Summary
LAM is a rare lung disease, found primarily in women of childbearing age, characterized by cystic lung destruction and abdominal tumors (e.g., renal angiomyolipoma, lymphangioleiomyoma). The disease results from proliferation of a neoplastic cell, termed the LAM cell, which has mutations in either of the tuberous sclerosis complex (TSC) 1 or TSC2 genes. Molecular phenotyping of LAM patients resulted in the identification of therapeutic targets for drug trials. Loss of TSC gene function leads to activation of mammalian target of rapamycin (mTOR), and thereby, effects on cell size and number. The involvement of mTOR in LAM pathogenesis is the basis for initiation of therapeutic trials of mTOR inhibitors (e.g., sirolimus). Occurrence of LAM essentially entirely in women is consistent with the hypothesis that anti-estrogen agents might prevent disease progression (e.g., gonadotropin-releasing hormone analogues). Levels of urinary matrix metalloproteinases (MMPs) were elevated in LAM patients, and MMPs were found in LAM lung nodules. In part because of these observations, effects of doxycycline, an anti-MMP, and anti-angiogenic agent, are under investigation. The metastatic properties of LAM cells offer additional potential for targets. Thus, insights into the molecular and biological properties of LAM cells and molecular phenotyping of patients with LAM have led to clinical trials of targeted therapies. Funded by the Intramural Research Program, NIH/NHLBI
Keywords: Lymphangioleiomyomatosis, Lymphatics, Metastasis, Mammalian target of rapamycin (mTOR), Tuberous sclerosis complex (TSC), Sirolimus
Lymphangioleiomyomatosis (LAM) is a rare disease that affects primarily women of childbearing age1–5 with an incidence of approximately 2.6 per 1 million women.3 Organs involved in LAM include the lung, kidney (e.g., angiomyolipomas (AMLs)), and axial lymphatics (e.g., lymphangioleiomyomas, adenopathy).1–5 Patients with LAM present most frequently with dyspnea and pneumothorax1–5; other symptoms evident at presentation or during the course of the disease include chylothorax, hemoptysis, and ascites.1–3,5 Patients usually first notice symptoms in their mid to late 30s,2–5 although diagnosis may be delayed for 5–6 years, in part due to similarities of the symptoms to those of more common lung diseases. Survival rate 10 years after diagnosis was approximately 85%6 or 90%.7 The frequency of LAM is elevated among patients with tuberous sclerosis complex (TSC), an inherited disorder resulting from mutations in either the TSC1 or TSC2 genes. The clinical phenotype results from proliferation of the neoplastic LAM cell, which contains the TSC mutation.
Characteristics of LAM
Pulmonary dysfunction
The characteristic pulmonary radiologic (computed tomography (CT)) finding in patients with LAM is thin-walled cysts spread diffusely throughout the lung parenchyma, with no apical or basilar dominance.1,5,6,8 Cystic changes, or the proliferation of LAM cells, are responsible for airflow obstruction and decreased lung diffusion capacity. FEV1 (forced expiratory volume in one second) and DLco (diffusion capacity of the lungs for carbon monoxide) are reduced in approximately 60% of patients.8 Evidence of air-trapping was observed on ventilation-perfusion scintigrams.2,9
Pathology
LAM results from the proliferation of abnormal smooth muscle-like cells (LAM cells), neoplastic cells that contain smooth muscle (α-smooth muscle actin (α-SMA)) and melanoma cell (gp100) antigens10 as well as tuberous sclerosis complex (TSC) gene mutations.11–13 Immunohistochemistry revealed two morphological types of LAM cells: epithelioid cells that react with HMB-45 (a monoclonal antibody that recognizes a splice variant of pmel-17 (gp100)) and spindle-shaped cells reactive with antibodies against proliferating cell nuclear antigen.14 LAM cells proliferate in the vicinity of blood and lymphatic vessels, near bronchioles, and in walls of the cystic lesions.10 In the lung, LAM cell nodules in the lung are traversed by slit-like lymphatic channels, whereas in extrapulmonary lesions, LAM cells are seen in fascicles, which form plump rod-shaped bundles separated by lymphatic channels.15 Lung and lymphatic lesions and AMLs contain smooth muscle-like LAM cells. AMLs differ, however, from other LAM lesions in that they contain also underdeveloped vasculature and adipose tissue.10,15–17 Hyperplastic type II pneumocytes are seen in LAM lung nodules.18
Pathogenesis
TSC is an autosomal dominant disorder, characterized by hamartomatous lesions, seizures, and mental retardation,19 resulting from mutations in the TSC1 or TSC2 genes, which encode hamartin or tuberin, respectively.20 Approximately one-third of women with TSC will present with pulmonary cystic lesions radiographically and histologically identical to those in LAM.21–23 TSC2 mutations are much more frequent than those of TSC1 in sporadic LAM patients.11–13 In patients with LAM, who have received a lung transplant, TSC mutations identical to those present in the explanted lung were observed in the donor lung, consistent with metastatic properties of LAM cells.24,25 Similarly consistent with a metastatic model of disease progression, LAM cells were also isolated from blood, urine, and chyle of LAM patients.26
LAM natural history study
More than 500 patients with LAM, primarily from the United States and Canada, but also from Europe and Southeast Asia, were enrolled in the LAM natural history protocol (NHLBI protocol 95-H-0186). In this longitudinal study, over 250 patients returned for five or more visits. Data on survival and disease progression from X-ray, biopsy, and/or physiological (e.g., pulmonary function tests) procedures were generated and collated.5
Predictors of time to death or transplantation
LAM histology scores
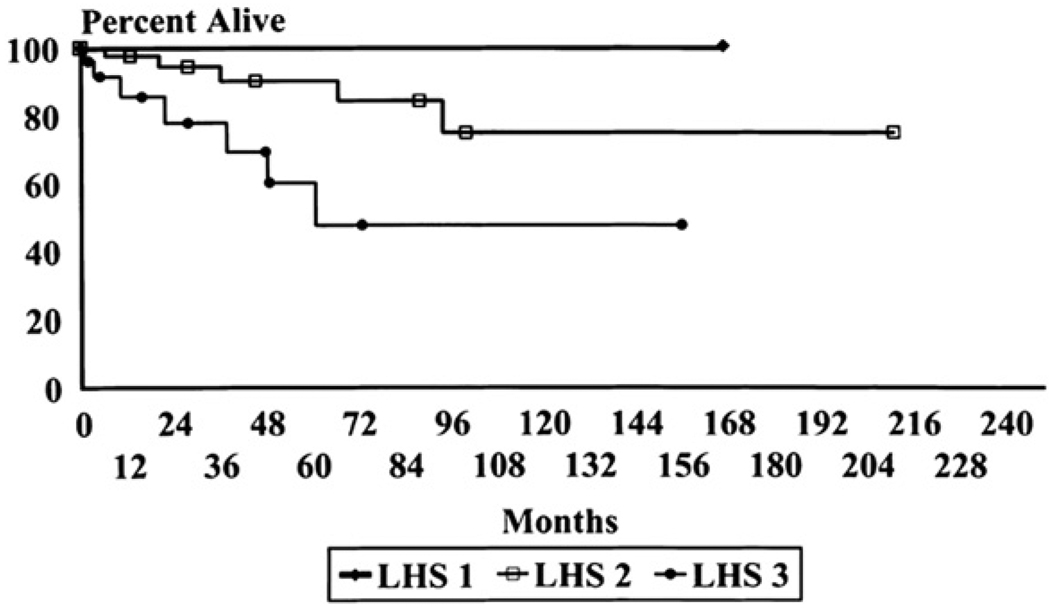
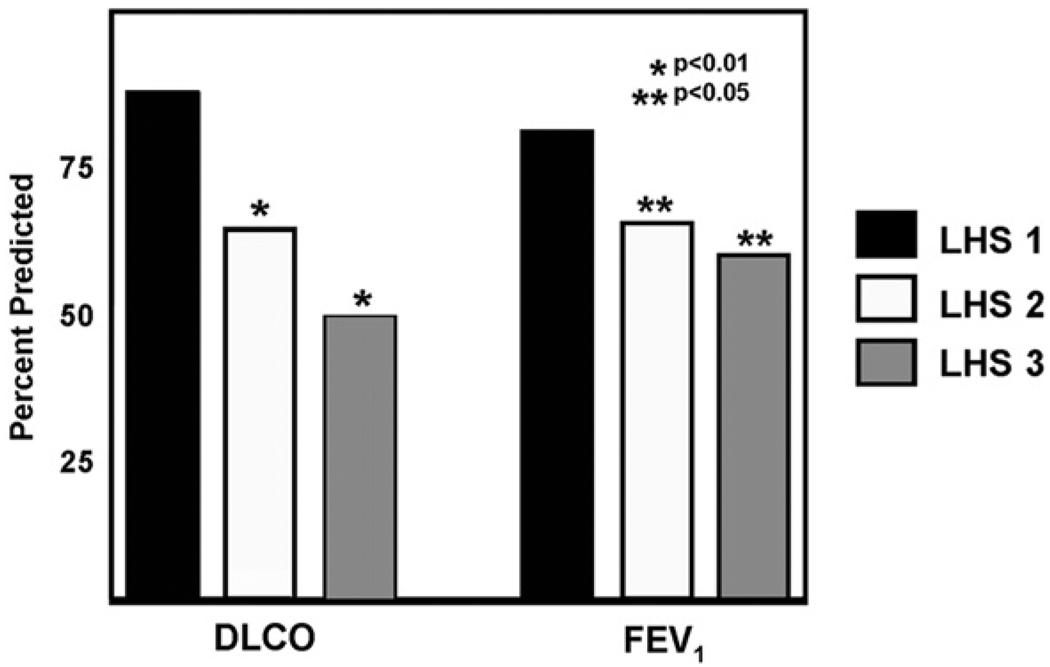
Severity of lung involvement in LAM was assessed in patients’ lung biopsies using the LAM Histology Score (LHS). LHS is based on the extent of replacement of normal lung tissue by cystic lesions and LAM cell infiltrates.27 The total percentage of tissue involvement by these two histologic patterns is graded as follows: LHS-1, <25%; LHS-2, 25% to 50%; and LHS-3, >50% of lung tissue involved. Using this grading method, significant differences in survival and time to transplantation for patients with LHS-1, -2, and -3 scores were observed (Fig. 1). The ten-year survival was found to be near 100% for LHS 1, 74.4% for LHS 2, and 52.3% for LHS 3.27 These data confirmed prior observations showing that patients with more cystic disease have worse prognosis, and are more likely to have lower DLco and more exercise-induced hypoxemia than those with more muscular, solid lesions.1 There was also a good correlation between DLco and FEV1 and LHS8 (Fig. 2).
