Abstract
The detection of salient or instrumental stimuli and the selection of cue-evoked responses are mediated by a fronto-parietal network that is modulated by cholinergic inputs originating from the basal forebrain. Visual cues that guide behavior are more strongly represented in the posterior parietal cortex (PPC) than are similar cues that are missed or task-irrelevant. Although the crucial role of cholinergic inputs in cue detection has been demonstrated by lesion studies, the role of PPC neurons in the cholinergic modulation of cue detection is unclear. We recorded extracellular spikes from PPC neurons of rats performing a sustained attention task, before and after selective removal of cholinergic inputs to the recording site. Visual cues that were subsequently detected evoked significant increases in the PPC firing rate. In the absence of cholinergic input, the activation of PPC neurons by detected cues was greatly diminished. When a visual distractor was introduced during task performance, a population of PPC neurons selectively responded to the distractor. As a result of cholinergic deafferentation, distractor-related neuronal activity was enhanced, and the detection-related activity was further suppressed. Thus, in deafferented subjects, the distractor lowered the signal-to-noise ratio of cue-evoked responses. This impairment in cue-evoked neuronal activity may have mediated the increased response latencies observed for detected cues in the presence of the distractor. Additional experiments demonstrated that the effects of cholinergic deafferentation were not confounded by extended practice or electrode depth. Collectively, these findings indicate that cholinergic inputs to PPC neurons amplify cue detection, and may also act to suppress irrelevant distractors.
Keywords: 192 IgG-saporin, acetylcholine (ACh), neuronal ensembles, posterior parietal cortex, rat
Introduction
Cue detection involves multiple cognitive operations, including shifting attention from the processing of task-irrelevant stimuli to cue detection and cue-evoked processing of response rules (Parikh & Sarter, 2008). Importantly, in this context, `detection' is defined as a cognitive process consisting of `the entry of information concerning the presence of a signal in a system that allows the subject to report the existence of the signal by an arbitrary response indicated by the experimenter' (Posner et al., 1980). The necessity of basal forebrain cortical cholinergic activity for cue detection in rats has been extensively documented (McGaughy et al., 1996, 2002; McGaughy & Sarter, 1998; Burk et al., 2002). Removal of cortical cholinergic input specifically reduces the detection of cues without affecting performance on non-signal trials (McGaughy et al., 1999). The performance of tasks that tax attentional resources is associated with increases in acetylcholine (ACh) efflux in the frontoparietal cortex (Dalley et al., 2001; Arnold et al., 2002). Furthermore, cholinergic dysfunction contributes to the severity of the cognitive decline in Alzheimer's disease (Mesulam, 2004).
The posterior parietal cortex (PPC) is an integral part of the frontoparietal network that mediates cue detection and attentional performance. The PPC is hypothesized to encode a salience map that ranks the significance of stimuli in the visual field (Gottlieb et al., 1998; Kusunoki et al., 2000; Gottlieb, 2007). This map is constructed of bottom-up visual signals that are modulated by top-down signals, depending on the task conditions. Neurophysiological studies in primates and rodents have demonstrated that salient distractors (Powell & Goldberg, 2000; Constantinidis & Steinmetz, 2005; Bisley & Goldberg, 2006) and instrumental cues for guiding action (Goodale & Milner, 1992; Constantinidis & Steinmetz, 2001; Broussard et al., 2006; Nitz, 2006) compete for representation in the PPC (Corbetta & Shulman, 2002; Constantinidis & Steinmetz, 2005). Goal-driven enhancement of PPC activity may reflect the shifting of attention from ongoing behavior and irrelevant stimuli to the processing of response rules that guide cue-evoked behavior (Bunge et al., 2002; Corbetta & Shulman, 2002; Yantis et al., 2002). Furthermore, the discrimination of relevant stimuli from irrelevant distractors may be mediated by circuitry involving the PPC, as indicated by stimulus discrimination impairments observed in patients with damage to the PPC (Driver & Vuilleumier, 2001; Friedman-Hill et al., 2003).
Pharmacological increases in cholinergic activity enhance parietal encoding and accelerate the latency of response to instrumental stimuli (Furey et al., 2000, 2008). At the cellular level, cholinergic activity has been proposed to enhance the processing of thalamic input while suppressing cortical feedback within the cortex (Hasselmo & Bower, 1992; Hasselmo, 1995; Sarter et al., 2005; Zinke et al., 2006). Although bilateral cholinergic parietal lesions have been previously shown to attenuate attentional performance in the presence of a distractor (Gill et al., 1999), the precise nature of the cholinergic contribution to parietal processing of cues at the level of the single cell is unclear. Owing to the sensitivity of the sustained attention task to cholinergic disruption, we hypothesized that cholinergic input to the PPC is necessary for the detection of cues in the presence of a distractor. In order to minimize the confounding behavioral effects that bilateral cortical deafferentation may have on our physiological results, we infused the cholinotoxin unilaterally into the PPC.
Materials and methods
Subjects and apparatus
Six-week-old male Long-Evans rats (n = 11, 250–300 g; Harlan, Indianapolis, IN, USA) were housed singly in climate-controlled cages on a 12 : 12-h light/ dark cycle (lights on at 06:00 h). Rats were handled extensively upon arrival, and provided with food and water ad libitum until 3 days before training, after which water was gradually restricted to 1 h/ day. Rats received a water reinforcer during daily training sessions, and received supplemental water following training. All animals were trained for 5–7 days a week. Animal care and experimentation were performed in accordance with protocols approved by the Ohio State University Institutional Animal Care and Use Committee, in accordance with NIH Guidelines for the Care and Use of Laboratory Animals.
The operant chambers have been described in detail elsewhere (Broussard et al., 2006). Briefly, three panel lights, three fixed levers and a house light near the ceiling of the chamber were present on one panel of the operant chamber. Only the center cue light and two side levers were operable. The opposite panel contained a recessed water port with a water dispenser and a tone generator on the outside of the operant chamber. All chambers were placed within a sound-attenuated shell. An operant chamber with a similar configuration fitted for neurophysiological recording and video recording was used for testing sessions.
Behavioral training
The sustained attention task was modified from the original task characterized by McGaughy & Sarter (1995). The current task uses fixed response levers, as opposed to retractable levers, in order to minimize electrical interference with the neurophysiological signal.
There were four stages of training in the sustained visual attention task. A house light was illuminated during all phases of training. Rats were initially trained to press each of two levers on an FR-1 schedule of reinforcement. Once rats had made at least 50 responses on each lever during a 1-h session for three consecutive days, they were trained in the sustained visual attention task.
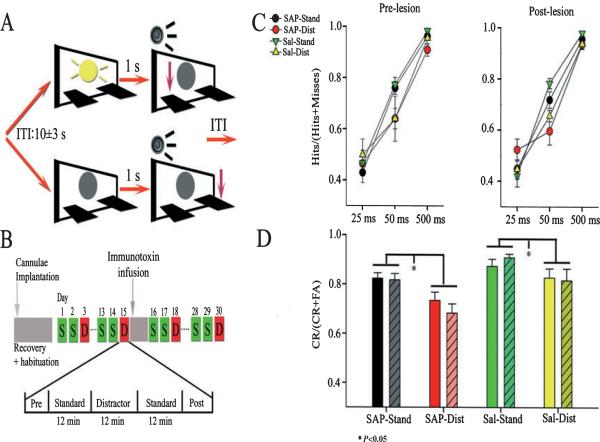
The rules of the sustained attention task were presented in the second stage of training (Fig. 1). The rules required rats to detect the presence of signal events (illumination of the central panel light for 500 ms) and correctly reject non-signal events (central panel light remained off). Both types of event were followed 1 s later by a 200-ms tone that initiated a 4-s response window. Following the presentation of the signal and subsequent tone, a left lever press was positively reinforced with a drop of water and scored as a hit. A right lever press on a signal trial was not reinforced, and was scored as a miss. On non-signal trials, a right lever press was positively reinforced and scored as a correct rejection, whereas a left lever press on non-signal trials was not reinforced and was scored as a false alarm. If a response was not made within 4 s, the trial was scored as an omission. Either a response or an omission initiated a variable intertrial interval (ITI; 9 ± 3 s). At this stage of training, an incorrect response led to a correction trial in which the same type of trial was repeated up to five consecutive times or until the rat made a correct response. Each behavioral training session included a 36-min task period preceded and followed by 5-min task-free periods.
