Abstract
The cyclopentane core of palau’amine has been constructed in optically pure form through the use of an asymmetric azomethine ylid [1,3]-dipolar cycloaddition reaction.
Keywords: Amino Acid, Dipolar cycloaddition, Spiro-guanidine
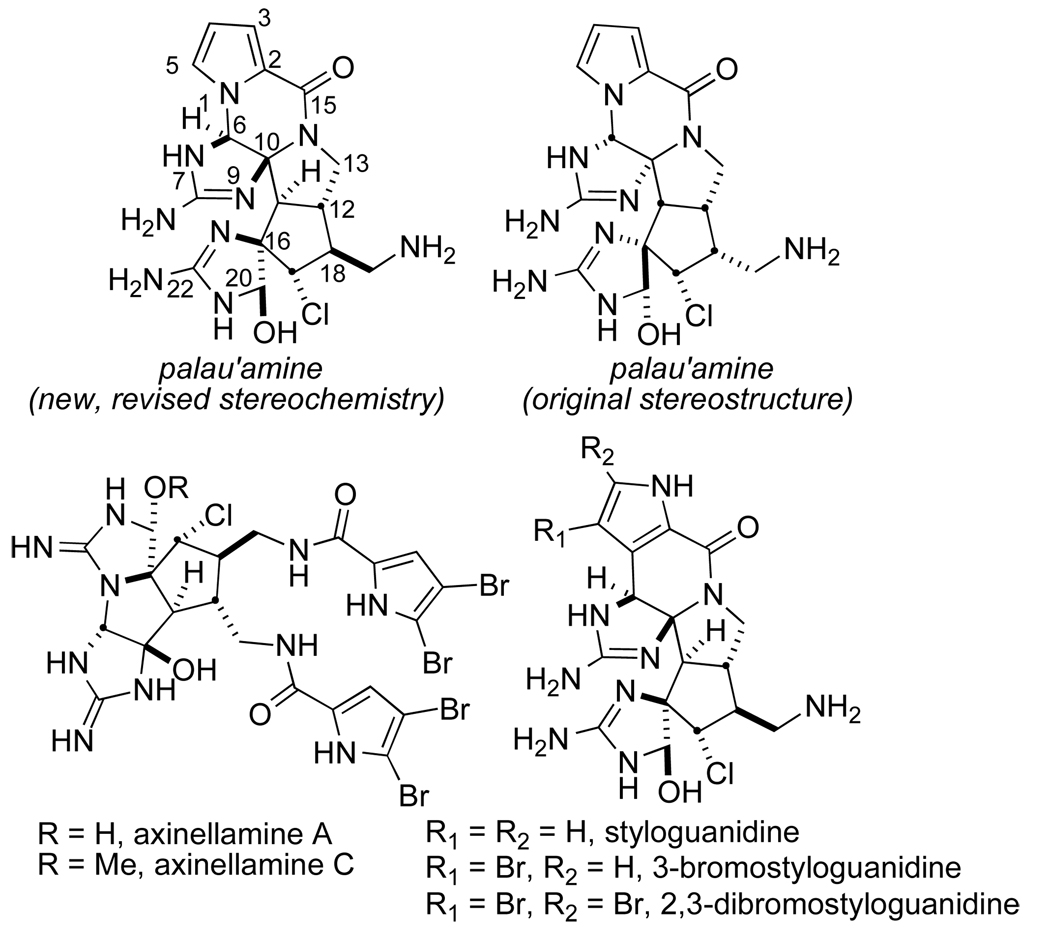
Palau’amine is a dauntingly complex hexacyclic bis-guanidine antibiotic obtained from the sponge Stylotella agminata that displays potent cytotoxic and immunosuppressive activities (Figure 1).1a,b This substance has attracted considerable attention from the synthetic community2 and a recent, brilliant total synthesis of racemic palau’amine by the Baran laboratory is particularly noteworthy.3 The relative stereochemistry of palau’amine was recently revised independently by Quinn4 and Köck5 to the stereostructure depicted in Figure 1 and turned out to be a fortuitous stereochemical array for the approach described herein. The related alkaloids, the styloguanidines were isolated from the marine sponge Stylotella aurantium and have been shown to be inhibitors of chitinase, an important enzyme in the molting of crustaceans.6 The axinellamines, isolated from the marine sponge Axinella sp., display moderate bactericidal activity against Helicobacter pylori.7
Figure 1.
Structures of palau’amine and congeners.
All of these substances share a structurally related cyclopentane core and thus far, only the Baran laboratory has been successful in total synthesis efforts in this family of alkaloids.8,9 The relative stereochemistry of the cyclopentane core of palau’amine and the styloguanidines is the same while the axinellamines dispose the aminomethyl substituent adjacent to the chlorine atom in an anti-relationship.
