Summary
Although clinical observations suggest that humans with amygdala damage have abnormal fear reactions and a reduced experience of fear [1–3], these impressions have not been systematically investigated. To address this gap, we conducted a new study in a rare human patient, SM, who has focal bilateral amygdala lesions [4]. To provoke fear in SM, we exposed her to live snakes and spiders, took her on a tour of a haunted house, and showed her emotionally evocative films. On no occasion did SM exhibit fear and she never endorsed feeling more than minimal levels of fear. Likewise, across a large battery of self-report questionnaires, three months of real-life experience sampling, and a life history replete with traumatic events, SM repeatedly demonstrated an absence of overt fear manifestations and an overall impoverished experience of fear. Despite her lack of fear, SM is able to exhibit other basic emotions and experience the respective feelings. The findings support the conclusion that the human amygdala plays a pivotal role in triggering a state of fear, and that the absence of such a state precludes the experience of fear itself.
Results and Discussion
The amygdala is involved in multiple aspects of fear processing, ranging from fear conditioning [5,6] to the modulation of attention and memory for fear-related stimuli [7–9] all the way to fear recognition [10] and the induction of fear-related behaviors [11–20]. Much less is known about the amygdala’s role in the conscious experience of fear, in large part because non-human animals with amygdala lesions are unable to verbally report on their internal subjective experience and humans with focal bilateral amygdala damage are extremely difficult to find. An exception is patient SM, a 44-year-old woman who is one of the best characterized human cases with bilateral amygdala damage [10] (see Figure S1 for a description of SM’s brain damage). SM’s neuropsychological profile has been stable for the past two decades. She performs within the normal range on standardized tests of IQ, memory, language and perception [10], yet is severely impaired in fear conditioning [21], in recognizing fear in facial expressions [4,10,22], and in aspects of social behavior thought to be mediated by emotions related to fear [23–25].
Importantly, none of the previous studies specifically assessed the induction and experience of fear in patient SM, and it is these two aspects of fear which form the basis for the current report. We define fear induction as the exposure to stimuli capable of triggering a state of fear. The success of an induction was measured by the presence of overt behavioral manifestations of fear1, especially signs of avoidance behavior or withdrawal in response to fear-provoking stimuli. Fear experience, on the other hand, is the subjective feeling of fear, and was measured by SM’s self-report of her internal experience. We predicted that without the amygdala, the action sequence that constitutes a state of fear would fail to be induced in SM, thereby preempting her experience of fear.
Fear induction
When exposed to dangerous stimuli, such as potential predators, animals with amygdala lesions typically display a lack of the behaviors normally associated with the action program of fear [11–20]. We used a comparable approach in SM by directly confronting her with fear-inducing stimuli and observing her behavior while also querying her subjective state. For ethical reasons, we chose three situations capable of inducing fear with little to no risk of direct harm to the subject: (1) visiting an exotic pet store with snakes and spiders, (2) walking through a haunted house, and (3) watching film clips of scary movies. SM provided her informed written consent to participate.
The first fear-inducing situation entailed direct exposure to snakes and spiders, two of the most commonly feared species in the animal kingdom. Interestingly, for many years, SM has repeatedly told us that she “hates” snakes and spiders and “tries to avoid them.” To test her real-life behavior, we took her to an exotic pet store and focused on probing for external manifestations of fear with a particular eye toward any signs of avoidance behavior. Upon entering the store, SM was spontaneously drawn to the snake terrariums and appeared visually captivated by the large collection of snakes. A store employee asked SM if she would like to hold a snake and she agreed (Figure 1A). SM held the snake for over three minutes while displaying a wide range of exploratory behaviors: she rubbed its leathery scales, touched its flicking tongue, and closely watched its movements as it slithered through her hands. Her verbal behavior revealed a comparable degree of fascination and inquisitiveness: she repeatedly commented, “This is so cool!” and asked the store employee numerous questions (e.g., “When they look at you, what do they see?”). During this time period, we asked SM to rate her fearfulness on a scale from 0 (no fear at all) to 10 (extreme fear). Her reported experience of fear never surpassed a rating of 2. Moreover, SM displayed a compulsive desire to want to “touch” and “poke” the store’s larger and more dangerous snakes, even though the store employee repeatedly told her that these snakes were not safe and could bite. In total, SM asked 15 different times if she could touch one of the larger snakes. She also attempted to touch a tarantula (Figure 1B), but had to be stopped due to the high risk of being bitten. When asked why she would want to touch something that she knows is dangerous and claims to hate, SM replied that she was overcome with “curiosity.” The disconnection between SM’s verbally stated aversion to snakes and spiders and her actual real-life behavior was striking. She did not display any signs of avoidance, but instead, exhibited an excessive degree of approach (a pattern highly reminiscent of the behavior in monkeys with Kluver-Bucy syndrome [12]). We note that SM’s behavior was not merely the result of her feeling comfortable in the relatively safe environment of the pet store since we later discovered that, in the past, SM encountered a large snake outdoors and behaved in a similar manner (see supplemental information).