Figure 1.
Kaplan–Meier survival curves of patients with pulmonary lymphangioleiomyomatosis staged according to the lymphangioleiomyomatosis histologic score (LHS). Patients with LHS-1 have nearly 100% survival. Patients with LHS-3 have the worst survival, and those with LHS-2 have an intermediate survival (p < 0.002) (From reference 27).
Figure 2.
Relationship between LHS and lung function at the time of biopsy. Patients with an LHS of 2 (white bars) or 3 (gray bars) have significantly lower DLCO than those with an LHS of 1 (black bars). Patients with an LHS of 2 (white bars) or 3 (gray bars) also have lower FEV1 than patients with an LHS of 1 (From reference 8).
Computed tomography
The severity of lung disease in LAM was graded semi-quantitatively by computed tomography,9,28 according to the percentage of the abnormal lung in three equal pulmonary zones, represented by scans of three equal portions of images, using the scale of: 0, absent; 1, less than 30% abnormal; 2, 30%–60% abnormal; 3, more than 60% abnormal. Although good correlation of computed tomography with lung function tests was demonstrated, a predictive value of time to death or transplantation is not yet shown.9,28
Quantitative grading CT scans correlated well with measures of gas exchange and exercise performance.29,30 Recent advances in computed tomography make possible the quantification of amount of lung parenchyma affected by lung cysts.31 Percentage of cyst volume correlated with expiratory flow (FEV1), residual volume and DLco.30,31 The value of this radiologic procedure for prediction of survival or time to transplantation remains to be determined.
Pulmonary function tests
Pulmonary function testing is the simplest method of assessing severity of lung disease in LAM.3,5,8,32 Abnormalities of pulmonary function were seen in >60% of patients. Both flow rates and lung diffusion can be normal, however, in as many as one third of patients. Although most patients have both airflow obstruction and impaired gas exchange, some may have normal flow or only mild airflow obstruction with a marked decrease in diffusion capacity. The severity of lung disease in those patients should be graded by assessment of gas exchange such as DLco, arterial blood gases, or alveolar-arterial oxygen (A-a/O2) gradient. These tests have limitations, however, as measurement of DLco is difficult to standardize and has a large intra-individual variability, whereas arterial blood gases at rest are very frequently normal.
No prospective study has established the levels of FEV1 and DLco below which a LAM patient should be referred for transplantation. As in other pulmonary diseases, lung function criteria are only one component of the pre-transplant evaluation, and despite severe functional impairment, LAM patients can be relatively asymptomatic at rest. In general, however, by the time LAM patients undergo lung transplantation, they are on supplemental oxygen and have percent-predicted FEV1 and/or DLco below 30% of predicted.33 Still, even for patients in the last group, survival rate without lung transplantation is not possible to predict.
Exercise testing
Exercise testing in LAM may demonstrate abnormalities of gas exchange and/or ventilation and abnormal cardiovascular function.33 Although lung function, especially DLco, can reasonably predict peak oxygen uptake (VO2max), VO2max is dependent on several additional factors, such as skeletal muscle function and cardiovascular fitness.33,34 Exercise testing in LAM is important in that it elicits abnormalities not readily apparent during resting lung function, such as exercise-induced hypoxemia and pulmonary hypertension.35 Because exercise-induced hypoxemia occurs in patients with mild impairment in lung function, cardiopulmonary exercise testing (CPET) or other types of exercise testing (e.g., six-minute walk test (6MWT)) may be useful measures of lung disease severity in LAM.30,33
An association between VO2max and LHS scores was demonstrated, and patients with more severe LHS had significantly lower VO2max.33 Since LHS is a predictor of death and time to transplantation, VO2max might also be a useful predictor of survival in patients with LAM.
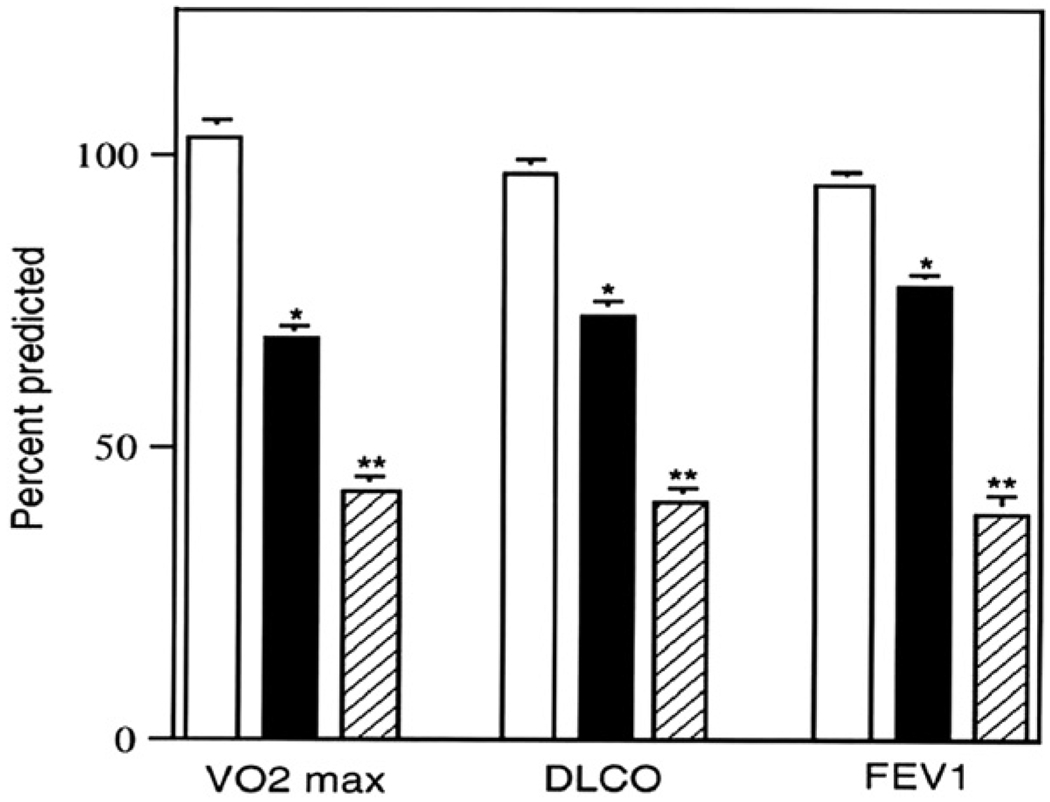
Good correlation between a quantitative CT severity index and A-a/O2 gradient, dead space/tidal volume ratio, and VO2max was reported.29 Further, we found that VO2max, DLco, and FEV1 correlated with the CT grade of disease severity33 (Fig. 3). Moreover, good correlation between quantitative scores of cystic-aerial lesions obtained from CT scans and the 6MWT test have also been observed.30 This is important because the 6MWT is a simple non-invasive test of proven value in assessing disease severity of patients with interstitial pulmonary fibrosis or pulmonary hypertension.36
Figure 3.
Relationship between VO2max, FEV1, and DLCO and CT grade of disease severity. Grade I, white bars; grade II, black bars; grade III, cross-hatched bars (From reference 33).
Rate of functional decline
The rate at which lung function declines in LAM indicate how aggressive the disease is and, in an individual patient, has prognostic significance.32 In one study involving 36 patients, the rate of FEV1 decline was 118 ± 21 ml/year and for DLco was 0.905 ± 0.26 ml/min/mmHg/year.37 Lazor et al.38 reported an average decline in FEV1 of 106 ± 35 ml/ year (3.4 ± 0.8 % predicted) in 31 patients followed for approximately five years. In a larger study of 275 patients followed for approximately four years,32 the average yearly rates of decline in FEV1 and DLco were 75 ± 9 ml (1.7 ± 0.4% predicted), and 0.69 ± 0.07 ml/min/mmHg (2.4 ± 0.4% predicted), respectively. Great variability in rates of disease progression is reflected in the large standard deviations. In some patients, LAM is a very aggressive disease with declines of FEV1 or DLco from 5–10% predicted per year. Hayashida et al.39 reported that in patients with FEV1 above one liter and DLco above 40% predicted, those patients who presented with dyspnea (n = 61), had a median decrease in FEV1 of 285 ml (range −582.9 to −71.3 ml) per year and a median decrease in percent predicted DLco of 11.9% (range −20.7 to −3.2%). In patients who presented with pneumothorax (n = 74), loss of function was much slower with median annual decline in FEV1 of 16.7 ml (range −157.1 to +46.8) or 1.6 % predicted (range −7.2 to +6.4%). Thus, LAM lung disease can sometimes progress quite slowly, taking decades to reach a level that interferes with activities of daily living or requires continuous oxygen therapy. In the absence of well-characterized markers of severity of this disease, FEV1 and DLco (monitored frequently) are now the best methods to assess severity and progression of pulmonary disease in LAM.
Hormonal interventions
At present, no treatment is known to reverse effectively the functional abnormalities and prevent ongoing lung damage in LAM. Most systematically employed drug regimens involved anti-estrogen therapy,5,32 based on the understanding of LAM as a disease predominantly of premenopausal women that may worsen during pregnancy40–42 or following the administration of estrogens. 43–48 Consequently, most women with LAM were treated either with oophorectomy or long-term hormonal therapy, or both. These treatments became a standard of care, but no controlled studies evaluated their effectiveness. Most publications included only a handful of patients or were retrospective reviews. Taylor et al.49 reported no improvement in 15 of 30 patients with LAM who had undergone bilateral oophorectomy; 11 patients worsened, and four were stable. The presence of estrogen or progesterone receptors in LAM lung lesions did not correlate with therapeutic response. Gonadotrophin-releasing hormone (GnRH) analogues were also found to be of benefit in the treatment of LAM,50–52 although other studies were inconclusive.53,54
In a retrospective study, Johnson and Tattersfield37 found that declines in FEV1 in patients treated with progesterone were not significantly smaller than those in untreated patients, although their initial levels of FEV1 and DLco were lower, and rates of decline over three years were not different. Decline in DLco was significantly less in 16 pre-menopausal patients treated with progesterone than in 13 pre-menopausal untreated patients, but the two groups were not matched. A decreased rate of functional decline was reported in five patients for whom pre- and post-treatment FEV1 and DLco data were available. Another retrospective analysis of 275 patients32 revealed that overall yearly rates of decline of FEV1 and DLco were not significantly different in patients treated with oral or intramuscular progesterone and patients who received no progesterone after adjusting for differences in initial lung function, age, and disease duration.