It should be noted that the absolute stereochemistry of these natural products has not been rigorously assigned, although based on CD similarities with monobromophakellin hydrochloride, Scheuer, et al., have postulated the absolute stereostructure shown.1 All of these agents represent an unusually challenging density of functionality and stereochemistry and provides a perhaps extreme test of suitable synthetic methodologies. While the landmark accomplishment by Baran and co-workers on the first total synthesis of palau’amine represents an important milestone in the chemistry of this fascinating family of alkaloids, the asymmetric synthesis of palau’amine remains an important synthetic objective.
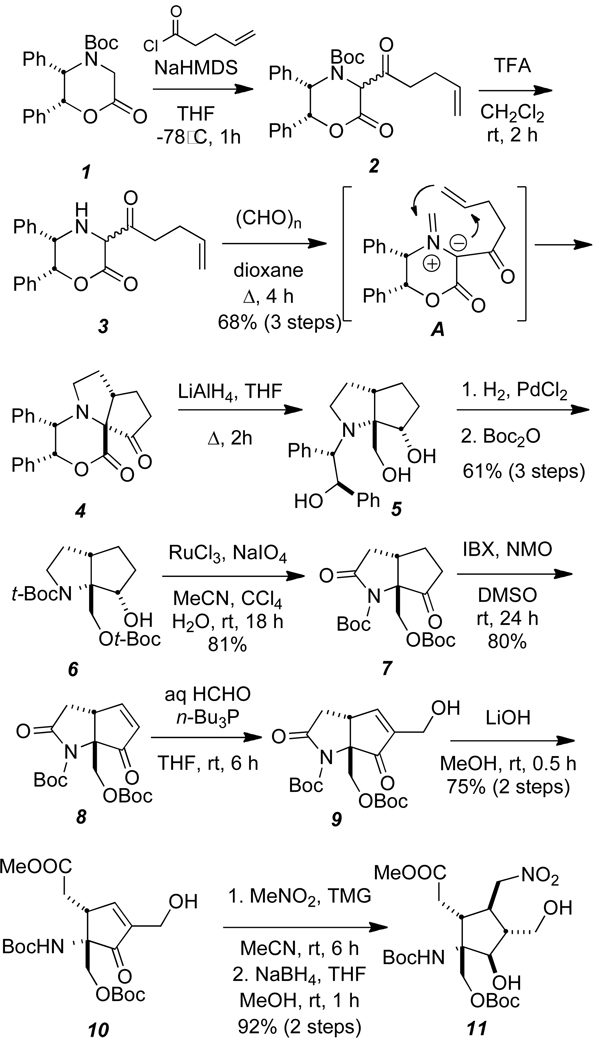
We have devised an efficient, stereocontrolled entry to the highly functionalized cyclopentane core of palau’amine by an asymmetric intramolecular azomethine ylide dipolar cycloaddition10, 11 as the key step and report these preliminary studies herein (Scheme 1).
Scheme 1.
Asymmetric synthesis of cyclopentane 11.
Our synthesis commences with the enolate acylation of commercially available oxazinone 111 to provide 2 (relative stereochemistry unassigned and inconsequential). Removal of the t-Boc group furnishes aminoketone 3 which was condensed with formaldehyde to generate the incipient azomethine ylid (A) that produced tricyclic intramolecular cycloaddition product 4 as a single diastereomer in 63% overall yield for the three steps. The intrinsic facial bias of this intramolecular cycloaddition remain unclear and constitute the subject of ongoing studies.
This substance was reduced with LiAlH4 to triol 5, followed by catalytic hydrogenation and t-Boc-protection to give 6 in 61% overall yield from 4. Ruthenium-mediated oxidation cleanly afforded ketoamide 7 in 81% yield that was converted into the unsaturated ketone by exposure to IBX and N-methylmorpholine N-oxide in DMSO delivering enone 8 in 80% yield.12
Stereoselective installation of the two one-carbon arms to enone 8 was accomplished in two stages beginning with a Morita-Baylis-Hillman type of hydroxymethylation that deployed aqueous formaldehyde and tri-N-butyl phosphine providing 9 in good yield.13 Methanolic lithium hydroxide treatment of 9 gave the monocyclic methyl ester 10 in 75% overall yield from 9. For the second stage, enone 10 was then treated with nitromethane and tetramethyl guanidine in acetonitrile at room temperature to give exclusively, the 1,2-anti product. Reduction of the ketone gave diol 11 which was subjected to extensive 1H NMR nOe experiments to secure the relative stereochemistry of this substance. The relative stereochemistry of the vicinal hydroxymethyl and nitromethyl substituents in 11 are set with the correct relative stereochemistry as per the newly reassigned stereochemistry of palau’amine.
