Abstract
Objective:
The aim of this study was to determine if there were focal cortical abnormalities in juvenile myoclonic epilepsy (JME) using neuropsychological investigations and MRI.
Methods:
Twenty-eight patients with JME and a large sample of healthy controls were assessed using a series of neuropsychological tests as well as structural and diffusion tensor MRI (DTI). DTI measures assessed fractional anisotropy (FA) within a white matter skeleton.
Results:
Neuropsychological testing indicated subtle dysfunctions in verbal fluency, comprehension, and expression, as well as nonverbal memory and mental flexibility. Utilizing whole-brain voxel-based morphometry for gray matter MRI data and tract-based spatial statistics for white matter diffusion MRI data, we found reductions in gray matter volume (GMV) in the supplementary motor area and posterior cingulate cortex and reductions in FA in underlying white matter of the corpus callosum. Supplementary motor area FA predicted scores in word naming tasks and expression scores. Posterior cingulate cortex GMV and FA predicted cognitive inhibition scores on the mental flexibility task.
Conclusions:
The neuropsychological, structural, and tractography results implicate mesial frontal cortex, especially the supplementary motor area, and posterior cingulate cortex in JME.
Juvenile myoclonic epilepsy (JME) is a common type of idiopathic generalized epilepsy (IGE), accounting for approximately 4%–11% of all epilepsies. It is characterized by an age-specific onset of epilepsy with myoclonic jerks, generalized tonic-clonic seizures, and, less frequently, absences.1
A typical abnormality detected by EEG is bilateral spike or polyspike and wave complexes, often strongest in frontocentral areas.2 Further focal or asymmetric abnormalities on EEG occur in approximately 30% of patients.3,4
Fifty years ago patients with JME were noted to have personality traits previously considered to characterize frontal lobe pathology.5 Studies examining neuropsychological aspects have identified a range of deficits, for example impaired performance on tasks of mental flexibility and cognitive speed.6 More recently, further impairments in executive functions have been highlighted.7–10
JME is also characterized by the absence of detectable structural brain abnormalities using MRI or CT.11 Focal abnormalities have been detected using volumetry or automated voxel-based morphometry (VBM) of structural MRI scans.12 These changes, however, have shown marked spatial variability,13 albeit with a tendency toward changes in frontal cortex.14–16 This frontal emphasis has been strengthened by recent findings using diffusion tensor imaging (DTI), showing decreased fractional anisotropy (FA) in a region corresponding to the anterior thalamic radiation.17
The purpose of the current study was to explore for additional evidence of focal cortical abnormalities in JME using neuropsychological tests and advanced structural neuroimaging techniques.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Kings College Hospital Research Ethics Committee (Ethics Ref 06/Q0703/124). After a full explanation of the methods involved, each subject gave written informed consent to take part in the study.
Participants.
Twenty-eight participants with JME (as determined by medical history, clinical features, and EEG, as set by the International League Against Epilepsy11) were identified using medical records (see table e-1 on the Neurology® Web site at www.neurology.org for detailed patient characteristics). A total of 55 healthy control subjects were recruited via a local volunteer's database.
Controls were excluded if they had any history of neurologic illness. JME patients were excluded if they had a history of any other neurologic illness or if they were currently using topiramate, as this has previously been shown to have an adverse affect on cognition, especially verbal abilities.18
All control subjects had a structural MRI (T1 volume) scan. Of these, 38 also had a diffusion MRI scan (DTI) and of these 24 also had neuropsychological assessment (table 1).
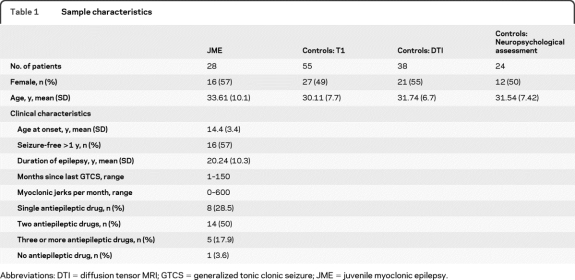
Table 1.
Sample characteristics
Abbreviations: DTI = diffusion tensor MRI; GTCS = generalized tonic clonic seizure; JME = juvenile myoclonic epilepsy.
Neuropsychological measures.
