Abstract
Mucins are high molecular weight glycoproteins that are involved in regulating diverse cellular activities both in normal and pathological conditions. Mucin activity and localization is mediated by several molecular mechanisms, including discrete interactions with other proteins. An understanding of the biochemistry behind the known interactions between mucins and other proteins, coupled with an appreciation of their pathophysiological significance, can lend insight into the development of novel therapeutic agents. Indeed, a recent study demonstrated that a cell permeable inhibitor, PMIP, which disrupts the MUC1–EGFR interaction, is effective in killing breast cancer cells in vitro and in tumor models.
Keywords: Mucin, glycoproteins, interacting proteins, therapeutic
Mucins and their interactions with other proteins
Mucins are high molecular weight glycoproteins which can confer normal physiological protection and lubrication to epithelial surfaces [1,2]. Based on their sub-cellular localization, mucins are grouped into two different categories, secreted and membrane-bound. The secreted type mucins (MUC2, MUC5AC, MUC5B, MUC6-8 and MUC19) lack a transmembrane domain, and are secreted into the extra-cellular space. By contrast, the membrane-bound mucins (MUC1, MUC3, MUC4, MUC12-17 and MUC20) are type I membrane-anchored proteins with one membrane spanning domain, an NH2-terminal extracellular region, and a COOH-terminal intracellular cytoplasmic tail. Membrane-bound mucins can be released from the plasma membrane due to cleavage; alternative splicing can also produce secreted variants [1,3]. Although all mucins are encoded by different genes, their primary protein structures share some characteristic similarities (Box 1). In addition to these similarities, however, the primary structures of different mucins also harbor many unique functional domains and motifs which are specific to an individual or group of mucins [1,2].
Box 1. General structure of mucins.
Mucins consist of a protein backbone, termed “apomucin”, covered with many O-linked oligosaccharides and a number of N-glycan chains. Mature mucin glycoproteins often undergo many post-translational modifications, including glycosylation, sialylation and sulfation, often in a cell-type specific manner. Early studies of mucin primary structure were complicated owing to this extensive glycosylation; recent advances, however, have allowed the structures of many mucin proteins to be solved. Analysis of the available mucin structures established that centrally located TR regions are the characteristic feature of all mucins, differentiating them from other membrane-bound glycoproteins. Due to the presence of many proline, threonine, and serine residues in the TR region, it is also known as a PTS domain [60]. Gendler et al. first showed that the variable number of tandem repeat (VNTR) polymorphisim found in the protein coding sequence of mucins leads to the expression of polypeptides of different lengths. [61]. However, it remains unknown how these mucin variants functionally differ from each other. The serine and threonine residues of TRs are potential sites for O-glycosylation, and proline residues might play a role in achieving a confirmation that allows the close packing of carbohydrate structures. In addition, many mucins contain carbohydrate structures in the regions surrounding the TRs; these sugar moieties constitute up to 80% of the mucins molecular mass and form potential binding sites for interacting partners. The non-glycosylated regions of mucins harbor many structural motifs and domains [1], which might be important for the pathophysiological role of mucins. The EGF-like domains and cytoplasmic tail (CT) domains of membrane-bound mucins are believed to direct the interactions with different proteins. Mucin EGF-like domains might direct heterodimerization with ErbB receptors. The presence of phosphorylation sites and other protein–protein interaction motifs like polybasic sequence of amino acids suggests that CTs might interact with kinases and with other cytoskeleton-associated proteins. Therefore, interactions between membrane-bound mucins and their protein partners serve as an outside-to-inside signal that has a role in cell survival, proliferation and differentiation [1,4,35].
Burgeoning evidence indicates that due to their specialized structures, different mucins interact with distinct protein partners. These interactions provide specificity to mucin-mediated molecular and cellular events. Importantly, in various cancers, the deregulated expression and structural modifications of mucins drive protein–protein interactions which can impact cancer progression. Given that major membrane-bound mucins regulate different cancer promoting signaling events [4], and that some mucins are co-expressed in the same cells, the identification of cross-talk between these signaling events should help to identify common target molecules. Of all the mucins, MUC1 is the best characterized with respect to its protein–protein interactions; however recent research has characterized many interactions involving other mucins. To date, the detailed biochemical nature of these interactions and their importance for the development of novel therapeutics have not been extensively addressed. Therefore, we review the biological significance and underlying biochemistry of these interactions as well as possible implications for therapeutic strategy.