In sum, although LAM is primarily a disease of pre-menopausal women, there is no clear evidence that treatments aimed at removing or antagonizing the effects of endogenous estrogens are effective.
Estradiol stimulated the growth of human angiomyolipoma TSC2−/− cells32 and the pulmonary metastasis of Tsc2−/− Eker rat uterine leiomyoma-derived smooth muscle cells (ELT3 cells) in mice.55 Enhancement by estrogens of the survival and growth of intravenously injected Tsc2−/− cells in mice55 was blocked by the MAPK/ERK kinase (MEK) inhibitor CI-1040.55 Estrogen receptor activation in LAM cells increased matrix metalloproteinase (MMP)-2 activity promoting their invasiveness.56 Estrogens also accelerated growth of angiomyolipoma cells in a xenograft tumor model.57 All of these data suggest blockade of the MEK pathway as a new potential approach to the treatment of LAM. From these findings, anti-estrogen therapy may have a role in the treatment LAM, but precluding LAM cell metastasis could be crucial for its safety and/or effectiveness.
Association of modifier genes and biomarkers with disease severity and progression in LAM
Biomarkers linked to diverse LAM cell functions or disease characteristics could be useful as surrogates to follow disease progression and/or responses to therapy. Therefore, the discovery of modifier genes and biomarkers that are closely associated with clinical phenotypes of LAM is an important goal of LAM research.
Extracellular matrix proteins and pneumothoraces
Pneumothorax is one of the most frequent presentations of patients with LAM,5 with a rate of recurrence surpassing that in any other pulmonary disease.5,58 In a study of 227 patients with LAM,59 the prevalence of pneumothorax was related to progression and severity of disease. Pulmonary function testing, CT grade, and cyst size of patients with LAM were compared to a history of pneumothoraces. Cyst size was graded by the average cyst diameter measured on high-resolution computed tomography (HRCT) using the following scale: size I < 0.5 cm; size II 0.5–1.0 cm; and size III > 1.0 cm. Patients with a history of pneumothorax were more likely to have larger cysts than those without. Multivariate analysis of a subgroup of LAM patients with mild disease and CT grade I revealed that FEV1 declined more rapidly among those with a history of pneumothorax than those who did not, suggesting that early disease progression may be related to the occurrence of pneumothoraces.59
Collagens I and III are principal components of the alveolar wall scaffolding in the lung parenchyma.60 Matrix metalloproteinase (MMP)-1 in LAM lung nodule is capable of degrading collagen and elastin.61–63 It was hypothesized that the occurrence of pneumothoraces might be related to differences in genes involved in extracellular matrix formation or function.59 Patients with LAM were genotyped for polymorphisms in collagens I and III and MMP-1 to associate with susceptibility to LAM or the occurrence of pneumothorax. No correlation of polymorphisms with susceptibility to LAM was found, but patients with and without pneumothoraces differed in frequencies of polymorphisms in types I and III collagen and MMP-1 genes. An association of collagen III polymorphism with cyst size, although only a trend, was suggestive of a relationship between cyst size, type III collagen, and pneumothorax. Types I and III collagen and MMP-1 might be modifier genes affecting changes in the extracellular matrix that result in a greater susceptibility to pneumothorax and rate of decline in lung function.59
Lymphatic involvement in LAM
Lymphatic involvement in LAM includes lymphangioleiomyomas, adenopathy, and chylous pleural effusions. Adenopathy on CT scans appears as round and/or elliptical, well-circumscribed solid masses with replacement of normal tissue by smooth muscle (LAM) cells.64 Avila et al.64 reported a 39% frequency of adenopathy in patients with LAM. Patients with abdominal adenopathy were more likely to have severe disease by CT grade.
Lymphangioleiomyomas, which occur in ca. 16%–21%64,65 of patients with LAM, appear on CT scans as thin or thick-walled encapsulated lobulated masses containing LAM cells, which can be seen microscopically infiltrating the adipose tissue surrounding the fibrous capsule of the lesion.15,64 Cysts contained fluid components. Lymphangioleiomyomas are thought to result from the proliferation of LAM cells, causing obstruction and subsequent dilatation of lymphatic vessels. Chylous ascites may occur from the rupture of distended lymphangioleiomyoma cysts. Lymphangioleiomyomas are usually found in conjunction with enlarged abdominal lymph nodes, pleural effusions, ascites, and dilatation of the thoracic duct.64 In two radiologic studies, using sonography and/or CT scans,65,66 12 of 13 and 21 of 22 patients with LAM demonstrated diurnal size changes in lymphangioleiomyomas. Observation of these changes on CT or sonography is helpful in the differential diagnosis of lymphangioleiomyomas and in the exclusion of lymphomas and other solid lymphatic masses that do not exhibit diurnal variation in size.
The identification of relatively specific lymphatic endothelial markers has facilitated analysis of the pathophysiologic role of lymphatic involvement in LAM. Examples are VEGFR-3 (FLT-4), a FMS-like tyrosine kinase 4 gene and a mediator of lymphangiogenesis67; VEGF-C and VEGF-D, both lymphatic growth factor ligands for VEGF-R368,69; podoplanin, a glomerular podocyte membrane mucoprotein recognized by antibody D2-40; and PROX-1, a transcription factor required for the differentiation of lymphatic endothelial cells.69,70
Immunohistochemical and immunocytochemical studies using specific lymphatic and vascular endothelial markers (e.g., CD31/PECAM, a platelet endothelial cell adhesion molecule and marker for vascular endothelial cells), as well as antibodies against HMB-45 and α-smooth muscle actin (LAM cell markers), demonstrated the extensive lymphatic involvement characteristic of LAM in the lungs and extrapulmonary tissue, including lymph nodes, uterus, and ovaries.71 LAM cells proliferated in the axial lymphatics, particularly the retroperitoneal area, including the diaphragm, thoracic duct, and left jugulosubclavian junction. 71,72 Anti-VEGFR-3 antibody reactivity of cells in the lining of the slit-like spaces within the LAM foci or nodules confirmed these structures as lymphatic tracts. Further, cells reactive with anti-VEGFR-3 antibodies, in mediastinal or retroperitoneal lymph nodes, appeared to segregate LAM cells into nodular or cluster-like arrangements. It was concluded that the observance of anti-VEGFR-3 reactive cells associated with LAM lung lesions indicated an active lymphangiogenic process linked to LAM. Consistent with these findings, microscopic observations revealed parts of LAM foci extending into the lymphatic lumen and clusters of LAM cells in lymphatic vessels.72 These LAM cell clusters (LCCs) were characterized as having an inner core of spindle-shaped cells, immunoreactive for anti-HMB-45, and an outer, single layer of flattened cells that react with anti-VEGFR-3 antibodies. LCCs were observed in chylous pleural and peritoneal effusions, and cystic lymphangioleiomyomas, as well as in the lung and in lymphatic channels in fat tissues surrounding lymph nodes containing LAM cells.72 Based on these results, it was proposed that the shedding of LCCs by lymphangiogenesis-mediated fragmentation of LAM lesions, and their subsequent entry into the lymphatic circulation was a mechanism for the dissemination of LAM cells.
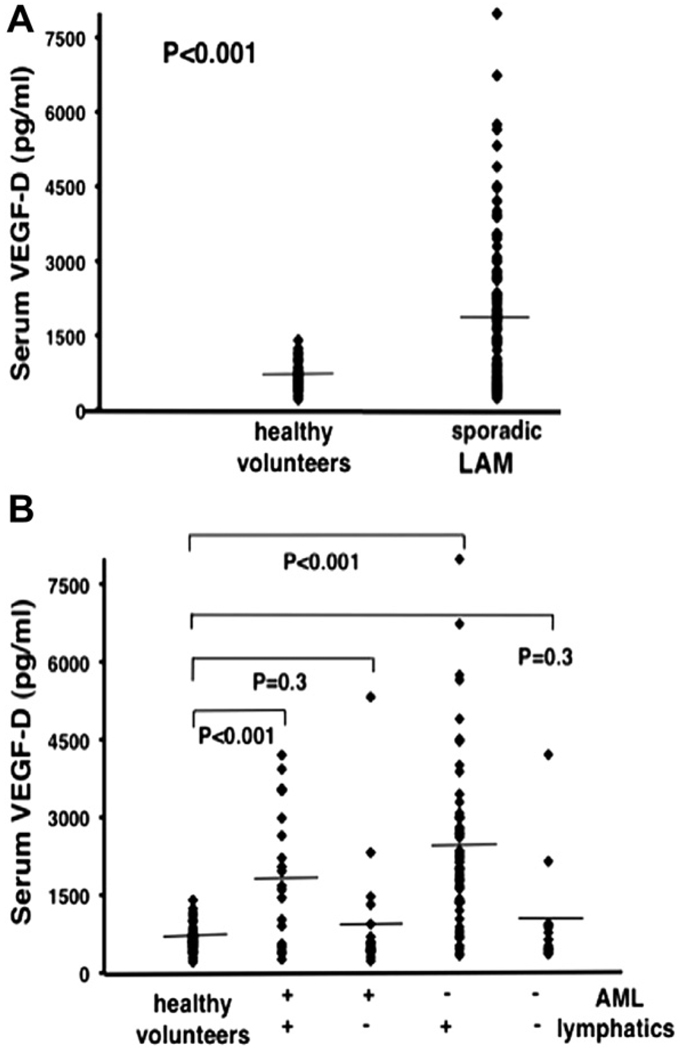
Immunoreactive VEGF-C and VEGF-D are both present in LAM cells.71,73 Serum levels of VEGF-C and VEGF-D quantified by enzyme-linked immunosorbent assay (ELISA) in 44 patients with sporadic LAM were compared to serum levels in 24 healthy volunteers. Serum of VEGF-C in patients with LAM was significantly lower than that in healthy volunteers. 73 In two separate studies,73,74 serum VEGF-D levels were significantly higher in LAM patients than in controls (Fig. 4A). The higher levels of VEGF-D correlated with more severe disease (CT grade),74 and worse FEV1/FVC ratio, DLco (adjusted for lung volune),73 and percent-predicted DLco,74 all consistent with a role for VEGF-D as a growth factor related to lymphangiogenesis in LAM.
Figure 4.
