Abstract
Mechanisms for meconium-induced inactivation of pulmonary surfactant as part of the meconium aspiration syndrome in newborn infants, to our knowledge, are not clearly understood. Here we have studied the biophysical mechanisms of how meconium affects surface activity of pulmonary surfactant and whether the membrane-perturbing effects of meconium can be mimicked by exposure of surfactant to a mixture of bile acids and cholesterol. Surface activity of pulmonary surfactant complexes purified from animal lungs was analyzed in the absence and in the presence of meconium in standard surface balances and in a captive bubble surfactometer. We have also evaluated accumulation of surfactant at the air-liquid interface by what we believe to be a novel microtiter plate fluorescent assay, and the effect of meconium components on surfactant membrane fluidity using Laurdan fluorescence thermotropic profiles and differential scanning calorimetry thermograms. Rapid interfacial adsorption, low surface tension upon film compression, efficient film replenishment upon expansion, and thermotropic properties of surfactant complexes are all adversely affected by meconium, and, in a similar manner, they are affected by cholesterol/taurocholate mixtures but not by taurocholate alone. We conclude that inhibition of surfactant by meconium can be mimicked by a bile salt-promoted incorporation of excess cholesterol into surfactant complexes. These results highlight the potential pathogenic role of cholesterol-mobilizing agents as a crucial factor resulting in cholesterol induced alterations of structure and dynamics of surfactant membranes and films.
Introduction
Pulmonary surfactant is a complex mixture of lipids and proteins lining the alveolar air-water interface. By lowering the surface tension, pulmonary surfactant stabilizes the respiratory surfaces against physical forces tending to collapse the air spaces (1,2). Lack or alteration of surfactant is associated with severe respiratory pathologies in neonates and adults (1–3). Premature newborn infants suffering from respiratory distress syndrome now receive treatment with clinical surfactants as the standard of care.
Meconium aspiration syndrome (MAS) remains an important cause of morbidity and mortality affecting >20,000 neonates per year in United States. MAS is characterized by airway obstruction, pneumonitis, pulmonary hypertension, ventilation/perfusion mismatch, acidosis, and hypoxemia (2). There is also underlying surfactant inactivation but the mechanisms are still not well understood. In controlled trials, some benefit has been shown with surfactant treatment, but the effect is less dramatic than the use of surfactant for premature infants with respiratory distress syndrome (4).
Meconium is a complex mixture that accumulates in the fetal gut during gestation. It contains proteins, sterols, bile salts, and inorganic molecules. The main sterol is cholesterol and bile salts include cholic acid, taurocholic acid, and glycocholic acid (5–7). It is normally excreted as the first stool of a newborn baby during the first hours of life. In ∼1–2% of deliveries, the fetus has aspirated meconium-laden amniotic fluid, and as a newborn, manifests meconium aspiration pneumonia, one aspect of which is inactivation of alveolar surfactant. Presumably better understanding of the nature of surfactant inactivation may lead to better treatments of this condition.
At least two different mechanisms have been proposed for surfactant inactivation after lung injuries causing respiratory distress (8,9). A first model involves blood proteins, inflammation proteins and other surface-active substances, which compete with surfactant complexes for reaching the interface (10). A second model hypothesizes that surfactant dysfunction results from the intrinsic impairment of surfactant complexes by small amphiphilic molecules such as free fatty acids, cholesterol, lysolipids, bile acids, and/or diacylglycerol. These substances, in part coming from the degradation of surfactant itself by inflammatory phospholipases, insert into the surfactant complexes rendering it dysfunctional (11).
In this article, we have characterized the inhibition of pulmonary surfactant by meconium using different biophysical techniques. We propose that inhibition of surfactant is produced by transfer of some components like cholesterol from meconium into surfactant complexes, facilitated by bile salts. To confirm this inhibitory mechanism, we have compared the effect of the exposure of surfactant to meconium with the effects of its exposure to a mixture of bile salts and cholesterol.
Materials and Methods
Materials
Native porcine lung surfactant was purified from bronchoalveolar lavage as previously described (12). Isolated surfactant was used without further organic extraction, in aqueous solution, and contains full complement of proteins SP-A, SP-B, and SP-C. It also maintains most of its original structure as routinely checked by electron microscopy (13). Surfactant concentration was measured by analysis of lipid phosphorus (14). Dilutions of the material to the required phospholipid concentration were made with 5 mM Tris buffer, pH 7, containing 150 mM NaCl. Incubation of native surfactant and meconium at the desired concentration was carried out before testing.
The organic extract of meconium was obtained following the standard Bligh & Dyer method. First-passed meconium from term infants was collected and lyophilized as a pool. Dry weight of meconium was used in mixtures with surfactant. BODIPY-PC or Laurdan (Invitrogen, San Diego, CA) surfactant labeling (1% dye/phospholipid mol/mol) was carried out as in Ravasio et al. (15). Cholesterol and taurocholic acid were obtained from Avanti Lipids (Birmingham, AL) and Sigma (St. Louis, MO), respectively. Complexes of cholesterol and methyl-β-cyclodextrine (MβCD) were obtained commercially as Cholesterol Water Soluble from Sigma.
Methods
Biochemical analysis of meconium
For all the analysis, meconium was prepared at a final concentration of 1 mg/mL. Total protein content was measured by the Lowry method (16) and the total phospholipid content as described by Rouser et al. (14). Cholesterol concentration was estimated using an enzymatic colorimetric method commercially provided by Spinreact (Girona, Spain) and total bile acids by an enzymatic colorimetric kit provided by Materlab (Madrid, Spain).
Surface balance measurements
Π-t isotherms
Surfactant adsorption was measured over time in a Teflon cup (Nima Technology, Coventry, UK) with 1.5 mL of stirred subphase at 25°C, as described (17). One-hundred-fifty micrograms of surfactant were injected into the subphase, that in some experiments contained meconium, and pressure-time kinetics were followed over 5 min.