Figure 1. Fear induction in patient SMM.

Still-frame pictures of (A) SM handling a snake, (B) the tarantula that SM tried to touch, and (C) the Waverly Hills Sanatorium Haunted House.
The second fear-inducing situation attempted to scare SM in a setting professionally designed for such a purpose. During Halloween, we took SM to the Waverly Hills Sanatorium (Figure 1C), ranked as one of the “most haunted” places in the world [26]. On an annual basis, the sanatorium hosts a haunted house, elaborately decorating the inside with eerie scenes, airing scary music and loud noises, and featuring people dressed as monsters, murderers, and ghosts. Upon arrival, SM and the research team were paired with a group of five women (all of whom were strangers). From the outset, SM voluntarily led the entire group through the haunted house, showing no signs of hesitation while walking around corners or into dark hallways. As the other members of the group lagged behind her, she would repeatedly call out, “This way guys, follow me!” The hidden monsters attempted to scare SM numerous times, but to no avail. She reacted to the monsters by smiling, laughing, or trying to talk to them. In contrast, their scare tactics typically elicited loud screams of fright from the other members of the group. More than showing a lack of fear, SM exhibited an unusual inclination to approach and touch the monsters. Ironically, SM scared one of the monsters when she poked it in the head because she was “curious” as to what it would feel like. Before, during, and after the haunted house, SM was queried about her current level of fear. She never reported experiencing any elevations in fear, her fear ratings being zero throughout. She did, however, report feeling a high level of excitement and enthusiasm. When asked to elaborate, she said her excitement was similar to the feeling she gets while riding on a rollercoaster, an activity which she claims to enjoy. In sum, SM was highly aroused by the haunted house, but did not feel any sense of fear, showed no signs of nervousness or apprehension while walking through dark passageways, and was never visibly frightened by any of the numerous attempts to scare her.
Lastly, we used a film induction procedure, widely considered one of the most effective and reliable ways to induce emotions in a laboratory setting [27,28]. SM viewed a set of 10 different fear-inducing film clips (Table S2). Interspersed between the fear clips were films aimed at inducing other types of emotion, including disgust, anger, sadness, happiness, and surprise. During the non-fear-related films, SM exhibited behaviors compatible with those emotions (e.g., laughter during happiness, shouts of revulsion during disgust) and reported experiencing high levels of the appropriate emotion (Figure S2). By contrast, SM exhibited no fear responses and reported experiencing little to no fear across the entire battery of fear-inducing films (Figure 2). Nonetheless, she found the fear films to be exciting and entertaining, and in one case, she inquired about the name of the movie so she could rent it from the video store later that day. Of note, SM commented that most people would likely feel scared by the content of the films, even though she did not; this provides evidence that her impoverished experience of fear can not be fully accounted for by a fear recognition deficit or a failure to understand the concept of fear (see supplemental information).
Figure 2. Fear induced by film clips.
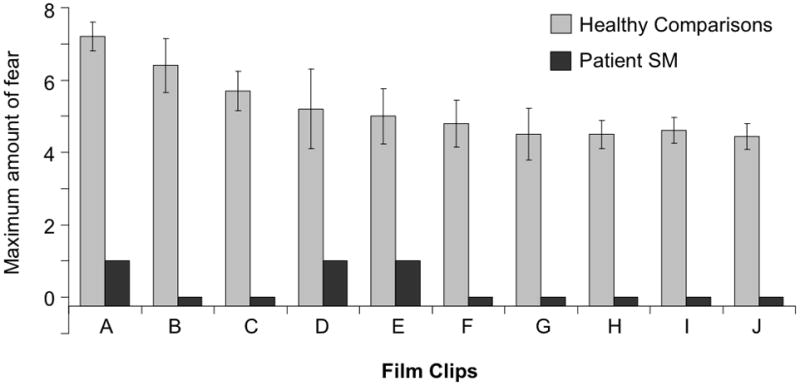
Subjective ratings for the maximum amount of fear induced while watching a series of 10 different scary film clips. Ratings were provided immediately after viewing each individual film clip using a modified visual analogue scale ranging from 0 (no fear) to 8 (extreme fear). Comparison data for films A–G were obtained from five females with no history of neurological or psychiatric illness. Comparison data for films H–J were derived from previous studies that tested large samples of healthy participants [28,41]. Descriptions of all film clips can be found in Table S2. Data for films inducing other emotions can be found in Figure S2. Error bars represent the standard error of the mean.