Mucin-interacting proteins: a large and diverse family
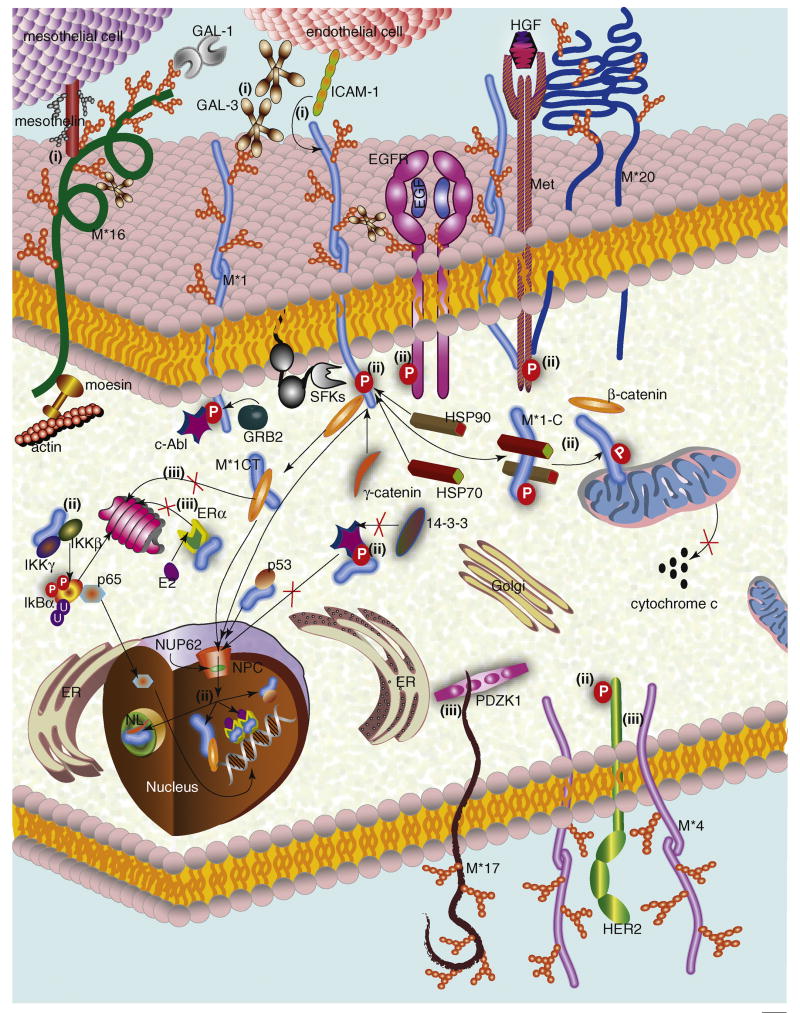
Membrane-bound mucins can interact with many different proteins present inside and/or outside of cells (Figure 1, Table 1). Additionally, they can also interact, both in cis and trans, with other transmembrane proteins. By contrast, the secreted mucins interact with other proteins only outside of cells (Figure 2, Table 1).
Figure 1. Transmembrane mucin-mediated cellular functions.
Interactions between transmembrane mucins and other proteins execute diverse functions by regulating different molecular and cellular events. (i) Cell–cell/protein–protein binding: MUC1– ICAM-1 and/or galectin-3 and MUC16–mesothelin interactions regulate different heterotypic cell-cell adhesions. The MUC1–galectin-3 interaction also bridges MUC1 and EGFR proteins. (ii) Signal transduction: Interactions between the MUC1 CT and different kinases [e.g., SFKs (c-Src/Lyn/Lck), IKK, EGFR, and Met)] regulate various downstream signaling events. Binding of the MUC1 CT with its specific partners promotes (for β-catenin, γ-catenin, p53, and ERα) or inhibits (for c-Abl) translocation to the nucleus (NC) and nucleolus (NL). The inhibition of c-Abl nuclear import restricts c-Abl-mediated apoptotic functions. The MUC1 CT–NUP62 interaction also regulates MUC1 nuclear import. In the nucleus, along with its interacting partners, the MUC1 CT regulates transcription of genes associated with cell-fate determination. The MUC1 CT–HSP70–HSP90 interaction facilitates translocation of MUC1-C to mitochondria and inhibits the release of the pro-apoptotic agent, cytochrome c. MUC20–Met and MUC4–HER2 interactions might also regulate downstream cell-signaling events. (iii) Protein stabilization: MUC17 CT–PDZK1 and MUC4–HER2 interactions stabilize MUC17 and HER2 on the cell surface. ERα and β-catenin proteasomal degradation is inhibited by their interaction with the MUC1 CT. Thus, stabilization of these proteins might regulate the cellular functions associated with these molecules. Abbreviations: E2-Estradiol, NPC-Nuclear Pore Complex, M*-mucin.
Table 1.
