SUMMARY
SETTING
Extensively drug-resistant tuberculosis (XDR-TB) has been documented worldwide, but reports of XDR-TB in children are extremely limited.
OBJECTIVE
To report the characteristics of pediatric XDR-TB patients in rural South Africa.
DESIGN
We retrospectively reviewed children with sputum culture-confirmed XDR-TB from Tugela Ferry, South Africa, from January 2006 to December 2007. Demographic, clinical and microbiologic data were abstracted from medical records.
RESULTS
Four children aged 6–8 years with XDR-TB were reviewed. Two had previous histories of TB. All were human immunodeficiency virus (HIV) infected orphans; three received highly active antiretroviral therapy (HAART) before XDR-TB diagnosis. All had clinical and radiographic improvement and sputum culture conversion while on standardized XDR-TB treatment and HAART. Two tolerated concomitant XDR-TB and HIV treatment well. Two experienced neuropsychiatric side effects related to cycloserine. All have survived >24 months and all were cured. Prior to XDR-TB diagnosis, the children had resided in the hospital’s pediatric ward for a median of 8 months (range 5–17), including a 3-month overlapping period.
CONCLUSIONS
XDR-TB is a microbiologic diagnosis that, even with HIV co-infection, can be successfully identified. Concurrent XDR-TB and HIV therapy is feasible and effective in children, although more research is needed into potential overlapping toxicities. Nosocomial transmission is suggested, calling for infection control policies in pediatric wards.
Keywords: extensively drug-resistant tuberculosis, drug-resistant tuberculosis, tuberculosis, pediatrics, HIV/TB, co-infection
Tuberculosis (TB) and human immunodeficiency virus (HIV) are among the leading infectious causes of mortality worldwide. Among the estimated 9.4 million annual active TB cases, children comprise 11%.1,2 In sub-Saharan Africa, the burden of pediatric TB is even greater, with up to 40% of all TB cases occurring among children.3
Drug-resistant TB is a growing global epidemic, with more than 500 000 cases occurring annually,1 which threatens gains in HIV treatment success in sub-Saharan Africa.4,5 Extensively drug-resistant tuberculosis (XDR-TB), defined as resistance to isoniazid (INH) and rifampin (RMP) (i.e., multidrug-resistant TB [MDR-TB]) plus additional resistance to a fluoroquinolone and a second-line injectable agent (kanamycin [KM], amikacin or capreomycin [CPM]), has now been reported from 57 countries worldwide.1 Growing numbers of drug-resistant TB cases among children have been reported, but global estimates are lacking. Epidemiologic surveys from South Africa demonstrate that pediatric MDR-TB has nearly tripled over the past 15 years, from 2.3% of all childhood TB cases during 1994–1998 to 6.7% during 2005–2007.6,7 The main mode of acquisition was primary transmission of a drug-resistant strain. Data for pediatric XDR-TB are extremely limited, with only two children having been reported, a non-HIV-infected infant and an HIV-infected 10-year-old, both of whom survived after initiating rescue regimens with linezolid.8,9
Although treatment success rates of 40–60% have been reported among adults with XDR-TB from low HIV prevalence settings,10–14 mortality among persons co-infected with HIV and XDR-TB in rural South Africa exceeds 80%.15 Apart from the two cases noted above, no information on treatment success in children with XDR-TB has been described.
Traditionally, drug-resistant TB is thought to occur through resistance acquired due to inadequate treatment. However, recent global data show that 57% of drug-resistant TB cases occur among new patients who have not previously received treatment.16 Among adults from rural South Africa, substantial evidence supports nosocomial transmission of XDR-TB as the primary mode of disease occurrence.17,18 Although transmission of drug-resistant TB strains from adults to children has been documented,19–21 including one report of a nosocomial outbreak of MDR-TB,22 nosocomial transmission of XDR-TB to or among children has not yet been described.
Given the limited published data about pediatric XDR-TB, we sought to describe the epidemiology, clinical features, treatment courses and outcomes of pediatric XDR-TB cases in Tugela Ferry, South Africa.
