Abstract
Type II diabetes, in its late stages, is often associated with the formation of extracellular islet amyloid deposits composed of islet amyloid polypeptide (IAPP or amylin). IAPP is stored before secretion at millimolar concentrations within secretory granules inside the β-cells. Of interest, at these same concentrations in vitro, IAPP rapidly aggregates and forms fibrils, yet within secretory granules of healthy individuals, IAPP does not fibrillize. Insulin is also stored within the secretory granules before secretion, and has been shown in vitro to inhibit IAPP fibril formation. Because of insulin's inhibitory effect on IAPP fibrillization, it has been suggested that insulin may also inhibit IAPP-mediated permeabilization of the β-cell plasma membrane in vivo. We show that although insulin is effective at preventing fiber-dependent membrane disruption, it is not effective at stopping the initial phase of membrane disruption before fibrillogenesis, and does not prevent the formation of small IAPP oligomers on the membrane. These results suggest that insulin has a more complicated role in inhibiting IAPP fibrillogenesis, and that other factors, such as the low pH of the secretory granule, may also play a role.
Introduction
Although the exact cause of β-cell death in Type II diabetes is unknown, there is strong evidence that human islet amyloid polypeptide (IAPP) plays an important role (1–3). It is well known through in vitro, tissue culture, and transgenic animal studies, as well as postmortem clinical examinations, that large β-sheet aggregates of IAPP, known as islet amyloid, are often associated with the development of Type II diabetes (1–3). Similar extracellular proteinaceous deposits composed of large aggregates of proteins in a characteristic cross β-sheet conformation are being found in a growing number of pathologies, including Alzheimer's, Huntington's, and Parkinson's disease among many others (4). An aggregated (but not necessarily fibrillar) form of the peptides and proteins implicated in these amyloid-associated diseases has been found to disrupt the integrity of cellular membranes to various degrees by allowing the uncontrolled influx of ions (in particular calcium) into the cell and subjecting the cell to membrane-associated stress (5,6).
The relevant factors that trigger IAPP aggregation and pancreatic β-cell membrane disruption in some individuals but not others are elusive, as the pathology of IAPP aggregation occurs most often with the wild-type peptide and is not associated with mutations of the IAPP sequence, except for the rare S20G polymorphism (7–10). Because wild-type IAPP is both aggressively amyloidogenic and toxic at low concentrations (<1 μM) when it is added exogenously, yet is stored in the secretory granule of β-cells at concentrations orders of magnitude higher (0.8–4 mM) without apparent dysfunction in nondiabetic individuals, it is unknown how IAPP is regulated to prevent both its aggregation and its inherent cytotoxicity. In fact, considering the high toxicity of IAPP in vitro, it may be more relevant to question what stops IAPP from permeabilizing β-cells in healthy individuals, rather than what triggers IAPP-induced toxicity to β-cells in diabetics.
Insulin, which is copackaged and cosecreted with IAPP within the secretory granule, has emerged as a possible regulator of IAPP fibrillogenesis. Insulin forms heterocomplexes with IAPP (11–13). Several studies have reported that these heterocomplexes can inhibit IAPP amyloid formation (12–15,17–20,22), although there is also evidence for either little change or a slight increase in the rate of fibril formation at lower ratios of IAPP to insulin (23). Because insulin suppresses IAPP amyloid fiber formation and is found in great excess over IAPP in the secretory granule, it is frequently assumed that insulin also inhibits the inherent toxicity of IAPP (11,14,18). However, studies on other amyloid inhibitors have shown that these two actions are not necessarily linked. For instance, compounds that bind to the end-stage of aggregation and consist mostly of amyloid fibrils are frequently unsuccessful in reducing the toxicity of amyloidogenic proteins, most likely due to the low toxicity of the end-stage amyloid fibers (24). A prime example of this effect is the anti-amyloid-β1-11 antibody, which was shown to inhibit amyloid-β1–42 fibrillization yet failed to prevent amyloid-β1–42 toxicity (25). Other compounds have been shown to actually increase the toxicity of amyloid proteins by inhibiting the formation of the relatively inert amyloid fibrils while increasing the amount of peptide in the small oligomeric, more toxic form. An example of this effect is clusterin (apolipoprotein J), a protein found in Alzheimer's disease plaques that inhibits Aβ1–42 from forming amyloid yet increases the cytotoxicity of the Aβ1–42 peptide (26–28). Conversely, other inhibitors have been shown to block the toxicity of these peptides without affecting or increasing amyloid formation. In this study, we show that insulin does not suppress the ability of IAPP to cause model membrane disruption, an effect that is believed to be one of the prime indicators of IAPP toxicity, in a simple manner. Instead, insulin appears to eliminate only later fiber-dependent membrane disruption, without affecting earlier events in the membrane permeabilization process.
Materials and Methods
IAPP and insulin preparation
Human IAPP was purchased from Anaspec (San Jose, CA). Recombinant human insulin expressed in yeast was purchased from Sigma-Aldrich (St. Louis, MO). The IAPP peptide used in this study was amidated at the C-terminus like the natural peptide. Preformed aggregates in both peptide preparations were disaggregated by first dissolving the peptide in a 75% acetonitrile/water solution at a concentration of 1 mg/mL. The peptides were then lyophilized and the lyophilized powder was redissolved in hexafluoroisopropanol (HFIP) at a concentration of 2–4 mg/mL. The HFIP was removed by lyophilization overnight at high vacuum (29). After lyophilization was completed, the peptide was first dissolved in pure water to a concentration of 25 μM in siliconized Eppendorf tubes and then diluted with 2× buffer to create the final working solution (final buffer composition: 10 mM sodium phosphate, pH 7.4, 100 mM NaCl) (30).