Fig. 1.
Depiction of task performance, timeline of main experimental events, and behavioral results. (A) At the start of each trial, cues (illuminations of a panel light at 25, 50 or 500 ms) are either presented (top sequence) or not (non-signal trial; bottom sequence). A 250-ms tone is initiated 1 s later, which opens a 4-s operant window. Animals were required to press one lever to report a hit and the other lever to report a correct rejection to receive a reward. A response or an omission (no response in 4 s) initiates a variable intertrial interval (ITI) (10 ± 3 s) for the next trial. (B) Single unit activity was recorded from task-performing animals for two standard sessions (S) and a distractor session (D; house light flashes at 0.5 Hz for the middle 12-min block of trials). After recordings had been collected from 15 sessions with intact PPC neurons, a 0.5-L bolus of the selective cholinotoxin 192-IgG saporin (SAP) (n = 5) or Dulbecco's saline (n = 2) was infused near the recording site of animals. Recordings were then collected from 15 post-infusion sessions (10 standard, five distractor) for each animal. (C and D) Behavioral performance as a function of signal duration, distractor and trial block. (C) Plots showing the relative number of hits during standard and distractor sessions. Cue detection was signal duration-dependent (F2,44 = 254.52, P < 0.05), and was hampered by the distractor (F2,44 = 6.5, P < 0.05). (D) The bar graphs show that the distractor (Dist) reduced the number of correct rejections relative to standard session performance (Stand) in lesioned and saline-infused animals (Dist; F2,44 = 18.82, *P < 0.05). Solid bars represent performance prior to SAP or saline infusions, and shaded bars represent post-infusion data. Cholinergic denervation did not affect accuracy on either signal or non-signal trials. CR, correct rejection; FA, false alarm.
In the third stage, correction trials were removed, and signal and non-signal trials were presented with equal probability throughout each 36-min session. For the animals to advance to the final stage of training, they were required to perform with an accuracy of 70% or higher on both signal and non-signal trials, with < 30% omissions for three consecutive days.
During the final stage of training, the task was modified to further tax attention. These modifications are based on findings in humans, in which variations in signal duration and increases in the event rate have been shown to challenge attentional performance (Parasuraman et al., 1987). Signals of three durations (25, 50 and 500 ms; Fig. 1A) were presented with non-signal trials, and the ITI was reduced to 10 ± 3 s. The criterion was raised to 75% correct responses on both types of trial and to < 25% omissions. Testing that took place using this task was called a standard session.
After reaching criterion performance for three consecutive days, rats performed in a distractor session, which was identical to the final stage of training except that a distractor was presented during the second 12-min block of the task period. The distractor consisted of turning off the house light, which is normally on, for a 1-s interval every 2 s, so that it alternated between on and off at 0.5 Hz. Previous studies using this task have demonstrated that this distractor impairs performance on signal and non-signal trials (Gill et al., 2000). Each rat received at least three distractor sessions that were separated by at least two standard sessions. Following the third distractor testing session, rats were transferred to an operant chamber equipped for electrophysiological recording, and required to reach criterion levels of performance on the final training stage. All rats reached criterion within 2–5 months of training. The rats underwent implantation surgery following three additional distractor sessions in this new environment.
Electrode and infusion cannula implantation
Two tetrodes were inserted into a 26-gauge cannula (15 mm) and extended ~1 mm beyond the distal end of the cannula. The cannula and tetrodes were affixed to a moveable headstage, as described in Broussard et al. (2006). The eight lead wires were soldered into separate channels of an eight-channel headstage (Plexon Inc., Dallas, TX, USA). Two separate Teflon-coated, stainless steel, 250-μm electrodes (A–M Systems, Everitt, WA, USA) were soldered into to the headstage as well, and served as a reference and ground for recordings. A 26-gauge, 17-mm infusion cannula was placed within ~1 mm of the electrode guide cannula for the purpose of infusing saline or 192-IgG saporin (SAP) near the recording site. In order to minimize damage to the cortex, the 26-gauge cannulae were placed directly above the cortex during surgery and, over the course of testing, were advanced at no more than 70 μm/day.
Rats trained to criterion performance were anesthetized with isoflurane gas mixed in oxygen. Body heat was maintained at approximately 37°C with a thermal pad (Deltaphase IV, Braintree, MA, USA). The tetrodes and a single infusion cannula were implanted unilaterally using the stereotaxic coordinates anteroposterior −4.5 mm, mediolateral ± 2.5 mm and dorsoventral −1.0 mm from the dura surface, in accordance with previous anatomical evidence of the rat homolog of PPC (Reep et al., 1994). Four additional burr holes were drilled into the skull, and machine screws were threaded into these. The reference electrode was placed in the contralateral somatosensory cortex, and the ground electrode was wrapped around a machine screw. The carrier, headstage and stainless steel electrodes were affixed to the skull with dental cement. Lidocaine and antibiotics were applied to the wound immediately after surgery. Rats were allowed 1 week to recover from surgery in their home cages with free access to food and water, after which access to water was reduced before resumption of behavioral testing and neurophysiological recording.
Neurophysiological recording sessions
Electrical potentials were collected with a head-mounted operational amplifier and sent via a cable to a commutator that relayed the signal to two differential amplifiers (A–M Systems, Carlsburg, WA, USA). The analog signals were amplified (×10 000), bandpass-filtered between 300 Hz and 5 kHz, and digitized with an analog-to-digital board at 250 kHz (CED Power 1401; Cambridge Electronics Design, Cambridge, UK). Signals that exhibited peak amplitudes exceeding a user-defined threshold on either electrode were sampled at 11 kHz using Spike 5 software (Cambridge Electronics Design). Multiple unit activity on each tetrode was separated into single units on the basis of the matching of templates automatically generated by Spike 5. The isolated single units were recorded during two consecutive standard sessions and one distractor session. The microdrive was advanced after distractor sessions, and a new population of neurons was recorded.
Following five distractor sessions of recording from intact PPC (15 sessions in total), rats were briefly anesthetized using isoflurane, a 30-gauge infusor was lowered 0.5 mm below the tip of the infusion cannula, and a 0.5-μL infusion of the cholinotoxin SAP (n =7) in Dulbecco's saline (ATS, San Diego, CA, USA; 0.15 μg/μL) was delivered. Rats in the sham group (n = 4) received 0.5-μL infusions of Dulbecco's saline. Behavioral testing was resumed 10 days post-infusion. After 1–2 days of re-training, animals were back to criterion levels of behavioral performance, and 15 more post-infusion testing sessions were recorded (see Fig. 2). Four subjects were withdrawn from the study; one animal in the SAP group showed no loss of acetylcholinesterase (AChE) staining, and three subjects lost their headsets before post-infusions recordings could take place.