METHODS
Setting
Tugela Ferry is a rural, 2000 km2 area that is home to approximately 200 000 Zulu people. The annual TB incidence in the province is >1000 per 100 000 population, with HIV co-infection rates reaching nearly 80%.23
The Church of Scotland Hospital (COSH), a 355-bed government district hospital in Tugela Ferry, has a centrally located pediatric ward with an open congregate design with numerous windows, but no extractor fans. There are 45 cots in the ward, including two located in an isolation room used for TB suspects.24 However, high demand may result in only temporary use of the isolation room during a TB suspect’s hospital admission. Overcrowding occasionally pushes the ward census to 60, requiring infants to share a cot. Mothers sleep on the floor alongside the cots, many for the duration of their child’s admission. School-aged children often play together and sleep two to a bed.
Study patients
This is a retrospective case series of children aged ≤10 years with sputum culture-confirmed XDR-TB diagnosed at COSH from January 2006 to December 2007. Patients were identified by reviewing all positive mycobacterial culture results reported to COSH from the provincial TB laboratory. Children were included if medical records were available for review. Demographic and clinical information were abstracted from medical records, including prior TB diagnosis, treatment course and contact history; HIV serostatus and treatment; previous hospitalizations, including location and duration of stay; current admission diagnoses and treatments; clinical presentation of TB; hospital course, including medication side effects; and social information. Recorded results included chest radiographs (CXRs), laboratory tests, microbiologic data and drug susceptibility testing (DST).
TB diagnosis
Cases were diagnosed with TB according to South African National Tuberculosis Control Programme Guidelines,25 including evaluation with CXRs. Microscopy, mycobacterial culture and DST of induced or expectorated sputum was performed on all cases in this series once they were considered to be drug-resistant TB suspects.
Detailed methodology regarding sputum collection, culture and DST has been described previously.17 Briefly, one specimen was prepared for onsite Ziehl-Neelsen smear, while another was sent to the provincial TB referral laboratory in Durban for culture using both the automated radiometric method (BACTEC Mycobacterial Growth Indicator Tube [MGIT] 960 system, Becton Dickinson, Sparks, MD, USA) and traditional Middlebrook 7H11 agar methods. Positive cultures were confirmed by niacin and nitrate reductase tests. DST was performed on all isolates using the 1% proportional method for six anti-tuberculosis drugs: INH (0.2 μg/ml), RMP (1 μg/ml), ethambutol (EMB) (7.5 μg/ml), streptomycin (2 μg/ml), ofloxacin (2 μg/ml) and KM (6 μg/ml).26 Results for the above first- and second-line drugs were reported simultaneously; rapid DST methods were not available at the time of this study. Additional DST for other second-line and third-line medications was not available.
TB management
Once diagnosed with TB, children were treated with first-line medications according to the national guidelines (Table 1).25 Once diagnosed with XDR-TB, children were referred to the provincial TB specialty hospital in Durban. Sputum specimens were repeated for culture and DST upon admission to the TB hospital and monthly thereafter. Standardized treatment regimens were initiated according to the national guidelines, based on the World Health Organization (WHO) principles of treatment for drug-resistant TB.27 The intensive phase consisted of one injectable drug (CM) and at least four oral drugs (ethionamide, cycloserine [CS], para-aminosalicylic acid and pyrazinamide). If the child was over 6 years of age, EMB was used. A combination of clarithromycin and amoxicillin/clavulanate was used when there was an insufficient number of drugs to which the isolate was susceptible. Linezolid was not available for pediatric or adult XDR-TB treatment in KwaZulu-Natal province. As per the 2006 treatment guidelines, the duration of the intensive phase extended at least 4 months after culture conversion, followed by a continuation phase with oral agents alone. The treatment guidelines were revised in 2008, extending the continuation phase from 12 to 18 months, making the total duration of XDR-TB treatment at least 24 months.
Table 1.