Π-t spreading kinetics
Adsorption of native or meconium-pretreated surfactant was also assessed after direct spreading at the interface of a 22.5 cm2 trough (Nima Technology) containing 14 mL of subphase.
Π-area isotherms
Native or meconium pretreated surfactant was spread onto a buffered (5 mM Tris pH 7, 150 mM NaCl) subphase in a surface balance equipped with a continuous Teflon ribbon barrier and a 200 cm2 trough (Nima Technology). After 10 min equilibration, the resulting films were compressed at 60 cm2/min. Langmuir-Blodgett films were prepared by transferring films onto glass coverslides using the COVASP method as described elsewhere (18). Epifluorescence microscopy images were obtained in an Axioplan II microscope (Zeiss, Dublin, CA) and analyzed with Image J software.
Captive bubble surfactometer
Surfactant performance at 37°C was evaluated by a modified captive bubble surfactometer as described previously (19,20). The chamber contained 5 mM Tris-HCl pH 7, 150 mM NaCl, 10% sucrose, where a small air bubble (0.035–0.040 cm3) was formed. Then, ∼150 nL of surfactant (25 mg/mL) was deposited at the bubble surface by a transparent capillary. A 5-min adsorption (film formation) period followed the introduction of surfactant into the chamber during which the change in surface tension (γ) was monitored from changes in the shape of the bubble (21). The chamber was then sealed and the bubble was rapidly (1 s) expanded to 0.15 cm3, to record postexpansion adsorption. Five minutes after expansion, quasistatic cycles started. The bubble size was first reduced and then enlarged in a stepwise fashion. There was 1-min intercycle delay between each of four quasistatic cycles and a further 1-min delay before starting dynamic cycles, in which the bubble was continuously varied for 20 cycles at 20 cycles/min. Data from initial and postexpansion adsorption are presented as averages from three experiments and quasistatic and dynamic cycles correspond to single representative experiments.
Fluorescence microplate assay
Accumulation of surfactant at the interface was evaluated in 96-well microtiter plates as described by Ravasio et al. (15), in a FLUORSTAR Optima Microplate Reader (BMG Labtech, Offenburg, Germany). In these experiments, meconium or meconium organic extract was mixed in 100 μL subphase. Three micrograms of native surfactant was applied at the bottom of each well, and the fluorescence intensity reaching the surface was followed for 2 h at 25°C. Data in these experiments are presented as the average of three replicates with their standard deviation, in relative fluorescence units (RFU-bg) corrected by subtraction of the measured background.
Laurdan thermotropic profiles
Laurdan fluorescence emission spectra of surfactant suspensions were obtained at different temperatures in a spectrofluorimeter SLM-Aminco AB2 (Urbana, IL). Emission spectra were recorded at 400–540 nm upon excitation at 370 nm. Laurdan emission is typically blue in the gel phase and green at the liquid crystalline phase (22). Spectral changes were analyzed using the generalized polarization (GP) function, defined as
with IB and IR being the intensities at the blue and red edges of the emission spectrum, respectively (22,23).
Differential scanning calorimetry
Differential scanning calorimetry (DSC) thermograms of native surfactant or surfactant suspensions preexposed to meconium or taurocholic acid (TA) and cholesterol (Chol), were obtained in a model No. MC-2 microcalorimeter (MicroCal, Amherst, MA) (20). Surfactant concentration in these experiments was 3 mg/mL, with or without the addition of 10 mg/mL meconium, or TA (10 μM) + 1.6% Cholesterol (w/w, Chol/phospholipid) against 5 mM Tris buffer 150 mM NaCl as a reference. Scans were obtained from 15 to 55°C, at 30°C/h. Data were analyzed with the software Origin (Origin Labs, Northampton, MA). Thermodynamic parameters were obtained from DSC scans of three independent batches of native surfactant.
Data reproducibility
When possible, the figures represent the mean ± SD after averaging data from 3–6 independent experiments, using at least two, and often three, different batches of surfactant. The behavior of different surfactant batches was consistent in the experiments.
Results
Interfacial adsorption
Native surfactant adsorbs in a few seconds from the subphase into the interface, rapidly reaching equilibrium surface pressures (πeq) of ∼45 mN/m (Fig. 1 A). Increasing concentrations of meconium produce a progressive reduction of interfacial adsorption, which keeps being fast at first instance, but reaches progressively lower equilibrium pressures at longer term. The inset in Fig. 1 A plots the maximum pressure reached during the time of the experiment versus the concentration of meconium into the subphase. The lowest meconium concentrations tested already produce a substantial decrease in the adsorption of surfactant.
Figure 1.
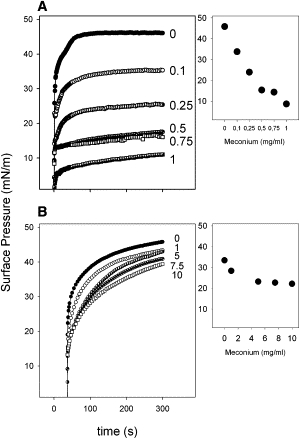
Interfacial adsorption of native surfactant exposed to different concentrations of meconium. (A) Interfacial adsorption of surfactant upon injection into the subphase of a Wilhelmy balance containing the indicated concentrations of meconium (mg/mL). (Inset) Surface pressure reached by the absorbed material at 100 s after injection versus the concentration of meconium. (B) Interfacial adsorption of surfactant preincubated with the indicated concentrations of meconium (mg/mL) once spread directly at the air-water interface of a surface balance. (Inset) Surface pressure reached by the spread material 100 s after spreading versus the concentration of meconium.
Adsorption of surfactant complexes from the subphase involves two processes: movement of surfactant particles through the bulk aqueous phase to reach subsurface compartments and transfer of surface active molecules from surface attached structures into the interface. To discriminate the effect of meconium in these two processes, we analyzed the effect of meconium exposure on the ability of surfactant to adsorb and spread after direct deposition at the interface. Fig. 1 B compares spreading kinetics of native surfactant onto a meconium-free subphase and onto subphases containing increasing concentrations of meconium. Exposure to progressively higher meconium concentrations also reduces the ability of surfactant to spread and adsorb at the interface, but to a much lesser extent than observed when injecting the surfactant sample into the bulk subphase.