Fear experience
We assessed SM’s general experience of fear using eight well-validated self-report questionnaires that cover topics ranging from phobias and panic symptoms all the way to fear in relation to specific situations such as public speaking or dying (Table S1). SM completed the questionnaires multiple times over the course of three years. A previous study [29] that used one of these questionnaires suggested that amygdala damage does not impair fear experience; however, the patients in this sample all had incomplete (and mostly unilateral) amygdala lesions. Despite the fact that most of these questionnaires were created for detecting abnormally high, rather than low, levels of fear, SM consistently scored near the floor level and well below the normative mean on all occasions (Figure 3 and Table S1). Together with our other data, these findings from self-report questionnaires corroborate a profound and reliable reduction in SM’s experience of fear.
Figure 3. Fear experience in patient SM.
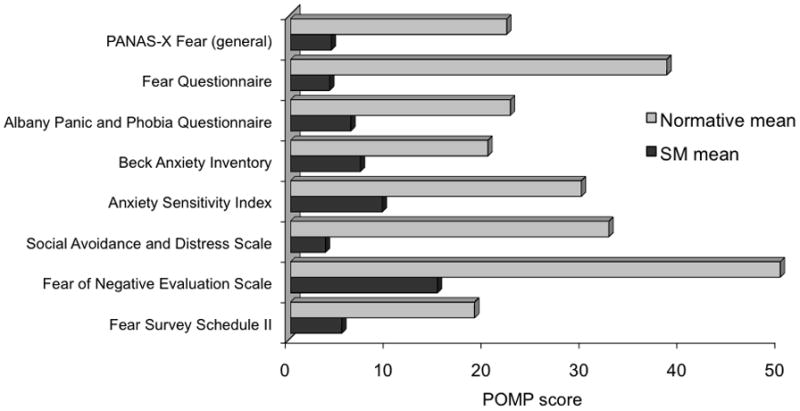
Self-report questionnaires comparing patient SM’s experience of fear to normative samples comprised of healthy individuals. All scores have been converted to POMP units [42] representing the “percent of maximum possible” for each questionnaire. Raw scores and additional information about the questionnaires can be found in Table S1. Data from the experience-sampling study can be found in Figure S3.
To further investigate SM’s emotional experiences in everyday life, we used the experience-sampling method, which captures emotional experiences in real-time as they unfold in the subject’s natural environment [30,31]. SM was provided with a hand-held computerized emotion diary that prompted her at three random times each day to rate her current emotional state using a set of 50 randomly presented emotion terms. The emotion terms covered a broad range of both positive and negative affect, and ratings were provided on a 5-point scale. Both the emotion terms and rating scale were derived from the Positive and Negative Affect Schedule – Expanded Form (PANAS-X) [32]. Across 156 sampling time points collected over a 3-month period, there were only 6 items (out of the 50) which SM consistently rated feeling at the lowest possible level: afraid, nervous, scared, guilty, ashamed, and fearful. Likewise, her average PANAS-X fear composite score was at the floor level (mean score = 0% of maximum possible) (Table S1). For all basic emotions other than fear, SM reported numerous instances of experiencing the emotion, with intensity levels varying from “a little” to “quite a bit” (Figure S3). Interestingly, out of the 50 different emotion terms, the item that received the highest average rating over the entire three month period was “fearless” (mean score = 45% of maximum possible). While we did not collect comparable experience-sampling data from healthy individuals, precluding quantitative statements about SM’s abnormality, the striking pattern observed and its consistency with the other questionnaires provides strong evidence that SM fails to experience fear, even though she can experience other emotions.
Fear in SM’s past
In modern day developed societies, fear-provoking situations are not commonly encountered [33]. To assess the possibility that SM’s lack of fear can be attributed to a lack of fear-provoking encounters, we queried her about past life experiences (including experiences during childhood; see supplemental information). As it turned out, SM has encountered numerous events that would be considered fear-inducing or even traumatic in nature. For instance, she has been held up at knife-point and at gun-point, she was once physically accosted by a woman twice her size, she was nearly killed in an act of domestic violence, and on more than one occasion she has been explicitly threatened with death (see supplemental information for a detailed account of one of these events). What stands out most is that in many of these situations SM’s life was in danger, yet her behavior lacked any sense of desperation or urgency. Police reports obtained from the local police department further corroborate SM’s recollection of these events and paint a picture of an individual who lives in a poverty-stricken area replete with crime, drugs, and danger. Of note, SM has never been convicted of any crime, but rather, has been the victim of numerous crimes. Moreover, it is evident that SM has great difficulty detecting looming threats in her environment and learning to avoid dangerous situations – features of her behavior that have in all likelihood contributed to her high incidence of life-threatening encounters.