Grouping and doses of anti-tuberculosis drugs used for the treatment of pediatric tuberculosis in KwaZulu-Natal, South Africa2,27,28
| Grouping, drug | Weight-based dose mg/kg daily | Maximum daily dose |
|---|---|---|
| First-line | ||
| Oral | ||
| Isoniazid | 4–12 | 300 mg |
| Rifampin | 8–12 | 600 mg |
| Ethambutol | 15–25 | 1.2 g |
| Pyrazinamide | 20–30 | 1.6 g |
| Injectable | ||
| Streptomycin | 12–18 | 1 g |
| Second-line | ||
| Oral | ||
| Ethionamide | 15–20* | 1 g |
| Cycloserine | 10–20* | 1 g |
| Terizidone | 10–20* | 1 g |
| Para-aminosalicylic acid | 150* | 12 g |
| Ofloxacin | 15–20 | 800 mg |
| Moxifloxacin | 7.5–10 | 400 mg |
| Ciprofloxacin | 20–30 | 1.5 g |
| Injectable | ||
| Kanamycin | 15–30 | 1 g |
| Amikacin | 15–22.5 | 1 g |
| Capreomycin | 15–30 | 1 g |
| Third-line† | ||
| Oral | ||
| Amoxicillin/clavulanate | 20–40‡ | Unknown |
| Clarithromycin | 7.5–15‡ | 1 g |
May be given in divided doses to facilitate tolerance.
Third-line agents have an unclear role in the treatment of drug-resistant TB and are not recommended by the WHO for routine use in MDR-TB patients. Insufficient information is known about their use for XDR-TB.27 A combination of amoxicillin/clavulanate and clarithromycin is used in KwaZulu-Natal when there is an insufficient number of drugs to which the TB isolate is susceptible. Optimal doses of third-line agents for drug-resistant TB are not established; the ranges listed here have been adapted from recommendations on adult dosing for XDR-TB and pediatric dosing for other indications (personal communication, Dr Iqbal Master, King George V Hospital, Durban, South Africa).
Should be given in divided doses, twice daily.
WHO = World Health Organization; MDR-TB = multidrug-resistant tuberculosis; XDR-TB = extensively drug-resistant tuberculosis.
Patients remained on the TB hospital’s in-patient ward for the entirety of the intensive phase, given the need for daily injections and lack of home-based treatment programs. Monitoring for adverse events as in-patients included baseline audiology assessment; baseline full blood count, electrolytes, and tests of renal, hepatic and thyroid function; weekly electrolytes and renal function; and regular clinical assessments. Once patients had completed intensive phase treatment, they were transitioned to out-patient care with monthly follow-up visits.
Definitions and outcome measures
A drug-resistant TB suspect was defined as clinically failing to improve on first-line TB treatment, including progression of disease on CXR, a history of defaulting TB treatment or history of contact with a drug-resistant TB case. The primary outcome measures were sputum culture conversion rate, time to culture conversion, medication side effects and 24-month survival. Culture conversion was defined as 2 consecutive months of negative sputum cultures, as per the 2006 treatment guidelines; survival was determined from time of diagnostic sputum collection date. Secondary outcomes included epidemiologic links among patients to determine possible transmission.
Ethical considerations
Ethical approval was granted from the University of KwaZulu-Natal, Yale University, the Albert Einstein College of Medicine and KwaZulu-Natal Department of Health.
RESULTS
During the study period, four children aged <10 years were identified with XDR-TB; all had charts available for review. All four children were HIV-infected orphans aged 6–8 years hospitalized in Tugela Ferry prior to XDR-TB diagnosis (Table 2). All had unstable social situations leading to prolonged hospitalizations, with a median in-patient stay of 8 months (range 5–17) prior to XDR-TB diagnosis. A timeline of each child’s hospitalizations and TB-related events is depicted in the Figure.
Table 2.
Baseline demographic and clinical characteristics of pediatric XDR-TB cases
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age at XDR-TB diagnosis, years* | 7.5 | 7 | 7.5 | 6 |
| Sex | Male | Male | Female | Female |
| Reason for initial admission | Kwashiorkor and cough | Kwashiorkor, cough and diarrhea | Cough and fever | Lymphadenopathy |
| TB history prior to admission (age at prior TB diagnosis, years) | Active PTB (5.5) | No | No | LTBI (3), scrofula (5) |
| Duration of drug-susceptible TB treatment prior to XDR-TB diagnosis, months* | 3 | 6 | 6 | Data unavailable |
| Duration as in-patient prior to XDR-TB diagnosis, months* | 17 | 8.5 | 7.5 | 5 |
| CD4 count at time of HAART initiation, cells/mm3 (% lymphocytes) | 420 (13) | 177 (9) | Data unavailable | 406 (20) |
| Time on HAART prior to XDR-TB treatment initiation, months | 16 | 0 | 9 | 6 |
Diagnosis is measured at the time the diagnostic sputum sample was sent.