Fig. 2.
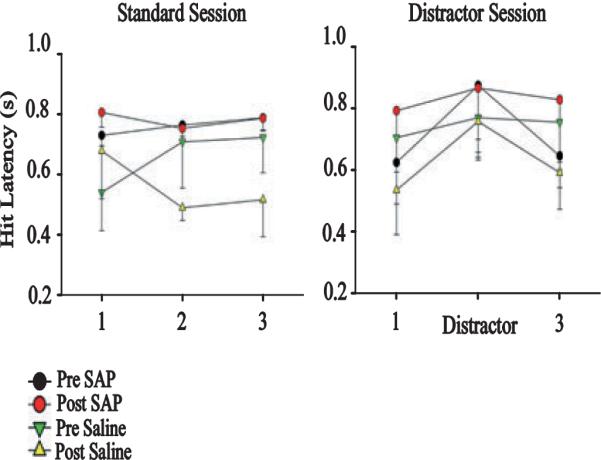
Cholinergic deafferentation delays hit latency during distractor sessions. During standard sessions, lesioned subjects show an initial, non-significant increase in latency (F2,44 = 0.26; P > 0.05). During distractor trials, cortically deafferented subjects display a significant increase in response latency during standard performance blocks 1 and 3 relative to saline-infused subjects (F1,22 = 5.88; P < 0.05). Distractors also delayed hit latency on both groups (F2,44 = 6.70; P < 0.05). SAP, 192-IgG saporin.
Histology
Following the final electrophysiological recording session, animals were anesthetized with sodium pentobarbital (100 mg/kg), and the final recording site was marked with a small electrolytic lesion (15 μA for 30 s on each channel of the two tetrodes), using a stimulator and stimulus isolation unit (Grass Instruments, Quincy, MA, USA). Rats were transcardially perfused with 0.9% cold saline followed by 4% paraformaldehyde. The brains were post-fixed for 24 h in 4% paraformaldehyde and transferred to a 30% sucrose solution in 0.1 m phosphate buffer (pH 7.4). Each brain was sliced into 50-μm sections; sections within 100 μm of the electrolytic lesion were stained for Nissl substance with cresyl violet, and the remaining PPC slices were stained for AChE-positive fibers (Tago et al., 1986). These procedures determined the extent of cholinergic loss due to SAP infusion. Sections were placed for 7–10 min in 0.1% H2O2, and then rinsed with 0.1 M maleate buffer (pH 6.2). Subsequent to rinsing, sections were incubated in a solution comprising 5 mg of acetylthiocholine, 0.147 g of sodium citrate, 0.075 g of copper sulfate and 0.0164 g of potassium ferrocyanide in 1.0 L of 0.1 M maleate buffer for 45 min. Following completion of this incubation, sections were rinsed using 50 mm Tris buffer (pH 7.6), and placed in a secondary incubation solution. This solution contained 0.05 g of diaminobenzidine and 0.375 g of nickel ammonium sulfate in 125 mL of 50 mm Tris buffer. After 10 min, 20 mL of 0.1% H2O2 was added for an additional 2 min to produce sufficient cortical layering of the stain. Sections were then rinsed in 5 mm Tris buffer, and mounted on gelatin-coated slides. After being dried overnight, sections were dehydrated in ethanol and de-fatted in xylene prior to cover slipping. Microphotographs were taken of the Nissl-stained and AChE-stained sections of the PPC, and analysed using standard thresholding techniques in ImageJ (Rasband, 1997–2009).
Behavioral measures
The behavioral measures generated for statistical analysis included response accuracy on signal trials [hits/(hits + misses)] and on non-signal trials [correct rejection/(correct rejections + false alarms)], response latency, and errors of omission. The behavioral measures were generated from distractor and baseline sessions. Baseline testing sessions with > 25% overall omissions from standard or distractor sessions were excluded from the statistical analyses. Percentage data were angularly transformed before analysis to correct for the skewed distribution of percentage scores (Zar, 2007).
Repeated-measures anovas were conducted on the behavioral data using session (baseline and distractor) and signal duration (25, 50, and 500 ms) as within-subjects factors for analysing the dependent measures of signal response accuracy, non-signal response accuracy, errors of omission and reaction time on signal and non-signal trials, as well as response lever side bias. A Huyn-Feldt correction was applied to all repeated measures to control for possible violations of the sphericity assumption of homogeneity of variances. The criterion for statistical differences was set at α < 0.05.
Neurophysiological measures
Single units exhibiting more than 800 total spikes during the recording sessions were used for the electrophysiological analysis (0.30 spikes/s). Nearly all cells detected exceeded this threshold, and the average firing rate was 2.46 ± 0.18 spikes/s (range, 0.092–22.29 spikes/s). The firing rate of the majority of these neurons (68%) was below 2 spikes/s, 22% of neurons had a firing rate between 2 and 5 spikes/s, 8% of neurons had a firing rate between 5 and 9.5 spikes/s, and 2% had a firing rate higher than 9.5 spikes/s. Fast-spiking neurons are considered to have a firing rate higher than 9.5 spikes/s and have low amplitude spikes relative to pyramidal cells (Constantinidis & Goldman-Rakic, 2002). Tetrode recordings are biased to recording from larger pyramidal cells, the action potentials from which remain above noise level for a greater distance from the cell itself (Logothetis, 2003). Posterior parietal unit activity was divided into 20-ms bins, and changes in activity were analysed in 2-s epochs. Analysis centered on stimulus presentation (e.g. signal light, tone, and distractor light) and behavioral responses (e.g. correct rejections, hits, misses, and false alarms), which were assessed using peri-event time histograms (PETHs). Because unit activity often violates the assumption of normality, Wilcoxon signed-rank tests were used to analyse pre-event and post-event epochs, with the criterion for statistical significance set at α < 0.01. A chi square analysis was applied to assess the proportion of neuron pairs exhibiting increases or decreases in task-related activity as a function of distractor or cholinergic deafferentation. Once the population of neurons exhibiting stimulus-driven activity had been determined, a calculation of the signal-to-noise ratio (SNR) was used to quantify how much the neuronal activity exceeded the background activity if a visual stimulus was present. The SNR was calculated for pre-lesion and post-lesion animals as the stimulus-driven response (Rstim) divided by the sum of the stimulus-driven response and background activity (Rspont) for each individual PETH [SNR = Rstim/(Rstim + Rspont)]. Values range from 0 to 1, in which a value of 0.5 indicates an equal distribution of spikes before and after the stimulus, and values approaching 1 indicate that the signal-evoked response is much higher than the background activity.