A battery of standardized neuropsychological measures was administered to the JME group and a subset of the healthy control participants, within 2 weeks of MRI scanning. The tasks focused on verbal processing abilities, memory, and executive functions (see table e-2). Healthy control and patient scores were compared using the general linear model, with age and gender as covariates. As multiple tasks were assessed, the false discovery rate was used to correct for multiple comparisons.19 A q value of 0.10 was used, indicating that approximately 10% of the rejected null hypotheses would be false positives.
Image acquisition.
Each participant was scanned using a GE Signa 3T HDx system (General Electric, Waukshua, WI), with actively shielded magnetic field gradients (maximum amplitude 40 mT m−1).
A 3-dimensional spoiled gradient echo (SPGR) volumetric scan was obtained. This T1-weighted SPGR image had an image matrix size of 256 × 256 × 196 voxels, with an isotropic voxel size of 1.1 mm (echo time/repetition time/inversion time = 2.8/6.6/450 msec, excitation flip angle 20°).
Each volume of DTI data was acquired using a multislice peripherally gated doubly refocused spin echo EPI sequence, optimized for precise measurement of the diffusion tensor in parenchyma, from 60 contiguous near-axial slice locations with isotropic (2.4 × 2.4 × 2.4 mm) voxels. The echo time was 104.5 msec while the effective repetition time varied between subjects in the range 12 and 20 RR intervals. The maximum diffusion weighting was 1,300 s mm−2. At each slice location, 4 images were acquired with no diffusion gradients applied, together with 32 diffusion-weighted images in which gradient directions were uniformly distributed in space.
Image preprocessing: T1 volume.
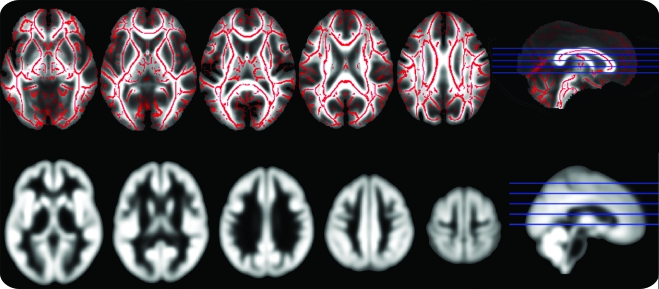
Segmentation of the SPGR images was performed using Statistical Parametric Mapping software (SPM8, revision 3408, Wellcome Department of Imaging Neurosciences, UCL, UK) running on Matlab 7.4 (Mathworks, Natick, MA). The unified segmentation routine was used for bias correction, registration to the Montreal Neurological Institute (MNI) template, and classification of brain tissue into gray matter, white matter, and CSF. The diffeomorphic anatomic registration through exponential lie algebra (DARTEL) toolbox was used to improve registration between volumes.20 The resulting tissue classification images were modulated by the Jacobian determinants derived from the registration step, allowing each subject's tissue volume to be preserved after warping. Finally, the images were smoothed by an 8-mm full width at half maximum isotropic Gaussian kernel (figure 1) to allow for interindividual gyral variation.
Figure 1. Mean normalized gray matter and fractional anisotropy (FA) images.
FA skeleton on which the statistical analysis was carried out, overlaid on the FA map taken from the average of all subjects (top row). Average gray matter map of all subjects after normalization and smoothing (bottom row).
Image preprocessing: Diffusion-weighted volumes.
Diffusion-weighted images were initially corrected for the effects of eddy-current induced distortion, and subject motion, using in-house software.21 FA images were created by fitting the diffusion tensor to this eddy-corrected, masked data using dtifit (part of the FMRIB MRI toolkit: http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_dtifit.html). The tract-based spatial statistics (TBSS) processing pipeline was used for voxel-wise analysis.22 All subjects' FA data were aligned into a common space (FSL's standard FA template, in approximate MNI space) using the nonlinear registration tool FNIRT.23 Next, a mean FA image was created from all the individual normalized FA images and thinned to create a mean FA skeleton (figure 1) which represents the centers of all tracts common to the group. Each subject's aligned FA data were then projected onto this skeleton.24
Statistical analysis.
A general linear model was designed with the program Glm (part of FSL 4.14, FMRIB www.fmrib.ox.ac.uk/fsl) to examine differences in gray matter volume (GMV) and FA between groups. Age and gender were included as covariates of no interest in the model. For gray matter VBM only, total brain volume (the sum of gray and white matter as classified during segmentation) was used as a further covariate of no interest. As the FA maps were unmodulated, inclusion of total brain volume in the model was unnecessary.