Mucins and their interacting partners
| Mucin | Interacting partner | Method of detection | Molecular/Cellular functions of the interactions | Reference |
|---|---|---|---|---|
| MUC1 | ICAM-1 | IPa | Promotes cancer metastasis (in vitro) | [26] |
| galectin-3 | IP and ACb | Promotes cancer metastasis (in vitro) | [16] | |
| EGFR | IP | Promotes tumorigenesis. Regulates proliferation and differentiation of normal epithelial cells (in vivo) | [20,62] | |
| Met | IP, IKAc and MS/MS | Reduces cancer metastasis (in vitro) | [23] | |
| FGFR3 | IP | Helps in MUC1 CT translocation to nucleus and mitochondria (in vitro). | [25] | |
| PDGFRβ | IKA | Promotes cancer metastasis (in vitro and in vivo) | [24] | |
| c-Src | IP and IKA | Promotes tumorigenesis (in vivo) | [37,63] | |
| Lyn | IP and IKA | Maturation and expansion of immune response (proposed) | [38] | |
| Lck | IP and IKA | T cell activation (proposed) | [36] | |
| c-Abl | IP and IKA | Promotes tumorigenesis (in vitro) | [41] | |
| IKKβ–IKKγ | IP, IBAd and IKA | Confers sustained induction of the IKK-NFκB p65 pathway (in vitro) | [44] | |
| GRB2 | IP and IBA | Promotes tumorigenesis (proposed) | [42] | |
| β-catenin | IP | Promotes tumorigenesis (In vivo) | [47,48] | |
| APC | IP | Possible role in breast cancer initiation and progression (proposed) | [51] | |
| GSK3β | IP, IKA and IBA | Inhibits MUC1-β-catenin interaction (in vitro) | [50] | |
| ERα | IP | Promotes tumorigenesis (in vitro) | [54] | |
| HSP70 | IP/MALDI-TOF-MSe | Targets the MUC1-C to mitochondria (in vitro) | [45] | |
| HSP90 | IP/MALDI-TOF-MS | Targets the MUC1-C to mitochondria (in vitro) | [45] | |
| p53 | IP | Promotes tumorigenesis (in vitro). | [53] | |
| NUP62 | IP | Promotes tumorigenesis (in vitro) | [52] | |
| MUC4 | HER2 | IP | Promotes tumorigenesis (proposed) | [19] |
| MUC5B | statherin and histatin 1, 3, and 5 | YTHf | Physiological protection (proposed) | [7,8] |
| MUC7 | α-amylase, PRP 2,3 and 4, statherin, histatin1 lactoferin, | YTH and FWBg FWB |
Physiological protection (proposed) Physiological protection (proposed) |
[10] |
| MUC16 | mesothelin | FWB | Promotes metastasis of ovarian cancer (in vitro). | [31] |
| galectin-1 | AC and MSh | Immune modulation (proposed) | [13] | |
| galectin-3 | AC | Physiological protection (in vitro) | [14] | |
| ERMs (moesin) | PDi | Cross-links MUC16 with the actin-cytoskeleton (proposed) | [55] | |
| MUC17 | PDZK1 | IP and MS | Stabilizes MUC17 at the cell surface (proposed) | [56] |
| MUC20 | Met | YTH and IP | Suppresses HGF-Met-mediated cell proliferation (in vitro and in vivo) | [22] |
Immunoprecipitation,
Affinity chromatography,
In vitro kinase assay,
In vitro binding assay,
Matrix Assisted Laser Desorption Ionization-Time Of Flight-Mass Spectroscopy,
Yeast Two Hybrid,
Far Western blot,
Mass spectrometry,
Pull-down assay.
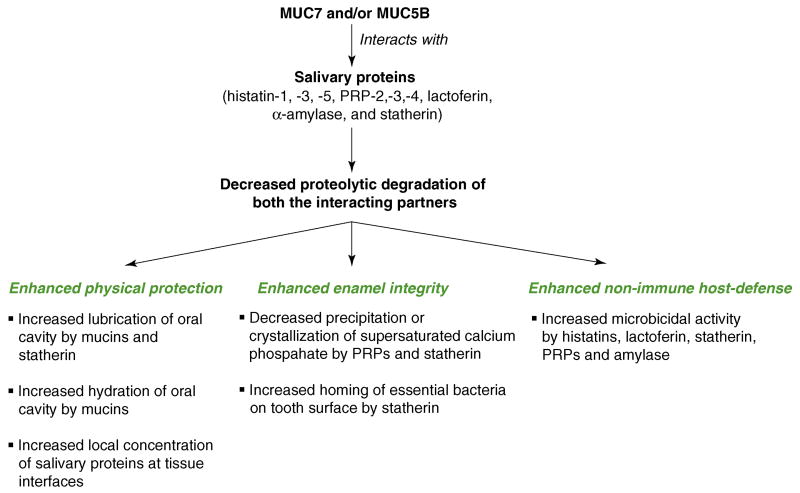
Figure 2. Secretory mucin-mediated cellular functions.
Interaction of secreted mucins with other proteins is most appreciated for its possible role in the maintenance of oral cavity health. The interaction of MUC5B with statherin and histatin-1,-3, and-5 might provide the oral cavity protection against different microorganisms. Likewise, interaction of MUC7 with α-amylase, PRP-2, PRP-3, PRP-4, statherin, histatin-1 and lactoferin might also contribute to oral microbicidal activity. Moreover, interactions with statherin might also guide helpful bacteria in attaching (homing) to the tooth surface, and promote maintenance of tooth enamel integrity. Along with PRPs, statherin also maintains enamel integrity by regulating oral calcium homeostasis. Like mucins, statherin might also provide lubrication and physical protection to the oral mucosal surface. Additionally, the existence of the heterotypic interaction of mucins to all these salivary proteins could protect both the partners from proteolysis and also promote the distribution of salivary proteins to different regions of the mouth (tooth surface, gum, crevices and other mucosal surfaces).
Extracellular mucin-interacting proteins
Extracellular mucin-interacting proteins are found in various body fluids and tissues, but most are present in saliva. Galectins are the only known non-salivary extracellular secreted proteins that interact with mucins.
Salivary proteins
Human salivary glands produce two types of secreted mucins: MG1 (also called MUC5B) and MG2 (also called MUC7). In addition to the presence of a tandem repeat (TR) region enriched with serine, threonine and proline residues, MUC5B and MUC7 harbor many cysteine residues in their primary structure [5,6]. In particular, the cysteine (Cys)-rich subdomains 1, 2 and 8a (Cys1, Cys2, and Cys8a) of MUC5B are predicted to have a high-order globular structure, which is suitable for[SC1] many protein–protein interactions [7]. Of all the known salivary mucin-interacting proteins, histatin and acidic proline-rich proteins (PRP) are found only in saliva, not in other body fluids. To date, more than 20 PRPs have been identified, and are grouped into three different types (acidic, basic and glycosylated). Immunological evidence demonstrates that MUC5B interacts with histatin and PRPs in human salivary fluid [8]. The MUC5B–histatin interaction is a direct protein–protein interaction, mediated by the MUC5B Cys1, Cys2, and Cys8a [7]. By contrast, the interaction sites for PRPs remain uncharacterized. Another secretory mucin, MUC7 also directly interacts with PRP2, an acidic PRP [9], via its N-terminal region [9].