Compression isotherms
Fig. 2 A compares the compression isotherms obtained from films formed by interfacial spreading of native surfactant or from native surfactant pretreated with meconium (10 mg/mL). Typical isotherms from native surfactant show increasing surface pressure as a function of area reduction from a variable lift-off molecular area—the area at which surface pressure starts to rise—up to reaching a plateau at ∼45 mN/m.
Figure 2.
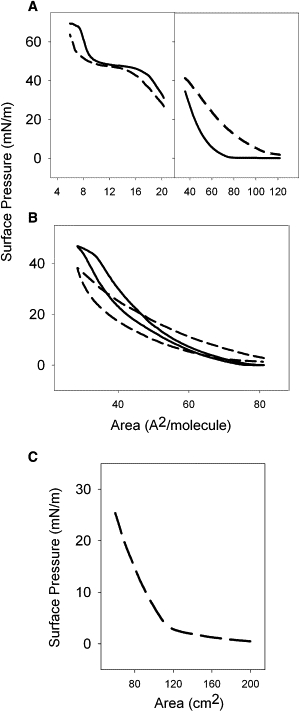
Compression isotherms of surfactant and meconium. (A) Low-pressure (right panel) and high-pressure (left panel) segments of the compression isotherm of films formed by native pulmonary surfactant without (solid line) or with (short dashed line) preexposure to meconium, once spread at the air-water interface. (B) Full compression-expansion cycling isotherms of films formed by meconium-free (solid line) or meconium-treated (dashed line) surfactant. (C) Compression isotherm of a film formed by spreading of meconium at the air-water interface.
Differences in apparent molecular areas of the surfactant isotherms are due to minor, uncontrollable, differences from sample to sample in the efficiency of transfer of spread surfactant into the interface. Compression beyond the plateau causes a sharp increase in pressure up to reach a collapse pressure of 70 mN/m. The isotherm from meconium pretreated surfactant differs. At low surface pressures, the isotherm exhibits an expansion to larger areas, meaning that the material transferred into the interface occupies a larger space than that taken by surfactant mixtures without meconium. However, meconium-treated surfactant requires a greater degree of compression to reach and maintain high surface pressures compared with meconium-free surfactant.
These data are consistent with meconium constituents inserting into surfactant and expanding the isotherm at low compression. This material from meconium may well have low collapse pressures and hence is desorbed from the interface at high pressures. The data also suggest that at high compression rates, desorption of meconium products may also remove some of the surfactant phospholipids.
Results in Fig. 2 B, obtained either from meconium-free or meconium-treated surfactant films, show that the expansion isotherm of meconium pretreated surfactant yields lower area/pressures, likely as a consequence of the irreversible loss of part of the material (surfactant + meconium) during compression. Fig. 2 C shows the compression isotherm of pure meconium, spread at the air-liquid interface. The collapse pressure of meconium film is 25 mN/m, indicating that most of its components are squeezed-out from the interface at pressures above that threshold. Interestingly, meconium isotherms exhibit segments with clearly distinct slopes, which may indicate the coexistence of components with different interfacial stability.
Captive bubble surfactometry
This technique better simulates the compression-expansion cycling that occurs at the breathing interface (8,19,21). Fig. 3 A compares interfacial adsorption kinetics of native surfactant and surfactant preexposed to meconium, as assessed after deposition at the interface of the captive air bubble in the captive bubble surfactometer. NS adsorbs to form a stable surface film with a minimum equilibrium surface tension of ∼23 mN/m within the first second after deposition. Pretreated surfactant adsorbs initially almost as fast as nontreated surfactant, but to a higher equilibrium tension of 25 mN/m. However, readsorption of excess material upon expansion of the bubble (see right panel in Fig. 3 A) occurs identically in meconium-treated and nontreated surfactant.
Figure 3.
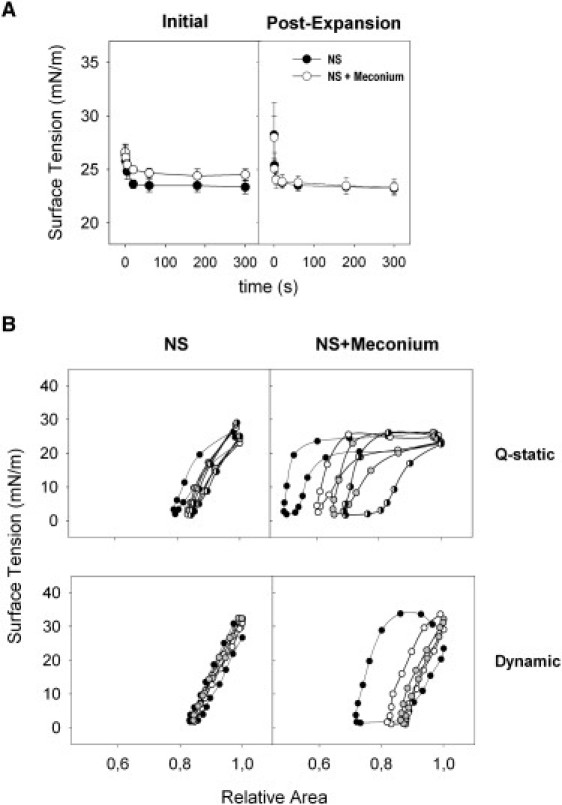
Effect of meconium on the surface activity of surfactant as evaluated in a captive bubble surfactometer. (A) Initial and postexpansion adsorption of native surfactant in the absence (solid circles) or in the presence (open circles) of 10 mg/mL meconium (representing 1.6% Chol w/w) (B) Quasistatic (Q-static, upper panels) and dynamic (lower panels) compression-expansion isotherms of surfactant films formed in the absence (left panels) or in the presence (right panels) of meconium; plotted are isotherms obtained from cycle 1 (solid), 2 (open), 3 (shaded), and 4 (half solid) for the quasistatic and 1 (solid), 10 (open), and 20 (shaded) for the dynamic cycling.