Results
Histology
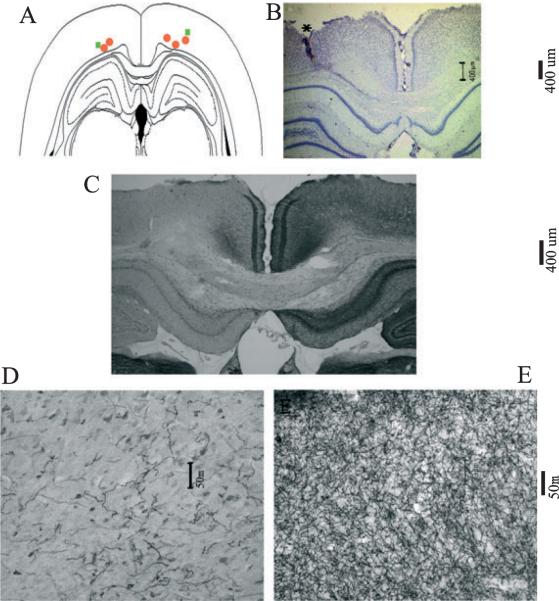
Verification of the placement of the recording electrodes was conducted on cresyl violet-stained brain sections. The final placement of the electrodes was determined by localizing the electrolytic lesion and reconstructing the dorsoventral path of the electrodes through the PPC. The coronal section represented in Fig. 3 illustrates that the final placement of recording electrodes in all rats was within the deep layers of the PPC (III-VI). As illustrated in Fig. 3B, damage to the superficial layers of the PPC was minimal, and tissue 150 μm from the center of the electrolytic lesion (Fig. 3C, left hemisphere) showed no damage to the superficial layers. Histological evaluation of the removal of cholinergic afferents within the PPC was conducted on AChE-stained brain sections. Images taken at ×20 magnification were analysed using a within-subjects ANOVA (lesion, F1,35 = 119.605, P < 0.05). Each of the five SAP-infused rats exhibited at least a 75% decrease in the density of AChE-positive fiber staining. The lesion destroyed cholinergic input to both medial and lateral aspects of the secondary occipital cortex (Paxinos & Watson, 1998), and area 1 of the parietal cortex. Together, these regions of the rat cortex correspond to the primate PPC, in that they receive input predominantly from the lateral dorsal and lateral posterior thalamus (Reep et al., 1994). Cholinergic deafferentation of the dorsal CA1 was also observed. The frontal, temporal and primary visual and other sensory cortices of the lesioned hemisphere retained cholinergic afferents, as did the entire contralateral cortex.
Fig. 3.
Electrode path and 192 IgG-saporin-induced acetylcholinesterase (AChE)-positive fiber loss within the posterior parietal cortex (PPC). (A) Schematic of a coronal section through the level of the PPC (4.3–4.5 mm posterior to bregma), illustrating the final recording sites of the recording electrodes in the left or right hemispheres. Rats were infused with 192-IgG saporin (SAP) (n = 5, red circles) or saline (n = 2, green squares). (B) Photomicrograph (×4) of the electrolytic lesion at the final recording site of Nissl-stained PPC. Electrolytic lesions from each animal were within PPC layers III–V of the left hemisphere. (C) Restricted loss of AChE-positive fibers to the PPC near the final recording site in the left parietal cortex. SAP lesions reduced the density of AChE-positive fibers by 75% as compared with the contralateral cortex (F1,35 = 119.605, P < 0.05). (D) Higher-magnification (×20) photomicrograph of AChE-positive fibers from the same site as in C. (E) High-magnification photomicrograph of the contralateral AChE fibers from the PPC of the same animal as in C.
Behavioral performance
Video monitoring of head position during task performance was performed simultaneously with neurophysiological recordings. Successful performance within a short latency required an animal to face the signal light and levers (Fig. 1). As seen in Figs 2 and 4, animals responded within 1.5 s in the majority of trials. After successful performance, subjects immediately entered the water port to retrieve the reward. The minimum 6-s ITI allowed animals to retrieve the water reward and reorient towards the signal light. The detection of visual cues during standard attention task performance was signal duration-dependent (F2,44 = 254.52, P < 0.05), with a significant linear trend (F1,22 = 447.30, P < 0.05). Analysis of latency on hit trials indicated a signal duration effect, with rats responding at shorter latencies to 500-ms cues (25 ms, 0.93 ± 0.05 s; 50 ms, 0.79 ± 0.04 s; 500 ms, 0.69 ± 0.04 s; F2,44 = 20.49, P < 0.05). In standard sessions, neither cued nor non-signal performance was affected by time on task (cue, F2,44 = 0.55, P > 0.05; non-signal, F2,44 = 1.064, P > 0.05).
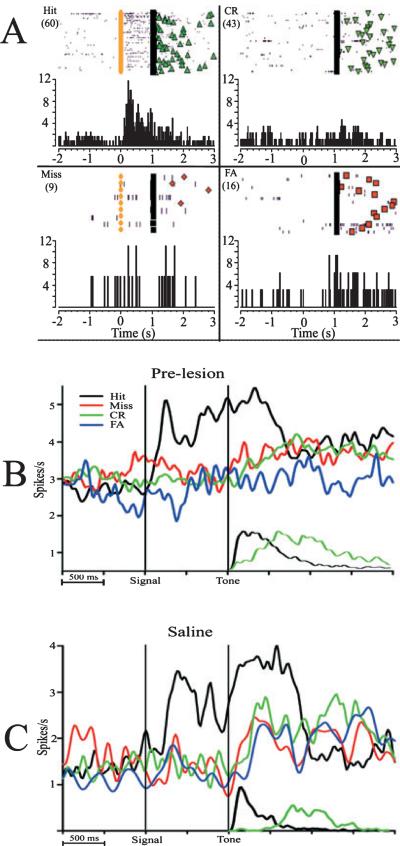
Fig. 4.
Behaviorally relevant cues elicit increases in firing rate from posterior parietal cortex (PPC) neurons. (A) Raster plot and histogram of a single PPC neuron during the four response types. Clockwise from top left: correct detection of a signal (Hit), incorrect rejection of a signal (Miss), correct rejection (CR) of non-signal trials, and incorrect detection of non-signal trials [false alarms (FAs]. The colored circles represent the onset of the visual signal; the black line represents the onset of the tone, opening the operant window on all trials. Symbols following the black line represent the behavioral response on each trial (green triangles, Hit; inverted green triangle, CR; orange diamond, Miss; orange square, FA). Rasters are organized by trial number, with the first trial on the top. The number of trials in each session is indicated in parentheses at the top of each graph. (B) Neural responses from 92 PPC neurons prior to infusions. The response to the visual cue initially peaks at 220 ms following cue presentation, and activation remains sustained until the hit response. The cue-evoked response was greater on hit trials than on miss trials. Smaller curves on the bottom represent the reaction time distributions for correct responses on cued and non-signal trials. False alarm and miss trials have a similar distribution and latency as CRs, and have been omitted for clarity. (C) Neural responses from 25 cue-driven PPC neurons following saline infusions exhibit a similar, detection-specific pattern.
As illustrated in Fig. 1, the presentation of the visual distractor decreased the accurate detection of cues (F2,44 = 6.5, P < 0.05) and correct rejection of non-signals (F2,44 = 18.82, P < 0.05). Post hoc analyses indicate that the distractor had no effect on performance on 25-ms cues (F2,44 = 1.84, P > 0.05), but impaired hit performance on the two longer cues (50 ms, F2,44 = 15.78, P < 0.05; 500 ms, F2,44 = 4.72, P < 0.05). The presence of the distractor significantly delayed latency of response to the cue (baseline latency, 0.74 ± 0.05 s; distractor latency, 0.88 ± 0.06 s; F2,44 = 3.63, P < 0.05), and the signal duration effect on latency, that is, slower responses to the shorter cues, was maintained in distractor sessions (F2,44 = 18.58, P < 0.05). Distractors did not significantly affect errors of omission (standard, 13.7% ± 0.69%; distractor, 11.8% ± 0.79%).