Serum Levels of VEGF-D in Lymphangioleiomyomatosis. In Panel A, serum VEGF-D levels in all patients with sporadic lymphangioleiomyomatosis (LAM) (n = 111) were compared to those of healthy volunteers (n = 40). Panel B shows patient samples compared on the basis of thoracic or abdominal lymphatic involvement (presence (n = 77) or absence (n = 34) of lymphangioleiomyomas and/or adenopathy) and the presence (n = 40) or absence (n = 71) of renal angiomyolipomas (AMLs). All groups were compared to healthy volunteers (n = 40). (+) = presence of, (−) = absence of. Each ♦ represents serum measurement of VEGF-D from one patient or healthy volunteer. Lines represent mean values (From reference 74).
When encountering a patient with pulmonary LAM in the absence of extrapulmonary manifestations (such as AMLs), distinguishing LAM from other cystic diseases can be problematical without performing a biopsy. Young et al.75 measured serum levels of VEGF-D in 38 LAM patients, 24 healthy controls, and 27 patients with other pulmonary diseases (Langerhans-cell histiocytosis, lymphangiomatosis, and emphysema). Serum levels of VEGF-D in patients with LAM were significantly higher (as much as 30 times) than those in the other groups. Based on these results, serum VEGF-D might seem a potential biomarker for LAM. In a study of a larger cohort of patients with LAM (LAM n = 111; healthy volunteers n = 40),74 however, statistic significance of the difference in serum VEGF-D levels of LAM versus controls was not maintained when the LAM population was divided into 2 groups based on lymphatic involvement (Fig. 4B). Serum VEGF-D remained significantly different only when normals were compared to LAM patients with lymphatic involvement, not when compared to patients with only pulmonary disease, regardless of the presence or absence of AMLs. Consequently, low serum VEGF-D levels may reflect confinement of the disease to the lung. Results of the lymphatic growth factor studies indicate that lymphatic involvement may occur with disease progression and that levels of VEGF-D likely reflect lymphatic involvement in LAM, but may not be useful for the diagnosis of LAM in patients who have only cystic disease. Given these data, we suggest that clinical therapies targeted at VEGF-D and/or VEGFR-3 may slow lymphatic proliferation in LAM and possibly disease progression. Potential therapies include a mouse monoclonal antibody (VD1) that competes with mature VEGF-D for binding to VEGFR-2 and VEGFR-3, thereby inhibiting its function.76 In a mouse tumor model, VD1 inhibited VEGF-D-induced formation of lymphatics and tumor growth. Development of a human version of this antibody is in progress, 69,77 and other drugs targeting the VEGFR-3 pathway (e.g., AZD2171, sorafenib, sunitinib) are in clinical trials.78
Metastasis and LAM
LAM is characterized by the proliferation of smooth muscle-like cells that are disseminated in blood, urine, and chylous effusions26 and may be able to form lung metastases.79 In other types of cancer, the multistep metastatic process or “invasion-metastatic cascade” starts with a primary neoplasm that undergoes progressive growth followed by vascularization, invasion, detachment, embolization, cell survival in the circulation, arrest, extravasation, evasion of host defenses and growth at metastatic sites.80 Since all of these steps are critical, blocking any of them could block the metastatic process, which involves “crosstalk” between the metastatic cell and its microenvironment.
The CD44 gene in chromosome 11 encodes 20 exons that undergo complex splicing to yield at least 10 different splice variants.81 The multiple interactions of CD44 proteins suggest its participation in cell binding to extracellular matrix, endothelial cells, and other cells in the neoplasm microenvironment.82 The metabolism and processing of CD44 presents interesting options for targeting. Splicing factors Sam68 and SRm160, which are activated by the Ras/MAPK pathway, regulate expression of CD44 forms83–85 and Ras activation appears to be regulated by CD44v6.83 Further investigation into the RAS/MAPK pathway downstream effects on CD44 splicing factors may elucidate therapeutic targets for controlling metastasis of LAM cells.
Osteopontin, an acidic 32.5-kDa phosphocytokine that is upregulated by TGF-β,86 EGF,87 and progesterone,88 not only binds CD44,89 but also regulates its splicing.90 Ostepontin has been correlated with several metastatic processes and CD44v6 expression. The finding of elevated osteopontin levels in serum of patients with pulmonary LAM,91 suggested that blocking its production could interfere with disease progression. Amounts of cleaved CD44 forms have been correlated with disease progression, and metalloproteases92 and ADAM1093 cleave CD44 to modulate cell attachment to extracellular matrix. Modification of CD44 enzymatic cleavage as well as of its splicing and turnover could involve several potential therapeutic targets.
Expression of the CD44v6, a splice variant of CD44, was detected in squamous cell carcinoma (head and neck, esophagus, lung, skin, cervix, vulva), adenocarcinoma (breast, Barrett’s esophagus, lung, gastric, pancreas, colon/rectum endometrium, prostate) and other tumors (thyroid carcinoma, small cell lung cancer, renal cell carcinoma, urinary bladder, ovarian cancer and basal carcinoma).82 The presence of CD44v6 in so many cancers spurred researchers to target the molecule94 using antisense technologies, antibodies (U36, chU36, BIWA 4),95,96 and peptides (patent JP 2006083122). The anti-CD44 antibody H90 was notably successful in eradicating acute myeloid leukemia.97 As most approaches were directed at the primary tumor, very little was done to target the actual metastatic cells. CD44v6 is found in LAM lesions that have TSC2 loss of heterozygosity, as well as in cultured cells grown from LAM lungs, similar to observations in other metastatic cells.91 Presence of the CD44v6 splice variant on circulating LAM cells means that interference with its specific interactions could prevent anchoring of the cell to a metastatic site.
Cancer cells, regulated by the interaction of chemokines and their receptors, localize to sites of metastasis. Production of chemoattractants mainly at the metastatic site was shown for breast cancer and melanoma cells.98 The metastatic cells display a defined group of receptors that are activated by chemokines produced by the normal stromal cells. LAM cells with identical mutations have been found in both lung and kidney of the same individual,11 consistent with metastasis, but the sites of origin were not determined. We have observed that LAM cells within the lung lesions reacted with antibodies raised against CCR2, CXCR4, CCR7, or CCR1, and cells that were selectively mobilized by CCL2 / MCP1 showed loss of heterozygosity at the TSC2 locus.99 In addition, tuberin appeared to alter CCL2 expression.100 These findings have led to the proposal of a positive feedback of selective chemoattraction. Multiple drugs that target CCR2101 are known. We speculate that blocking the CCL2/CCR2 interaction via drug therapy might prevent metastasis of LAM cells and, therefore, disease progression. Multiple approaches, including the use of truncated MCP-1, anti-MCP-1 and aptamers, have been used to inhibit the function of CCL2.102,103
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases critical in physiological embryogenesis and tissue remodeling. Dissociation of the cysteine-zinc complex produces an active zymogen capable of degrading extracellular matrix substrates.104 This activation is inhibited by endogenous tissue inhibitors of metalloproteinases (TIMPs).104 MMP-9 and MMP-2 are able to degrade a variety of matrix substrates including gelatin, collagen I, collagen IV, laminin, and elastin.104 MMP activity is believed to facilitate tumor cell invasion by disrupting basement membranes.105 Concentrations of MMP-9 (in serum/plasma, and protein activity) have consistently correlated with the presence of cancer106,107 or metastasis108,109 and disease progression. 110,111 Levels of MMP-2 and MMP-9 activity in urine were predictive of disease status in a variety of cancers.112 Degradation of elastic fibers in areas of smooth muscle proliferation was found in light and electron microscopic immunohistochemical observations of LAM lung biopsy specimens.61 In agreement, immunohistochemical analysis of LAM tissue from lung biopsies indicated greater reactivity of MMP-2 and MMP-9, but not of TIMP-1 and TIMP-2, in LAM cells than in normal lung tissue.62 MMP-2 and type IV collagen colocalized in some LAM cells.62 In another study63 MT1-MMP, an activator of MMP-1, was associated with proliferating LAM cells, all suggesting from these studies a role for metalloproteinases in the cystic lung destruction of LAM. Because doxycycline is a MMP inhibitor that affects growth and migration of neoplastic cells, angiogenesis, lymphangiogenesis, and smooth muscle cell growth,113,114 MMP-9 and MMP-2 were proposed as biomarkers to measure disease progression during doxycycline therapy in a patient with LAM.115 Levels of MMP-2, MMP-9, and MMP-9 complexed with neutrophil gelatinase-associated lipocalin (NGAL), which inhibits MMP-9 degradation,116 were elevated before initiation of doxycycline treatment. After seven months of treatment with doxycycline, patient lung function had improved and MMP levels had decreased.115
Cyclin-dependent kinase 2 (CDK2) inhibitors
CDK2 inhibitors are potentially another therapeutic option for LAM.117 Tuberin is a negative regulator of cell cycle progression that shortens the G1 phase of the cell cycle by binding p27KIP1, a cyclin-dependent kinase inhibitor, preventing its degradation and leading to inhibition of the cell cycle. In the absence of tuberin, p27 is mislocalized in the cytoplasm, allowing progression of the cell cycle.118–121 Tuberin prevented degradation of p27, a major regulator of cell cycle progression, increasing the amount of p27 bound to CDK2. CDK2 inhibitors such as roscovitine, a new potential anti-cancer therapy,122 may have a role in the therapy of LAM.
