Abstract
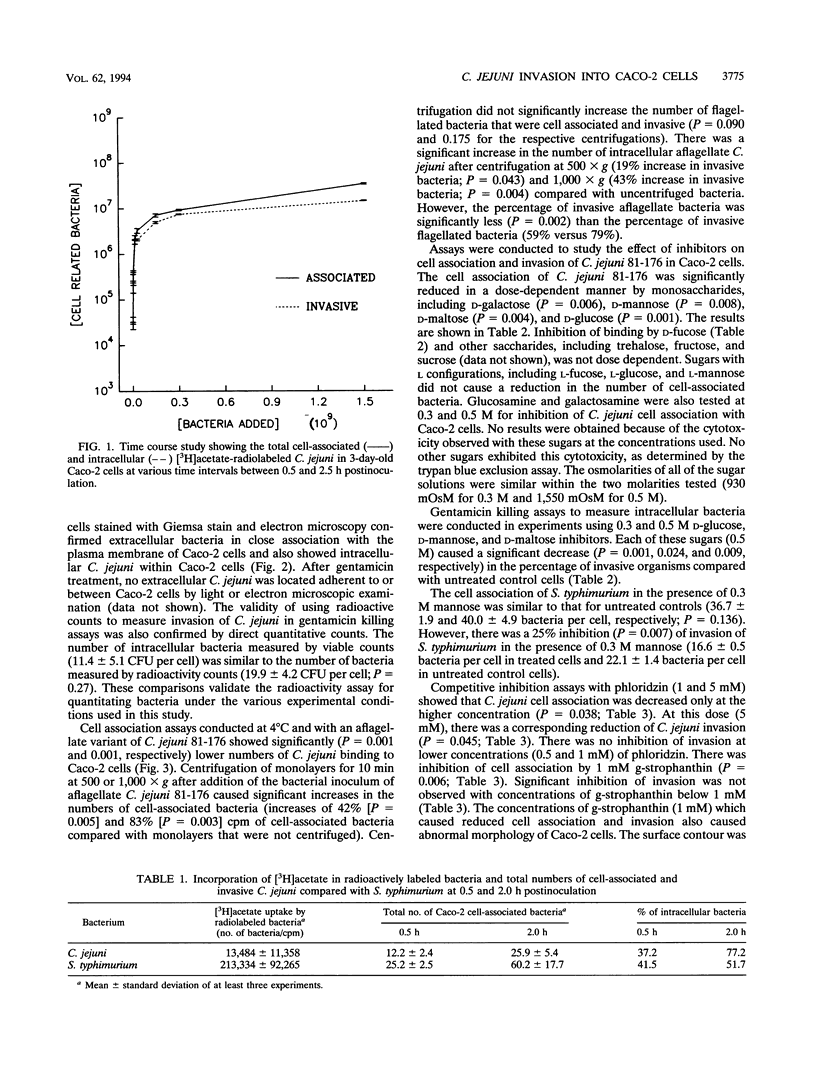
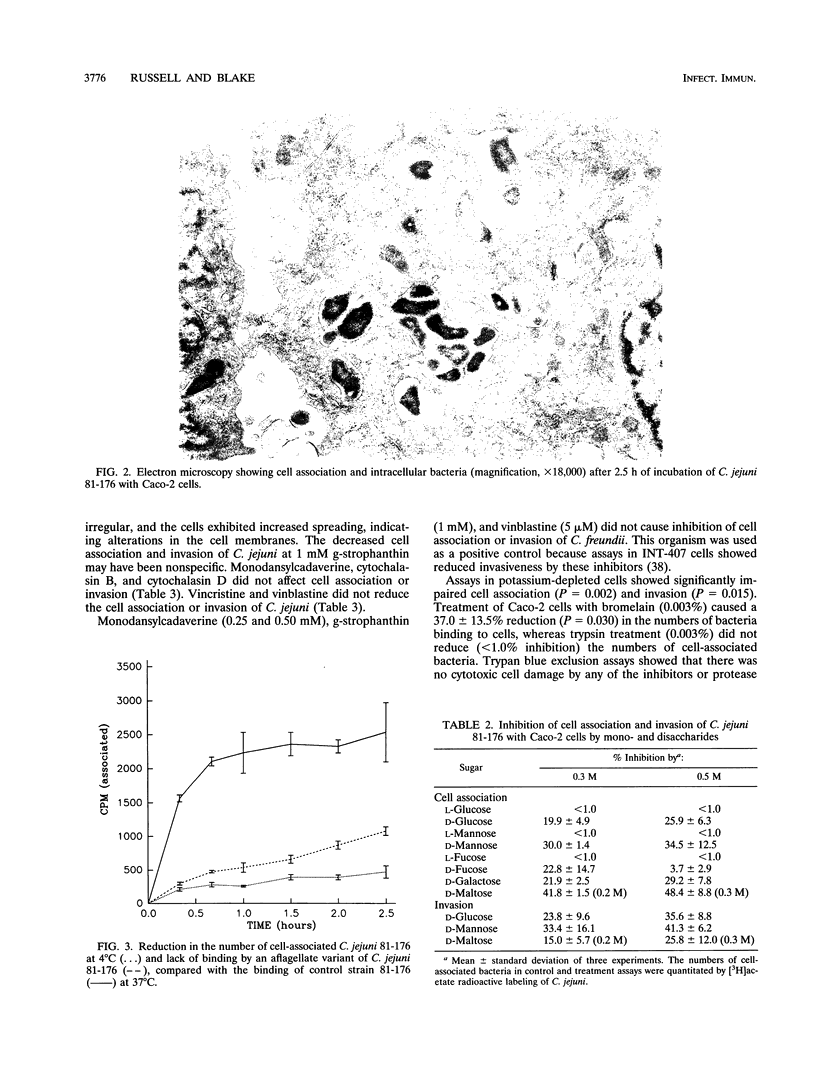
Adherence and invasion studies were conducted in monolayers of Caco-2 cells. Three-day-old monolayers were inoculated with Campylobacter jejuni 81-176 at a bacterium/cell ratio of 1,000:1. Saturation studies demonstrated time- and dose-dependent saturation curves for C. jejuni cell association and invasion into Caco-2 cells. Electron microscopy revealed intracellular C. jejuni located within membrane-bound vacuoles. Cell association and invasion were inhibited by 0.3 and 0.5 M concentrations of various sugars, including D-glucose, D-mannose, and D-fucose. However, there was no inhibition with the corresponding L-sugars, indicating physiological specificity. The inhibition of cell association with phloridzin was less pronounced. There was no inhibition of bacterial entry with monodansylcadaverine or g-strophanthin, indicating that it was unlikely that coated-pit formation is important in the invasion of C. jejuni into Caco-2 cells. Furthermore, there was no inhibition with cytochalasin D, vincristine, or vinblastine. Inhibition of cell association was demonstrated at 4 degrees C. Significantly decreased cell association and invasion were seen in potassium-depleted cells. Treatment of cells with bromelain also caused reduction in the number of C. jejuni binding to cells. A nonmotile aflagellate variant of C. jejuni also showed reduced invasion. The results of this study are consistent with energy-dependent invasion mechanisms. The results do not support an endocytic method of invasion for C. jejuni into Caco-2 cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamse S. L., Bindels R. J., van Os C. H. The colon carcinoma cell line Caco-2 contains an H+/K(+)-ATPase that contributes to intracellular pH regulation. Pflugers Arch. 1992 Sep;421(6):591–597. doi: 10.1007/BF00375056. [DOI] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnir B., Lee H. S., Hediger M. A., Wright E. M. Expression and characterization of the intestinal Na+/glucose cotransporter in COS-7 cells. Biochim Biophys Acta. 1990 Jan 30;1048(1):100–104. doi: 10.1016/0167-4781(90)90028-z. [DOI] [PubMed] [Google Scholar]