Cyclopentane 11 embodies the stereochemistry and relevant functionality to constitute a viable intermediate for the asymmetric synthesis of palau’amine and congeners. Efforts to complete an asymmetric total synthesis of palau’amine from cyclopentane 11 are currently under investigation in these laboratories.
Supplementary Material
Acknowledgment
We are grateful to the National Institutes of Health (GM068011) for financial support. We also acknowledge fellowship support from the JSPS (to K.N.) and the Uehara Foundation (to M.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information Available. Complete experimental details and spectroscopic characterization of all new compounds.
References and Notes
- 1.(a) Kinnel RB, Gehrken H-P, Scheuer PJ. J. Am. Chem. Soc. 1993;115:3376–3377. [Google Scholar]; (b) Kinnel RB, Gehrken H-P, Swali R, Skoropowski G, Scheuer PJ. J. Org. Chem. 1998;63:3281–3286. [Google Scholar]
- 2. Overman LE, Rogers BN, Tellew JE, Trenkle WC. J. Am. Chem. Soc. 1997;119:7159–7160. Starr JT, Koch G, Carreira EM. J. Am. Chem. Soc. 2000;122:8793–8794. Dilley AS, Romo D. Org. Lett. 2001;3:1535–1538. doi: 10.1021/ol015864j. Lovely CJ, H. Du H, Dias HVR. Org. Lett. 2001;3:1319–1322. doi: 10.1021/ol015687m. Jacquot DEN, Hoffmann H, Polborn K, Lindel T. Tetrahedron Lett. 2002;43:3699–3702. Poullennec KG, Kelly AT, Romo D. Org. Lett. 2002;4:2645–2648. doi: 10.1021/ol026140q. Belanger G, Hong FT, Overman LE, B. N. Rogers BN, Tellew JE, Trenkle WC. J. Org. Chem. 2002;67:7880–7883. doi: 10.1021/jo026282y. Koenig SG, Miller SM, Leonard KA, Lowe RS, Chen BC, Austin DJ. Org. Lett. 2003;5:2203–2206. doi: 10.1021/ol0344063. He Y, Chen Y, Wu H, Lovely CJ. Org. Lett. 2003;5:3623–3626. doi: 10.1021/ol0352862. Poullennec KG, Romo D. J. Am. Chem. Soc. 2003;125:6344–6345. doi: 10.1021/ja034575i. Katz JD, Overman LE. Tetrahedron. 2004;60:9559–9568. Lovely CJ, Du H, Y Y, Dias HVR. Org. Lett. 2004;6:735–738. doi: 10.1021/ol036403w. Garrido-Hernandez H, Nakadai M, Vimolratana M, Li Q, Doundoulakis T, Harran PG. Angew. Chem., Int. Ed. 2005;44:765–769. doi: 10.1002/anie.200462069. Dransfield PJ, Wang S, Dilley A, Romo D. Org. Lett. 2005;7:1679–1682. doi: 10.1021/ol0473602. Jacquot DEN, Lindel T. Curr. Org. Chem. 2005;9:1551–1565. Dransfield PJ, Dilley AS, Wang S, Romo D. Tetrahedron. 2006;62:5223–5247. Wang S, Dilley AS, Poullennec KG, Romo D. Tetrahedron. 2006;14462:7155–7161. Gergely J, Morgan JB, Overman LE. J. Org. Chem. 2006;71:9144–9152. doi: 10.1021/jo061537j. Nakadai M, Harran PG. Tetrahedron Lett. 2006;47:3933–3935. Dransfield PJ, Dilley AS, Wang S, Romo D. Tetrahedron. 2006;62:5223–5247. Schroif-Gregoire C, Travert N, Zaparucha A, Al-Mourabit A. Org. Lett. 2006;8:2961–2964. doi: 10.1021/ol0608451. Tan X, Chen C. Angew. Chem. Int. Ed. 2006;45:4345–4348. doi: 10.1002/anie.200601208. Lanman BA, Overman LE. Heterocycles. 2006;70:557–570. doi: 10.3987/com-06-s(w)16. Du H, He Y, Sivappa R, Lovely CJ. Synlett. 2006:965–992. Sivappa R, Hernandez NM, He Y, Lovely CJ. Org. Lett. 2007;9:3861–3864. doi: 10.1021/ol0711568. Tang L, Romo D. Heterocycles. 2007;74:999–1008. Lanman BA, Overman LE, R. Paulini R, White NS. J. Am. Chem. Soc. 2007;129:12896–12900. doi: 10.1021/ja074939x. Cernak TA, Gleason L. J. Org. Chem. 2008;73:102–110. doi: 10.1021/jo701866g. Wang S, Romo D. Angew. Chem. Int. Ed. 2008;47:1284–1286. doi: 10.1002/anie.200703998. Bultman MS, Ma J, Gin DY. Angew. Chem. Int. Ed. 2008;47:6821–6824. doi: 10.1002/anie.200801969. Zancanella MA, D. Romo D. Org. Lett. 2008;10:3685–3688. doi: 10.1021/ol801289b. Hudon J, Cernak TA, Ashenhurst JA, Gleason JL. Angew. Chem. Int. Ed. 2008;47:8885–8888. doi: 10.1002/anie.200803344. Namba K, Kaihara Y, Yamamoto H, Imagawa H, Tanino K, Williams RM, M. Nishizawa M. Chem. Eur. J. 2009;15:6560–6563. doi: 10.1002/chem.200900622. Li Q, Hurley P, Ding H, Roberts AG, Akella R, Harran PG. J. Org. Chem. 2009;74:5909–5919. doi: 10.1021/jo900755r. Sivappa R, Mukherjee S, Dias HVR, Lovely CJ. Org. Biomol. Chem. 2009;7:3215–3218. doi: 10.1039/b909482b. Feldman KS, Nuriya AY. Org. Lett. 2010;12 0000 ASAP. Ma Z, Lu J, Wang X, Chen CJ. Chem. Soc. Chem. Comm. 2011 0000, ASAP. (ll) for references to Ph.D. theses describing synthetic approaches to palau’amine, please see ref. 3.