XDR-TB = extensively drug-resistant tuberculosis; TB = tuberculosis; PTB = pulmonary TB; LTBI = latent TB infection; HAART = highly active antiretroviral therapy.
Figure.
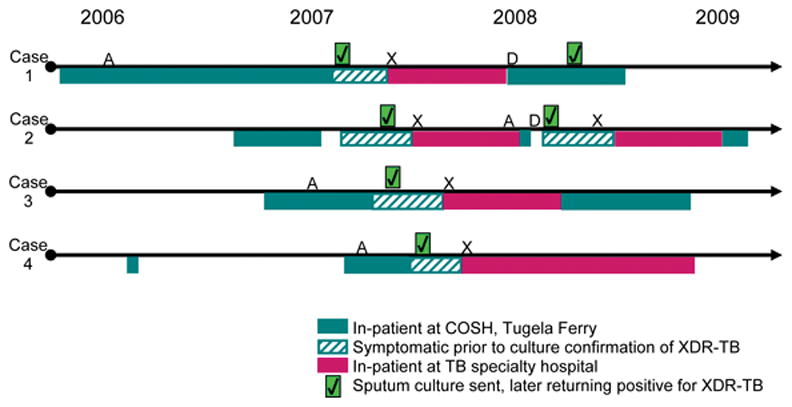
Timeline of hospitalizations and XDR-TB–related clinical events. Case 1 was inadvertently given partial XDR-TB treatment for the first 5 months of the continuation phase. Although asymptomatic, surveillance cultures grew XDR-TB; the full continuation phase of XDR-TB treatment was restarted and cultures re-converted within 4 weeks and remained negative thereafter. Case 2 defaulted during the second month of the continuation phase. He returned 6 weeks later with TB symptoms and was restarted on continuation phase XDR-TB treatment. Cultures returned positive for XDR-TB, prompting readmission to restart intensive phase treatment. The sputum culture re-converted to negative after 8.5 weeks. A = ART initiation; X = XDR-TB treatment initiation; D = treatment default; COSH = Church of Scotland Hospital; XDR-TB = extensively drug-resistant tuberculosis. This image can be viewed online in colour at http://www.ingentaconnect.com/content/iuatld/ijtld/2010/00000014/00000010/art00005
Two children had a history of clinically diagnosed TB disease and had successfully completed treatment with first-line TB medications (Table 2). Upon hospitalization, three children were started on first-line TB medications, again based on a clinical TB diagnosis. Despite initially responding, all three clinically deteriorated after 3–6 months of treatment. No child had documented prior exposure to second-line anti-tuberculosis drugs.
Despite an initial sputum smear-negative result for all cases (Table 3), two cases eventually had acid-fast bacilli (AFB) smear-positive sputum samples. The median time to receiving culture and DST results revealing XDR-TB was 8.75 weeks (range 6–13). Standardized XDR-TB treatment was initiated after DST confirmation of XDR-TB, resulting in clinical and radiographic improvement. All children were sputum culture-negative after a median of 10.5 weeks (range 5–13). Cases 1 and 2 had substantial TB treatment lapses which led to culture reversion; however, cultures re-converted to and remained negative after resumption of appropriate treatment (Figure).
Table 3.