As the design of the study was unbalanced in terms of n per group and the normality of the data were unknown, a nonparametric approach was taken. Randomize v2.5 (also part of FSL 4.14) was used to test the general linear model. Randomize corrects for multiple comparisons by using the null distribution of the maximum cluster size across the image and can be considered more appropriate when the assumptions underlying a parametric approach are not met.25 This same method of statistical analysis was applied to the DARTEL modulated gray matter images and the TBSS normalized FA-skeleton images. For both modalities, differences were considered significant at a cluster size threshold of t >3 and tested for significance at p < 0.05 corrected for multiple comparisons.
To further elucidate the anatomic definition of the regions identified from the TBSS analysis, a probabilistic tractography analysis, seeded from regions of significant intergroup difference, was performed using FSL's Bedpostx (Bayesian estimation of diffusion parameters obtained using sampling techniques) looking at the JME group only. Bedpostx runs Markov Chain Monte Carlo sampling to build up distributions on diffusion parameters at each voxel. From this, probabilistic tractography was performed using FSL's probtrackx, with seed masks defined from group differences in the TBSS analysis. This generated a connectivity distribution from voxels in the seed mask. The resulting tractograms were thresholded at a value corresponding to 1% of the total tracks generated and then nonlinearly warped from native space to MNI space using the warp field generated in the TBSS analysis. Results were then binarized, averaged across subjects, and displayed thresholded so each voxel was reached in at least 50% of all subjects.
Neuropsychological and anatomic relationships.
To investigate the relationship between structural changes and neuropsychological/clinical variables in the JME group, a series of stepwise linear regressions were carried out to test the predictive power of structural changes for changes in neuropsychological function. These regressions were calculated separately for each significantly different neuropsychological measure, using mean structural MRI values (GMV and FA) in regions of anatomic difference, as well as age and gender, as possible predictors. Results were considered significant at p < 0.05, Bonferroni corrected.
RESULTS
Control and JME groups did not differ significantly in age or gender distribution (table 1).
Neuropsychological test results.
Neuropsychological test results are detailed in table e-3. Groups did not differ on IQ, as estimated by the NART scale (t = 1.07, df = 49, p > 0.29). Both groups performed well, and were within the normal range on the majority of tests administered. Differences were detected, however, on the following measures, with the JME group performing less well: Letter Fluency (F = 4.54, df = 47,1, p < 0.038), Graded Naming task (F = 5.64, df = 47,1, p < 0.022), Similarities (F = 10.27, df = 47,1, p < 0.003), Trail Making Test interference score (F = 4.73, df = 46,1, p < 0.035), and the post interference aspect of the Design Learning task (F = 4.23, df = 46,1, p < 0.029).
Gray matter VBM results.
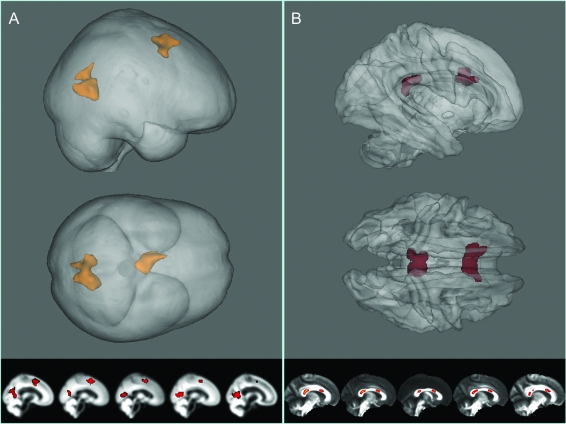
We detected focal reductions of gray matter volume in the JME group relative to controls, bilaterally in 2 mesial cortical regions (figure 2): the supplementary motor area (SMA) (p < 0.04 corrected, t-max = 4.37, [x, y, z] = −7.5, 10.5, 40.5) and posterior cingulate (p < 0.011 corrected, t-max = 4.22, [x, y, z] = 13.5, −60, −3). Coordinates are reported in mm, in MNI space.
Figure 2. Results of the voxelwise analyses.