Liposome preparation
1-Palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (POPG) and 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) dissolved in chloroform were purchased from Avanti (Alabaster, AL). To make liposomes, chloroform lipid stocks were dried with a stream of nitrogen and then placed under vacuum overnight to remove residual solvent. The lipid films were then rehydrated for 1–2 h with 10 mM NaPi buffer at pH 7.4 containing 100 mM NaCl. The rehydrated lipids were vortexed and then subjected to eight freeze-thaw cycles. The resulting multilamellar vesicles were extruded 21 times through two 100 nm polycarbonate Nucleopore membrane filters (Whatman) to create unilamellar vesicles.
To prepare liposomes with dye encapsulated inside, we created vesicles as above except that the buffer also contained 50 mM 5(6)-carboxyfluorescein. Free dye was separated from the dye-encapsulated lipid vesicles by running the sample through a 10 mL Sephadex G-50 gel filtration column (Sigma-Aldrich). Lipid concentrations were determined using the Stewart method (31). Liposomes with encapsulated dye were mixed with liposomes without dye in a 1:20 ratio to create the final stock solution.
Dye leakage experiments with direct addition of IAPP/insulin samples
IAPP (10 μM concentration) was directly mixed with 200 μM POPG/POPC liposomes (1:1 molar ratio) in 50 mM sodium phosphate buffer pH 7.4, 100 mM NaCl. The fluorescence was then recorded using a Biotek Synergy 2 plate reader equipped with 494 nm excitation filter with a 2 nm bandpass, and a 512 nm emission filter with a 2 nm bandpass for 400 min at 25°C under fast shaking.
The fluorescence signal recorded was normalized against the value at 100% permeabilization by adding Triton-X detergent to 0.2% v/v after 400 min, which effectively permeabilized any remaining intact dye-encapsulated large unilamellar vesicles (LUVs). Dye leakage was plotted according to the following equation:
Dye leakage experiments with preincubation of IAPP/insulin
IAPP or IAPP/insulin was preincubated for the indicated length of time at a 25 μM concentration in siliconized Eppendorf tubes at 25°C without shaking, and then diluted and added to a concentrated POPG vesicle stock solution to initiate membrane permeabilization. The final concentrations for the assay were 1 μM IAPP, 200 μM POPG, and 0, 1, or 10 μM insulin.
Thioflavin T assay
Peptides were initially solubilized with deionized water. Buffer and Thioflavin T (ThT) were then added to make the final concentrations 5 μM ThT, 25 μM IAPP, and 25 or 250 μM insulin in 100 mM NaCl, 10 mM NaPi, pH 7.4 buffer. The aggregation reaction was performed without shaking in sealed Corning 96-well, clear-bottom, half-area, nonbinding surface plates at a constant temperature of 25°C. The fluorescence from the ThT reporter dye was recorded by a Biotek Synergy 2 plate reader using a 440 excitation filter equipped with a 30 nm bandpass, and a 485 emission filter with a 20 nm bandpass.
Glutaraldehyde cross-linking and Western blotting
IAPP (25 μM) and IAPP/insulin (25 μM: 25 μM) were incubated at room temperature with POPG/POPC LUVs (1:1 molar ratio, 200 μM) in 100 mM NaF, 10 mM NaPi, pH 7.4, in siliconized Eppendorf tubes at 25°C without shaking. After incubation, 0.01% (v/v) glutaraldehyde was added to initiate cross-linking at each time point. The cross-linking reaction was allowed to continue for 30 min and was then quenched by the addition of 100 mM lysine. Then, 3× sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added and the samples were placed on a heat block at 90°C for 2 min. The samples were electrophoresed on 10–20% (w/v) polyacrylamide SDS Tris-Tricine Ready Gel (Bio-Rad, Hercules, CA), transferred with CAPs transfer buffer, pH 11.0, immunoblotted on nitrocellulose with rabbit polyclonal α-IAPP antibody (Anaspec, San Jose, CA). The primary antibody was visualized with a horseradish peroxidase conjugated α-rabbit antibody and enhanced chemiluminescence (Bio-Rad, Hercules, CA).
Results
The kinetics of membrane disruption by IAPP in the absence of insulin is biphasic, and the second phase is associated with the maturation of IAPP amyloid fibrils
One of the primary mechanisms of IAPP-induced toxicity is believed to be disruption of the cellular membrane (5,6,32–38), which leads to dysregulation of calcium homeostasis, mitochondrial overload, and eventually apoptosis. We used a dye leakage assay to measure the ability of IAPP to permeabilize model membranes. A self-quenching dye, (6)-carboxyfluorescein, was entrapped at high concentrations in POPC/POPG vesicles (1:1 molar ratio, 200 μM lipid concentration) and incubated with either IAPP alone or IAPP with varying concentrations of insulin. Disruption of the integrity of the membrane surface releases the vesicle contents into solution, where dilution of the self-quenching dye results in a large increase in fluorescence.
A time-trace of dye leakage induced by 10 μM IAPP is shown in Fig. 1. In the absence of insulin, membrane permeabilization by IAPP occurs in two distinct stages. Typically, membrane-disrupting agents (e.g., antimicrobial peptides) cause rapid, near-exponential increases in fluorescence as transient pores form on the membrane (39,40). The final normalized fluorescence value as t→∞ may not approach one, as would be expected for total disruption of all vesicles (graded leakage). This phenomenon has been observed for antimicrobial peptides and reported for IAPP (38), and is believed to result from a relaxation process that relieves mechanical stress on the membrane, resulting in mass imbalance on the outer leaflet of the membrane caused by the binding of the peptide (41). Several mechanisms have been proposed for the relaxation process, including translocation of the peptide across the membrane (42,43), which may occur through the formation of toroidal pores (44) or by the removal of IAPP-lipid adducts from the membrane (33,36,45,46).
Figure 1.