Diagnosis, management and outcomes of pediatric XDR-TB cases
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Sputum smear result* | Positive on 2nd specimen | Positive on 4th specimen | All 5 specimens negative | All 2 specimens negative |
| Culture result* | Positive on 1st specimen | Positive on 3rd specimen | Positive on 3rd specimen | Positive on 2nd specimen |
| DST result | All resistant to INH, RMP, SM, EMB, CFX, KM | |||
| Time to DST result, weeks† | 10 | 6 | 13 | 7.5 |
| Chest radiograph findings at time of XDR-TB diagnosis | Right ML infiltrate and right UL cavity | Extensive bilateral infiltrates | Left UL/LL infiltrates; right perihilar lymphadenopathy | Left UL/LL consolidation |
| Intensive phase regimen | Backbone of ETH, PAS, PZA, CS, CM | |||
| EMB, CLA,‡ AM/CL‡ | CLA, AM/CL | EMB, CLA, AM/CL | EMB, CLA, AM/CL | |
| Time to culture conversion, weeks§ | 13¶ | 13¶ | 5¶ | 8¶ |
| Medication side effects | None | None | Acute psychosis, likely due to CS and efavirenz | Behavioral changes due to CS |
| Weight gain, kg# | 4.8 | 6.0 | 6.0 | 2.8 |
| Change in CD4 count in cells/mm3 (% change in lymphocytes)** | +356 (+7.1) | (+6.0) | Baseline data not available | +60 (+6.0) |
| 24-month survival | Alive with mild bronchiectasis | Thriving | Thriving | Alive with bronchiectasis and chronic lung disease |
| XDR-TB treatment outcome | Cured | Cured | Cured | Cured |
Specimens obtained prior to initiation of XDR-TB treatment.
Interval between date of diagnostic sputum sample collection and receipt of DST results showing XDR-TB.
Added during month 5 of treatment due to impending drug shortages.
Culture conversion: 2 successive months of sputum cultures without growth of Mycobacterium tuberculosis while on XDR-TB treatment.
Initial culture conversion; Cases 1 and 2 had relapse of XDR-TB following initial culture conversion after defaulting from treatment; Cases 2–4 had an isolated positive sputum culture after culture conversion while remaining on full treatment, then re-converted to negative.
Weight gain: measured as difference in weight over 24-month survival period.
Measured after 7–15 months of initiating HAART, based on available data.
XDR-TB = extensively drug-resistant tuberculosis; DST = drug susceptibility testing; INH = isoniazid; RMP = rifampin; SM = streptomycin; EMB = ethambutol; CFX = ciprofloxacin; KM = kanamycin; ML = middle lobe; UL = upper lobe; LL = lower lobe; ETH = ethionamide; PAS = para-aminosalicylic acid; PZA = pyrazinamide; CS = cycloserine; CM = capreomycin; CLA = clarithromycin; AM/CL = amoxicillin/clavulanate.
All children were HIV-infected, with a median baseline CD4 count of 406 cells/mm3 (13% lymphocytes, Table 2). Three were receiving highly active anti-retroviral therapy (HAART) with lamivudine, stavudine and efavirenz prior to XDR-TB diagnosis; the fourth child started HAART after completing 6 months of XDR-TB treatment. All four responded well clinically, with suppressed viral loads (Table 3).
Cases 1 and 2 tolerated concomitant XDR-TB and HIV treatment well (Table 3). Case 3 developed acute psychosis after 3 weeks of starting XDR-TB treatment, which was attributed to interactions between CS and efavirenz; symptoms resolved after stopping CS and replacing efavirenz with nevirapine. Case 4 developed hyperactivity and mood lability after 4 weeks of treatment for both XDR-TB and HIV that resolved after stopping CS.
As of September 2010, all cases were alive and all had been successfully cured. Two children had evidence of bronchiectasis, one of whom had moderate chronic lung disease.
All cases overlapped on the ward in Tugela Ferry for a period of 3 months, during which Cases 1–3 sequentially developed TB symptoms (Figure). Cases 1 and 2 had smear-positive sputum samples with cavitary disease and extensive infiltrates on CXRs, respectively. Case 4, the last to develop symptoms, overlapped with Case 3 for 3 months prior to becoming symptomatic; although sputum smear-negative, Case 3 had extensive multilobar pulmonary disease. All cases had the same DST profile showing six-drug resistance. Cases 1–3 had no known TB contacts outside the pediatric ward. Case 4 had an XDR-TB contact: her mother had AFB smear-negative, culture-confirmed pulmonary XDR-TB in early 2006 and died before starting treatment.