As predicted, unilateral cholinergic deafferentation of the PPC did not affect the rats' ability to detect cues in either standard sessions (cue, F1,22 = 0.16, P > 0.05; non-signal, F1,54 = 0.32, P > 0.05) or distractor sessions (cue, F1,22 = 0.002, P > 0.05; non-signal, F1,22 = 0.47, P > 0.05). Furthermore, distractor-induced decreases in the detection of cues and correct rejection of non-signals was unaffected by unilateral cholinergic deafferentation. The latencies of all responses under standard conditions were not significantly different following the lesion (hit, F1,41 = 1.08, P > 0.05, all other responses P > 0.05). Cholinergic deafferentation produced longer latencies on hit trials during the distractor sessions (pre-lesion, 0.74 s; post-lesion, 0.85 s; F1,22 = 4.94, P < 0.05), an effect not found on other response types (all P > 0.05). Further analysis of the distractor sessions by block indicated that the delayed response latency occurred during the non-distractor blocks of this task (Fig. 2). Saline infusions had no effect on latency or accuracy (all P > 0.05).
Significant activation of PPC neuronal activity during the detection of cues
Multiple unit activity from the PPC was recorded for each 46-min session. During each session, 2–24 units were isolated from multiple unit activity using principal component analysis of aspects of the waveform and template matching using Spike 5 software (Cambridge Electronics Design). Prior to cholinergic deafferentation, 350 units were isolated during standard testing sessions and 195 units were isolated during distractor sessions.
Neuronal responses in standard sessions were characterized by increases in unit activity following the presentation of the signal light on hit trials. Cue-evoked activity was the most prevalent behavioral correlate, as 26% of neurons (92/ 350; Tables 1 and 2) exhibited significant activation relative to ITI activity (P < 0.01, Wilcoxon signed-rank test). The data shown in Fig. 4A exemplify the increases in firing rate of a single PPC neuron in response to the cue on hit trials, but not non-signal trials or missed trials. This population exhibited a 53% increase in firing rate during the 1-s epoch following the cue (baseline, 2.74 ± 0.15 spikes/s; post-cue, 4.24 ± 0.69 spikes/s), with a peak latency of 220 ms post-cue (Fig. 4). Following infusions of saline and further testing, 36 of 131 (27%) of parietal units were driven by the visual cue. This population exhibited a 54% increase in firing rate during the 1-s post-cue epoch (baseline, 1.42 ± 0.03 spikes/s; post-cue, 2.59 ± 0.08 spikes/s). Importantly, as predicted, neurons in this population were only modulated during detections of the visual cue.
Table 1.
Behavioral correlates of posterior parietal cortex unit activity during sustained visual attention
| Response | Pre-lesion | Saline | SAP |
|---|---|---|---|
| Hit (Cue-evoked) | 26 (92) | 27 (37) | 18 (62)* |
| Miss | 8 (28) | 4 (5) | 8 (27) |
| Correct rejection | 8 (28) | 7 (9) | 8 (28) |
| False alarm | 5 (19) | 8 (11) | 5 (22) |
Data are expressed as the percentage of units (absolute number noted in parentheses) displaying significant behavioral correlates from the total number of units recorded [pre-lesion, 350; saline-infused 136, 192-IgG saporin (SAP)-infused, 331].
P < 0.05, compared with both both pre-lesion and unlesioned (chi square test).
Table 2A.
Cue-evoked correlates to the visual cue: saline group
| Subject number | Pre-saline standard | Pre-saline distractor | Post-saline standard | Post-saline distractor |
|---|---|---|---|---|
| Saline 1 | 23 (17/75) | 11 (6/56) | 14 (6/44) | 21 (10/47) |
| Saline 2 | 36 (20/56) | 55 (10/18) | 37 (19/52) | 22 (7/32) |
Data are expressed as the percentage and number of neurons exhibiting significant cue-evoked correlates by condition. The total numbers of neurons collected from subjects are noted on the right of the divisor.
Effects of signal duration on cue-evoked PPC activity
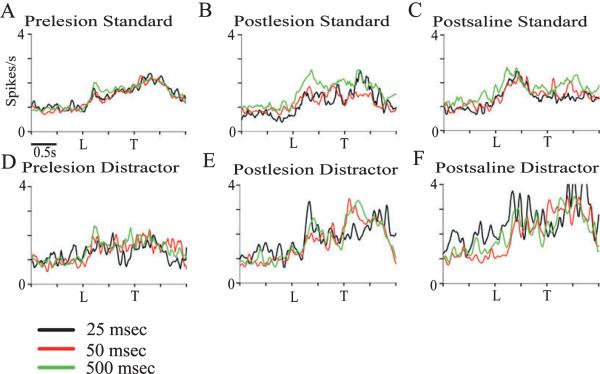
In order to assess the effects of signal duration on PPC neuronal activity, those neurons that exhibited significant cue-evoked activity (92/ 350 of pre-SAP neurons, and 37/ 136 of pre-saline neurons) were further analysed by signal duration. The average firing rate of this population increased at each duration; detected 25-ms cues evoked a 39% increase in firing rate, 50-ms cues evoked a 40% increase in firing rate, and 500-ms cues evoked a 45% increase in firing rate. There was an effect of signal duration on average firing rate of the prelesion population under standard task conditions (F2,236 = 7.17, P < 0.05). Statistical analysis of each neuron indicated that longer cues activated a greater proportion of neurons, with 25-ms cues eliciting significant activation from 41/ 129 cells (31%), 50-ms cues from 67/ 129 cells (52%), and 500-ms cues from 92/ 129 cells (71%). In each condition, 25-ms signals tended to evoke fewer correlates than longer signals (Table 3). However, in the remaining five conditions, signal duration did not modulate parietal responses (all P > 0.05; Fig. 5). In order to further investigate whether PPC activity was a function of signal duration, the SNR was calculated from the firing rate of each cell at all durations. Analysis of the SNR of these neurons indicated no significant effect of signal duration (P > 0.05, Friedman test).
Table 3.
Cue-evoked correlates analysed by signal duration
| Task condition | Total cells | 25 ms | 50 ms | 500 ms |
|---|---|---|---|---|
| Pre-lesion standard | 129 | 32 (41/129) | 52 (67/129) | 71 (92/129) |
| Post-lesion standard | 62 | 39 (24/62) | 52 (32/62) | 60 (37/62) |
| Post-saline standard | 25 | 52 (13/25) | 84 (21/25) | 80 (20/25) |
| Pre-lesion distractor | 58 | 43 (25/58) | 45 (26/58) | 43 (25/58) |
| Post-lesion distractor | 18 | 22 (4/18) | 38 (7/18) | 77 (14/18) |
| Post-saline distractor | 17 | 47 (8/17) | 88 (15/17) | 82 (14/17) |
Data are expressed as the percentage and number of neurons exhibiting significant cue-evoked correlates by condition.
Fig. 5.
Cue-driven activity at 25-ms, 50-ms and 500-ms signal durations, normalized to the pre-signal average firing rate. The top panels represent the cue-evoked firing rate of all hit-related neurons for each standard session condition; the bottom panels represent the firing rate of hit-related neurons during distractor conditions. (A) Prior to saline or saporin infusions 500-ms cues elicited a larger neurophysiological response (see text). (B) Cue-driven activity in standard sessions following cholinotoxin lesions. All durations evoked a similar response from cue-driven neurons (F2,122 = 2.08, P > 0.05). (C) Cue-driven activity in standard sessions following saline infusions (F2,48 = 1.14, P > 0.05). (D) Pre-lesion cue-driven activity in distractor sessions (F2,98 = 0.05, P > 0.05). (E) Cue-driven activity in distractor sessions following cholinotoxin lesions (F2,34 = 0.34, P > 0.05). (F) Cue-driven activity in distractor sessions following infusions of saline (F2,24 = 1.35, P > 0.05). L, signal light; T, tone.