Cycling isotherms obtained from native surfactant films show practically no compression/expansion hysteresis along the successive quasistatic or dynamic cycles (Fig. 3 B). Only the first quasistatic cycle exhibits a marginal hysteresis, which is lost in the subsequent cycles. Native surfactant isotherms reach surface tensions ≤2 mN/m with <20% compression (see summary of data in Table S1 in the Supporting Material). In contrast, isotherms of meconium-treated surfactant films exhibit a considerable hysteresis. Under quasistatic conditions, both compression and expansion isotherms show marked plateaus at the equilibrium pressure, which are progressively reduced in the subsequent cycles.
These plateaus practically disappear upon dynamic cycling, likely due to completion of film depuration, i.e., once rapid cycling to the highest pressures promotes the squeeze-out and the subsequent desorption of the components inserted from meconium into the surfactant films. However, complete refining of the isotherms requires much larger compression ratios (∼50%) than required by meconium-free surfactant. Meconium addition does not increase the maximum surface tensions, reached upon expansion, again confirming that meconium does not impair the respreading potential of surfactant that is already associated with the interface.
Structure of the surface film
Evidence suggests that meconium incorporates constituent molecules into surfactant films and that meconium-treated surfactant films require compression-driven depuration before becoming competent to reach the lowest tensions. This finding may indicate that meconium also affects the structure of surfactant films. Under epifluorescence microscopy, the lateral structure of native surfactant films subjected to compression, exhibits features that have been well characterized (25,26) (Fig. 4 A). At pressures higher than 10 mN/m there is a progressive segregation of small condensed domains, which grow with compression to reach maximum size at ∼30 mN/m and then decrease upon higher compression (see quantitation of domain morphologies in Fig. 4, B–D). Dark condensed domains always showed a rounded shape and were homogeneously distributed, as dispersed in the fluorescent, presumably liquid-disordered, film matrix.
Figure 4.
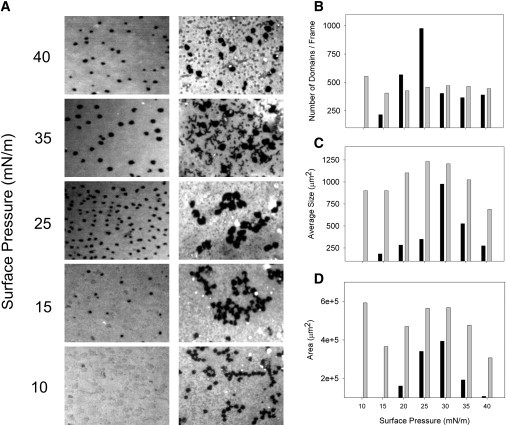
Effect of meconium on the structure of surfactant films. (A) Epifluorescence microscopy images from interfacial films of native surfactant containing a trace of the fluorescent probe BODIPY-PC, in the absence (left pictures) or in the presence (right pictures) of meconium, obtained upon transfer to glass at the indicated surface pressures. Graphs on the right correspond to the quantitative analysis of the density of condensed domains (B), their average size (C), and the total area occupied by the condensed phase (D) versus the surface pressure, for meconium-free (solid bars) and meconium-treated (shaded bars) surfactant films.
In contrast, morphology of films from meconium-treated surfactant was different. Overall, meconium surfactant mixtures showed higher proportions of condensedlike phase at any pressure. This could be related with a condensing effect caused by the components inserted into surfactant by meconium. Also in contrast to natural surfactant films, condensed domains showed a strong tendency to aggregate in meconium-exposed layers, producing a relatively heterogeneous morphology in these films. Segregation of large clusters of condensed phase from large areas devoid of condensed domains could be an important source of instability for the films as a whole. Meconium-treated films also showed the presence of numerous bright spots, likely corresponding to material that has been already squeezed-out from the interface during compression due to its low interfacial stability.
Biochemical analysis of meconium
Table S2 summarizes the amounts of total protein, phospholipids, cholesterol, and bile acids in meconium. Still, most of meconium weight seems to consist of nondetermined components, possibly salts, heme byproducts, and others. Particularly remarkable is the presence of a substantial amount of cholesterol and bile acids, which may perturb the structure and function of surfactant. We have therefore compared the effect of meconium with a mixture of cholesterol and taurocholic acid (Chol/TA). TA was used because it is one of the most abundant human bile acids (27).
Accumulation of surfactant at the interface
A method recently developed allows evaluation of the amount of surfactant material accumulating at the interface (15). We therefore tested whether meconium and Chol/TA produced similar alteration in the ability of surfactant to form surface films. Fig. 5 A shows how native surfactant rapidly reaches the interface and stably remains associated with it, whereas its exposure to increasing concentrations of meconium produces a progressive reduction in the amount of interfacial surfactant.
Figure 5.