Results from fractional anisotropy (FA) tract-based spatial statistics and gray matter voxel-based morphometry (VBM) analyses overlaid on mean images. Gray matter VBM results show relative gray matter volume decreases in the juvenile myoclonic epilepsy group in the supplementary motor area and posterior cingulate region (A). Reduced FA is reduced in associated regions of the corpus callosum (B). Statistics overlaid on average images are shown at the bottom of each panel.
White matter (FA) TBSS results.
Two focal decreases in fractional anisotropy were detected within the corpus callosum. One region lay within the anterior aspect of the splenium (p < 0.004 corrected, t-max = 4.73, x, y, z = 10, −32, 10). The other region was in the rostral body of the corpus callosum (p < 0.003 corrected, t-max = 4.97, [x, y, z] = 5, 14, 20). Again, coordinates are reported in mm and in the approximate MNI space of the FSL FA template. As can be seen in figure 2, both clusters are bilateral.
Post hoc probabilistic tractography.
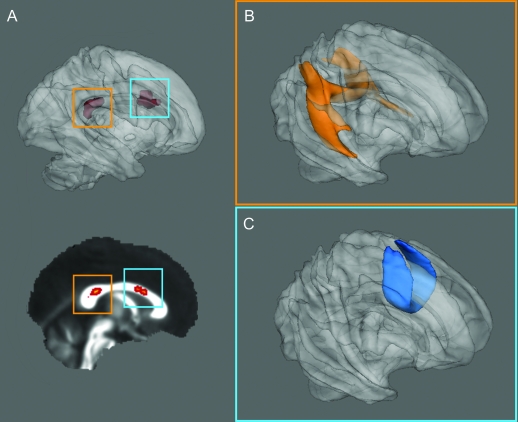
Results of the probabilistic tractography analysis across all subjects are displayed in figure 3. Fibers from the anterior corpus callosal region project to superior frontal regions including the SMA. Fibers from the posterior callosal region project to posterior parietal regions and to inferior mesial temporal regions. The fibers projecting to the mesial temporal lobe may represent short fibers traveling within cingulum bundle; the fibers projecting to the posterior parietal regions represent part of the splenium of the corpus callosum.
Figure 3. Probabilistic tractography of the anterior and posterior regions of change.
Probabilistic tractography group maps (healthy controls and patients with juvenile myoclonic epilepsy) seeded from posterior (A, orange box) and anterior (B, blue box) corpus callosal regions identified in tract-based spatial statistics analysis. (B) Connectivity from the posterior callosal region; projections include the temporo-parietal projections of the cingulum and splenial fibers. (C) Group average connectivity to anterior superior frontal regions from the anterior callosum.
Neuropsychological and brain region relationships.
Two test scores were predicted by regional anatomic differences. The FA values in the anterior SMA regions positively predicted scores on the McKenna picture naming task (F = 6.49, df = 1,24, p < 0.005, R2 = 0.264). The FA values in the posterior cingulate region as well as the corresponding GMV values negatively predicted scores on the trail making task (F = 6.331, df = 2,24, p < 0.007, R2 = 0.308). Neither age nor gender predicted performance.
DISCUSSION
The aim of this study was to investigate the putative focal nature of JME using a multimodal neuroimaging approach that took into account structural, connectional, and cognitive characteristics. Focal differences were detected in the 2 MRI modalities employed, implicating posterior brain regions in addition to frontal lobe dysfunction.
We found that gray matter volumes in superior midline frontal regions were reduced, whereas others have reported increases in comparable areas.26 Reductions in gray matter volume in the posterior cingulate in patients with JME, demonstrated here, are novel. These gray matter changes are given increased credibility by the finding that SMA and the posterior cingulate regional decreases have spatially corresponding decreases in FA. In the case of the anterior callosum region, tractography results show the region to represent callosal tracks connecting the SMA and pre-SMA. Tractography of the posterior white matter region represents not only posterior callosal regions but also cingulum connections to the temporal cortex27 and posterior parietal regions representing part of the splenium. Previously reported extrafrontal changes were found in the occipital lobes of patients with JME, and then only in those who were photosensitive.28
Though the structural changes may not overlap with previous VBM studies, the anatomic changes show a plausible relationship with the neuropsychological changes in the same group. Values of FA underlying the SMA predicted scores on the naming task and a combination of FA and gray matter volume values predicted scores on the trail making task.