- Black R. E., Levine M. M., Clements M. L., Hughes T. P., Blaser M. J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988 Mar;157(3):472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Bukholm G. Effect of cytochalasin B and dihydrocytochalasin B on invasiveness of entero-invasive bacteria in HEp-2 cell cultures. Acta Pathol Microbiol Immunol Scand B. 1984 Jun;92(3):145–149. doi: 10.1111/j.1699-0463.1984.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Clerc P. L., Sansonetti P. J. Evidence for clathrin mobilization during directed phagocytosis of Shigella flexneri by HEp2 cells. Microb Pathog. 1989 Nov;7(5):329–336. doi: 10.1016/0882-4010(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Melo M. A., Gabbiani G., Pechère J. C. Cellular events and intracellular survival of Campylobacter jejuni during infection of HEp-2 cells. Infect Immun. 1989 Jul;57(7):2214–2222. doi: 10.1128/iai.57.7.2214-2222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson S. R., Bickerstaff G. F. Properties of immobilised bromelain. Biochem Soc Trans. 1992 Feb;20(1):23S–23S. doi: 10.1042/bst020023s. [DOI] [PubMed] [Google Scholar]
- Everest P. H., Goossens H., Butzler J. P., Lloyd D., Knutton S., Ketley J. M., Williams P. H. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J Med Microbiol. 1992 Nov;37(5):319–325. doi: 10.1099/00222615-37-5-319. [DOI] [PubMed] [Google Scholar]
- Fauchere J. L., Rosenau A., Veron M., Moyen E. N., Richard S., Pfister A. Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect Immun. 1986 Nov;54(2):283–287. doi: 10.1128/iai.54.2.283-287.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Ruschkowski S., Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J Cell Sci. 1991 Jun;99(Pt 2):283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Heffron F., Finlay B. B., Falkow S. Invasion and replication of Salmonella typhimurium in animal cells. Infect Immun. 1990 Feb;58(2):443–448. doi: 10.1128/iai.58.2.443-448.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Anderson R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989 Feb;108(2):389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Nilsson L. Endocytosis of Salmonella typhimurium 395 MS and MR10 by HeLa cells. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):322–328. doi: 10.1111/j.1699-0463.1977.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Konkel M. E., Mead D. J., Hayes S. F., Cieplak W., Jr Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. J Infect Dis. 1992 Aug;166(2):308–315. doi: 10.1093/infdis/166.2.308. [DOI] [PubMed] [Google Scholar]
- Korlath J. A., Osterholm M. T., Judy L. A., Forfang J. C., Robinson R. A. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985 Sep;152(3):592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- Larkin J. M., Brown M. S., Goldstein J. L., Anderson R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983 May;33(1):273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Madshus I. H., Tønnessen T. I., Olsnes S., Sandvig K. Effect of potassium depletion of Hep 2 cells on intracellular pH and on chloride uptake by anion antiport. J Cell Physiol. 1987 Apr;131(1):6–13. doi: 10.1002/jcp.1041310103. [DOI] [PubMed] [Google Scholar]