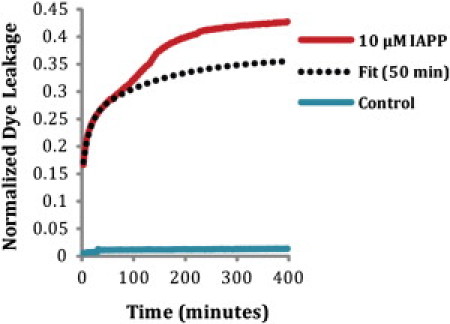
Mean (n = 5) kinetic trace of membrane disruption of 10 μM IAPP added to 200 μM POPG/POPC vesicles (1:1 molar ratio). The initial phase (t = 0–50 min) of membrane disruption was fit to a double exponential curve: Vesicles were stable over the time course of the experiment in the absence of IAPP, as shown by the negligible dye leakage that occurred in control liposomes.
The early stage of membrane disruption by IAPP (t = 0 to ∼50 min) indeed displays the double exponential kinetics that is typical of antimicrobial peptides (Fig. 1, dashed line) and readily apparent on this timescale, with the initial rise in fluorescence followed by the gradual decrease in the rate-of-efflux from the vesicle. However, a second phase is also apparent after ∼50 min in which the fluorescence begins to rise once again, as shown in Fig. 1. This second phase has been linked to damage to the membrane through the growth of amyloid fibers on the membrane, because the kinetic profile of the second phase of dye leakage is consistent with the kinetic profile of amyloid formation (see Fig. 3), as previously shown for IAPP (33). The fitted curve is the same for both Figs. 1 and 2.
Figure 3.
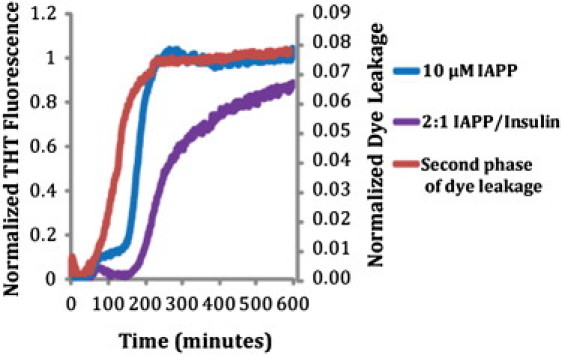
Amyloid fiber formation in relation to the second phase of membrane disruption. Amyloid fiber formation by 10 μM IAPP and 10 μM IAPP/5 μM insulin as tracked by ThT fluorescence is plotted on the left axis, and the second phase of membrane disruption is plotted on the right axis. The second phase of membrane disruption was defined as the difference between the observed fluorescence values and those predicted by a double exponential fit of the first 50 min (see Fig. 1). Higher concentrations of insulin led to high background signals of THT fluorescence, possibly due to nonspecific binding of THT to nonamyloid forms of insulin (data not shown).
Figure 2.
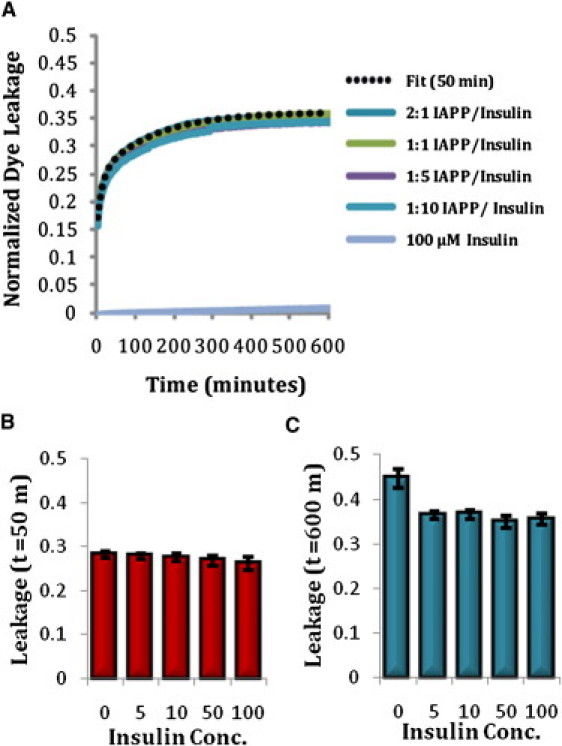
(A) Mean (n = 5) kinetic traces of membrane disruption by IAPP/insulin mixtures. The double exponential fit to the initial (t = 0–50 min) phase of membrane disruption by IAPP alone is shown for comparison (dashed line, ). Insulin by itself did cause membrane disruption at any concentration over the course of the experiment (data for 5, 10, and 50 μM insulin not shown). (B) Membrane disruption caused by IAPP/insulin mixtures at t = 50 min. Error bars indicate mean ± SE (n = 5). (C) Membrane disruption caused by IAPP/insulin mixtures at t = 600 min. Error bars indicate mean ± SE (n = 5).
Insulin blocks late fibril-dependent membrane disruption but not initial membrane permeabilization by IAPP
If fibril formation correlates with the later phase of membrane disruption, molecules that block fibril formation should also inhibit or block membrane permeabilization. Because insulin has been shown to block fibril formation in both aqueous and membrane environments (14–20), it is likely that it will also block fibril-dependent membrane damage. Fig. 3 shows that even substoichiometric amounts of insulin (2:1 IAPP/insulin molar ratio) block the second phase of membrane disruption. The total amount of membrane disruption in the presence of insulin is similar to that expected based on a model of the first phase alone (Fig. 3 B), a strong indication that suppression of IAPP amyloid formation by insulin also eliminates damage to the membrane associated with the amyloid formation process. This is in agreement with previous results that showed a decrease in the total membrane leakage by IAPP in 7:3 POPC/POPS liposomes after 1000 min in the presence of insulin (33).