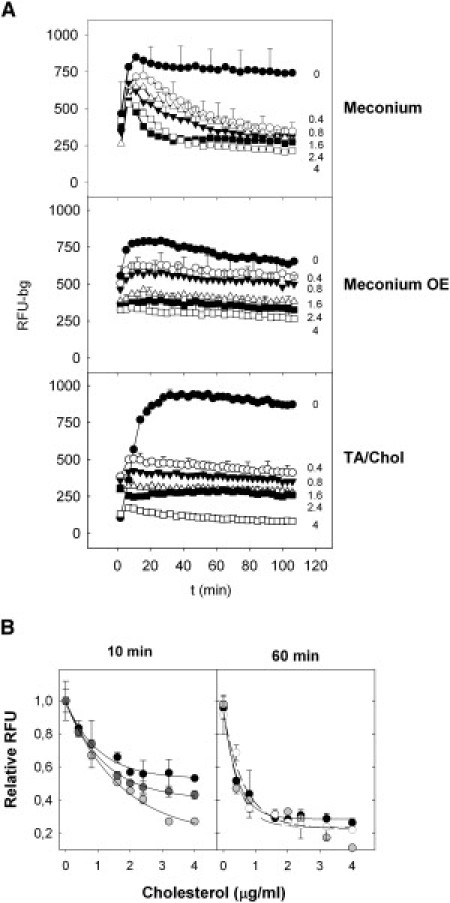
Effect of meconium on the interfacial accumulation of surfactant. (A) Accumulation of material into the interface upon adsorption of fluorescently labeled native surfactant in the presence of the indicated concentrations of meconium in μg/mL (top), of the organic extract (OE) from meconium (center) or of a mixture of taurocholic acid and cholesterol (TA/Chol, bottom), all of them expressed as μg/mL of cholesterol added. (B) Relative accumulation of labeled surfactant into the interface as a function of the amount of meconium (solid), meconium organic extract (open), or TA + cholesterol (shaded), 10 min (left panel) or 60 min (right panel) after injection of surfactant into the subphase. Lines are mere guides to eye, illustrating the similar trends of the three types of samples.
A similar inhibiting effect was observed when native surfactant was exposed to a suspension prepared from a chloroform/methanol extract of meconium (see Fig. 5 A, middle panel), indicating that the inhibiting compounds are extractable by organic solvents. The lower panel in Fig. 5 A shows how the inhibition profile of surfactant by meconium can be fully mimicked by exposure of surfactant to a mixture Chol/TA containing quantities of cholesterol comparable to those found in meconium. Fig. 5 B illustrates that the concentration dependence of surfactant inhibition by meconium is matched by meconium organic extract or the Chol/TA mixture, particularly at long term, when the maximal amount of surface-associated material is reached. The larger differences observed at short times likely highlight the different kinetics of inhibition once surfactant is exposed to the three substances. Meconium could be the slowest inhibiting substance due to its complexity.
Mimicking of meconium inhibition by cholesterol mobilizing complexes
Fig. 6 shows that exposure of surfactant to Chol/TA has similar inhibiting effects as meconium. The mixture Chol/TA reduces only slightly the initial adsorption rate of surfactant with no effect in adsorption postexpansion. Compression-expansion isotherms of Chol/TA-treated surfactant were, however, substantially affected, in a manner entirely comparable to the effect of meconium (compare Fig. 6 with Fig. 3). Much larger compression was required for surfactant to reach the minimal tensions if preexposed to Chol/TA, particularly during the quasistatic regime. The hysteresis of the isotherms was progressively reduced during the subsequent cycles presumably as a result of the progressive depuration of spurious components. Exposure to Chol/TA combinations did not increase the maximal surface tension, as occurred upon exposure to meconium.
Figure 6.
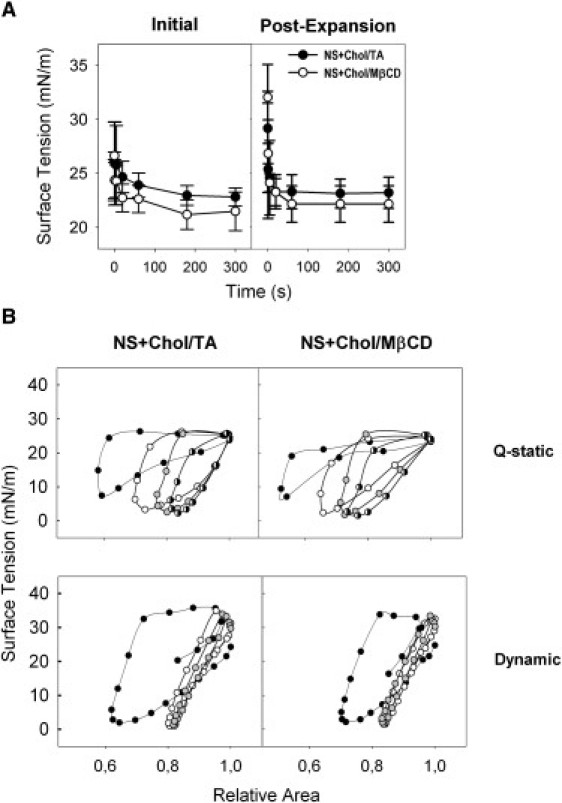
Effect of cholesterol-mobilizing complexes on the surface activity of surfactant as evaluated in a captive bubble surfactometer. (A) Initial and postexpansion adsorption of native surfactant in the presence of a mixture of TA and cholesterol (1.6% Cholesterol w/w) (solid circles) or in the presence of complexes of cholesterol and MβCD (open circles) representing a final proportion of cholesterol of 1.6% w/w. (B) Quasistatic (Q-static, upper panels) and dynamic (lower panels) compression-expansion isotherms of surfactant films formed in the presence of Chol/TA (left panels) or Chol/MβCD (right panels) complexes; plotted are isotherms obtained from cycle 1 (solid), 2 (open), 3 (shaded), and 4 (half solid) for the quasistatic and 1 (solid), 10 (open), and 20 (shaded) for the dynamic cycling.
In some experiments, we preincubated surfactant with dried cholesterol films, prepared by evaporation of organic solutions of cholesterol and, in contrast to the effect of Chol/TA mixtures, we did not detect significant effects on surfactant surface activity (not shown). This suggests a potential role of bile acids as cholesterol mobilizing agents to facilitate transference of cholesterol into surfactant complexes and so to mediate inhibition. Fig. 6 shows how the exposure of surfactant to the combination of cholesterol with another well-known steroid-mobilizing agent, methyl-β-cyclodextrin, produces perturbations on surfactant surface activity that are practically indistinguishable from those induced by meconium or Chol/TA complexes.