Distractor-induced modulation of cue-evoked activation of PPC neurons
The presentation of a visual distractor decreased the proportion of neurons exhibiting cue-evoked activation. Specifically, the relative number of neurons exhibiting cue-evoked activity (36/ 208, 17%) declined significantly relative to standard sessions [92/ 350 (26%), χ2 = 9.9, P < 0.05; Table 4]. Distractors similarly reduced the proportion of neurons exhibiting cue-evoked activity following saline infusions (saline-infused subjects: standard session, 37/ 136, 27%; distractor, 16/ 74, 21%; χ2 = 28.17, P < 0.05). Furthermore, a second population of neurons was recruited by the visual distractor. As illustrated in Fig. 6, the visual distractor was the house light flashing at 0.5 Hz. This population of neurons (40/ 208) exhibited a 20% increase in firing rate during the 1-s period for which the house light was off. Cue-evoked (36/ 208) and distractor-driven (40/ 208) neurons comprised two distinct populations; only three neurons were activated by both the signal and the distractor light.
Table 4.
Behavioral correlates of posterior parietal cortex unit activity during distractor sessions
| Response | Pre-lesion | Saline | SAP |
|---|---|---|---|
| Hit | 17 (36)‡ | 21 (16) | 10 (18)† |
| Miss | 2 (5) | 3 (2) | 7 (13) |
| Correct rejection | 6 (13) | 1 (1) | 6 (10) |
| False alarm | 8 (16) | 6 (5) | 7 (13) |
| House light | 19 (40) | 11 (8) | 31 (52)* |
Data are expressed as the percentage of units (absolute number noted in parentheses) displaying significant behavioral correlates from the total number of units recorded [pre-lesion, 208; saline, 74; 192-IgG saporin (SAP), 167].
P < 0.05, compared with both both pre-lesion and unlesioned (chi square test).
P < 0.05, compared with pre-lesion distractor performance and post-lesion standard performance.
P < 0.05, compared with standard sessions.
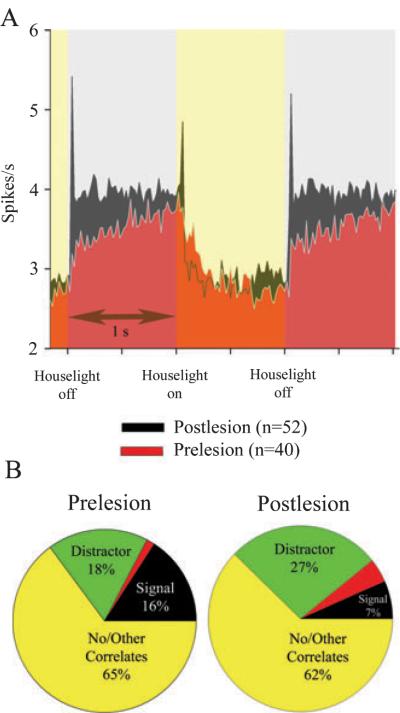
Fig. 6.
Cholinergic lesions increase the proportion of posterior parietal cortex (PPC) neurons responsive to the visual distractor. (A) Population of neurons that are significantly activated when the distractor light is off. (B) Distribution of significant correlates for pre-lesion and post-lesion sessions. The distractor activates 40/208 neurons prior to infusion of 192-IgG saporin. The peak activation of this population took place 20 ms after the distractor light was turned off. Following cholinergic deafferentation, a greater proportion of PPC neurons are activated by the off phase of the distractor (52/167, 31%; (χ2 = 20.32, P < 0.05), and fewer neurons are activated by the signal light. In both graphs, neurons that have mixed correlates are activated by both the signal and the distractor.
Cholinergic deafferentation increased distractor-related PPC unit activity
Cholinergic deafferentation significantly increased the proportion of neurons exhibiting distractor-related increases in unit activity. A population of 52/ 167 neurons (31%) increased their firing rate by 27% when the distractor light was off, a significantly larger proportion of neurons relative to the pre-lesion population (v2 = 20.32, P < 0.05; Fig. 6). Unlike the pre-lesion findings, in which two distinct populations of PPC neurons correlated with either the distractor light or signal light onset, 38% (7/ 18) of deafferented PPC neurons exhibiting signal-evoked activation were also activated by the distractor. By contrast, the number of distractor-related neurons following saline infusions dropped to 11% of the total population (Table 4).
Cholinergic deafferentation reduced cue-evoked PPC unit activity
Cholinergic deafferentation of the PPC significantly reduced the proportion of neurons exhibiting cue-evoked activation in standard sessions. During standard task performance, 65/331 (19%) neurons exhibited cue-evoked activation on hit trials, as opposed to 92/350 (26%) neurons from the pre-lesion population (x2 = 8.174, P < 0.05). Cholinergic deafferentation did not significantly reduce the overall firing rate of all PPC neurons (pre-lesion, 2.34 ± 0.22 spikes/s; post-lesion, 2.38 ± 0.21 spike/s; t = 0.70, P > 0.05). This effect was not a function of electrode depth or number of sessions, as data from saline-infused animals showed a similar proportion of cue-evoked activity from the pre-lesion PPC population (Tables 1, 2 and 4).
Cholinergic deafferentation also significantly reduced cue-evoked activation of PPC neurons in the presence of the visual distractor. Only 10.9% of total neurons isolated (18 of a total of 167) exhibited cue-evoked activation. This is the product of two main effects. As presented earlier, the distractor significantly reduced the proportion of cue-evoked neurons, and cholinergic deafferentation further reduced the magnitude of cue-evoked responses of PPC neurons. This proportion of neurons is significantly smaller than the proportion of pre-lesion neurons exhibiting cue-evoked activation in the presence of the distractor (pre-lesion distractor, 36/208, 17%; post-lesion distractor, 18/167, 10.9%; x2 = 9.9, P < 0.05). Furthermore, this subset of neurons is significantly smaller than the proportion of neurons exhibiting cue-evoked activation on standard sessions following cholinergic deafferentation (post-lesion standard, 65/331, 19%; post-lesion distractor, 18/167, 10.9%; x2 = 126.88, P < 0.05). These results suggest that ACh is necessary for optimization of the cue-evoked response under increased attentional demand of the distractor.
Cholinergic modulation of the SNR under attentionally challenging conditions
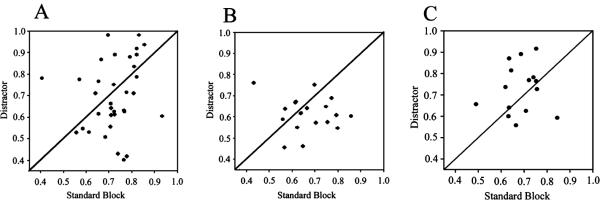
In order to test whether cholinergic input to the PPC was necessary to filter out distractor stimuli, cue-evoked activity was analysed by block of trials. We calculated the median SNR for each cell during a standard task trial block and in the presence of the visual distractor, and compared the two conditions. As presented earlier, the SNR was not affected by the distractor prior to lesion (Fig. 7; Z = −0.833; P > 0.01, Wilcoxon), or following saline infusion (Z = )1.306; P > 0.01), but there was a trend towards a significant decrease in SNR following cholinergic deafferentation (Z = −1.851, P = 0.06), suggesting that ACh neurotransmission is necessary to maintain a higher SNR in the presence of a distractor.