Of interest, although the second phase of membrane disruption is blocked, the initial rise in fluorescence is almost entirely unaffected by insulin (insulin by itself only negligibly affects the stability of the dye-loaded vesicles). For this lipid composition, insulin apparently does not block association of IAPP with the membrane, in agreement with previous attenuated total reflectance Fourier transform infrared (ATR-FTIR) results that showed a reduced, but still substantial, attachment of IAPP in 7:3 POPC/POPS liposomes in the presence of insulin (20). In addition, once it is bound to the membrane, IAPP apparently is still able to affect membrane disruption.
Insulin slows the time-dependent decrease in the ability of IAPP to cause membrane disruption associated with the formation of amyloid fibrils in solution
The varying ability of different oligomeric forms of amyloid proteins to cause membrane disruption has been the subject of intensive research. Although the exact toxicity of particular oligomeric species has been the subject of much controversy, mature amyloid fibrils that are fully preformed in solution before addition to the membrane have been found almost universally to be less damaging to the membrane than oligomeric forms of the same peptides. For this reason, some investigators have hypothesized that the formation of amyloid fibrils serves as a protective mechanism against the toxic effects of smaller oligomeric species (47).
Because the amyloid form of the peptide is less effective at disrupting membranes than the freshly dissolved peptide, the ability of IAPP to permeabilize membranes declines with incubation time in solution before addition to the membrane as the percentage of preformed amyloid in the sample increases. We monitored this effect by adding IAPP (with or without insulin) to fresh lipid preparations at each time point to measure how membrane disruption changed with the growth of amyloid fibrils (Fig. 4). IAPP's ability to permeabilize liposomes in the absence of insulin declined rapidly after 5 h of preincubation in solution before it was added to the liposomes, which roughly corresponds with the start of exponential fibril growth in solution measured by ThT fluorescence at ∼15 h (Fig. 4 B). This result is in agreement with numerous reports that have shown mature IAPP amyloid fibrils to be nondisruptive to membranes and nontoxic to cells, in contrast to earlier forms of the peptide.
Figure 4.
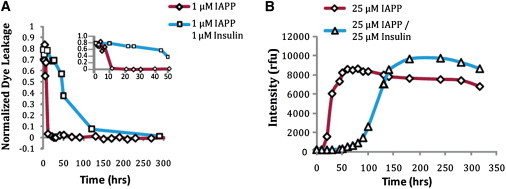
(A) Time course of membrane disruption by IAPP preincubated with insulin. First, 25 μM IAPP were incubated in solution with 0, 25, or 250 μM insulin in the absence of lipids for the indicated time points. A 1:25 dilution (1 μM IAPP and 0, 1, or 10 μM insulin) was then added to 200 μM POPG vesicles and the dye leakage that was induced after 100 s was recorded. (B) Amyloid fiber formation by 25 μM IAPP incubated with 0, 25, or 250 μM insulin in the absence of lipids as shown by ThT fluorescence.
In contrast to the rapid decrease in permeabilization activity with preincubation time in the absence of insulin, the ability of the IAPP/insulin mixture to permeabilize liposomes declined only a little over the course of the experiment. The membrane disruptive activity of 1:1 IAPP/insulin did not fully disappear until after ∼50 h, when it started to slowly polymerize (Fig. 4 B). At a higher concentration of 250 μM (10× the equimolar concentration to IAPP), insulin completely prevented the time-dependent decrease in permeabilization over the course of the experiment. Insulin by itself did not cause membrane disruption over the course of the experiment.
Insulin does not prevent the formation of small oligomeric species of IAPP
We performed glutaraldehyde cross-linking to detect populations of small oligomeric species of IAPP bound to the membrane at several time points (at 0, 2, 3, and 4 h) during membrane-catalyzed aggregation of IAPP, using an IAPP-specific antibody to identify IAPP species. In the absence of insulin, we detected bands that corresponded to monomers, dimers, trimers, and tetramers, along with a strong band that was identified as a high molecular weight (MW) species that exceeded the resolving power of gel. Over time, we were able to detect a decrease in the intensity of the monomer band and a corresponding increase in the oligomer bands by comparing the relative intensities of bands from the initial time point (lane 1) and later time points (lanes 2–4), indicating time-dependent aggregation. Of interest, an equimolar amount of insulin only inhibited the formation of the high-MW species near the top of the gel, and showed little to no ability to block the formation of the small oligomeric species of IAPP in the IAPP/insulin sample (lanes 5–8 in Fig. 5). To summarize, insulin blocks fibril formation, but is ineffective at blocking the formation of small membrane-active oligomers, and can actually increase membrane disruption in some circumstances by suppressing the tendency of IAPP to form membrane-inert amyloid fibers when IAPP is incubated in solution before being bound to the membrane.
Figure 5.
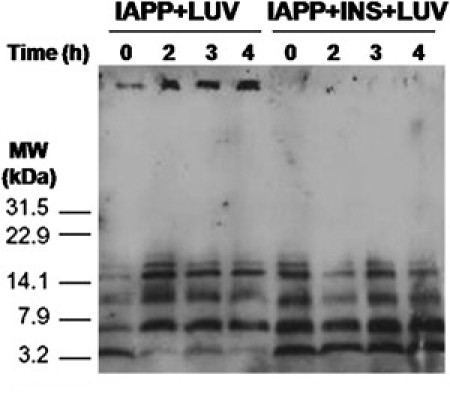
Cross-linking of IAPP and insulin bound to POPG/POPC LUVs. IAPP (25 μM) or IAPP/insulin (25 μM/25 μM) was incubated with POPG/POPC vesicles (1:1 molar ratio, 200 μM) at room temperature for the indicated time points. At each time point, aliquots of each sample were removed and cross-linked with 0.01% (v/v) glutaraldehyde. The reactions were quenched with the addition of lysine and loading buffer, boiled, and separated by SDS-PAGE. The gel was transferred and then immunoblotted with a rabbit polyclonal α-IAPP antibody.