The reductions in performance in these neuropsychological tests, in addition to those detected on the letter fluency and similarities tasks, are concordant with prior research showing frontal lobe dysfunction in JME.6,7,9,29,30 However, they also demonstrate performance reductions that are consistent with extrafrontal dysfunction. Although our JME group performed less well on specific cognitive measures, they were otherwise cognitively high functioning. The reduced scores are indicative of a subtle dysfunction, in keeping with some of the original observations made of patients with JME.5
The structural changes also show good consistency with previous functional and neurochemical studies. Functional MRI studies with simultaneous EEG have shown blood oxygenation level–dependent activity positively correlated with spike-and-wave activity in the SMA and thalamus, and negatively correlated with activity in the posterior cingulate, the region of decreased gray matter in our JME group.31 A further study corroborated this, showing 2 additional regions of decorrelated activity in the lateral posterior parietal lobe, a region connected anatomically by our posterior corpus callosal region of decreased FA.32
Research using magnetic resonance spectroscopy (MRS), a method that can measure brain metabolites, also shows good correspondence with our results. Reductions in cortical N-acetylaspartate (NAA) plus N-acetylaspartylglutamate and increases in glutamate and glutamine (GLX) in prefrontal cortex have been detected in regions that, on visual inspection, overlap with the SMA gray matter reductions reported here.33 As NAA is a compound found exclusively in neurons in the adult brain, a reduction therein is thought to be a measure of neuronal dysfunction or loss. An increase in GLX could be considered a measure of increased neuronal excitability. However, this study did not investigate any other cortical regions and did not look specifically at JME, with only a subset of 7 out of 21 patients with IGE having JME. A study considering only patients with JME showed mesial prefrontal-specific reductions in NAA, though the region tested was anterior and inferior relative to the results reported here.34 No differences were found in the thalamus, cerebellum, or occipital regions tested. A more recent study investigating a large network of regions implicated in JME using MRS, including the medial prefrontal cortex, primary motor cortex, occipital cortex, and posterior cingulate cortex, further demonstrated decreased NAA in primary motor cortex, medial prefrontal cortex, and reduced GLX to creatine-phosphocreatine ratios (a proxy measure of neuronal excitability) in the posterior cingulate, overlapping with the regions reported here in the VBM analyses.35
In line with the guidelines of Pell and colleagues36 on maximization of detection power in group comparison VBM, our control group size varied across analyses. However, a subset of participants was kept constant across all 3 analyses. In addition, statistical analysis on the magnetic resonance measures was carried out non-parametrically, thereby making less assumption of the normality of the data.25
As with most studies in epilepsy, separating out the effect of pharmacology on cognition is difficult, so medication represents a significant confounding variable. This is further complicated by the fact that the one of the greatest predictors of pharmacoresistance is the occurrence of multiple seizure types, indicating a more severe form of JME.37 However, the sample of patients with JME reported here was otherwise well-matched with the healthy controls in terms of age, gender, and IQ. Moreover, no patients were using topiramate for seizure control, a compound that has been shown to affect neuropsychological function.38
There has been a longstanding debate in epileptology regarding the concept of “idiopathic generalized” epilepsies and seizures. Studies in animal models strongly suggest focal cortical onset of classic “primary generalized” absence seizures,39 and such findings are beginning to receive support from human investigations.40 We believe our findings add further support to the notion that specific brain networks, implicating focal cortical regions, may underlie idiopathic generalized epilepsies. We anticipate that further studies will elucidate detailed mechanisms at fault in these focal regions, potentially allowing future development of new interventions.