In human saliva, α-amylase (a glycoside hydrolase) also interacts with MUC5B and MUC7 [8,9]. The N-terminal region of MUC7 participates in the direct interaction between these two proteins. However, the nature of the α-amylase–MUC5B interaction, which was suggested to be non-covalent, remains incompletely characterized. Like histatins, statherin, another salivary protein, directly interacts with MUC5B [8]; the MUC5B Cys8a-subdomain forms the binding site for statherin [7]. The involvement of the MUC5B Cys1-, Cys2- and Cys8a-subdomains in directing interactions with statherin and histatins suggests that these proteins undergo a glycosylation-independent interaction, as these regions are predicted to have a low number of potential glycosylaton sites in their primary structure. However, further studies will be needed to rule out the involvement of glycosylation or phosphorylation in these binding events [7].
In an effort to identify additional MUC7 interacting proteins, Soares R.V. et al., demonstrated that lactoferrin, an iron-binding protein, directly interacts with MUC7 in a glycosylation-independent manner [10]. Additionally, a peptide (KLADFALLCLDGKRK) corresponding to residues 587-601 of lactoferrin interacts with MUC7, but the exact minimal region essential for this interaction remains unidentified [10].
Interactions between secretory mucins and the aforementioned salivary proteins seems to be very important in the maintenance of oral physiology [1,11,12]. Before discussing the significance of these interactions, it is appropriate to mention some of the important functions of salivary mucin-interacting proteins (Figure 2) [11]. Experimental evidence indicates that PRP and statherin promote salivary calcium homeostasis. Statherins are also believed to promote binding of helpful bacteria to the enamel surface. By contrast, the antimicrobial activities of histatins and lactoferins represent a major component of the non-immune host-defense system in the human oral cavity. Therefore, interactions between salivary mucins and various salivary proteins might help to enhance their stability and function (details in Figure 2).
Galectins
Carbohydrate structures present on the highly glycosylated TR region or outside the TR region of mucins make them potential candidates to interact with the galectin family of carbohydrate binding proteins (β-galactoside-specific lectins). Among all the 14 known galectins, galectin-3 interacts with both MUC16 (also called CA125) and MUC1, and galectin-1 interacts with MUC16. The interaction of both of the galectins with MUC16 are MUC16-O-glycosylation dependent [13,14]. However, the binding affinity of MUC16 to galectin-1 is higher than galectin-3 [13]. Although the exact biological significance of the galectin-1–MUC16 interaction is not known, a possible role in promoting the adhesion of ovarian cancer cells to natural killer (NK) cells has been envisioned [15]. Further studies are required to clarify this speculation. Recent findings indicate that the interaction between galectin-3 and MUC16 contributes to the ocular surface epithelial barrier, which is essential to maintain corneal smoothness and clarity [14].
Like MUC16, MUC1 present on the surface of cancer cells also interacts with galectin-3 in the serum of cancer patients [16]. The TF (Thomsen-Friedenreich)-antigens present on the surface of MUC1 serve as the binding site for galectin-3 [16]. Experimental evidence also shows that the MUC1 carboxyl terminal region (MUC1-C), glycosylated at Asn-36, is essential for direct binding of galectin-3 [17]. This interaction bridges MUC1 and epidermal growth factor receptor (EGFR); here, galectin-3 acts as a scaffold [17]. Additionally, the galectin-3 CRD (carbohydrate recognition domain) is essential for its interaction with the MUC1-C. Particularly in breast cancer, the MUC1–galectin-3 interaction might have functional roles in transformation and metastasis. Galectin-3-mediated MUC1-C–EGFR binding might elicit EGFR-mediated downstream signaling pathways which are involved in tumorigenesis and cancer cell growth [17]. Furthermore, interactions between MUC1 present on the surface of cancer cells and serum-localized galectin-3 promotes strong adhesion (locking) of tumor cells on the endothelial surface, thus promoting cancer cell metastasis [16].
Membrane-bound mucin-interacting proteins
Most of the interactions between transmembrane mucins and their membrane-bound partners occur in pathological conditions such as cancer. Such binding partners typically belong to families of receptor tyrosine kinases or adhesion molecules.
ErbB/HER receptor tyrosine kinases
The ErbB (also called HER; human growth factor receptor) family of protein kinases comprises EGFR (also called ErbB1 or HER1), ErbB2 (also called HER2 or neu), ErbB3 (also called HER3), and ErbB4 (also called HER4). The interactions between these receptors and their specific ligands lead to receptor activation; however, no soluble ligand has been identified for HER2. In addition to activation by canonical ligands, ErbB receptor activation can be potentiated by proteins, including MUC1 and MUC4, which contain EGF-like domains.