Thermotropic profiles of surfactant membranes
Laurdan is a fluorescent probe sensitive to the level of hydration of the headgroup region of phospholipid membranes, which depends on lipid packing and membrane phase (28). The thermotropic profile of the fluorescence of a Laurdan trace incorporated into native surfactant membranes provides information on the effect of temperature on surfactant structure. Fig. 7 A shows that native surfactant membranes manifest a broad transition from ordered, relatively dehydrated states at low temperatures to more disordered and loosely packed configurations at high temperatures, with a melting temperature (calculated as the temperature at which Laurdan GP is halfway from that of ordered to that of disordered membranes) of ∼35°C.
Figure 7.
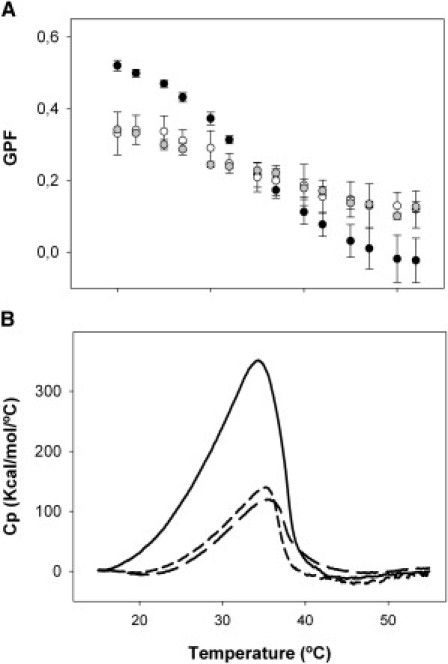
Effect of meconium on the thermotropic properties of surfactant membranes. (A) Thermotropic profiles of generalized polarization (GP) fluorescence obtained from the fluorescence spectra of Laurdan-labeled native surfactant (solid) or surfactant preexposed to meconium (open), or to TA + cholesterol (shaded). (B) DSC thermograms from native surfactant (solid line) or surfactant preexposed to meconium (long dashed line) or to TA + cholesterol (short dashed line).
Fluorescence of Laurdan inserted into surfactant treated with meconium indicates that these membranes have become more hydrated/disordered at low temperatures and more ordered/dehydrated at high temperatures than the membranes that were not exposed to meconium. A practically identical broadening of the thermotropic transition of surfactant membranes was observed upon exposure of surfactant to the Chol/TA mixture. On the other hand, if only taurocholic acid was used as surfactant perturbing compound, the thermotropic profile of surfactant membranes was only affected at low temperatures, where the bile acid seems to produce an apparent disordering effect over the surfactant membrane ordered states (data not shown).
To confirm the nature of the perturbations introduced by meconium into surfactant membranes, we compared differential scanning calorimetry thermograms of native surfactant and surfactant treated with meconium or the Chol/TA mixture. Natural surfactant shows a complex and broad calorimetric peak between 15 and 40°C with a Tm of ∼35°C (Fig. 7 B) as it has been reported before (29). The thermogram from surfactant exposed to meconium exhibits also a similarly broaden thermotropic peak, but with a substantially reduced associated enthalpy (calculated as the area under the peak, see summary of calorimetric parameters in Table S3). The thermotropic behavior of surfactant treated with the Chol/TA mixture reproduces that of meconium-treated surfactant, including a similar reduction of enthalpy. The reduction in enthalpy caused by the combined action of cholesterol and the bile acid therefore parallels the decrease in the amplitude of the thermotropic transition as reported by Laurdan.
Discussion
In the past, inhibition by meconium had been found with clinical surfactants such as Curosurf (27), Surfactant-TA (30–32), or others (33), but no data were available on the inhibition of natural pulmonary surfactant by meconium, a process actually occurring with MAS. The data presented here support that the inhibitory action of meconium is mediated by a direct incorporation of meconium components into surfactant membranes and films and their subsequent perturbation. The nature of the perturbations observed are compatible with cholesterol being an important inhibitory element that alters the properties of surfactant membranes and films in defined and recognizable ways.
The inhibitory action of the presence of excess cholesterol in surfactant complexes has been widely reported and largely studied. The early finding that cholesterol was deleterious for surfactant function, at least as assessed in vitro, led to the removal of cholesterol from most of the surfactant preparations currently used for the treatment of respiratory pathologies (34). Recent studies have also reported that excess of cholesterol could be also a pathogenic factor in ARDS (35). How much cholesterol is essential as a constituent of pulmonary surfactant is under much discussion in recent reports, as a minimal amount of cholesterol is required for natural pulmonary surfactant membranes to adopt their characteristic lateral structure (36) and proper dynamics (29). Therefore the amount of cholesterol in surfactant must be carefully regulated to maintain the proportions that optimize surfactant performance whereas excessive cholesterol has deleterious consequences (8). Moreover, regulation of the amount of cholesterol in surfactant may be a physiological adaptive response to certain environmental changes (37).
Our findings indicate that the effect of cholesterol contained in meconium as an inhibitor of surfactant, could be mediated by the bile acids in this material. Cholesterol alone failed to reproduce the effects caused by meconium (not shown), probably because the low solubility of cholesterol in aqueous medium prevent its extensive transfer into surfactant complexes. Bile acids may act to enable insertion of cholesterol into surfactant membranes resulting in surfactant dysfunction as occur upon exposure to complexes of methyl-β-cyclodextrine and cholesterol. Inhibition by meconium could therefore constitute a particular case of inhibition by excess cholesterol facilitated by the presence of bile salts as cholesterol-mobilizing agent.
Facilitated transfer of cholesterol could be also a significant contribution in other pathological alterations of surfactant such as in the increase of neutral lipids associated with surfactant inhibition in patients with ARDS (35). The increase of cholesterol in surfactant as a consequence of serum leakage into airspaces could be dependent on the cholesterol-mobilizing properties of lipoproteins. The notion of the effect of cholesterol-mobilizing agents on structure and activity of pulmonary surfactant is also behind the idea proposed by Gunasekara et al. (38) that cyclodextrine could be used as an additive in therapeutic surfactants, where it could aid to deplete the excess of cholesterol from endogenous inhibited surfactant. Our results suggest that incorporation of cholesterol-mobilizing agents such as cyclodextrine into exogenous surfactants might even have the opposite, negative, effect and promote further inhibition if more extensive mobilization of cholesterol occurs.