Regulation of TSC1- and TSC2-dependent pathways in LAM cells by mTOR
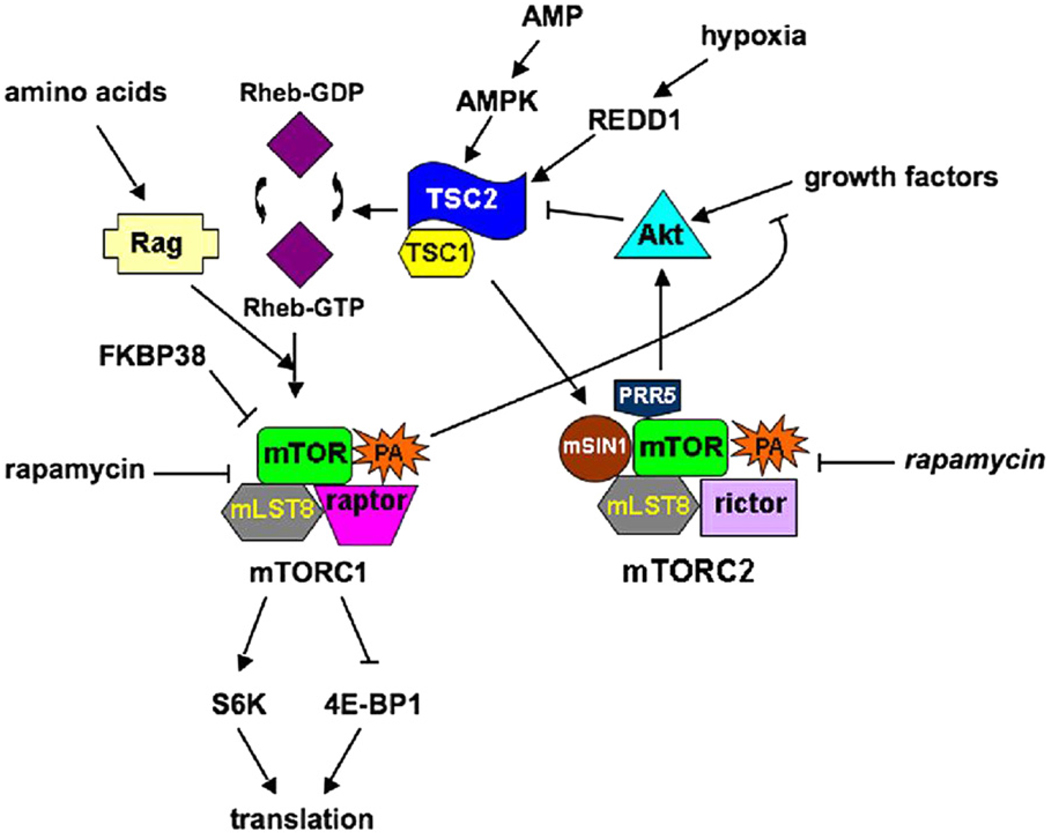
In LAM cells, mutations in the TSC2 or TSC1 genes resulted in defective regulation of a key protein kinase, mTOR. Further investigations of TSC1- and TSC2-dependent signal transduction pathways have led to promising pharmacological targets. mTOR (mammalian target of rapamycin) is an intracellular serine/threonine kinase that is responsible for mediating signals of energy availability and growth factor stimulation to regulate cell growth and metabolism (Fig. 5). Two different complexes contain mTOR: mTORC1, comprising mTOR, mLST8 (mammalian lethal with SEC13 protein 8), and raptor (regulatory associated protein of TOR); and mTORC2, containing mTOR, mLST8, rictor (rapamycin-insensitive companion of TOR), mSIN1 (mammalian stress-activated-protein-kinase-interacting protein 1), and PRR5 (proline-rich protein 5).123–125 The substrate specificity of mTORC1 is determined by raptor.126 mTORC1 is acutely sensitive to rapamycin, whereas mTORC2 is sensitive only to prolonged rapamycin treatment.127,128
Figure 5.
mTOR dependent pathways. Two different complexes contain mTOR: mTORC1, which is acutely sensitive to rapamycin, and mTORC2, which is inhibited by rapamycin (in italics) after prolonged exposure. Activation of mTORC1 leads to protein translation, and thereby cell growth and proliferation, while activation of mTORC2 results in the phosphorylation of Akt. Growth factors, hypoxia, and energy stress affect the status of the TSC1/2 complex, leading to inactivation of the tumor suppressor complex in the presence of growth factors and activation in the presence of stress. TSC1/2 inhibits mTORC1 through its Rheb-GAP activity: Rheb-GTP is necessary for the activation of mTORC1, preventing the interaction of MTOR with its endogenous inhibitor FKBP38. The presence of amino acids stimulates the localization of mTORC1 in Rheb-containing compartments through the actions of Rag-GTPases. mTORC1 is also involved in a negative feedback loop, which blocks growth factor-stimulated phosphorylation of Akt. TSC1/2 activates mTORC2, which then phosphorylates Akt.
TSC1 and TSC2 form a cytoplasmic complex129–131 that acts upstream of mTOR to integrate growth factor, energy, and stress signals and thereby regulate protein synthesis and cell growth and proliferation. TSC1, on chromosome 9q34,132 encodes the ca. 130-kDa protein hamartin, which regulates levels of Rho-GTP. It interacts with proteins of the ezrin-radixin-moezin family and with tuberin, the TSC2 protein,130,133–135 encoded by TSC2 on chromosome 16p13.3.136 The 198-kDa tuberin protein molecule contains a region with significant similarity to GTPase-activating proteins and a region that binds hamartin,130,133,137–139 which stabilizes it and prevents its ubiquitin-mediated degradation.140
The TSC1/2 complex is a negative regulator of mTORC1. TSC2 is a GAP (GTPase-activating protein) for the GTPase Rheb (Ras homolog enriched in brain), that accelerates its Rheb conversion from the active Rheb-GTP to an inactive Rheb-GDP.137–139 Rheb-GTP activates mTORC1, resulting in phosphorylation of substrates such as ribosomal S6 kinases, e.g., S6K1, and eukaryotic initiation factor 4E-binding proteins, e.g., 4E-BP1.141 These phosphorylations lead to S6 kinase activation and 4E-BP inhibition, resulting in enhanced translation of 5′ TOP (terminal oligopyrimidine tract)-containing RNAs and cap-dependent translation. In response to growth factors, Akt phosphorylates and inactivates TSC2,142,143 which relieves the TSC1/2 inhibition of Rheb, allowing mTORC1 activation and promoting translation. 144–146 Under poor growth conditions, FKBP38 binds mTORC1 and inhibits its activity.147 Rheb-GTP enables activation of mTORC1 by preventing the interaction of FKBP38 and mTOR.147 Phosphorylation of TSC2 by Akt may lead to its binding of 14-3-3 and its resulting inactivation.148
Growth factors cannot stimulate mTORC1 without a supply of amino acids or cellular energy (in the form of ATP) sufficient to fuel translation. Under conditions of energy stress as detected by increased intracellular levels of AMP, activated AMPK (AMP-dependent protein kinase) phosphorylates TSC2.149 This phosphorylation activates TSC2149 and thereby inhibits mTORC1. Hypoxia also causes AMPK activation, as oxygen is required for aerobic mitochondrial production of ATP by oxidative phosphorylation. Hypoxia stimulates HIFα (hypoxia-inducible factor α), which increases transcription of REDD1 that binds 14-3-3 proteins and can facilitate dissociation of Akt-induced TSC2/14-3-3 complexes, thereby activating TSC2 and inhibiting mTORC1.148
Ample supply of amino acids is needed also to permit localization of mTORC1 correctly for activation by Rheb. Amino acids stimulate GTP binding by Rag (Ras-related small GTP-binding protein)-GTPase heterodimers, which are able to bind raptor, and result in localization of mTORC1 in compartments where Rheb is able to activate mTORC1.150 This translocation of mTORC1 stimulated by amino acids allows the mTORC1 activation and mRNA translation only when substrate sufficient for translation is present.
The TSC1/2 complex is a positive regulator of mTORC2, resulting in phosphorylation of Akt.151,152 TSC1/2 physically interacts with mTORC2, and levels of TSC2 expression are correlated with increases in Akt phosphorylation.151 Loss of TSC1/2 in disease leads to an inability to activate Akt, through both the inactivation of mTORC2 and through a negative feedback loop created by activated mTORC1, which blocks growth factor-stimulated phosphorylation of Akt.123,151 As Akt is an important kinase in tumor progression, an inability to activate Akt may result in a more “benign” disease such as TSC, where activation of mTORC1 may initially result in tumor formation, but the negative feedback loop on Akt phosphorylation and inactivation of mTORC2 may limit disease progression.151
Rapamycin was approved in 1997 for immunosuppression in kidney transplant patients, and in 2003 for use in cardiovascular stents to prevent smooth muscle cell proliferation. 153 Rapamycin and FKBP-12 (FK506 binding protein-12) forms a complex that binds and inhibits mTORC1.154 mTORC1 responds acutely to rapamycin, whereas prolonged rapamycin treatment can inhibit mTORC2. The differences in sensitivity have been proposed to be due to phospholipase D (PLD) action and the phosphatidic acid (PA) product.155,156 PLD activity generating PA from phosphatidylcholine hydrolysis was elevated in human cancer cell lines.157–159 PA, which is required for the formation/stabilization of mTOR complexes, binds the FKBP12-rapamycin domain, competing with rapamycin for FKBP12 binding.160 It was proposed that the apparent differences in sensitivity of the two mTOR complexes reflect a difference in the affinities for PA, such that mTORC2/PA is a more stable complex than mTORC1/PA, rendering mTORC1 more sensitive to rapamycin and requiring a more prolonged treatment with rapamycin to inhibit mTORC2.155,156
Effects of rapamycin or sirolimus have been investigated on tumors or renal cysts in mouse and rat models of TSC. CCI-779 (temsirolimus, a rapamycin analog) reduced the number of cystadenomas per kidney in Tsc2+/− mice.161 Rapamycin treatment of the Eker rat, which carries a germline mutation of Tsc2 that is functionally null, diminished tumors of the kidney and suppressed phosphorylation of S6 and 4E-BP1.162 There were, however, rare persistent renal tumors with hyperphosphorylated S6 proteins after rapamycin treatment, consistent with resistance to rapamycin.
Several ongoing clinical trials are assessing the effect of rapamycin or its analogs on AML size in TSC and/or LAM patients. Some of the trials evaluate also effects on pulmonary function in LAM patients. A 24-month nonrandomized study163 of TSC and/or LAM patients examined the effects of 12 months of sirolimus on AML volume and pulmonary function (in the LAM patients), and then followed the patients another 12 months without drug. AML volume was smaller at 12 months (53% of baseline), whereas, without sirolimus, the AMLs were 86% larger (86% at 24 months). Some improvement in airflow and gas trapping was found in LAM patients although there was no effect on DLCO. Whereas the effects of sirolimus tended not to be permanent, improvements in spirometry of some LAM patients persisted after treatment stopped. Information about clinical trials of rapamycin and its analogs on disease progression in TSC and LAM patients can be found at www.clinicaltrials.gov.
Conclusion
The discovery of an effective treatment for LAM is thus far elusive. Data from a longitudinal natural history study defined predictors of time to transplantation or death and identified extra-pulmonary manifestations of LAM. Molecular insights and the discovery of possible biomarkers in LAM have led to potential therapeutic options and means to monitor effects of therapy. Detection of estrogen receptors in LAM lesions and differences in post- and pre-menopausal disease progression suggest that hormonal intervention may offer benefits, and further study is warranted to establish the effectiveness of specific interventions. The isolation of LAM cells from blood and other body fluids offers a possible non-invasive diagnostic tool. VEGF-D may be a marker for lymphatic involvement in LAM and anti-VEGF-D therapies may slow lymphatic proliferation in LAM. Elevation of MMP-2/MMP-9 levels suggests doxycycline as a useful treatment, and CD44v6 may represent an effective target for cytotoxic agents. Elucidation of the effects of TSC gene mutations on the mTOR pathway has led to a clinical trial using sirolimus.