DISCUSSION
Treatment outcomes among XDR-TB and HIV co-infected adults have been dismal, with survival rates of 2–17%, but few cases of pediatric XDR-TB and treatment outcomes have been reported. In this study, we describe a series of four successfully identified and treated children with XDR-TB and HIV co-infection. The concomitant administration of HAART with resultant immune restoration likely contributed to the favorable responses. With a median baseline CD4 count of 406 cells/mm3 (13% lymphocytes), these co-infected children were not as profoundly immunosuppressed as adults with XDR-TB and HIV co-infection from previously published studies.15,17 Furthermore, three children were responding to HAART prior to becoming symptomatic with XDR-TB. This observation mirrors the increasing evidence that survival with XDR-TB and HIV co-infection in adults is associated with treatment of both diseases29 and the well-established role of HAART in improving drug-susceptible TB outcomes.4
Several additional findings from this study are noteworthy. A high degree of suspicion for drug-resistant TB and aggressive pursuit of culture diagnosis by the responsible clinicians was critical to the favorable outcomes. The diagnosis of XDR-TB can only be made using mycobacterial culture and DST. Such tests, however, are infrequently performed in children due to difficulties in obtaining sputum samples and their historically low yield: smears are positive in 10–15% of cases and cultures in approximately 30%.30 These children required multiple specimens for diagnosis, and this positive laboratory identification allowed for initiation of XDR-TB treatment, leading to therapeutic success.
The children’s outcomes may also have been influenced by their nutritional intake while hospitalized. Although two of the children were severely mal-nourished on hospital admission, during the months immediately prior to XDR-TB diagnosis none were subject to the food insecurities common in the region. Paradoxically, although their long in-patient stay may have placed these children at risk for XDR-TB infection, stable access to micro- and macro-nutrients provided on the ward may have tempered the severity of their active XDR-TB disease.
Another important finding from this series relates to the most likely mode of infection of XDR-TB. The sequential time-course of development of XDR-TB in the four cases, coupled with the highly infectious disease of at least two cases and the prolonged overlap in residence on the pediatric ward, strongly suggests nosocomial transmission. These children were likely infected by one another or by an undiagnosed patient, accompanying mother or staff member. In contrast to adults, children often progress rapidly from infection to disease when exposed to TB. The majority of children reported with non-XDR, drug-resistant TB are believed to have primary or transmitted disease.31 Although all cases in this series were exposed to first-line TB medications prior to the collection of the XDR-TB sputum sample, none had received second-line TB drugs, making it unlikely that they acquired drug resistance while on TB therapy.
Outbreaks of MDR-TB have been described in children; most resulted from an index adult or adolescent who was symptomatic with AFB-positive sputum or cavitary disease on CXR.32–34 Although reports of child-to-child transmission of TB are less common, such transmission does occur from children with reactivation TB, including cavitary disease or extensive infiltrates, children who are immunocompromised, and infants with congenital TB.32 Although there has been increased recognition of the need for improved airborne infection control measures in health care facilities in resource-limited settings,16,35 this has focused mainly on adult patients and staff. The clinical and epidemiological characteristics of these four cases illustrate that similar emphasis should be placed on pediatric facilities, with special attention to children with extensive TB disease and parents or visitors who may have active, infectious TB.
This series has several limitations. Other pediatric cases of XDR-TB may have occurred during the study period without culture and DST confirmation or may not have survived long enough to prompt suspicion of drug-resistant TB. The children in this series were of similar age; their clinical course may not be representative of disease in younger children. Furthermore, all children in this series were residing in the pediatric ward prior to becoming symptomatic with XDR-TB, allowing for regular assessments by health care staff and greater access to diagnostic evaluation. The cases in this series may therefore be subject to detection bias and may not fully represent the spectrum of pediatric XDR-TB or XDR-TB and HIV co-infection.