- 3.Seiple IB, Su S, Young IS, Lewis CA, Yamaguchi J, Baran PS. Angew. Chem. Int. Ed. 2010;49:1095–1098. doi: 10.1002/anie.200907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan MS, Carroll AR, Addepalli R, Avery VM, Hooper JNA, Quinn RJ. J. Org. Chem. 2007;72:2309–2317. doi: 10.1021/jo062007q. [DOI] [PubMed] [Google Scholar]
- 5.Grube A, Köck M. Angew. Chem. Int. Ed. 2007;46:2320–2324. doi: 10.1002/anie.200604076. [DOI] [PubMed] [Google Scholar]
- 6.Kato T, Shizuri Y, Izumada H, Yokoyama A, Endo M. Tetrahedron Lett. 1995;36:2133–2136. [Google Scholar]
- 7.Urban S, Leone PDA, Carroll AR, Fechner G, Smith J, Hooper JNA, Quinn RJ. J. Org. Chem., 1999;64:731–735. doi: 10.1021/jo981034g. [DOI] [PubMed] [Google Scholar]
- 8.(a) Yamaguchi J, Seiple IB, Young IS, O’Malley MP, Maue M, Baran PS. Angew. Chem. Int. Ed. 2008;47:3578–3580. doi: 10.1002/anie.200705913. [DOI] [PubMed] [Google Scholar]; (b) O’Malley DP, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2008;47:3581–3583. doi: 10.1002/anie.200801138. [DOI] [PubMed] [Google Scholar]
- 9.Su S, Seiple IB, Young IS, Baran PS. J. Am. Chem. Soc. 2008;130:16490–16491. doi: 10.1021/ja8074852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For relevant examples of azomethine ylid reactions derived from 1, see: Williams RM, Zhai W, Aldous DJ, Aldous SC. J. Org. Chem. 1992;57:6527–6532. Sebahar PR, Williams RM. J. Am. Chem. Soc. 2000;122:5666–5667. Sebahar PR, Osada H, Usui T, Williams RM. Tetrahedron. 2002;58:6311–6322. Sebahar P, Williams RM. Heterocycles. 2002;58:563–575. Onishi T, Sebahar PR, Williams RM. Org. Lett. 2003;5:3135–3137. doi: 10.1021/ol0351910. Onishi T, Sebahar PR, Williams RM. Tetrahedron. 2004;60:9503–9515. Ahrendt KA, Williams RM. Org. Lett. 2004;6:4539–4541. doi: 10.1021/ol048126e. Williams RM, Burnett CM. ACS Symp. Ser; 2009. pp. 420–442.
- 11.For reviews utilizing 1, see: Williams RM. Aldrichimica Acta. 1992;25:11–25. Asymmetric Synthesis of a-Amino Acids. Williams RM. In: Advances in Asymmetric Synthesis. Hassner A, editor. Volume 1. JAI Press; 1995. pp. 45–94. Asymmetric Synthesis of a-Amino Acids. Burnett C, Williams RM. Strategies and Tactics in Organic Synthesis. 2008;Vol.7(Chapter 8):268–327.
- 12.Nicolaou KC, Montagnon T, Baran PS, Zhong Y-L. J. Am. Chem. Soc. 2002;124:2245–2248. doi: 10.1021/ja012127+. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 13.For a review, see: Basavaiah D, Rao AJ, Satyanarayana T. Chem. Rev. 2003;103:811–892. doi: 10.1021/cr010043d.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.