When asked to recollect how she felt during the aforementioned situations, SM denied feeling fear, but did report feeling upset and angry about what had happened. Without fear, it can be said that SM’s distress lacks the deep heartfelt intensity endured by most survivors of trauma. Such an interpretation is consistent with a previous study [34], where two experienced clinical psychologists interviewed SM without having any knowledge of her condition. To the psychologists, SM came across as a “survivor”, as being “resilient” and even “heroic” in the way that she had dealt with adversity in her life. Taken together, this evidence illuminates the possibility that because of her amygdala damage, SM is immune to the devastating effects of Posttraumatic Stress Disorder (PTSD) – an intriguing hypothesis that has recently found support in war veterans with amygdala lesions [35].
Conclusions
Taken together, the findings from this study indicate that patient SM, a woman with focal bilateral amygdala lesions, has a profound and pervasive impairment in the induction and experience of fear across a wide range of situations and measures. By contrast, SM appears entirely capable of triggering and feeling emotions other than fear (see Figures S2 and S3). Her inability to generate fear across the range of situations probed in this study supports the conclusion that the amygdala is a critical brain region for triggering a state of fear when an individual encounters threatening stimuli in the external environment. There is no reason to expect that fear, or even panic, induced by internal stimuli (e.g., the interoceptively conveyed pain caused by myocardial infarction) would be mediated by the amygdala. On the contrary, structures in the brainstem would likely be the direct trigger region for interoceptive fear-inducing stimuli, a prediction that our group is in the process of investigating and for which there is some factual support [36]. Such a conclusion is consistent with what is known about the functional neuroanatomy of the amygdala. Sensory and association cortices required for representing external stimuli are intact in SM’s brain, as is the brainstem and hypothalamic circuitry necessary for orchestrating the action program of fear. SM’s amygdala lesions in effect disconnect these two components, making it improbable, if not impossible, for sensory representations to trigger fear responses. Our framework for thinking about emotion and feeling argues that many different cognitive, autonomic, and behavioral changes comprise a state of fear, and the induction of such a state is required in order to experience a feeling of fear. In short, we view SM’s lack of experienced fear as a direct consequence of her failure to mount a normal fear response (see supplemental information for additional explanation).
Interestingly, SM’s reaction to fear-inducing stimuli was not characterized by a loss of responsiveness, but rather manifested as a heightened arousal and interest in the face of a near-complete lack of avoidance and caution. Moreover, SM’s lack of avoidance was often accompanied by an excess of exploratory approach behavior which she verbally described as an overwhelming feeling of “curiosity.” This striking pattern of behavior is consistent with reports in amygdala-lesioned monkeys [20], but not easily reconciled with emerging accounts of the amygdala as critical in detecting the saliency of stimuli. At a minimum, our findings argue that fear-inducing stimuli are still capable of eliciting changes in attention and arousal through structures other than the amygdala [37].
Finally, our findings suggest that the amygdala’s role in the induction and experience of emotion is specific to fear [38]. To say that SM is emotionless or unable to feel emotion is simply false. Her emotional deficit is primarily circumscribed to the behaviors and experiences that characterize a state of fear. While this study has several limitations inherent to any case study (see supplemental information), the results are remarkably consistent with previous work in non-human animals [11–20], as well as with other case reports documenting diminished fear in humans with amygdala damage [1–3]. The unique case of patient SM provides a rare glimpse into the adverse consequences of living life without the amygdala. For SM, the consequences have been severe. Her behavior, time and time again, leads her back to the very situations she should be avoiding, highlighting the indispensable role that the amygdala plays in promoting survival by compelling the organism away from danger [39,40]. Indeed, it appears that without the amygdala, the evolutionary value of fear is lost.
Supplementary Material
Acknowledgments
We are greatly indebted to SM for her continued commitment to brain research. We would also like to thank Christopher Kovach for his invaluable help during some of the testing and Joel Bruss for his assistance with SM’s brain scans. This research was supported by grants from the National Institutes of Health (NINDS P50 NS19632, NIDA R01 DA022549, and NIMH R01 MH080721), and a National Science Foundation Graduate Research Fellowship.