The use of pharmacological agents against these molecular targets has advantages as well as raising questions. Side effects of anti-estrogen therapy, such as loss of bone mineral density, may preclude the use in most patients of those presently available. MMP inhibitors, that are not selective for a particular enzyme, may produce off-target effects. The effectiveness of any treatment may depend on disease stage or many other physiological variables for each individual. All of these factors should be considered in designing treatment studies.
Acknowledgments
This review was supported by the Intramural Research Program of the National Institutes of Health, NHLBI. We thank Dr. Martha Vaughan for helpful discussions and critical review of the manuscript. We thank patients with LAM for their commitment and inspiration.
Footnotes
Conflict of interest statements
The authors have no financial conflicts of interest to disclose.
Contributor Information
Connie G. Glasgow, Email: glasgowc@nih.gov.
Wendy K. Steagall, Email: steagalw@nhlbi.nih.gov.
Angelo Taveira-DaSilva, Email: dasilvaa@nhlbi.nih.gov.
Gustavo Pacheco-Rodriguez, Email: pachecog@nhlbi.nih.gov.
Xiong Cai, Email: caix@nhlbi.nih.gov.
Souheil El-Chemaly, Email: elchemalys@nhlbi.nih.gov.
Marsha Moses, Email: Marsha.Moses@childrens.harvard.edu.
Thomas Darling, Email: tdarling@usuhs.mil.
Joel Moss, Email: mossj@nhlbi.nih.gov.
References
- 1.Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995 Feb;151(2 Pt 1):527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 2.Chu SC, Horiba K, Usuki J, Avila NA, Chen CC, Travis WD, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999 Apr;115(4):1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 3.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Medicine (Baltimore) 1999 Sep;78(5):321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax. 2000 Dec;55(12):1052–1057. doi: 10.1136/thorax.55.12.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006 Jan 1;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006 Oct;13(4):276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SR, Whale CI, Hubbard RB, Lewis SA, Tattersfield AE. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax. 2004 Sep;59(9):800–803. doi: 10.1136/thx.2004.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taveira-DaSilva AM, Hedin C, Stylianou MP, Travis WD, Matsui K, Ferrans VJ, et al. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2001 Sep 15;164(6):1072–1076. doi: 10.1164/ajrccm.164.6.2102125. [DOI] [PubMed] [Google Scholar]
- 9.Avila NA, Chen CC, Chu SC, Wu M, Jones EC, Neumann RD, et al. Pulmonary lymphangioleiomyomatosis: correlation of ventilation-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology. 2000 Feb;214(2):441–446. doi: 10.1148/radiology.214.2.r00fe41441. [DOI] [PubMed] [Google Scholar]
- 10.Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, Avila NA, et al. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch. 2000 Oct;67(5):311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- 11.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998 Apr;62(4):810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000 May 23;97(11):6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato T, Seyama K, Fujii H, Maruyama H, Setoguchi Y, Iwakami S, et al. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J Hum Genet. 2002;47(1):20–28. doi: 10.1007/s10038-002-8651-8. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto Y, Horiba K, Usuki J, Chu SC, Ferrans VJ, Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999 Sep;21(3):327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- 15.Matsui K, Tatsuguchi A, Valencia J, Yu Z, Bechtle J, Beasley MB, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum Pathol. 2000 Oct;31(10):1242–1248. doi: 10.1053/hupa.2000.18500. [DOI] [PubMed] [Google Scholar]
- 16.Kelly J, Moss J. Lymphangioleiomyomatosis. Am J Med Sci. 2001 Jan;321(1):17–25. doi: 10.1097/00000441-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Maziak DE, Kesten S, Rappaport DC, Maurer J. Extrathoracic angiomyolipomas in lymphangioleiomyomatosis. Eur Respir J. 1996 Mar;9(3):402–405. doi: 10.1183/09031936.96.09030402. [DOI] [PubMed] [Google Scholar]
- 18.Matsui K, W Kr Hilbert SL, Yu ZX, Takeda K, Travis WD, et al. Hyperplasia of type II pneumocytes in pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000 Nov;124(11):1642–1648. doi: 10.5858/2000-124-1642-HOTIPI. [DOI] [PubMed] [Google Scholar]
- 19.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 20.Sampson JR, Harris PC. The molecular genetics of tuberous sclerosis. Hum Mol Genet. 1994;3 doi: 10.1093/hmg/3.suppl_1.1477. Spec No:1477–80. [DOI] [PubMed] [Google Scholar]
- 21.Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001 Aug 15;164(4):661–668. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 22.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001 Aug 15;164(4):669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 23.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000 Jun;75(6):591–594. doi: 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 24.Bittmann I, Rolf B, Amann G, Lohrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol. 2003 Jan;34(1):95–98. doi: 10.1053/hupa.2003.50. [DOI] [PubMed] [Google Scholar]
- 25.Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003 Apr 1;167(7):976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 26.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP, Jr, Wang JA, Kumaki F, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui K, Beasley MB, Nelson WK, Barnes PM, Bechtle J, Falk R, et al. Prognostic significance of pulmonary lymphangioleiomyomatosis histologic score. Am J Surg Pathol. 2001 Apr;25(4):479–484. doi: 10.1097/00000478-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Avila NA, Kelly JA, Dwyer AJ, Johnson DL, Jones EC, Moss J. Lymphangioleiomyomatosis: correlation of qualitative and quantitative thin-section CT with pulmonary function tests and assessment of dependence on pleurodesis. Radiology. 2002 Apr;223(1):189–197. doi: 10.1148/radiol.2231010315. [DOI] [PubMed] [Google Scholar]
- 29.Crausman RS, Lynch DA, Mortenson RL, King TE, Jr, Irvin CG, Hale VA, et al. Quantitative CT predicts the severity of physiologic dysfunction in patients with lymphangioleiomyomatosis. Chest. 1996 Jan;109(1):131–137. doi: 10.1378/chest.109.1.131. [DOI] [PubMed] [Google Scholar]
- 30.Paciocco G, Uslenghi E, Bianchi A, Mazzarella G, Roviaro GC, Vecchi G, et al. Diffuse cystic lung diseases: correlation between radiologic and functional status. Chest. 2004 Jan;125(1):135–142. doi: 10.1378/chest.125.1.135. [DOI] [PubMed] [Google Scholar]
- 31.Schmithorst VJ, Altes TA, Young LR, Franz DN, Bissler JJ, McCormack FX, et al. Automated algorithm for quantifying the extent of cystic change on volumetric chest CT: initial results in Lymphangioleiomyomatosis. AJR Am J Roentgenol. 2009;192(4):1037–1044. doi: 10.2214/AJR.07.3334. [DOI] [PubMed] [Google Scholar]
- 32.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004 Dec;126(6):1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 33.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Kristof AS, Avila NA, Rabel A, et al. Maximal oxygen uptake and severity of disease in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2003 Dec 15;168(12):1427–1431. doi: 10.1164/rccm.200206-593OC. [DOI] [PubMed] [Google Scholar]
- 34.Crausman RS, Jennings CA, Mortenson RL, Ackerson LM, Irvin CG, King TE., Jr Lymphangioleiomyomatosis: the pathophysiology of diminished exercise capacity. Am J Respir Crit Care Med. 1996 Apr;153(4 Pt 1):1368–1376. doi: 10.1164/ajrccm.153.4.8616568. [DOI] [PubMed] [Google Scholar]
- 35.Taveira-DaSilva AM, Hathaway OM, Sachdev V, Shizukuda Y, Birdsall CW, Moss J. Pulmonary artery pressure in lymphangioleiomyomatosis: an echocardiographic study. Chest. 2007 Nov;132(5):1573–1578. doi: 10.1378/chest.07-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005 May 15;171(10):1150–1157. doi: 10.1164/rccm.200405-578OC. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999 Aug;160(2):628–633. doi: 10.1164/ajrccm.160.2.9901027. [DOI] [PubMed] [Google Scholar]
- 38.Lazor R, Valeyre D, Lacronique J, Wallaert B, Urban T, Cordier JF. Low initial KCO predicts rapid FEV1 decline in pulmonary lymphangioleiomyomatosis. Respir Med. 2004 Jun;98(6):536–541. doi: 10.1016/j.rmed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Hayashida M, Seyama K, Inoue Y, Fujimoto K, Kubo K. The epidemiology of lymphangioleiomyomatosis in Japan: a nationwide cross-sectional study of presenting features and prognostic factors. Respirology. 2007 Jul;12(4):523–530. doi: 10.1111/j.1440-1843.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- 40.Colley MH, Geppert E, Franklin WA. Immunohistochemical detection of steroid receptors in a case of pulmonary lymphangioleiomyomatosis. AmJ Surg Pathol. 1989 Sep;13(9):803–807. doi: 10.1097/00000478-198909000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Berger U, Khaghani A, Pomerance A, Yacoub MH, Coombes RC. Pulmonary lymphangioleiomyomatosis and steroid receptors. An immunocytochemical study. Am J Clin Pathol. 1990 May;93(5):609–614. doi: 10.1093/ajcp/93.5.609. [DOI] [PubMed] [Google Scholar]
- 42.Ohori NP, Yousem SA, Sonmez-Alpan E, Colby TV. Estrogen and progesterone receptors in lymphangioleiomyomatosis, epithelioid hemangioendothelioma, and sclerosing hemangioma of the lung. Am J Clin Pathol. 1991 Oct;96(4):529–535. doi: 10.1093/ajcp/96.4.529. [DOI] [PubMed] [Google Scholar]
- 43.Yockey CC, Riepe RE, Ryan K. Pulmonary lymphangioleiomyomatosis complicated by pregnancy. Kans Med. 1986 Oct;87(10):277–278. 93. [PubMed] [Google Scholar]
- 44.Brunelli A, Catalini G, Fianchini A. Pregnancy exacerbating unsuspected mediastinal lymphangioleiomyomatosis and chylothorax. Int J Gynaecol Obstet. 1996 Mar;52(3):289–290. doi: 10.1016/0020-7292(95)02619-3. [DOI] [PubMed] [Google Scholar]
- 45.Shen A, Iseman MD, Waldron JA, King TE. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous estrogens. Chest. 1987 May;91(5):782–785. doi: 10.1378/chest.91.5.782. [DOI] [PubMed] [Google Scholar]
- 46.Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax. 2002 Dec;57(12):1085–1086. doi: 10.1136/thorax.57.12.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oberstein EM, Fleming LE, Gomez-Marin O, Glassberg MK. Pulmonary lymphangioleiomyomatosis (LAM): examining oral contraceptive pills and the onset of disease. J Womens Health (Larchmt) 2003 Jan–Feb;12(1):81–85. doi: 10.1089/154099903321154176. [DOI] [PubMed] [Google Scholar]