The first interaction between a transmembrane mucin and a member of the ErbB family was observed between ErbB2 and the trans-membrane subunit of rat MUC4 (ASGP-2) [18]. Of the two EGF-like domains that are present in ASGP-2, only one takes part in this interaction, which is thought to be direct [18]. We recently demonstrated that human MUC4 associates with and stabilizes HER2 at the cell surface of pancreatic cancer cells. Such stabilization might play a significant role in HER2-mediated oncogenic signaling in pancreatic cancer cells [19]. It is not known whether MUC4 and HER2 interact directly.
Full-length MUC1 interacts with all four ErbB receptors [4,20]; the MUC1–EGFR interaction remains the best characterized [20,21]. This interaction inhibits EGFR down-regulation and promotes the transforming ability of EGFR [21]. Additionally, EGFR phosphorylates the YEKV motif of the MUC1 cytoplasmic tail (CT) [4]. A previous report revealed a direct interaction between the MUC1 CT and the EGFR kinase domain [20]. More recently, however, extracellular galectin-3 was implicated in bridging the interaction between EGFR and MUC1 [17]. Further studies are needed to clarify this discordance.
Non-ErbB type receptor tyrosine kinases
Several mucins, including MUC20 and MUC1, can interact with Met, the bonafide receptor for HGF (hepatocyte growth factor; also called scatter factor [SF]). MUC20, which is overexpressed in patients with immunoglobulin A nephropathy, directly interacts with Met, via its C-terminal 53 residues [22]. Additionally, MUC20 can oligomerize, thereby augmenting MUC20–Met binding. The MUC20–Met interaction suppresses the HGF-induced growth factor receptor-bound protein 2 (GRB2)-Ras pathway, and ultimately impairs cell proliferation [22]. Met also interacts with and catalyzes the tyrosine phosphorylation of the YHPM motif present in the MUC1 CT [23]. Although tandem mass spectrometry (MS/MS) analysis of MUC1 CT-interacting proteins identified a FTVKVADFGLAR fragment within the Met CT [23], the minimal region of the MUC1 CT that is required for this interaction has not been mapped. Like Met, in pancreatic adenocarcinoma cells, platelet-derived growth factor receptor β (PDGFRβ) phosphorylates the tyrosine residue present in the MUC1 CT HGRYVPP and RDTYHPM motifs, both in vitro and in vivo [24]; however the details about the minimal regions of the MUC1 CT that direct this interaction remain incomplete. PDGFRβ- and/or Met-mediated phosphorylation of the MUC1 CT modulate signaling events related to the motility and invasion of pancreatic adenocarcinoma cells [23,24]. Fibroblast growth factor receptor-3 (FGFR3) also interacts with MUC1 in a FGF1-dependent manner [25]. Stimulation of cells with FGF-1 activates FGFR3, which further activates c-Src, and thus induces c-Src-dependent phosphorylation of the MUC1 CT on the YEKV motif [25]. This interaction induces binding of the MUC1 CT with both β-catenin and the molecular chaperone HSP90 (discussed later) in a mutually exclusive manner, thereby targeting the MUC1 CT either into the nucleus or to the mitochondrial outer membrane [25]. Although this information points to a possible mechanism though which FGFR3 promotes MUC1 CT phosphorylation, the underlying biochemistry of the FGFR3–MUC1 CT interaction remains unclear.
Adhesion molecules
Owing to the presence of extracellular domains, membrane-bound mucins can interact (either in cis or trans) with other membrane-anchored molecules, especially those involved in cell–cell adhesion. Among these, intercellular cell adhesion molecule-1 (ICAM-1), which is present on the surface of endothelial cells, interacts with MUC1 [26,27]. The ICAM-1 binding site on MUC1 lies within the peptide core, which is exposed due to cancer-associated MUC1 hypoglycosylation [28]. Moreover, the inhibition of cell–cell aggregation between MUC1 and ICAM-1 expressing cells mediated via an anti-MUC1 antibody against the MUC1 TR core protein suggests that the mucin core protein of the MUC1 TR region interacts with ICAM-1 [29]. Specifically, loss of restricted apical expression, overexpression and hypoglycosylation of MUC1 in breast cancer cells directs the MUC1 backbone to interact with ICAM-1 on the surface of endothelial and fibroblast cells. The interaction of MUC1, present on the surface of tumor cells, with ICAM-1 on the surface of stimulated endothelial cells facilitates the adhesion of circulating tumor cells to endothelial cells, thus promoting metastasis [26,30].
Much like the MUC1–ICAM-1 interaction, a direct interaction occurs between mesothelin, a glycosylphosphatidylinositol-linked cell surface protein present on mesothelial/ovarian cancer cells, and MUC16 (also called CA125) [31,32]. This interaction relies on MUC16-N-glycosylation [31]. A recent study mapped mesothelin residues 296-359 as the minimal region for MUC16 binding [33]. Antibodies generated against the MUC16 TR region abrogate the MUC16–mesothelin interaction, thus pointing to a role for the MUC16 TRs in this interaction [34]. The interaction of the MUC16 present on the surface of ovarian cancer cells with mesothelin present on the surface of mesothelial and other ovarian cancer cells facilitates cell–cell adhesion, which promotes the metastasis of ovarian cancer cells [3,31].
Intracellular mucin-interacting proteins
Among the known intracellular mucin-interacting proteins, MUC1 CT-interacting proteins are the best characterized. Due to the diversified function of MUC1 in both normal and malignant cells, several structure-function studies have been performed. In this endeavor, several potential phosporylation and protein-binding sites have been identified in the MUC1 CT. Importantly, different functional studies point to the ability of the MUC1 CT to interact with a battery of proteins, thereby eliciting diverse downstream signaling events [4,35].