The small number of cases limits our ability to identify specific patient or treatment characteristics that impacted outcomes. Nonetheless, these data provide support for further evaluation of co-treatment of XDR-TB and HIV in resource-limited settings. Although caution has been voiced about the potential for additive toxicities with HAART and second-line TB drugs in adults,5 less is known about drug interactions in children. Of note, EMB was well-tolerated without ocular toxicity in three cases, consistent with recent WHO recommendations for its use in children of all age groups.36 Lastly, while child-to-child transmission is strongly suggested, molecular epidemiology is currently unavailable for confirmation.
CONCLUSIONS
These successfully identified and treated children with XDR-TB illustrate the importance of bacteriologic diagnosis and DST for children with suspected TB. Treatment for pediatric XDR-TB is complex, but even with HIV co-infection, successful outcomes for both diseases are possible. Their orphan status predisposed them to long-term hospitalization, increasing their risks for nosocomial exposure to XDR-TB. This may, however, have improved their nutritional and immune status and bolstered their ability to successfully combat XDR-TB and HIV co-infection. Strengthened infection control measures in pediatric wards with high TB and HIV prevalence is needed, together with greater efforts to diagnose and treat XDR-TB in children worldwide.
Acknowledgments
The authors thank the patients and their families for making this study possible. They also thank the paediatric ward staff at the Church of Scotland Hospital and King George V Hospital for their dedication. SVS is supported by the Fogarty International Clinical Research Scholars Support Center, National Institutes of Health; SKH is supported by the Burroughs Wellcome Fund and American Society of Tropical Medicine and Hygiene; NRG, NSS and GF are supported by the Doris Duke Charitable Foundation, Howard Hughes Medical Institute and the US President’s Emergency Plan for AIDS Relief; GF is also supported by The Gilead Foundation and the Irene Diamond Fund.
References
- 1.World Health Organization. WHO/HTM/TB/2009.426. Geneva, Switzerland: WHO; 2009. Global tuberculosis control: a short update to the 2009 report. [Google Scholar]
- 2.World Health Organization. WHO/HTM/TB/2006.371. Geneva, Switzerland: WHO; 2006. Guidance for national tuberculosis programmes on the management of tuberculosis in children. [PubMed] [Google Scholar]
- 3.Donald PR. Childhood tuberculosis: out of control? Curr Opin Pulm Med. 2002;8:178–182. doi: 10.1097/00063198-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenoi S, Heysell S, Moll A, Friedland G. Multidrug-resistant and extensively drug-resistant tuberculosis: consequences for the global HIV community. Curr Opin Infect Dis. 2009;22:11–17. doi: 10.1097/QCO.0b013e3283210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaaf H, Gie R, Beyers N, Sirgel F, de Klerk P, Donald P. Primary drug-resistant tuberculosis in children. Int J Tuberc Lung Dis. 2000;4:1149–1155. [PubMed] [Google Scholar]
- 7.Schaaf HS, Marais BJ, Hesseling AC, Brittle W, Donald PR. Surveillance of antituberculosis drug resistance among children from the Western Cape Province of South Africa—an upward trend. Am J Public Health. 2009;99:1486–1490. doi: 10.2105/AJPH.2008.143271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaaf H, Willemse M, Donald P. Long-term linezolid treatment in a young child with extensively drug-resistant tuberculosis. Pediatr Infect Dis J. 2009;28:748–750. doi: 10.1097/INF.0b013e31819bc491. [DOI] [PubMed] [Google Scholar]
- 9.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom W. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest. 2008;134:187–192. doi: 10.1378/chest.07-1988. [DOI] [PubMed] [Google Scholar]
- 10.Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–1409. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 11.Mitnick C, Shin S, Seung K, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–574. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migliori GB, Besozzi G, Girardi E, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–626. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 13.Shah NS, Pratt R, Armstrong L, Robison V, Castro KG, Cegielski JP. Extensively drug-resistant tuberculosis in the United States, 1993–2007. JAMA. 2008;300:2153–2160. doi: 10.1001/jama.300.18.2153. [DOI] [PubMed] [Google Scholar]