- 48.Wahedna I, Cooper S, Williams J, Paterson IC, Britton JR, Tattersfield AE. Relation of pulmonary lymphangioleiomyomatosis to use of the oral contraceptive pill and fertility in the UK: a national case control study. Thorax. 1994 Sep;49(9):910–914. doi: 10.1136/thx.49.9.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor JR, Ryu J, Colby TV. RaffinLymphangioleiomyo matosis TA. Clinical course in 32 patients. N Engl J Med. 1990 Nov 1;323(18):1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 50.Rossi GA, Balbi B, Oddera S, Lantero S, Ravazzoni C. Response to treatment with an analog of the luteinizing-hormone-releasing hormone in a patient with pulmonary lymphangioleiomyomatosis. Am Rev Respir Dis. 1991 Jan;143(1):174–176. doi: 10.1164/ajrccm/143.1.174. [DOI] [PubMed] [Google Scholar]
- 51.Desurmont S, Bauters C, Copin MC, Dewailly D, Tonnel AB, Wallaert B. Treatment of pulmonary lymphangioleiomyomatosis using a GnRH agonist. Rev Mal Respir. 1996 Jul;13(3):300–304. [PubMed] [Google Scholar]
- 52.Medeiros P., Jr Evaluation of functional pulmonary response to gonadotrophin releasing hormone agonist (goserelin) in the treatment of lymphangioleiomyomatosis. Am J Respir Crit Care Medicine. 2002;165:A701. [Google Scholar]
- 53.de la Fuente J, Paramo C, Roman F, Perez R, Masa C, de Letona JM. Lymphangioleiomyomatosis: unsuccessful treatment with luteinizing-hormone-releasing hormone analogues. Eur J Med. 1993 Jun–Jul;2(6):377–378. [PubMed] [Google Scholar]
- 54.Harari S, Cassandro R, Chiodini J, Taveira-DaSilva AM, Moss J. Effect of a gonadotrophin-releasing hormone analogue on lung function in lymphangioleiomyomatosis. Chest. 2008 Feb;133(2):448–454. doi: 10.1378/chest.07-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu JJ, Robb VA, Morrison TA, Ariazi EA, Karbowniczek M, Astrinidis A, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glassberg MK, Elliot SJ, Fritz J, Catanuto P, Potier M, Donahue R, et al. Activation of the estrogen receptor contributes to the progression of pulmonary lymphangioleiomyomatosis via matrix metalloproteinase-induced cell invasiveness. J Clin Endocrinol Metab. 2008 May;93(5):1625–1633. doi: 10.1210/jc.2007-1283. [DOI] [PubMed] [Google Scholar]
- 57.Clements D, Asprey SL, McCulloch TA, Morris TA, Watson SA, Johnson SR. Analysis of the oestrogen response in an angiomyolipoma derived xenograft model. Endocr Relat Cancer. 2009 Mar;16(1):59–72. doi: 10.1677/ERC-08-0123. [DOI] [PubMed] [Google Scholar]
- 58.Almoosa KF, McCormack FX, Sahn SA. Pleural disease in lymphangioleiomyomatosis. Clin Chest Med. 2006 Jun;27(2):355–368. doi: 10.1016/j.ccm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Steagall WK, Glasgow CG, Hathaway OM, Avila NA, Taveira-Dasilva AM, Rabel A, et al. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L800–L808. doi: 10.1152/ajplung.00176.2007. [DOI] [PubMed] [Google Scholar]
- 60.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol. 2005 May;98(5):1892–1899. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 61.Fukuda Y, Kawamoto M, Yamamoto A, Ishizaki M, Basset F, Masugi Y. Role of elastic fiber degradation in emphysema-like lesions of pulmonary lymphangiomyomatosis. Hum Pathol. 1990 Dec;21(12):1252–1261. doi: 10.1016/s0046-8177(06)80039-0. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi T, Fleming MV, Stetler-Stevenson WG, Liotta LA, Moss J, Ferrans VJ, et al. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM) Hum Pathol. 1997 Sep;28(9):1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- 63.Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000 Feb;124(2):267–275. doi: 10.5858/2000-124-0267-RFAOMM. [DOI] [PubMed] [Google Scholar]
- 64.Avila NA, Kelly JA, Chu SC, Dwyer AJ, Moss J. Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology. 2000 Jul;216(1):147–153. doi: 10.1148/radiology.216.1.r00jl42147. [DOI] [PubMed] [Google Scholar]
- 65.Avila NA, Bechtle J, Dwyer AJ, Ferrans VJ, Moss J. Lymphangioleiomyomatosis: CT of diurnal variation of lymphangioleiomyomas. Radiology. 2001 Nov;221(2):415–421. doi: 10.1148/radiol.2212001448. [DOI] [PubMed] [Google Scholar]
- 66.Avila NA, Dwyer AJ, Murphy-Johnson DV, Brooks P, Moss J. Sonography of lymphangioleiomyoma in lymphangioleiomyomatosis: demonstration of diurnal variation in lesion size. AJR Am J Roentgenol. 2005 Feb;184(2):459–464. doi: 10.2214/ajr.184.2.01840459. [DOI] [PubMed] [Google Scholar]
- 67.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996 Dec;122(12):3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 69.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001 Feb;7(2):186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 70.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002 Jul;82(3):673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 71.Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004 Aug;28(8):1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 72.Kumasaka T, Seyama K, Mitani K, Souma S, Kashiwagi S, Hebisawa A, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005 Oct;29(10):1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 73.Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;4(3):143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 74.Glasgow CG, Avila NA, Lin JP, Stylianou MP, Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009 May;135(5):1293–1300. doi: 10.1378/chest.08-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008 Jan 10;358(2):199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Achen MG, Roufail S, Domagala T, Catimel B, Nice EC, Geleick DM, et al. Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur J Biochem. 2000 May;267(9):2505–2515. doi: 10.1046/j.1432-1327.2000.01257.x. [DOI] [PubMed] [Google Scholar]
- 77.Stacker SA, Achen MG. From anti-angiogenesis to anti-lymphangiogenesis: emerging trends in cancer therapy. Lymphat Res Biol. 2008;6(3–4):165–172. doi: 10.1089/lrb.2008.1015. [DOI] [PubMed] [Google Scholar]
- 78.Hanrahan EO, Heymach JV. Vascular endothelial growth factor receptor tyrosine kinase inhibitors vandetanib (ZD6474) and AZD2171 in lung cancer. Clin Cancer Res. 2007 Aug 1;13(15 Pt 2):s4617–s4622. doi: 10.1158/1078-0432.CCR-07-0539. [DOI] [PubMed] [Google Scholar]
- 79.Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosomes Cancer. 2003 Dec;38(4):376–381. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- 80.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007 May;28(3):297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 81.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003 Jan;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 82.Heider KH, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother. 2004 Jul;53(7):567–579. doi: 10.1007/s00262-003-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006 Jul 1;20(13):1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng C, Sharp PA. Regulation of CD44 alternative splicing by SRm160 and its potential role in tumor cell invasion. Mol Cell Biol. 2006 Jan;26(1):362–370. doi: 10.1128/MCB.26.1.362-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matter N, Herrlich P, Konig H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002 Dec 12;420(6916):691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 86.Noda M, Yoon K, Prince CW, Butler WT, Rodan GA. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J Biol Chem. 1988 Sep 25;263(27):13916–13921. [PubMed] [Google Scholar]
- 87.Atkins KB, Simpson RU, Somerman MJ. Stimulation of osteopontin mRNA expression in HL-60 cells is independent of differentiation. Arch Biochem Biophys. 1997 Jul 15;343(2):157–163. doi: 10.1006/abbi.1997.0151. [DOI] [PubMed] [Google Scholar]
- 88.Omigbodun A, Ziolkiewicz P, Tessler C, Hoyer JR, Coutifaris C. Progesterone regulates osteopontin expression in human trophoblasts: a model of paracrine control in the placenta? Endocrinology. 1997 Oct;138(10):4308–4315. doi: 10.1210/endo.138.10.5431. [DOI] [PubMed] [Google Scholar]
- 89.Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine–glycine–aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999 Jan 1;59(1):219–226. [PubMed] [Google Scholar]
- 90.Gao C, Guo H, Downey L, Marroquin C, Wei J, Kuo PC. Osteopontin-dependent CD44v6 expression and cell adhesion in HepG2 cells. Carcinogenesis. 2003 Dec;24(12):1871–1878. doi: 10.1093/carcin/bgg139. [DOI] [PubMed] [Google Scholar]
- 91.Pacheco-Rodriguez G, Steagall WK, Crooks DM, Stevens LA, Hashimoto H, Li S, et al. TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis. Cancer Res. 2007 Nov 1;67(21):10573–10581. doi: 10.1158/0008-5472.CAN-07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004 Dec;95(12):930–935. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderegg U, Eichenberg T, Parthaune T, Haiduk C, Saalbach A, Milkova L, et al. ADAM10 is the constitutive functional sheddase of CD44 in human melanoma cells. J Invest Dermatol. 2009 Jun;129(6):1471–1482. doi: 10.1038/jid.2008.323. [DOI] [PubMed] [Google Scholar]
- 94.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 95.Reeder JA, Gotley DC, Walsh MD, Fawcett J, Antalis TM. Expression of antisense CD44 variant 6 inhibits colorectal tumor metastasis and tumor growth in a wound environment. Cancer Res. 1998 Aug 15;58(16):3719–3726. [PubMed] [Google Scholar]
- 96.Harada N, Mizoi T, Kinouchi M, Hoshi K, Ishii S, Shiiba K, et al. Introduction of antisense CD44S CDNA down-regulates expression of overall CD44 isoforms and inhibits tumor growth and metastasis in highly metastatic colon carcinoma cells. Int J Cancer. 2001 Jan 1;91(1):67–75. doi: 10.1002/1097-0215(20010101)91:1<67::aid-ijc1011>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 97.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nature Med. 2006;12(10):1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 98.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001 Mar 1;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 99.Pacheco-Rodriguez G, Kumaki F, Steagall WK, Zhang Y, Ikeda Y, Lin JP, et al. Chemokine-enhanced chemotaxis of lymphangioleiomyomatosis cells with mutations in the tumor suppressor TSC2 gene. J Immunol. 2009 Feb 1;182(3):1270–1277. doi: 10.4049/jimmunol.182.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li S, Takeuchi F, Wang JA, Fuller C, Pacheco-Rodriguez G, Moss J, et al. MCP-1 overexpressed in tuberous sclerosis lesions acts as a paracrine factor for tumor development. J Exp Med. 2005 Sep 5;202(5):617–624. doi: 10.1084/jem.20042469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu X, Lee VC, Chevalier E, Hwang ST. Chemokine receptors as targets for cancer therapy. Curr Pharm Des. 2009;15(7):742–757. doi: 10.2174/138161209787582165. [DOI] [PubMed] [Google Scholar]
- 102.Dawson J, Miltz W, Mir AK, Wiessner C. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin Ther Targets. 2003 Feb;7(1):35–48. doi: 10.1517/14728222.7.1.35. [DOI] [PubMed] [Google Scholar]