Intracellular kinases which interact with the MUC1 CT
The MUC1 CT is targeted by many kinases. Furthermore, phosphorylation of the MUC1 CT by these kinases regulates the interactions between the MUC1 CT and many of its other binding partners. Of the nine known members of the Src-family kinases (SFKs), c-Src, Lyn and Lck interact with the MUC1 CT [36-40]. These three SFKs bind and phosphorylate Tyr46 within the MUC1 CT YEKV motif, thereby regulating other MUC1 protein–protein interactions. The CQCR sequence present in the MUC1 CT forms the predicted Lck binding site [36], and the interaction between the MUC1 CT and Lck and Zeta-chain-associated protein kinase 70 (ZAP-70) is envisioned to contribute to signaling events associated with T-cell activation [36]. Likewise, the MUC1–Lyn interaction might participate in the maturation and expansion of the immune response [38]. Similarly, the pro-apoptotic kinase c-Abl directly interacts with the anti-apoptotic MUC1 CT [41]; this interaction leads to phosphorylation of Tyr-60 in the MUC1 CT, which in turn functions as a binding site for the c-Abl Src homology 2 (SH2) domain. Moreover, the interaction between the MUC1 CT inhibits c-Abl nuclear import (Figure 1). The same site (pY60TNP) is also involved in a direct interaction between GRB2, a scaffolding protein, and the MUC1 CT [42], but the way in which the interactions are coordinated in the same cell remains unclear. The MUC1 CT, in a complex with GRB2 and SOS (son of sevenless), might promote activation of the Ras-MEK-ERK2 signaling pathway, thereby promoting cell growth and differentiation [42,43].
MUC1 also interacts with components of IκB kinase (IKK) complex, which contains two catalytic subunits, IKK-α and IKK-β, and a regulatory subunit, IKK-γ (also called as NEMO) [44]. In mammalian cells, the association between IKK-β and IKK-γ is necessary and sufficient for phosphorylation of inhibitory κBα (IκBα) in the classical NF-κB pathway. The MUC1 CT binds both IKK-β and IKK-γ, thereny enhancing the interaction between these two IKKs. This interaction is directed by residues 1-45 of the MUC1 CT and 1-458 of the IKK-β N-terminus. Likewise, MUC1 CT residues 46-72 interact with residues 187-419 of IKK-γ C-terminus; amino acid substitutions within the MUC1 CT serine-rich motif (SRM, aa 50-59) are sufficient to abrogate the IKK-γ–MUC1 CT interaction. The IKK-β–MUC1 CT interaction, however, is not affected by these changes. The MUC1–IKK complex interaction promotes IKK-β phosphorylation on serine-181 (present in the activation loop), which is required for IKK-β activity. Moreover, IKK-β activation induces phosphorylation and degradation of IκBα, an event which targets NF-κB p65 to the nucleus, thereby enhancing NF-κB p65 transcriptional activity (Figure 1). NF-κB p65 induces the transcription of many genes; most serve anti-apoptotic functions and induce cellular transformation.
Molecular chaperones which interact with MUC1 CT
Heat shock protein 90 (HSP90), an essential molecular chaperone, interacts with the MUC1 CT; this interaction relies on prior c-Src-mediated MUC1 CT phosphorylation at Y46 [45]. The HSP90 binding site on MUC1 CT lies between amino acids 46-72: however, some additional sequences N-terminal to 46th amino acid also participate in this interaction. MUC1 also interacts with HSP70 in a phosphorylation independent manner; the MUC1 CT (amino acids 46-72) also forms the HSP70 interaction site. Both HSP90 and HSP70 can separately interact with the MUC1 CT in vitro. The interaction of the MUC1 CT with the HSP70–HSP90 complex promotes only mitochondrial translocation of only the MUC1 carboxyl terminal region (MUC1-C), and not the MUC1-amino terminal region (MUC1-N). The mitochondrial membrane-associated MUC1-C might inhibit the intrinsic apoptotic pathway by attenuating the release of mitochondrial apoptogenic factors such as cytochrome c (Figure 1) [46].
Components of the Wnt-signaling pathway which interact with the MUC1 CT
The MUC1 CT interacts with β-catenin (a component of Wnt-signaling pathway) through its β-catenin binding motif (SXXXXXL) [47]. This interaction participates in in vivo mammary gland transformation [48,49]. Although this interaction occurs both in primary and metastatic tumors, it is significantly enhanced in metastatic tumors, and promotes cancer cells metastasis [49]. The affinity for this interaction is regulated by phosphorylation at specific sites and by binding of other proteins, such as glycogen synthase kinase-3β (GSK-3β) and HSP90. GSK-3β, an important component of the Wnt-signaling pathway, directly interacts with and phosphorylates the MUC1 CT at the serine within the TDRSP motif. GSK-3β-mediated phosphorylation of the MUC1 CT decreases MUC-1–β-catenin binding in vitro and in vivo [50]. Likewise, the HSP90–MUC1 CT interaction can competitively inhibit the MUC1 CT–β-catenin interaction [4,25,35,36,50]. In breast cancer cells, MUC1 also interacts with adenomatosis polyposis coli (APC), another component of the Wnt-signaling pathway [51]. However, the detailed biochemical nature and biological significance of this interaction remains uncertain.