An effective surfactant must optimize three main properties: rapid interfacial adsorption, very low surface tension upon film compression, and efficient film replenishment upon expansion (9,39). Meconium alters these three properties, impairing surfactant performance. It reduces substantially accumulation of surfactant at the interface. Perhaps some of the elements incorporated by meconium into surfactant are directly altering the molecular structures responsible for their attachment to the interface.
Meconium itself has a relatively low interfacial collapse pressure, suggesting that it desorbs easily from the interface. Perhaps the accumulation of meconium components into surfactant membranes may also lead to a facilitated desorption, which reduces the amounts of surfactant accumulated at the interface. The detergent properties of bile acids may be responsible for facilitated desorption of surfactant complexes from the interface. The effects of bile acids as a single inhibitory component had been previously studied with a clinical surfactant, Curosurf (27). This study identified some similarities between taurocholic acid and meconium, but in this study, taurocholic acid by itself did not reproduce all the effects observed after exposure of surfactant to meconium.
The alteration of the structure of surfactant as a consequence of the exposure to meconium, could explain the altered ability of meconium-treated surfactant to reach very low surface tensions upon compression. Bile acid-promoted incorporation of cholesterol into surfactant could be responsible of the partial fluidization of the condensed states of membranes and films and the requirement of much larger compression to depurate surface films before reaching the lowest tensions. Our experiments in the captive bubble surfactometer indicate that during compression-expansion cycling, surface films may lose deleterious components of meconium, but this could depend on the total amount of material incorporated. It is particularly intriguing that meconium increases the fraction of surface films occupied by condensed domains as observed by epifluorescence microscopy, especially considering that meconium seems to fluidize/decrease packing in surfactant membranes. We speculate that incorporation of cholesterol, and perhaps bile salts, into the condensed domains would increase their fractional area while decreasing their internal molecular packing. This would also explain the reduction of melting enthalpy of surfactant membranes that had been exposed to meconium. An altered composition of condensed surfactant domains could be responsible for their abnormal propensity to cluster, which, in turn, could have consequences on the lack of stability of meconium contaminated films.
The nature of the inhibitory mechanisms of meconium revealed here suggests strategies to improve the surfactant treatment of several lung diseases. In the case of meconium aspiration pneumonia, the use of surfactant preparations free of cholesterol would appear logical based on our in vitro results. A cholesterol-free preparation might resist meconium inhibition to a greater degree than a cholesterol-containing surfactant. However, a complete lack of cholesterol may adversely affect the beneficial effects offered by physiological amounts of cholesterol. Supplementing clinical surfactants with antagonists of bile acids is an alternative approach. Ursodeoxicholic acid, for instance, is a hydrophilic bile acid which has been proposed as a membrane-protective agent useful in the treatment of cholestasis (40). Similar strategies may be useful in ARDS wherein increased amounts of cholesterol have been found with surfactant obtained from these patients (35). Targeting of surfactant inhibitors by adding specific inhibitor antagonists to therapeutic surfactants may improve clinical responses in patients with surfactant dysfunction who heretofore have not responded well to conventional surfactant therapy.
Acknowledgments
This research has been supported by grants from the Spanish Ministry of Science (No. BIO2009-09694 and No. CSD2007-00010), Community of Madrid (No. S0505/MAT/0283), and the National Institutes of Health (No. HLBI RO1 HL 66410).
Supporting Material
References
- 1.Been J.V., Zimmermann L.J. What's new in surfactant? A clinical view on recent developments in neonatology and pediatrics. Eur. J. Pediatr. 2007;166:889–899. doi: 10.1007/s00431-007-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nkadi P.O., Merritt T.A., Pillers D.-A. M. An overview of pulmonary surfactant in the neonate: genetics, metabolism, and the role of surfactant in health and disease. Mol. Genet. Metab. 2009;97:95–101. doi: 10.1016/j.ymgme.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin B.K., Tomkiewicz R.P., Easa D. The surface and transport properties of meconium and reconstituted meconium solutions. Pediatr. Res. 1996;40:834–838. doi: 10.1203/00006450-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 4.El Shahed A.I., Dargaville P., Soll R.F. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database Syst. Rev. 2007;3:CD002054. doi: 10.1002/14651858.CD002054.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Lindenskov P.H., Castellheim A., Saugstad O.D. Meconium induced IL-8 production and intratracheal albumin alleviated lung injury in newborn pigs. Pediatr. Res. 2005;57:371–377. doi: 10.1203/01.PDR.0000153870.66197.D1. [DOI] [PubMed] [Google Scholar]
- 6.Righetti C., Peroni D.G., Zancanaro C. Proton nuclear magnetic resonance analysis of meconium composition in newborns. J. Pediatr. Gastroenterol. Nutr. 2003;36:498–501. doi: 10.1097/00005176-200304000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Tølløfsrud P.A., Medbø S., Saugstad O.D. Albumin mixed with meconium attenuates pulmonary dysfunction in a newborn piglet model with meconium aspiration. Pediatr. Res. 2002;52:545–553. doi: 10.1203/00006450-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Gunasekara L., Schoel W.M., Amrein M.W. A comparative study of mechanisms of surfactant inhibition. Biochim. Biophys. Acta. 2008;1778:433–444. doi: 10.1016/j.bbamem.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Y.Y., Veldhuizen R.A.W., Possmayer F. Current perspectives in pulmonary surfactant—inhibition, enhancement and evaluation. Biochim. Biophys. Acta. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Fernsler J.G., Zasadzinski J.A. Competitive adsorption: a physical model for lung surfactant inactivation. Langmuir. 2009;25:8131–8143. doi: 10.1021/la8039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwanicki J.L., Lu K.W., Taeusch H.W. Hyaluronan decreases pulmonary surfactant inactivation by phospholipase A2 in vitro. Exp. Lung Res. 2010;36:167–174. doi: 10.3109/01902140903234186. [DOI] [PubMed] [Google Scholar]