Discussion
Although insulin has been demonstrated to be an in vitro inhibitor of IAPP fibrillization, its effects on IAPP membrane disruption and toxicity are less understood. To test the impact of insulin on IAPP toxicity, we observed the ability of insulin to inhibit IAPP fibril formation, as well as IAPP's ability to disrupt model membranes under buffer conditions that approximate the pH and ionic strength found after secretion. We found that in POPC/POPG vesicles, 1), insulin does not inhibit the rapid permeabilization that occurs immediately after addition of IAPP to membranes; 2), insulin blocks the later rise in membrane permeabilization that is associated with fiber formation; 3), insulin only inhibits amyloid fibrillization, and not the formation of small oligomeric species of IAPP on the membrane; 4), the ability of IAPP to permeabilize membranes decreases rapidly with the time the peptide is incubated in solution in the absence of membranes; and 4), insulin arrests the time-dependent decrease in permeabilization activity, and actually maintains IAPP in such a state that it is able to permeabilize liposomes for a far longer period of time.
Membrane disruption is believed to be one of the primary causes of cell death caused by amyloidogenic proteins (32,37,48). Although there is very strong evidence that hIAPP has a deleterious effect on cell membranes, the molecular mechanisms by which this occurs are not understood. Studies so far have been conflicting: some have provided evidence for the in vitro formation of discrete ion-channel-like pores, whereas others have provided evidence for the total disruption of the bilayer through membrane fragmentation. Strong evidence for nonspecific membrane disruption through fibril growth at the membrane comes from Engel et al. (33), who showed that the time course of calcein leakage from vesicles was strongly correlated with amyloid fibril formation, and that the kinetics of dye-leakage could be strongly altered by seeding amyloid formation, implying a direct link between fibril formation and membrane disruption. Furthermore, electron micrographs of giant unilamellar vesicles in the presence of IAPP showed the vesicles to be severely distorted (33), indicating that membrane permeabilization occurs nonspecifically by mechanical fragmentation of the membrane, as has been observed in other studies (36,45,46). On the other hand, channel-like structures have also been observed in membranes by atomic force microscopy, and discrete electrical conductances suggestive of channels have been recorded that could be reversibly blocked by zinc and other ligands (35,49,50). In addition, a fragment of IAPP comprising the putative membrane active domain caused a rapid rise in intracellular calcium when added to islet cells, but did not form amyloid fibrils when bound to the membrane (6,51,52). It is difficult to account for these findings with the membrane-damage-through-fibril-growth model alone, suggesting a role for nonfibril-dependent membrane damage in the early stages of membrane disruption. Indeed, early membrane permeabilization events were observed in the study by Engel et al. (33), albeit with a smaller degree of disruption compared with our findings (∼10% vs. ∼30). This difference is most probably due to the higher anionic content of the vesicles used here (50% vs. 30%), and their use of calcein (MW 622.6) versus carboxyfluorescein (MW 376.3), which permits leakage through smaller pores. It is likely that both channel-like and nonspecific peremabilization occurs when IAPP is added to membranes, with the amount of each being dependent on other factors such as the IAPP concentration and lipid environment, as has been proposed for the related Aβ peptide (53). In particular, the ratio of disruption in the early- to late-phase disruption has been shown to depend on the ratio of charged to zwitterionic lipids in binary POPC/POPS mixtures, with the fiber-dependent late-phase disruption dominating at the low charged to zwitterionic ratios typical of cells (33,35). Channel-like activity has also been shown to be diminished in the presence of cholesterol (3,5). On the other hand, in more complex raft-like lipid mixtures, complete disruption of the membrane occurred immediately upon addition of IAPP, even in the absence of anionic lipids and despite a much slower fibrillization rate in comparison with mixed POPC/POPS membranes (54).
Insulin was found to inhibit only the later fibril-dependent process, in agreement with a study by Larson and Miranker (14) that showed that insulin strongly inhibits the elongation step of monomer addition to existing fibrils at substoichiometric concentrations. This inhibition of the elongation process can be expected to strongly affect membrane disruption through mechanical stress caused by fiber growth at the membrane. On the other hand, the lack of effect on the initial membrane permeabilization activity is consistent with ATR-FTIR studies showing that insulin does not completely suppress the binding of IAPP to 7:3 POPC/POPS liposomes (20). The initial rise in membrane permeabilization could be linked to formation of channel-type structures; however, definite identification is inconclusive and outside the scope of this study.
Among amyloid peptides, IAPP is somewhat unusual in that the highest amount of membrane disruption is detected immediately after solubilization and then decreases monotonically (Fig. 4) (55,56). This is in contrast to most other amyloid proteins, in which membrane disruption first increases with time, as toxic membrane-binding oligomers form in solution, and then decreases as the protofibrillar species are converted into relatively inert mature amyloid fibrils (57–66). The transient nature of the protofibrillar species limits the amount of time the β-cell is exposed to the highly toxic IAPP species, because IAPP either diffuses away from the secretion point, where it rapidly disassociates below nonaggregating concentrations, or is sequestered in less toxic amyloid fibrils (38,67–69).