Importin and nucleoporin proteins which interact with the MUC1 CT
Additionally, the MUC1 CT interacts with importin-β and nucleoporin p62 (NUP62); this protein plays an important role in nucleo-cytoplasmic transport [52]. The NUP62 T-rich linker region and the C-terminal coiled coil region direct its interaction with MUC1. Whereas the interaction between the MUC1 CT and the NUP62 C-terminal domain is importin-β-mediated, the interaction with the T-rich linker is direct. MUC1 CT–NUP62–importin-β binding is facilitated by CQC motif-mediated MUC1 CT oligomerization. This interaction promotes MUC1 CT [SC2]nuclear import, thus regulating MUC1-mediated transcription events.
Transcription factors which interact with the MUC1 CT
In response to various external stimuli, the MUC1 CT specifically interacts with certain transcription factors, and the complexes subsequently translocate to the nucleus where they can regulate gene expression programs. By example, the interaction between the p53 tumor suppressor and the MUC1 CT, via the p53 regulatory domain (amino acids 363-393), modulates p53-mediated gene expression [53]. Chromatin immunoprecipitation (ChIP) assays revealed that MUC1 co-precipitates with p53 on the p53-responsive elements present within the cyclin-dependent kinase inhibitor 1A (CDKN1A; also called p21) promoter. Thus, in part, MUC1 regulates p53-dependent growth arrest and apoptotic signaling in response to DNA damage [53]. The DNA-binding domain of estrogen receptor α (ERα) also directly interacts with the N-terminal region of the MUC1 CT (amino acids 9-51) in a 17β-estradiol (E2)-dependent manner [54]. A ChIP experiment has confirmed the presence of MUC1 in the active ERα transcription complex. MUC1 CT–ERα binding stabilizes ERα by blocking its proteasomal degradation, thereby enhancing ERα-mediated gene transcription that directs the growth and survival of breast cancer cells.
ERM and PDZ-domain-containing proteins
The ERM protein family consists of three closely related proteins: ezrin, radixin and moesin. The N-terminal domain of ERM proteins interacts with the polybasic sequence present in membrane-bound proteins, and the C-terminal domain interacts with actin filaments. Knowing that the MUC16 cytoplasmic tail has a polybasic sequence of amino acids (RRRKK) in its cytoplasmic tail, Blalock et al. were the first to test for, and demonstrate, the direct interaction between the MUC16 CT and moesin in vitro (Figure 1) [55]. The MUC16-bnding site is present in the moesin N-terminal region (amino acids 1-332). The biological significance of this interaction remains poorly understood, but interestingly, this interaction is only specific for the MUC16 CT; neither the MUC4 CT nor the MUC1 CT binds moesin. Although other membrane-bound mucins’ CT lack a polybasic sequence of amino acids, further experimental evidence will be needed to rule out any possible direct/indirect interaction with ERM proteins.
Due to the presence of multiple PDZ domains, a given PDZ domain-containing protein can interact with multiple proteins which contain having PDZ-domain-binding sites. Thus, in a cell, PDZ domain-containing proteins can act as scaffolds. Three of the membrane-bound mucins (MUC3, MUC17 and MUC12) have C-terminal sequences typical of PDZ-domain-binding proteins. Malmberg et al., first identified a direct interaction between the MUC17 CT and PDZK1 (PDZ domain containing 1) in vitro (Figure 1) [56]. The MUC17–PDZ interaction site is capable of binding to three of the four PDZ domains present in PDZK1. Interestingly, this interaction was detected for MUC17, but not for MUC3, and was very weak for MUC12. Further analysis of PDZ protein arrays has also shown a very weak interaction between Na+/H+ exchange regulatory factor-1 (NHERF1) and both MUC17 and the MUC12 CT. A study in Pdzk1-/- mice suggests that PDZK1 might play a role in stabilizing MUC3 (orthologue of human MUC17) in the apical membrane of small intestine enterocytes. This interaction might facilitate mucin-mediated regulation of epithelial cell surface homoeostasis; however, future studies are needed to clarify its role in human physiology.
Pharmacological standpoints
The inhibition of the cancer-promoting interactions between mucins and other proteins might provide novel therapeutic targets. To develop specific pharmacological tools against these interactions, it is essential to characterize the interacting domains in each partner protein (Table 2).
Table 2.
Mucin-interacting partners and their interaction site/structure present in corresponding mucins
| Mucin | Binding partner | Interaction site of specific mucins | Reference |
|---|---|---|---|
| MUC1a | β-catenin | SAGNGGSSL (SRM; serine rich motif) present in the CT | [47] |
| HSP90 | SAGNGGSSL (SRM; serine rich motif) present in the CT | [25] | |
| HSP70 | CT (amino acids 46-72) | [45] | |
| FGFR3 | CT (amino acids 46-72) | [25] | |
| γ-catenin | SAGNGGSSL (SRM; serine rich motif) present in the CT | [64] | |
| ER α | CT (amino acids 9-46) | [54] | |
| GRB2 | pYTNP motif in the CT | [42] | |
| p53 | CT (amino acids 9-46) | [53] | |
| IKKβ | CT (amino acids 1-45) | [44] | |
| IKKγ | CT (amino acids 46-72) specifically within the SAGNGGSSLS motif (SRM) | [44] | |
| galectin-3 | TF-antigen | [16] | |
| MUC17 | PDZK1 | TTSF motif present in the CT | [56] |
| MUC16 | moesin | RRRKK, the polybasic sequence present in the CT | [55] |
| galectin-3 | O-glycans | [14] | |
| galectin-1 | O-glycans | [13] | |
| MUC20 | Met | CT (amino acids 451-503) | [22] |
| MUC5B | histatin1 statherin and histatin 3 and 5 |
Cysl and Cys2 (in Tandem repeat region) and Cys8a (in C-terminus) Cys8a (in C-terminus) |
[7] |
| MUC7 | α-amylase, PRP2, PRP3, PRP4, statherin, histatin 1 | N-terminal 144 residues of MUC7 | [9] |
For the individual kinases that interact with the MUC1 CT, the phosphorylation site is considered to be the site of interaction.