Fig. 7.
Lesions caused by 192-IgG saporin (SAP) showed a trend towards reducing the signal-to-noise ratio (SNR) of cue-evoked posterior parietal cortex (PPC) neurons in distractor blocks. (A) Prior to the administration of SAP, cue-driven PPC neuron activity is equivalent in both the distractor and standard trial blocks. The SNR [SNR = Rstim/(Rstim+Rspont)] was calculated from each of the 36 cue-driven neurons during each trial block; the SNR of each neuron in standard blocks is plotted with respect to the SNR during the distractor block. The cue-driven activity was equivalent in both trial blocks (Z = −0.833; P > 0.05, Wilcoxon). (B) Following infusions of SAP, the SNR of 18 cue-driven neurons in standard trial blocks remained elevated, but the SNR was relatively lower in the distractor block, with a trend towards significance (Z = −1.851, P = 0.06). (C) The SNR was equivalent for both the standard and distractor trial blocks following saline infusions (Z = −1.306; P > 0.05).
Correct vs. incorrect trials
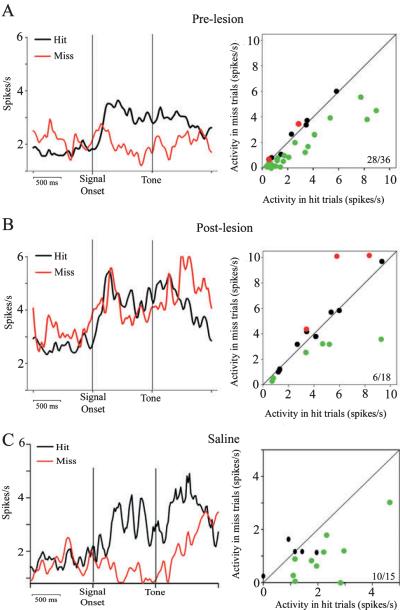
As seen in Fig. 8A, pre-lesion PPC activity indicated stronger cue-evoked activity on hit trials than on miss trials. This pattern of activation was consistent in saline-infused PPC in both standard sessions (Z = 5.638, P < 0.01; Wilcoxon) and in the presence of the distractor (Z = 3.959, P < 0.01; Fig. 8C). Cholinergically deafferented PPC maintained this pattern of activation in standard sessions (Z = 5.527, P < 0.01). However, examination of cholinergically deafferented PPC activity in the presence of the visual distractor indicated that the magnitude of the cue-evoked activity was similar for both hit and miss trials (Z = 0.283, P > 0.01; Fig. 8B).
Fig. 8.
Detection-related PPC neurons respond similarly on hit and miss trials following cholinergic lesions. (A, left) Stimulus-locked population peri-event time histograms (PETHs) for hit and miss trials (20-ms bins, Gaussian filtered over three bins) of 36 cue-evoked neurons from pre-lesion recordings. Peak activation of these neurons on hit trials occur at an average of 220 ms following the cue, and the firing rate remains elevated through the 1-s delay and response. (A, right) The average firing rate of each neuron during the 1-s epoch following the cue, with hit trials plotted against miss trials; 28/36 neurons (green dots) have a significantly higher firing rate on hit trials (all P < 0.05). Only 2/36 neurons (red dots) have a significantly higher firing rate on miss trials. (B, left) A stimulus-locked PETH from 18 cholinergically lesioned cue-driven neurons. PPC neurons are activated on both hit and miss trials. (B, right) The average firing rate of each neuron during the 1-s epoch following the cue; 6/18 neurons have a higher firing rate on hit trials than on miss trials, and 3/18 neurons have a higher firing rate on miss trials. (C, left) Following saline infusions, PPC neurons are activated on hit trials, but not miss trials. Right: 10/15 neurons have a higher firing rate in the 1-s epoch following the cue on hit trials than on miss trials.
Discussion
This study examined whether cholinergic innervation of the PPC contributes to the visual cue-detection correlates observed in PPC neurons in attention task-performing rats. The evidence supported the hypothesis that cholinergic input to the PPC is necessary for the discrimination of relevant cues in the presence of irrelevant distractors. First, we found that many PPC neurons showed robust increases in firing rate in response to task-relevant visual cues on detected but not missed trials (Fig. 4), which is consistent with our previous findings (Broussard et al., 2006). A separate population of PPC neurons was modulated by the distractor stimuli, albeit to a much lesser extent than cue-driven PPC activity (i.e. a 100% or greater increase in firing rate in Fig. 5, relative to a 33% increase in Fig. 6). Cholinergic denervation nearly eliminated detection-specific firing in the PPC (Figs 7 and 8), and increased the response latencies for hit responses in distractor sessions (Fig. 2), showing that cholinergic modulation of firing rate is crucial, as the predictive value of cues is obscured by visual distractors. Importantly, the relative number of hits and correct rejections was not affected by cholinergic deafferentation. Thus, the neurophysiological results are not confounded by decreases in detection accuracy.
Signals of longer duration produced more consistent increases in the firing rate of signal-evoked PPC neurons (Table 3). The average population firing rate and the SNR of the signal-evoked PPC population showed little differentiation between signals of different duration (Fig. 5). The lack of significance at the level of individual neurons, then, may be due to fewer hit trials at shorter signals, as PETH data based on fewer than 15 trials varied considerably (Clayton et al., 2004).
The distractor produced a significant increase in neuronal activity in a large population of PPC neurons. In our previous study (Broussard et al., 2006), we implanted electrodes in a wider mediolateral range of the parietal cortex (2.0–4.3 mm), in which lateral placements had longer latency responses (> 0.5 s to the visual signal), and medial placements (2–2.5 mm) had a faster rise time in response to the visual signal. Because of these properties, we restricted recordings to this medial range in the current study, with the result that the neurons were more responsive to visual input.
In the presence of a distractor, cholinergic deafferentation further attenuated neurophysiological activity evoked by cues. Because the context in which a stimulus occurs is thought to be part of the information by which the stimulus is encoded (Pearce & Hall, 1980), the effects of cholinergic lesion on cue processing in the distractor task is consistent with the view that PPC cholinergic activity mediates the surprise-induced enhancement of attention seen at the behavioral level in other tasks (Bucci, 2009).
Separate populations of neurons encoded the visual distractor and signal light. Following cholinergic deafferentation, the proportion of distractor-encoding neurons increased dramatically. The presence of the distractor dominated the response of cholinergically deafferented PPC neurons. The conflicting effects of cholinergic deafferentation on neuronal activity evoked by distractor and signal stimuli may be explained by the nature of these two stimuli. The distractor flashes approximately 365 times during the 12-min block of trials; in contrast, the signal is randomly presented approximately 27 times during the same period. The reduction of cue-evoked activation of cholinergically deafferented PPC neurons suggests that top-down processes act via cholinergic mechanisms to optimize signal processing. In the absence of this input, PPC neurons maintain a sensory response, as reflected by the increase in evoked response to the distractor.
A limitation of the current study is that cholinergic input to the dorsal hippocampus directly ventral to the PPC was affected in deafferented animals. However, the limited retention interval of this task (1 s) and restricted space within the operant chamber minimizes the demands on working memory and spatial navigation, the two main functions of the hippocampus in rats (Morris, 2007). Furthermore, cue-evoked correlates begin at brief latencies, indicating that attenuation of these correlates is a direct effect of cholinergic deafferentation of the PPC.