In the act of blocking IAPP fibril formation, insulin actually prolonged the lifetime of membrane-damaging IAPP species long past its normal span in the absence of insulin (Fig. 4). In the absence of insulin, IAPP formed fibrils in solution, and the ability of IAPP to cause membrane disruption was rapidly reduced after 5 h (Fig. 4). However, in the presence of an equimolar amount of insulin, the ability of IAPP to induce membrane disruption was maintained for far longer and only decreased slowly after ∼50 h (Fig. 4). The ability of insulin to suppress the formation of high-MW species of IAPP, but not small oligomers of IAPP on the membrane, was confirmed by time-lapse cross-linking of IAPP and IAPP/insulin solutions added to POPG/POPC liposomes. Small oligomers could be detected in both samples regardless of whether insulin was present with IAPP. The IAPP monomer quickly disappeared in the sample without insulin, aggregating to larger oligomeric species that were trapped at the top of the gel, and were not seen when IAPP was coincubated with insulin (Fig. 5). Of note, the bands appeared in the same MW range regardless of the presence of insulin, indicating that insulin did not form efficient heterocomplexes with IAPP in the lipid environment under these experimental conditions.
It is notoriously difficult to extrapolate from results on model systems to the in vivo situation, especially for a system as complex as IAPP/insulin/membrane. Taking these difficulties into consideration, it is worthwhile to consider what other factors besides insulin may be responsible for maintaining IAPP in a nontoxic state in normal individuals in the secretory granule and immediately after secretion. The anionic content of the membrane, which is known to change in type II diabetics (70), is likely to be an important factor (71). The changes in membrane cholesterol and free fatty acid levels that occur in the development of type II diabetes have been shown to have a complex effect on Aβ toxicity (72). Preliminary results suggest that a similar effect may exist for IAPP as well (35,54,73). Of significance, hIAPP transgenic mice only develop symptoms of diabetes when fed a high-fat diet (74), indicating a possible role of lipid metabolism in the etiology of IAPP in type II diabetes. An additional important factor may be the acidic pH of the vesicle. Acidic pH is known to strongly inhibit the fibrillogenesis of the IAPP peptide (75,76), and an acidic pH has been shown to significantly reduce the amount of membrane disruption by fragments of the IAPP peptide by altering the position of IAPP fragment within the membrane, causing it to occupy a more surface-associated topology than it would at neutral pH (6,52). Other contents of the β-cell secretory granule, such as zinc, which has been shown to reversibly blockade IAPP channels, may suppress the remaining disruptive activity that is unaffected by insulin (50). Further work is needed, particularly in live-cell systems, to clarify these matters.
Acknowledgments
This work was supported by National Institutes of Health grants R21DK074714 (to A.G.) and DK078885 to A.R., and American Diabetes Association grant 7-06-RA-48 (to A.G.). E. L. Lee was supported by National Institutes of Health training grant T32-AG000114.
References
- 1.Höppener J.W.M., Ahrén B., Lips C.J.M. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 2000;343:411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 2.Kahn S.E., Andrikopoulos S., Verchere C.B. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 3.Hull R.L., Westermark G.T., Kahn S.E. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 4.Harrison R.S., Sharpe P.C., Fairlie D.P. Amyloid peptides and proteins in review. Rev. Physiol. Biochem. Pharmacol. 2007;159:1–77. doi: 10.1007/112_2007_0701. [DOI] [PubMed] [Google Scholar]
- 5.Demuro A., Mina E., Glabe C.G. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 6.Brender J.R., Hartman K., Ramamoorthy A. A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry. 2008;47:12680–12688. doi: 10.1021/bi801427c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esapa C., Moffitt J.H., Clark A. Islet amyloid polypeptide gene promoter polymorphisms are not associated with type 2 diabetes or with the severity of islet amyloidosis. Biochim. Biophys. Acta. 2005;1740:74–78. doi: 10.1016/j.bbadis.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Pildal J., Lajer M., Pedersen O. Studies of variability in the islet amyloid polypeptide gene in relation to type 2 diabetes. Diabet. Med. 2003;20:491–494. doi: 10.1046/j.1464-5491.2003.00951.x. [DOI] [PubMed] [Google Scholar]
- 9.Novials A., Rojas I., Gomis R. A novel mutation in islet amyloid polypeptide (IAPP) gene promoter is associated with type II diabetes mellitus. Diabetologia. 2001;44:1064–1065. doi: 10.1007/s001250100599. [DOI] [PubMed] [Google Scholar]
- 10.Sakagashira S., Hiddinga H.J., Eberhardt N.L. Compared to wild-type human amylin, S20G mutant amylin exhibits increased cytotoxicity in transfected COS-1 cells and increased amyloidogenicity in vitro. Diabetes. 2000;49 doi: 10.1016/S0002-9440(10)64848-1. A401–A401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei L., Jiang P., Pervushin K. Residual structure in islet amyloid polypeptide mediates its interactions with soluble insulin. Biochemistry. 2009;48:2368–2376. doi: 10.1021/bi802097b. [DOI] [PubMed] [Google Scholar]
- 12.Wiltzius J.J., Sievers S.A., Eisenberg D. Atomic structures of IAPP (amylin) fusions suggest a mechanism for fibrillation and the role of insulin in the process. Protein Sci. 2009;18:1521–1530. doi: 10.1002/pro.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight J.D., Williamson J.A., Miranker A.D. Interaction of membrane-bound islet amyloid polypeptide with soluble and crystalline insulin. Protein Sci. 2008;17:1850–1856. doi: 10.1110/ps.036350.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson J.L., Miranker A.D. The mechanism of insulin action on islet amyloid polypeptide fiber formation. J. Mol. Biol. 2004;335:221–231. doi: 10.1016/j.jmb.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Gilead S., Wolfenson H., Gazit E. Molecular mapping of the recognition interface between the islet amyloid polypeptide and insulin. Angew. Chem. Int. Ed. Engl. 2006;45:6476–6480. doi: 10.1002/anie.200602034. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted in proof.