Supplementary Material
Supplemental data at www.neurology.org
- DTI
- diffusion tensor MRI
- FA
- fractional anisotropy
- GLX
- glutamine
- GMV
- gray matter volume
- IGE
- idiopathic generalized epilepsy
- JME
- juvenile myoclonic epilepsy
- MNI
- Montreal Neurological Institute
- MRS
- magnetic resonance spectroscopy
- NAA
- N-acetylaspartate
- SMA
- supplementary motor area
- SPGR
- spoiled gradient echo
- TBSS
- tract-based spatial statistics
- VBM
- voxel-based morphometry
DISCLOSURE
J. O'Muircheartaigh and Dr. Vollmar report no disclosures. Prof. Barker serves on a scientific advisory board for and has received funding for travel and speaker honoraria from GE Healthcare and receives research support from the Medical Research Council UK, the Wellcome Trust, Guy's and St Thomas, Epilepsy Research UK, and the Baily Thomas Charitable Fund. Prof. Kumari and Dr. Symms report no disclosures. Dr. Thompson serves on the editorial board of Seizure and receives research support from the Wellcome Trust. Prof. Duncan has served on scientific advisory boards for GE Healthcare, Eisai Inc., and Sanofi-Aventis; has received funding for travel from Janssen-Cilag; serves on the editorial boards of Seizure, Epilepsy Research, and Epilepsia; may accrue revenue on a patent re: A miniaturized wearable apnoea detector ; receives royalties from the publication of Eyelid Myoclonia and Typical Absences (Libbey, 1995); has received speaker honoraria from UCB and Eisai Inc.; has an active practice in epilepsy surgery; and receives research support from the Medical Research Council UK and the Wellcome Trust. Prof. Koepp has served on scientific advisory boards for GE Healthcare; has received funding for travel from Desitin Pharmaceuticals, GmbH, UCB, and Pfizer Inc.; serves on the editorial boards of Epilepsy Research and Epileptic Disorders; receives research support from MRC, Wellcome Trust, and EU-Framework 7 programme; and he and his spouse own stock in GlaxoSmithKline. Prof. Richardson has served on scientific advisory boards for Schwarz Pharma and UCB; has received funding for travel from Funding Janssen Cilag, UCB, and Eisai Inc.; serves on the editorial board of the Journal of Neurology, Neurosurgery and Psychiatry; and receives research support from the Medical Research Council UK, the Wellcome Trust, Epilepsy Research UK, the Charles Sykes Memorial Fund, King's Medical Research Trust, and the Getty Family Foundation.
REFERENCES
- 1. Genton P, Gelisse P. Juvenile myoclonic epilepsy. Arch Neurol 2001;58:1487–1490 [DOI] [PubMed] [Google Scholar]
- 2. Montalenti E, Imperiale D, Rovera A, et al. Clinical features, EEG findings and diagnostic pitfalls in juvenile myoclonic epilepsy: a series of 63 patients. J Neurol Sci 2001;184:65–70 [DOI] [PubMed] [Google Scholar]
- 3. Panayiotopoulos CP. Idiopathic generalized epilepsies: a review and modern approach. Epilepsia 2005;11;46:1–6 [DOI] [PubMed] [Google Scholar]
- 4. Aliberti V, Grünewald RA, Panayiotopoulos CP, Chroni E. Focal electroencephalographic abnormalities in juvenile myoclonic epilepsy. Epilepsia 1994;35:297–301 [DOI] [PubMed] [Google Scholar]
- 5. Janz D, Christian W. Impulsiv-petit mal. J Neurol 1957;176:346–386 [Google Scholar]
- 6. Devinsky O, Gershengorn J, Brown E, et al. Frontal functions in juvenile myoclonic epilepsy. Neuropsychiatry Neuropsychol Behav Neurol 1997;10:243–246 [PubMed] [Google Scholar]
- 7. Iqbal N, Caswell HL, Hare DJ, et al. Neuropsychological profiles of patients with juvenile myoclonic epilepsy and their siblings: a preliminary controlled experimental video-EEG case series. Epilepsy Behav 2009;14:516–521 [DOI] [PubMed] [Google Scholar]
- 8. Kim S, Hwang Y, Lee H, et al. Cognitive impairment in juvenile myoclonic epilepsy. J Clin Neurol 2007;3:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, et al. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav 2007;10:263–267 [DOI] [PubMed] [Google Scholar]
- 10. Pulsipher DT, Seidenberg M, Guidotti L, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia 2009;50:1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Classification CO, Epilepsy TOTILA. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389–399 [DOI] [PubMed] [Google Scholar]
- 12. Koepp MJ. Juvenile myoclonic epilepsy: a generalized epilepsy syndrome? Acta Neurol Scand Suppl 2005;181:57–62 [DOI] [PubMed] [Google Scholar]
- 13. Roebling R, Scheerer N, Uttner I, et al. Evaluation of cognition, structural, and functional MRI in juvenile myoclonic epilepsy. Epilepsia 2009;50:2456–2465 [DOI] [PubMed] [Google Scholar]