- 103.Dol F, Martin G, Staels B, Mares AM, Cazaubon C, Nisato D, et al. Angiotensin AT1 receptor antagonist irbesartan decreases lesion size, chemokine expression, and macrophage accumulation in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2001 Sep;38(3):395–405. doi: 10.1097/00005344-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 104.Nagase H. Matrix metalloproteinases. In: HN M, editor. Zinc metalloproteases in health and disease. London: Taylor and Frances; 1996. pp. 153–204. [Google Scholar]
- 105.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43 Suppl:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 106.La Rocca G, Pucci-Minafra I, Marrazzo A, Taormina P, Minafra S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. Br J Cancer. 2004 Apr 5;90(7):1414–1421. doi: 10.1038/sj.bjc.6601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koc M, Ediger D, Budak F, Karadag M, Oral HB, Uzaslan E, et al. Matrix metalloproteinase-9 (MMP-9) elevated in serum but not in bronchial lavage fluid in patients with lung cancer. Tumori. 2006 Mar–Apr;92(2):149–154. doi: 10.1177/030089160609200211. [DOI] [PubMed] [Google Scholar]
- 108.O-Charoenrat P, Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001 Jul;127(7):813–820. [PubMed] [Google Scholar]
- 109.Tamura M, Oda M, Matsumoto I, Tsunezuka Y, Kawakami K, Ohta Y, et al. The combination assay with circulating vascular endothelial growth factor (VEGF)-C, matrix metalloproteinase-9, and VEGF for diagnosing lymph node metastasis in patients with non-small cell lung cancer. Ann Surg Oncol. 2004 Oct;11(10):928–933. doi: 10.1245/ASO.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 110.Tutton MG, George ML, Eccles SA, Burton S, Swift RI, Abulafi AM. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int J Cancer. 2003 Nov 20;107(4):541–550. doi: 10.1002/ijc.11436. [DOI] [PubMed] [Google Scholar]
- 111.Mylona E, Nomikos A, Magkou C, Kamberou M, Papassideri I, Keramopoulos A, et al. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology. 2007 Feb;50(3):338–347. doi: 10.1111/j.1365-2559.2007.02615.x. [DOI] [PubMed] [Google Scholar]
- 112.Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998 Apr 1;58(7):1395–1399. [PubMed] [Google Scholar]
- 113.Gilbertson-Beadling S, Powers EA, Stamp-Cole M, Scott PS, Wallace TL, Copeland J, et al. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother Pharmacol. 1995;36(5):418–424. doi: 10.1007/BF00686191. [DOI] [PubMed] [Google Scholar]
- 114.Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol. 2006;4(2):83–100. doi: 10.1089/lrb.2006.4.83. [DOI] [PubMed] [Google Scholar]
- 115.Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006 Jun 15;354(24):2621–2622. doi: 10.1056/NEJMc053410. [DOI] [PubMed] [Google Scholar]
- 116.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001 Oct 5;276(40):37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 117.Burgstaller S, Rosner M, Lindengrun C, Hanneder M, Siegel N, Valli A, et al. Tuberin, p27 and MTOR in different cells. Amino Acids. 2009 Feb;36(2):297–302. doi: 10.1007/s00726-008-0066-1. [DOI] [PubMed] [Google Scholar]
- 118.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. MTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008 Jun 20;30(6):701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 119.Short JD, Houston KD, Dere R, Cai SL, Kim J, Johnson CL, et al. AMP-activated protein kinase signaling results in cytoplasmic sequestration of p27. Cancer Res. 2008 Aug 15;68(16):6496–6506. doi: 10.1158/0008-5472.CAN-07-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosner M, Hengstschlager M. Tuberin binds p27 and negatively regulates its interaction with the SCF component Skp2. J Biol Chem. 2004 Nov 19;279(47):48707–48715. doi: 10.1074/jbc.M405528200. [DOI] [PubMed] [Google Scholar]
- 121.Soucek T, Rosner M, Miloloza A, Kubista M, Cheadle JP, Sampson JR, et al. Tuberous sclerosis causing mutants of the TSC2 gene product affect proliferation and p27 expression. Oncogene. 2001 Aug 9;20(35):4904–4909. doi: 10.1038/sj.onc.1204627. [DOI] [PubMed] [Google Scholar]
- 122.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009 Mar;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 123.Polak P, Hall MN. MTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009 Apr;21(2):209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 124.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002 Sep;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 125.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of MTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004 Jul 27;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 126.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002 Jul 26;110(2):177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 127.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits MTORC2 assembly and Akt/PKB. Mol Cell. 2006 Apr 21;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 128.Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, et al. Rapamycin derivatives reduce MTORC2 signaling and inhibit AKT activation in AML. Blood. 2007 Apr 15;109(8):3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plank TL, Yeung RS, Henske EP. Hamartin, the product of the tuberous sclerosis 1 (TSC1) gene, interacts with tuberin and appears to be localized to cytoplasmic vesicles. Cancer Res. 1998 Nov 1;58(21):4766–4770. [PubMed] [Google Scholar]
- 130.van Slegtenhorst M, Nellist M, Nagelkerken B, Cheadle J, Snell R, van den Ouweland A, et al. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet. 1998 Jun;7(6):1053–1057. doi: 10.1093/hmg/7.6.1053. [DOI] [PubMed] [Google Scholar]
- 131.Nellist M, van Slegtenhorst MA, Goedbloed M, van den Ouweland AM, Halley DJ, van der Sluijs P. Characterization of the cytosolic tuberin-hamartin complex. Tuberin is a cytosolic chaperone for hamartin. J Biol Chem. 1999 Dec 10;274(50):35647–35652. doi: 10.1074/jbc.274.50.35647. [DOI] [PubMed] [Google Scholar]
- 132.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science (New York, NY) 1997;277(5327):805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 133.Rosner M, Freilinger A, Hengstschlager M. Proteins interacting with the tuberous sclerosis gene products. Amino Acids. 2004 Oct;27(2):119–128. doi: 10.1007/s00726-004-0119-z. [DOI] [PubMed] [Google Scholar]
- 134.Lamb RF, Roy C, Diefenbach TJ, Vinters HV, Johnson MW, Jay DG, et al. The TSC1 tumour suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nat Cell Biol. 2000 May;2(5):281–287. doi: 10.1038/35010550. [DOI] [PubMed] [Google Scholar]
- 135.Fukuhara S, Gutkind JS. A new twist for the tumour suppressor hamartin. Nat Cell Biol. 2000 May;2(5):E76–E78. doi: 10.1038/35010506. [DOI] [PubMed] [Google Scholar]
- 136.The European Chromosome 16 tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75(7):1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 137.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003 Aug 29;278(35):32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 138.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control MTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003 Aug 5;13(15):1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003 Jun;5(6):578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 140.Benvenuto G, Li S, Brown SJ, Braverman R, Vass WC, Cheadle JP, et al. The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene. 2000 Dec 14;19(54):6306–6316. doi: 10.1038/sj.onc.1204009. [DOI] [PubMed] [Google Scholar]
- 141.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by MTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002 Jun 15;16(12):1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses MTOR signalling. Nat Cell Biol. 2002 Sep;4(9):648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 143.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002 Jul;10(1):151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 144.Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002 Sep;4(9):699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 145.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002 Aug 23;277(34):30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 146.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (MTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, et al. Rheb activates MTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007 Nov 9;318(5852):977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 148.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-MTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008 Jan 15;22(2):239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003 Nov 26;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 150.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to MTORC1. Science. 2008 Jun 13;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1–TSC2 complex is required for proper activation of MTOR complex 2. Mol Cell Biol. 2008 Jun;28(12):4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-MTOR complex. Science. 2005 Feb 18;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 153.Plas DR, Thomas G. Tubers and tumors: rapamycin therapy for benign and malignant tumors. Curr Opin Cell Biol. 2009 Apr;21(2):230–236. doi: 10.1016/j.ceb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 154.Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995 Oct 5;377(6548):441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 155.Foster DA. Phosphatidic acid signaling to MTOR: Signals for the survival of human cancer cells. Biochim Biophys Acta. 2009 Mar 2; doi: 10.1016/j.bbalip.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of MTORC1 and MTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009 Mar;29(6):1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Noh DY, Ahn SJ, Lee RA, Park IA, Kim JH, Suh PG, et al. Overexpression of phospholipase D1 in human breast cancer tissues. Cancer Lett. 2000 Dec 20;161(2):207–214. doi: 10.1016/s0304-3835(00)00612-1. [DOI] [PubMed] [Google Scholar]
- 158.Zhao Y, Ehara H, Akao Y, Shamoto M, Nakagawa Y, Banno Y, et al. Increased activity and intranuclear expression of phospholipase D2 in human renal cancer. Biochem Biophys Res Commun. 2000 Nov 11;278(1):140–143. doi: 10.1006/bbrc.2000.3719. [DOI] [PubMed] [Google Scholar]
- 159.Uchida N, Okamura S, Kuwano H. Phospholipase D activity in human gastric carcinoma. Anticancer Res. 1999 Jan–Feb;19(1B):671–675. [PubMed] [Google Scholar]
- 160.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of MTOR signaling. Science. 2001 Nov 30;294(5548):1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 161.Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer. 2005 Mar;42(3):213–227. doi: 10.1002/gcc.20118. [DOI] [PubMed] [Google Scholar]
- 162.Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005 Jan;57(1):67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- 163.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008 Jan 10;358(2):140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]