- 17.Kudva Y.C., Mueske C., Eberhardt N.L. A novel assay in vitro of human islet amyloid polypeptide amyloidogenesis and effects of insulin secretory vesicle peptides on amyloid formation. Biochem. J. 1998;331:809–813. doi: 10.1042/bj3310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaikaran E.T., Nilsson M.R., Clark A. Pancreatic β-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation. Biochem. J. 2004;377:709–716. doi: 10.1042/BJ20030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui W., Ma J.W., Li Y.M. Insulin is a kinetic but not a thermodynamic inhibitor of amylin aggregation. FEBS J. 2009;276:3365–3371. doi: 10.1111/j.1742-4658.2009.07061.x. [DOI] [PubMed] [Google Scholar]
- 20.Sellin D., Yan L.M., Winter R. Suppression of IAPP fibrillation at anionic lipid membranes via IAPP-derived amyloid inhibitors and insulin. Biophys. Chem. 2010;150:73–79. doi: 10.1016/j.bpc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Reference deleted in proof.
- 22.Westermark P., Li Z.C., Steiner D.F. Effects of β cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 1996;379:203–206. doi: 10.1016/0014-5793(95)01512-4. [DOI] [PubMed] [Google Scholar]
- 23.Janciauskiene S., Eriksson S., Ahrén B. B cell granule peptides affect human islet amyloid polypeptide (IAPP) fibril formation in vitro. Biochem. Biophys. Res. Commun. 1997;236:580–585. doi: 10.1006/bbrc.1997.7014. [DOI] [PubMed] [Google Scholar]
- 24.Shoval H., Weiner L., Lichtenberg D. Polyphenol-induced dissociation of various amyloid fibrils results in a methionine-independent formation of ROS. Biochim. Biophys. Acta. 2008;1784:1570–1577. doi: 10.1016/j.bbapap.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Mamikonyan G., Necula M., Agadjanyan M.G. Anti-Aβ 1-11 antibody binds to different β-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils but not the most toxic oligomers. J. Biol. Chem. 2007;282:22376–22386. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert M.P., Barlow A.K., Klein W.L. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda T., P. Wals, Osterburg H.H., Finch C.E. Clusterin (apoJ) alters the aggregation of amyloid β-peptide (A β 1-42) and forms slowly sedimenting A β complexes that cause oxidative stress. Exp. Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 28.Klein W.L., Krafft G.A., Finch C.E. Targeting small Aβ oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 29.Capone R., Quiroz F.G., Mayer M. Amyloid-β ion channels in artificial lipid bilayers and neuronal cells. Neurotox. Res. 2008;15:608–650. doi: 10.1007/s12640-009-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higham C.E., Jaikaran E.T., Clark A. Preparation of synthetic human islet amyloid polypeptide (IAPP) in a stable conformation to enable study of conversion to amyloid-like fibrils. FEBS Lett. 2000;470:55–60. doi: 10.1016/s0014-5793(00)01287-4. [DOI] [PubMed] [Google Scholar]
- 31.Stewart J.C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 32.Engel M.F. Membrane permeabilization by islet amyloid polypeptide. Chem. Phys. Lipids. 2009;160:1–10. doi: 10.1016/j.chemphyslip.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Engel M.F., Khemtémourian L., Höppener J.W. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc. Natl. Acad. Sci. USA. 2008;105:6033–6038. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kayed R., Sokolov Y., Glabe C.G. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J. Biol. Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 35.Mirzabekov T.A., Lin M.C., Kagan B.L. Pore formation by the cytotoxic islet amyloid peptide amylin. J. Biol. Chem. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 36.Sparr E., Engel M.F.M., Killian J.A. Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Lett. 2004;577:117–120. doi: 10.1016/j.febslet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 37.Hebda J.A., Miranker A.D. The interplay of catalysis and toxicity by amyloid intermediates on lipid bilayers: insights from type II diabetes. Annu. Rev. Biophys. 2009;38:125–152. doi: 10.1146/annurev.biophys.050708.133622. [DOI] [PubMed] [Google Scholar]
- 38.Knight J.D., Hebda J.A., Miranker A.D. Conserved and cooperative assembly of membrane-bound α-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 39.Rex S., Schwarz G. Quantitative studies on the melittin-induced leakage mechanism of lipid vesicles. Biochemistry. 1998;37:2336–2345. doi: 10.1021/bi971009p. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz G., Robert C.H. Kinetics of pore-mediated release of marker molecules from liposomes or cells. Biophys. Chem. 1992;42:291–296. doi: 10.1016/0301-4622(92)80021-v. [DOI] [PubMed] [Google Scholar]
- 41.Almeida P.F., Pokorny A. Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48:8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregory S.M., Pokorny A., Almeida P.F.F. Magainin 2 revisited: a test of the quantitative model for the all-or-none permeabilization of phospholipid vesicles. Biophys. J. 2009;96:116–131. doi: 10.1016/j.bpj.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory S.M., Cavenaugh A., Almeida P.F. A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide cecropin A. Biophys. J. 2008;94:1667–1680. doi: 10.1529/biophysj.107.118760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith P.E., Brender J.R., Ramamoorthy A. Induction of negative curvature as a mechanism of cell toxicity by amyloidogenic peptides: the case of islet amyloid polypeptide. J. Am. Chem. Soc. 2009;131:4470–4478. doi: 10.1021/ja809002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brender J.R., Dürr U.H.N., Ramamoorthy A. Membrane fragmentation by an amyloidogenic fragment of human islet amyloid polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. Biochim. Biophys. Acta. 2007;1768:2026–2029. doi: 10.1016/j.bbamem.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Domanov Y.A., Kinnunen P.K.J. Islet amyloid polypeptide forms rigid lipid-protein amyloid fibrils on supported phospholipid bilayers. J. Mol. Biol. 2008;376:42–54. doi: 10.1016/j.jmb.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 47.Treusch S., Cyr D.M., Lindquist S. Amyloid deposits: protection against toxic protein species? Cell Cycle. 2009;8:1668–1674. doi: 10.4161/cc.8.11.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khemtémourian L., Killian J.A., Engel M.F. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in β-cell death in type 2 diabetes mellitus. Exp. Diabetes Res. 2008;2008:421287. doi: 10.1155/2008/421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quist A., Doudevski I., Lal R. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirakura Y., Yiu W.W., Kagan B.L. Amyloid peptide channels: blockade by zinc and inhibition by Congo red (amyloid channel block) Amyloid. 2000;7:194–199. doi: 10.3109/13506120009146834. [DOI] [PubMed] [Google Scholar]