Footnotes
Since much of the testing occurred in real-world settings, we did not have the opportunity to collect complementary psychophysiological data. However, we note that previous studies [21,43] have shown impairments in SM’s conditioned skin conductance response and startle reflex.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sprengelmeyer R, Young AW, Schroeder U, Grossenbacher PG, Federlein J, Buttner T, Przuntek H. Knowing no fear. Proc R Soc Lond B. 1999;266:2451–2456. doi: 10.1098/rspb.1999.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurlemann R, Schlaepfer TE, Matusch A, Reich H, Shah NJ, Zilles K, Maier W, Bauer A. Reduced 5-HT2A receptor signaling following selective bilateral amygdala damage. SCAN. 2009;4:79–84. doi: 10.1093/scan/nsn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac CL, Mayes AR, Hodges JR, Montaldi D, Cezayirli E. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia. 1998;36:59–70. doi: 10.1016/s0028-3932(97)00105-x. [DOI] [PubMed] [Google Scholar]
- 4.Adolphs R, Tranel D, Damasio H, Damasio AR. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 5.LeDoux J. The amygdala. Current Biology. 2007;17:868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 7.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 8.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 9.Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- 10.Adolphs R, Tranel D. Emotion recognition and the human amygdala. In: Aggleton JP, editor. The amygdala: A functional analysis. New York: Oxford University Press; 2000. pp. 587–630. [Google Scholar]
- 11.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 12.Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 13.Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 14.Aggleton JP, Passingham R. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) J Comp Physiol Psychol. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- 15.Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 16.Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- 17.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 22.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy DP, Gläscher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nat Neurosci. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. PNAS. 2010;107:3788–3792. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waverly Hills Sanatorium. Wikipedia, The Free Encyclopedia. 2009 Retrieved from http://en.wikipedia.org/w/index.php?title=Waverly_Hills_Sanatorium&oldid=324971912.
- 27.Gerrards-Hesse A, Spies K, Hesse FW. Experimental inductions of emotional states and their effectiveness: A review. Br J Psychol. 1994;85:55–78. [Google Scholar]
- 28.Rottenberg J, Ray RR, Gross JJ. Emotion elicitation using films. In: Coan JA, Allen JJB, editors. Handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007. pp. 9–28. [Google Scholar]
- 29.Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci. 2002;14:709–720. doi: 10.1162/08989290260138618. [DOI] [PubMed] [Google Scholar]
- 30.Barrett DJ, Barrett LF. The Experience-Sampling Program (ESP) 2000. [Google Scholar]
- 31.Christensen TC, Barrett LF, Bliss-Moreau E, Lebo K, Kaschub C. A practical guide to experience-sampling procedures. Journal of Happiness Studies. 2003;4:53–78. [Google Scholar]
- 32.Watson D, Clark LA. University of Iowa; 1994. The PANAS-X: Manual for the positive and negative affect schedule-expanded form http://www.psychology.uiowa.edu/Faculty/Watson/PANAS-X.pdf) [Google Scholar]
- 33.Watson D. Mood and Temperament. New York: The Guilford Press; 2000. [Google Scholar]
- 34.Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cognitive Neuropsychiatry. 2006;11:219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- 35.Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nature Neuroscience. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiest G, Lehner-Baumgartner E, Baumgartner C. Panic attacks in an individual with bilateral selective lesions of the amygdala. Arch Neurol. 2006;63:1798–1801. doi: 10.1001/archneur.63.12.1798. [DOI] [PubMed] [Google Scholar]
- 37.Tranel D, Damasio H. Intact electrodermal skin conductance responses after bilateral amygdala damage. Neuropsychologia. 1989;27:381–390. doi: 10.1016/0028-3932(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 38.Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nature Reviews Neuroscience. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 39.Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 40.Dicks D, Myers RE, Kling A. Uncus and amygdala lesions: Effects on social behavior in the free-ranging rhesus monkey. Science. 1969;165:69–71. [PubMed] [Google Scholar]
- 41.Hewig J, Hagemann D, Seifert J, Gollwitzer M, Naumann E, Bartussek D. A revised film set for the induction of basic emotions. Cognition and Emotion. 2005;19:1095–1109. [Google Scholar]
- 42.Cohen P, Cohen J, Aiken LS, West SG. The problem of units and the circumstance for POMP. Multivar Behav Res. 1999;34:315–346. [Google Scholar]
- 43.Buchanan TW, Tranel D, Adolphs R. Anteromedial temporal lobe damage blocks startle modulation by fear and disgust. Behav Neurosci. 2004;118:429–437. doi: 10.1037/0735-7044.118.2.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