- 12.Taeusch H.W., Bernardino de la Serna J., Zasadzinski J.A. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys. J. 2005;89:1769–1779. doi: 10.1529/biophysj.105.062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravasio A., Olmeda B., Pérez-Gil J. Lamellar bodies form solid three-dimensional films at the respiratory air-liquid interface. J. Biol. Chem. 2010;285:28174–28182. doi: 10.1074/jbc.M110.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouser G., Siakotos A.N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966;1:85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- 15.Ravasio A., Cruz A., Haller T. High-throughput evaluation of pulmonary surfactant adsorption and surface film formation. J. Lipid Res. 2008;49:2479–2488. doi: 10.1194/jlr.D800029-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Lowry O.H., Rosebrough N.J., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Cruz A., Worthman L.A.D., Pérez-Gil J. Microstructure and dynamic surface properties of surfactant protein SP-B/dipalmitoylphosphatidylcholine interfacial films spread from lipid-protein bilayers. Eur. Biophys. J. 2000;29:204–213. doi: 10.1007/pl00006647. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Cruz A., Mendelsohn R. Langmuir-Blodgett films formed by continuously varying surface pressure. Characterization by IR spectroscopy and epifluorescence microscopy. Langmuir. 2007;23:4950–4958. doi: 10.1021/la063139h. [DOI] [PubMed] [Google Scholar]
- 19.Schürch S., Green F.H.Y., Bachofen H. Formation and structure of surface films: captive bubble surfactometry. Biochim. Biophys. Acta. 1998;1408:180–202. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Gil L., Schürch D., Pérez-Gil J. Pulmonary surfactant protein SP-C counteracts the deleterious effects of cholesterol on the activity of surfactant films under physiologically relevant compression-expansion dynamics. Biophys. J. 2009;97:2736–2745. doi: 10.1016/j.bpj.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoel W.M., Schürch S., Goerke J. The captive bubble method for the evaluation of pulmonary surfactant: Surface tension, area, and volume calculations. Biochim. Biophys. Acta. 1994;1200:281–290. doi: 10.1016/0304-4165(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 22.Parasassi T., De Stasio G., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991;60:179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parasassi T., De Stasio G., Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990;57:1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted in proof.
- 25.Nag K., Perez-Gil J., Keough K.M. Phase transitions in films of lung surfactant at the air-water interface. Biophys. J. 1998;74:2983–2995. doi: 10.1016/S0006-3495(98)78005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Discher B.M., Maloney K.M., Hall S.B. Lateral phase separation in interfacial films of pulmonary surfactant. Biophys. J. 1996;71:2583–2590. doi: 10.1016/S0006-3495(96)79450-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross T., Zmora E., Berman A. Lung-surfactant-meconium interaction: in vitro study in bulk and at the air-solution interface. Langmuir. 2006;22:3243–3250. doi: 10.1021/la0521241. [DOI] [PubMed] [Google Scholar]
- 28.Parasassi T., Di Stefano M., Gratton E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys. J. 1994;66:120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardino de la Serna J., Orädd G., Perez-Gil J. Segregated phases in pulmonary surfactant membranes do not show coexistence of lipid populations with differentiated dynamic properties. Biophys. J. 2009;97:1381–1389. doi: 10.1016/j.bpj.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae C.-W., Takahashi A., Sasaki M. Morphology and function of pulmonary surfactant inhibited by meconium. Pediatr. Res. 1998;44:187–191. doi: 10.1203/00006450-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Oh M.H., Bae C.W. Inhibitory effect of meconium on pulmonary surfactant function tested in vitro using the stable microbubble test. Eur. J. Pediatr. 2000;159:770–774. doi: 10.1007/pl00008344. [DOI] [PubMed] [Google Scholar]
- 32.Park K.-H., Bae C.-W., Chung S.-J. In vitro effect of meconium on the physical surface properties and morphology of exogenous pulmonary surfactant. J. Korean Med. Sci. 1996;11:429–436. doi: 10.3346/jkms.1996.11.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herting E., Rauprich P., Robertson B. Resistance of different surfactant preparations to inactivation by meconium. Pediatr. Res. 2001;50:44–49. doi: 10.1203/00006450-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Blanco O., Pérez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat respiratory distress syndrome: role of the different components in an efficient pulmonary surfactant. Eur. J. Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 35.Markart P., Ruppert C., Guenther A. Patients with ARDS show improvement but not normalization of alveolar surface activity with surfactant treatment: putative role of neutral lipids. Thorax. 2007;62:588–594. doi: 10.1136/thx.2006.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernardino de la Serna J., Perez-Gil J., Bagatolli L.A. Cholesterol rules: direct observation of the coexistence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J. Biol. Chem. 2004;279:40715–40722. doi: 10.1074/jbc.M404648200. [DOI] [PubMed] [Google Scholar]
- 37.Orgeig S., Daniels C.B. The roles of cholesterol in pulmonary surfactant: insights from comparative and evolutionary studies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;129:75–89. doi: 10.1016/s1095-6433(01)00307-5. [DOI] [PubMed] [Google Scholar]
- 38.Gunasekara L.C., Pratt R.M., Amrein M.W. Methyl-[β]-cyclodextrin restores the structure and function of pulmonary surfactant films impaired by cholesterol. Biochim. Biophys. Acta. Biomembranes. 2010;1798:986–994. doi: 10.1016/j.bbamem.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Serrano A.G., Pérez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem. Phys. Lipids. 2006;141:105–118. doi: 10.1016/j.chemphyslip.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y., Doyen R., Lichtenberger L.M. The role of membrane cholesterol in determining bile acid cytotoxicity and cytoprotection of ursodeoxycholic acid. Biochim. Biophys. Acta. 2009;1788:507–513. doi: 10.1016/j.bbamem.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