- 51.Brender J.R., Lee E.L., Ramamoorthy A. Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide. J. Am. Chem. Soc. 2008;130:6424–6429. doi: 10.1021/ja710484d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nanga R.P.R., Brender J.R., Ramamoorthy A. Structures of rat and human islet amyloid polypeptide IAPP(1-19) in micelles by NMR spectroscopy. Biochemistry. 2008;47:12689–12697. doi: 10.1021/bi8014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schauerte J.A., Wong P.T., Gafni A. Simultaneous single-molecule fluorescence and conductivity studies reveal distinct classes of Aβ species on lipid bilayers. Biochemistry. 2010;49:3031–3039. doi: 10.1021/bi901444w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weise K., Radovan D., Winter R. Interaction of hIAPP with model raft membranes and pancreatic β-cells: cytotoxicity of hIAPP oligomers. ChemBioChem. 2010;11:1280–1290. doi: 10.1002/cbic.201000039. [DOI] [PubMed] [Google Scholar]
- 55.Anguiano M., Nowak R.J., Lansbury P.T., Jr. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 56.Porat Y., Kolusheva S., Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry. 2003;42:10971–10977. doi: 10.1021/bi034889i. [DOI] [PubMed] [Google Scholar]
- 57.Smith D.P., Tew D.J., Cappai R. Formation of a high affinity lipid-binding intermediate during the early aggregation phase of α-synuclein. Biochemistry. 2008;47:1425–1434. doi: 10.1021/bi701522m. [DOI] [PubMed] [Google Scholar]
- 58.El-Agnaf O.M.A., Nagala S., Austen B.M. Non-fibrillar oligomeric species of the amyloid ABri peptide, implicated in familial British dementia, are more potent at inducing apoptotic cell death than protofibrils or mature fibrils. J. Mol. Biol. 2001;310:157–168. doi: 10.1006/jmbi.2001.4743. [DOI] [PubMed] [Google Scholar]
- 59.Shankar G.M., Li S.M., Selkoe D.J. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Townsend M., Shankar G.M., Selkoe D.J. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J. Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cleary J.P., Walsh D.M., Ashe K.H. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 62.Bitan G., Lomakin A., Teplow D.B. Amyloid β-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J. Biol. Chem. 2001;276:35176–35184. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- 63.Gong Y., Chang L., Klein W.L. Alzheimer's disease-affected brain: presence of oligomeric A β ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang T.H.J., Yang D.S., Chakrabartty A. Structural studies of soluble oligomers of the Alzheimer β-amyloid peptide. J. Mol. Biol. 2000;297:73–87. doi: 10.1006/jmbi.2000.3559. [DOI] [PubMed] [Google Scholar]
- 65.Lesné S., Koh M.T., Ashe K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 66.Hepler R.W., Grimm K.M., Joyce J.G. Solution state characterization of amyloid β-derived diffusible ligands. Biochemistry. 2006;45:15157–15167. doi: 10.1021/bi061850f. [DOI] [PubMed] [Google Scholar]
- 67.Soong R., Brender J.R., Ramamoorthy A. Association of highly compact type II diabetes related islet amyloid polypeptide intermediate species at physiological temperature revealed by diffusion NMR spectroscopy. J. Am. Chem. Soc. 2009;131:7079–7085. doi: 10.1021/ja900285z. [DOI] [PubMed] [Google Scholar]
- 68.Engel M.F.M., Yigittop H., Antoinette Killian J. Islet amyloid polypeptide inserts into phospholipid monolayers as monomer. J. Mol. Biol. 2006;356:783–789. doi: 10.1016/j.jmb.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 69.Vaiana S.M., Ghirlando R., Hofrichter J. Sedimentation studies on human amylin fail to detect low-molecular-weight oligomers. Biophys. J. 2008;94:L45–L47. doi: 10.1529/biophysj.107.125146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rustenbeck I., Matthies A., Lenzen S. Lipid composition of glucose-stimulated pancreatic islets and insulin-secreting tumor cells. Lipids. 1994;29:685–692. doi: 10.1007/BF02538912. [DOI] [PubMed] [Google Scholar]
- 71.Knight J.D., Miranker A.D. Phospholipid catalysis of diabetic amyloid assembly. J. Mol. Biol. 2004;341:1175–1187. doi: 10.1016/j.jmb.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 72.Schengrund C.L. Lipid rafts: keys to neurodegeneration. Brain Res. Bull. 2010;82:7–17. doi: 10.1016/j.brainresbull.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Cho W.J., Trikha S., Jeremic A.M. Cholesterol regulates assembly of human islet amyloid polypeptide on model membranes. J. Mol. Biol. 2009;393:765–775. doi: 10.1016/j.jmb.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 74.Matveyenko A.V., Gurlo T., Butler P.C. Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced β-cell endoplasmic reticulum stress. Diabetes. 2009;58:906–916. doi: 10.2337/db08-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abedini A., Raleigh D.P. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- 76.Jaikaran E.T., Higham C.E., Fraser P.E. Identification of a novel human islet amyloid polypeptide β-sheet domain and factors influencing fibrillogenesis. J. Mol. Biol. 2001;308:515–525. doi: 10.1006/jmbi.2001.4593. [DOI] [PubMed] [Google Scholar]