The role of cholinergic input to the PPC in attention remains a complex issue (Bucci, 2009). It has been shown that performance of the five-choice serial reaction time task does not require cholinergic input to the PPC (Muir et al., 1996; Maddux et al., 2007). However, cholinergic input to the PPC is required when the reliable, predictive relationship between stimuli is violated in a serial conditioning task (Bucci & Macleod, 2007; Bucci, 2009). On the surface, this evidence seems to contradict our results, in which neurophysiological correlates of cue detection decline in the absence of cholinergic input under standard task conditions. Although cues are consistently predictive in our task, as in the five-choice serial reaction time task, the sustained attention task in this experiment includes the interleaved presentation of cued and non-cued trials, presumably requiring processing mode shifts between these two types of trial. Thus, by raising the level of expected uncertainty on any given trial (Yu & Dayan, 2005), even a consistently predictive cue may still require cholinergic parietal processing for detection.
Accumulating evidence has demonstrated that basal forebrain ACh neurons modulate neocortical activity at multiple time-scales (Parikh et al., 2007, 2008; Parikh & Sarter, 2008). One recent finding indicated that pre-cue cortical ACh levels fostered cue detection, and that detected cues elicited transient increases in medial prefrontal ACh levels (Parikh et al., 2007). Because prefrontal efferent projections mediate top-down modulation of cholinergic activity in the PPC (Zaborszky, 2002; Nelson et al., 2005; Sarter et al., 2006), phasic ACh levels in the medial prefrontal cortex may serve to dynamically modulate cholinergic input to the PPC. In the present study, loss of endogenous cholinergic input resulted in a lower SNR of PPC neurons during distractor sessions (Fig. 7), but not standard sessions. Attention requires a subject to disengage from task-irrelevant behavior and stimuli, and allocate resources to the processing of relevant stimuli. The elevated firing rate in the pre-cue interval (Fig. 8) indicates that ACh levels during the ITI are crucial for proper detection and evaluation of signals. Furthermore, the deafferentation-induced increase in the processing of distractor stimuli is consistent with the hypothesis that cholinergic input to the PPC is necessary for subjects to learn to disengage from irrelevant stimuli in cue detection tasks. These results suggest that cholinergic input to the PPC may be necessary for the detection of visual cues under attentionally challenging conditions.
Table 2B.
Cue-evoked correlates to the visual cue: saporin group
| Subject number | Pre-saline baseline | Pre-saline distractor | Post-saline baseline | Post-saline distractor |
|---|---|---|---|---|
| SAP 1 | 24 (22/92) | 27 (13/47) | 7 (6/81) | 14 (6/44) |
| SAP 2 | 17 (7/42) | 14 (5/37) | 8 (6/69) | 0 (0/27) |
| SAP 3 | 42 (25/60) | 30 (11/36) | 25 (22/88) | 19 (8/42) |
| SAP 4 | 15 (14/92) | 4 (2/47) | 17 (13/78) | 4 (2/45) |
| SAP 5 | 37 (24/64) | 42 (11/26) | 37 (13/35) | 12 (2/16) |
SAP, 192-IgG saporin. Data are expressed as the percentage and number of neurons exhibiting significant cue-evoked correlates by condition. The total numbers of neurons collected from subjects are noted on the right of the divisor.
Acknowledgements
This research was supported by PHS grants NS37026 and KO2MH01072 (M. Sarter). Sharmila Venugopal provided valuable programming assistance.
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ITI
intertrial interval
- PETH
peri-event time histogram
- PPC
posterior parietal cortex
- Rspont
background activity
- Rstim
stimulus-driven response
- SAP
192-IgG saporin
- SNR
signal-to-noise ratio
References
- Arnold HM, Burk JA, Sarter M, Bruno JP. Cortical acetylcholine release in rats performing an operant sustained attention task or operant control procedures. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neural correlates of attention and distractibility in the lateral intraparietal area. J. Neurophysiol. 2006;95:1696–1717. doi: 10.1152/jn.00848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard J, Sarter M, Givens B. Neuronal correlates of signal detection in the posterior parietal cortex of rats performing a sustained attention task. Neuroscience. 2006;143:407–417. doi: 10.1016/j.neuroscience.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ. Posterior parietal cortex: an interface between attention and learning? Neurobiol Learn Mem. 2009;91:114–120. doi: 10.1016/j.nlm.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Macleod JE. Changes in neural activity associated with a surprising change in the predictive validity of a conditioned stimulus. Eur. J. Neurosci. 2007;26:2669–2676. doi: 10.1111/j.1460-9568.2007.05902.x. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE. Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Burk JA, Herzog CD, Porter MC, Sarter M. Interactions between aging and cortical cholinergic deafferentation on attention. Neurobiol. Aging. 2002;23:467–477. doi: 10.1016/s0197-4580(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J. Neurosci. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J. Neurophysiol. 2002;88:3487–3497. doi: 10.1152/jn.00188.2002. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple-stimulus displays: I. neurons encode the location of the salient stimulus. Cereb. Cortex. 2001;11:581–591. doi: 10.1093/cercor/11.7.581. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Posterior parietal cortex automatically encodes the location of salient stimuli. J. Neurosci. 2005;25:233–238. doi: 10.1523/JNEUROSCI.3379-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J. Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG. Posterior parietal cortex and the filtering of distractors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4263–4268. doi: 10.1073/pnas.0730772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TM, Masters J, Sarter M, Givens B. The role of acetylcholine within the medial prefrontal and posterior parietal cortices during sustained visual attention in the rat. Abstr. -Soc. Neurosci. 1999;25:1895. [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J. Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Natur. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav. Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J. Neurophysiol. 1992;67:1222–1229. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Gottlieb J, Goldberg ME. The lateral intraparietal area as a salience map: the representation of abrupt onset, stimulus motion, and task relevance. Vision Res. 2000;40:1459–1468. doi: 10.1016/s0042-6989(99)00212-6. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JM, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behav. Neurosci. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav. Neurosci. 1998;112:1519–1525. doi: 10.1037//0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav. Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144:175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J. Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Morris R. Theories of hippocampal function. In: Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford; New York, NY: 2007. pp. 591–654. [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb. Cortex. 1996;6:470–480. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Nelson C, Sarter M, Bruno J. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Nitz DA. Tracking route progression in the posterior parietal cortex. Neuron. 2006;49:747–756. doi: 10.1016/j.neuron.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Warm JS, Dember WN. Vigilance: taxonomy and utility. In: Mark LS, Warm JS, Huston RL, editors. Ergonomics and Human Factors: Recent Research. Springer Verlag; New York, NY: 1987. pp. 11–39. [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann. NY Acad. Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J. Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. Academic Press; New York, NY: 1998. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol. Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Posner MI, Snyder C, Davidson B. Attention and detection of signals. J. Exp. Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J. Neurophysiol. 2000;84:301–310. doi: 10.1152/jn.2000.84.1.301. [DOI] [PubMed] [Google Scholar]
- Rasband W. ImageJ. US National Institutes of Health; Bethesda, MA: 1997 2009. [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp. Brain Res. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo M, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res. Brain Res. Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J. Histochem. Cytochem. 1986;34:1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat. Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog. Brain Res. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Prentiss Hall; Englewood Cliffs, NJ: 2007. [Google Scholar]
- Zinke W, Roberts MJ, Guo K, McDonald JS, Robertson R, Thiele A. Cholinergic modulation of response properties and orientation tuning of neurons in primary visual cortex of anaesthetized Marmoset monkeys. Eur. J. Neurosci. 2006;24:314–328. doi: 10.1111/j.1460-9568.2006.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]