- 14.Kwon YS, Kim YH, Suh GY, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008;47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi NR, Shah NS, Andrews JR, et al. HIV co-infection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO report 2009. WHO/HTM/TB/2009.411. Geneva, Switzerland: WHO; 2009. Global tuberculosis control: epidemiology, planning, financing. [Google Scholar]
- 17.Gandhi N, Moll A, Sturm A, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 18.Andrews J, Gandhi N, Moodley P, et al. Exogenous re-infection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis. 2008;198:1582–1589. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- 19.Reves R, Blakey D, Snider DJ, Farer L. Transmission of multiple drug-resistant tuberculosis: report of a school and community outbreak. Am J Epidemiol. 1981;113:423–435. doi: 10.1093/oxfordjournals.aje.a113110. [DOI] [PubMed] [Google Scholar]
- 20.Ridzon R, Kent J, Valway S, et al. Outbreak of drug-resistant tuberculosis with second-generation transmission in a high school in California. J Pediatr. 1997;131:863–868. doi: 10.1016/s0022-3476(97)70034-9. [DOI] [PubMed] [Google Scholar]
- 21.Schaaf HS, Van Rie A, Gie RP, et al. Transmission of multidrug-resistant tuberculosis. Pediatr Infect Dis J. 2000;19:695–699. doi: 10.1097/00006454-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Nivin B, Nicholas P, Gayer M, Frieden T, Fujiwara P. A continuing outbreak of multidrug-resistant tuberculosis, with transmission in a hospital nursery. Clin Infect Dis. 1998;26:303–307. doi: 10.1086/516296. [DOI] [PubMed] [Google Scholar]
- 23.Institute Superiore di Sanita, Cooperazione Italiana. Lessons learnt from South Africa, 2005–2009. Pietermaritzburg, KwaZulu-Natal, South Africa: ISS & Cooperazione Italiana, in partnership with Umzinyathi District Management; 2009. Tuberculosis MDR/XDR: the Msinga experience. [Google Scholar]
- 24.Parsons SA, Abbott G. TB risk assessment report: Church of Scotland Hospital. Pretoria, South Africa: COSH; 2009. [Google Scholar]
- 25.Department of Health, South Africa. The South African National Tuberculosis Control Programme practical guidelines, 2004. Pretoria, South Africa: DoH; 2004. [Google Scholar]
- 26.Mitchison D. Drug resistance in tuberculosis. Eur Respir J. 2005;25:376–379. doi: 10.1183/09031936.05.00075704. [DOI] [PubMed] [Google Scholar]
- 27.Stop TB Department, World Health Organization. WHO/HTM/TB/2008.402. Geneva, Switzerland: WHO; 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. [Google Scholar]
- 28.McIlleron H, Willemse M, Werely CJ, et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48:1547–1553. doi: 10.1086/598192. [DOI] [PubMed] [Google Scholar]
- 29.Shenoi S, Gandhi NR, Moll AP, et al. Clinical characteristics of survivors of XDR-TB and HIV co-infection in rural KwaZulu-Natal, South Africa. Cape Town, South Africa: International AIDS Society; 2009. [Google Scholar]
- 30.Zar H, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365:130–134. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 31.Schaaf H. Drug-resistant tuberculosis in children. S Afr Med J. 2007;97:995–997. [PubMed] [Google Scholar]
- 32.Starke JR. Transmission of Mycobacterium tuberculosis to and from children and adolescents. Sem Pediatr Infect Dis. 2001;12:115–123. [Google Scholar]
- 33.Weinstein J, Barrett C, Baltimore R, Hierholzer WJ. Nosocomial transmission of tuberculosis from a hospital visitor on a pediatrics ward. Pediatr Infect Dis J. 1995;14:232–234. doi: 10.1097/00006454-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Aznar J, Safi H, Romero J, Alejo A, Gracia A, Palomares J. Nosocomial transmission of tuberculosis infection in pediatrics wards. Pediatr Infect Dis J. 1995;14:44–48. doi: 10.1097/00006454-199501000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Basu S, Andrews J, Poolman E, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization, Stop TB Department & Department of Child and Adolescent Health and Development. WHO/HTM/TB/2006.365. Geneva, Switzerland: WHO; 2006. Ethambutol efficacy and toxicity: literature review and recommendations for daily and intermittent dosage in children. [Google Scholar]