- 14. Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI in patients with idiopathic generalized epilepsy: evidence of widespread cerebral structural changes. Brain 1998;121:1661–1667 [DOI] [PubMed] [Google Scholar]
- 15. Kim JH, Lee JK, Koh S, et al. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. Neuroimage 2007;37:1132–1137 [DOI] [PubMed] [Google Scholar]
- 16. Betting LE, Mory SB, Li LM, et al. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage 2006;32:498–502 [DOI] [PubMed] [Google Scholar]
- 17. Deppe M, Kellinghaus C, Duning T, et al. Nerve fiber impairment of anterior thalamocortical circuitry in juvenile myoclonic epilepsy. Neurology 2008;71:1981–1985 [DOI] [PubMed] [Google Scholar]
- 18. Thompson PJ, Baxendale SA, Duncan JS, Sander JWAS. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry 2000;69:636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300 [Google Scholar]
- 20. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113 [DOI] [PubMed] [Google Scholar]
- 21. Jones DK, Griffin LD, Alexander DC, et al. Spatial normalization and averaging of diffusion tensor MRI data sets. NeuroImage 2002;17:592–617 [PubMed] [Google Scholar]
- 22. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 2006;31:1487–1505 [DOI] [PubMed] [Google Scholar]
- 23. Andersson JLR, Jenkinson M, Smith SM. Non-linear Registration, aka Spatial Normalization: FMRIB Technical Report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007 [Google Scholar]
- 24. Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protocols 2007;2:499–503 [DOI] [PubMed] [Google Scholar]
- 25. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain 1999;122:2101 [DOI] [PubMed] [Google Scholar]
- 27. Seltzer B, Pandya DN. Posterior cingulate and retrosplenial cortex connections of the caudal superior temporal region in the rhesus monkey. Exp Brain Res 2009;195:325–334 [DOI] [PubMed] [Google Scholar]
- 28. Lin K, Jackowski AP, Carrete H, et al. Voxel-based morphometry evaluation of patients with photosensitive juvenile myoclonic epilepsy. Epilepsy Res 2009;86:138–145 [DOI] [PubMed] [Google Scholar]
- 29. Piazzini A, Turner K, Vignoli A, Canger R, Canevini MP. Frontal cognitive dysfunction in juvenile myoclonic epilepsy. Epilepsia 2008;49:657–662 [DOI] [PubMed] [Google Scholar]
- 30. Sonmez F, Atakli D, Sari H, Atay T, Arpaci B. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav 2004;5:329–336 [DOI] [PubMed] [Google Scholar]
- 31. Gotman J, Grova C, Bagshaw A, et al. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA 2005;102:15236–15240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamandi K, Salek-Haddadi A, Laufs H, et al. EEG-fMRI of idiopathic and secondarily generalized epilepsies. NeuroImage 2006;31:1700–1710 [DOI] [PubMed] [Google Scholar]
- 33. Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton MRS reveals frontal lobe metabolite abnormalities in idiopathic generalized epilepsy. Neurology 2003;61:897–902 [DOI] [PubMed] [Google Scholar]
- 34. Savic I, Lekvall A, Greitz D, Helms G. MR spectroscopy shows reduced frontal lobe concentrations of N-acetyl aspartate in patients with juvenile myoclonic epilepsy. Epilepsia 2000;41:290–296 [DOI] [PubMed] [Google Scholar]
- 35. Lin K, Carrete H, Lin J, et al. Magnetic resonance spectroscopy reveals an epileptic network in juvenile myoclonic epilepsy. Epilepsia 2009;50:1191–1200 [DOI] [PubMed] [Google Scholar]
- 36. Pell GS, Briellmann RS, Chan CH, et al. Selection of the control group for VBM analysis: Influence of covariates, matching and sample size. NeuroImage 2008;41:1324–1335 [DOI] [PubMed] [Google Scholar]
- 37. Gelisse P, Genton P, Thomas P, et al. Clinical factors of drug resistance in juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry 2001;70:240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meador KJ, Loring DW, Vahle VJ, et al. Cognitive and behavioral effects of lamotrigine and topiramate in healthy volunteers. Neurology 2005;64:2108 [DOI] [PubMed] [Google Scholar]
- 39. Meeren H, Pijn J, Van Luijtelaar E, Coenen A, Lopes da Silva F. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci 2002;22:1480–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vaudano A, Laufs H, Kiebel S, et al. Causal hierarchy within the thalamo-cortical network in spike and wave discharges. PLoS One 2009;4:e6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.