- Manninen K. I., Prescott J. F., Dohoo I. R. Pathogenicity of Campylobacter jejuni isolates from animals and humans. Infect Immun. 1982 Oct;38(1):46–52. doi: 10.1128/iai.38.1.46-52.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Burr D. H., Walker R. I. Intestinal mucus gel and secretory antibody are barriers to Campylobacter jejuni adherence to INT 407 cells. Infect Immun. 1987 Jun;55(6):1431–1435. doi: 10.1128/iai.55.6.1431-1435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985 Aug;131(8):1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- Oelschlaeger T. A., Guerry P., Kopecko D. J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F., Barker I. K., Manninen K. I., Miniats O. P. Campylobacter jejuni colitis in gnotobiotic dogs. Can J Comp Med. 1981 Oct;45(4):377–383. [PMC free article] [PubMed] [Google Scholar]
- Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986 Sep;68(9):1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Blaser M. J., Sarmiento J. I., Fox J. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect Immun. 1989 May;57(5):1438–1444. doi: 10.1128/iai.57.5.1438-1444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. G., O'Donnoghue M., Blake D. C., Jr, Zulty J., DeTolla L. J. Early colonic damage and invasion of Campylobacter jejuni in experimentally challenged infant Macaca mulatta. J Infect Dis. 1993 Jul;168(1):210–215. doi: 10.1093/infdis/168.1.210. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Wassenaar T. M., Bleumink-Pluym N. M., van der Zeijst B. A. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991 Aug;10(8):2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. J., Levine S., Donowitz M., Montrose M. H. Kinetics and regulation of a polarized Na(+)-H+ exchanger from Caco-2 cells, a human intestinal cell line. Am J Physiol. 1991 Aug;261(2 Pt 1):G229–G238. doi: 10.1152/ajpgi.1991.261.2.G229. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Turk E., Zabel B., Mundlos S., Dyer J. Molecular genetics of intestinal glucose transport. J Clin Invest. 1991 Nov;88(5):1435–1440. doi: 10.1172/JCI115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K., Biwersi J., Periasamy N., Verkman A. S. Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J Cell Biol. 1992 Oct;119(1):99–110. doi: 10.1083/jcb.119.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]