Wamsley JK. Autoradiographic localization of high-affinity [3H]kainic acid binding sites in the rat forebrain. Eur J Pharmacol. 1983;86:361–371. doi: 10.1016/0014-2999(83)90185-1. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Velasco M, Brito F, Jimenez F, Gallegos M, Velasco AL, Velasco F. Effect of fentanyl and naloxone on a thalamic induced painful response in intractable epileptic patients. Stereotact Funct Neurosurg. 1998;71:90–102. doi: 10.1159/000029653. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Marini G, Tredici G. Anatomical organization of the telencephalic connections of the parafascicular nucleus in adult and developing rats. Eur J Neurosci. 2003;18:275–289. doi: 10.1046/j.1460-9568.2003.02743.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB. Cingulate cortex and disease models. In: Paxinos G, editor. The Rat Nervous System. San Diego: Elsevier Academic Press; 2004. pp. 705–727. [Google Scholar]
- Wang HC, Chai SC, Wu YS, Wang CC. Does the medial thalamus play a role in the negative affective component of visceral pain in rats? Neurosci Lett. 2007;420:80–84. doi: 10.1016/j.neulet.2007.04.064. [DOI] [PubMed] [Google Scholar]
- Weigel R, Krauss JK. Center median-parafascicular complex and pain control. Review from a neurosurgical perspective. Stereotact Funct Neurosurg. 2004;82:115–126. doi: 10.1159/000079843. [DOI] [PubMed] [Google Scholar]
- Whittle IR, Jenkinson JL. CT-guided stereotactic antero-medial pulvinotomy and centromedian-parafascicular thalamotomy for intractable malignant pain. Br J Neurosurg. 1995;9:195–200. doi: 10.1080/02688699550041548. [DOI] [PubMed] [Google Scholar]
- Willoch F, Gamringer U, Medele R, Steude U, Tolle TR. Analgesia by electrostimulation of the trigeminal ganglion in patients with trigeminopathic pain: a PET activation study. Pain. 2003;103:119–130. doi: 10.1016/s0304-3959(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Wilson HD, Uhelski ML, Fuchs PN. Examining the role of the medial thalamus in modulating the affective dimension of pain. Brain Res. 2008;1229:90–99. doi: 10.1016/j.brainres.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: Observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Yang JW, Shih HC, Shyu BC. Intracortical circuits in rat anterior cingulate cortex are activated by nociceptive inputs mediated by medial thalamus. J Neurophysiol. 2006;96:3409–3422. doi: 10.1152/jn.00623.2006. [DOI] [PubMed] [Google Scholar]
- Yen CP, Kung SS, Su YF, Lin WC, Howng SL, Kwan AL. Stereotactic bilateral anterior cingulotomy for intractable pain. J Clin Neurosci. 2005;12:886–890. doi: 10.1016/j.jocn.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Yen CT, Fu TC, Chen RC. Distribution of thalamic nociceptive neurons activated from the tail of the rat. Brain Res. 1989;498:118–122. doi: 10.1016/0006-8993(89)90405-8. [DOI] [PubMed] [Google Scholar]
- Yu L-C, Han J-S. Habenula as a relay in the descending pathway from nucleus accumbens to periaqueductal grey subserving antinociception. Intern J Neurosci. 1990;54:245–251. doi: 10.3109/00207459008986640. [DOI] [PubMed] [Google Scholar]
- Zhao DC, Xu T, Sun MZ. The effects of acetylcholine on the electric activities of pain reaction neurons in nucleus parafascicularis of thalamus and midbrain reticular formation in rats. Sheng Li Xue Bao. 1988;40:326–334. [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The rat nervous system. vol. 2. San Diego: Academic Press; 1995. pp. 649–685. [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]