Recent reports showed that a cell penetrating peptide, PMIP [Protein transduction domain (PTD4)+ MUC1 inhibitory peptide (MIP)], could effectively inhibit MUC1–β-catenin and MUC1–EGFR interactions [57]. In addition, PMIP also induces a ligand-dependent reduction of EGFR levels, which might be due to the inhibition of the MUC1–EGFR interaction, as MUC1 protects ligand-induced EGFR degradation [21]. Importantly, these PMIP-mediated inhibitory events have a significant antitumor activity, in vitro and in vivo [57]. To date, clinical trials using EGFR kinase inhibitors or an anti-EGFR antibody have had little to no effect on breast cancer survival. Out of all the possible reasons behind these failures, MUC1-mediated EGFR internalization and alteration in its sub-cellular localization might be a potential cause [21]. Therefore, any compound, like the PMIP peptide, which can effectively abrogate cancer-promoting MUC1-mediated protein–protein interactions, holds promise as a potential anti-cancer agent.
In two different in vitro studies, the synthetic peptides GGSSLSY and STDRSPYE effectively inhibited interactions between the MIC1 CT and β-catenin and GSK3β, respectively [47,50]. In another study, 4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo (3, 4-d) pyrimidine (PP2), a small molecule inhibitor of Src, blocked FGF1-induced phosphorylation of the MUC1 CT on Tyr46 [25] in ZR-75-1 breast cancer cells, thereby preventing MUC1–β-catenin complex formation and subsequent translocation into the nucleus. Likewise, 17-(allylamino)-17-demethoxygeldanamycin (17-AAG), a small molecule inhibitor of HSP90, blocked FGF1-induced targeting of MUC1-C to the mitochondria [25]. In the same context, an anti-MUC1 Ab (B27.29) or a synthetic MUC1 peptide comprising six tandem repeats of MUC1 (each repeat containing 120-aa) inhibit the MUC1–ICAM-1 interaction [58]. Therefore, these reagents might be able to inhibit the MUC-1–ICAM-1 interaction, along with its downstream effects, thereby targeting cancer metastasis.
MUC1 CT oligomerization is a potential therapeutic target. MUC1 CT oligomerization enhances MUC1 CT–NUP62–importin-β binding, and thus promotes MUC1 CT nuclear translocation. Moreover, nuclear MUC1 CT can mediate cellular transformation by regulating the transcription of numerous target genes. Accordingly, a recent study showed that a peptide derived from the NH2-terminal region of MUC1, which contains the CQC motif (GO-201), could successfully attenuate MUC1 CT-nuclear-targeting and lead to regression of tumor growth in vivo [59].
In addition, recent findings about the MUC16–mesothelin interaction and its importance in ovarian cancer metastasis have spawned interest in its potential therapeutic exploitation. Antibodies against MUC16 can be used as potential agents to inhibit the MUC16–mesothelin interaction, thus inhibiting ovarian cancer cell metastasis [34]. Furthermore, the identification of the MUC16-interacting region in mesothelin has aided in the design of agents that can inhibit the MUC16–mesothelin interaction. By example, a fusion protein which consists of the mesothelin MUC16 binding domain and the crystallizable portion of immunoglobin (Fc-IgG) competitively inhibits the interaction of mesothelin-expressing cells with MUC16-expressing cells [33]. However, the in vivo effectiveness of all these reagents has not been determined.
Concluding remarks
As our knowledge on mucin-interacting proteins accumulates, the relationship between their structure and function becomes clearer. In the context of translational research, however, much of this information remains relatively immature. Therefore, we must limit ourselves, in the short term, to identifying interacting partners and placing more emphasis on understanding the physiological significance of these interactions. Moreover, for therapeutic purposes, biochemical and genetic studies will be instrumental in the accurate mapping of domains involved in these interactions. Clearer delineation of the interaction sites between mucins and other proteins will help to design effective and specific targeting/therapeutic agents. Although the specificity of interactions between some mucins with their interacting partners is known, many others still need to be defined. Without this information, the development of effective and safe therapeutics will be difficult. The use of specific inhibitors against protein–protein interactions that are involved in pathological conditions might have the potential to selectively inhibit the disease promoting effects of mucins and not their normal beneficial functions. At the same time, knowledge about the advantageous effects of salivary mucins interactions with other salivary proteins might be exploited to formulate artificial saliva for use in conditions such as Xerostomia (dry mouth).
Acknowledgments
The authors on this work are supported by grants from the National Institutes of Health (CA78590, CA111294, CA133774 and CA131944) and Department of Defense (PC081409 and W81XWH-08-1-0541). We thank Ms. Kristi L. Berger for editing the manuscript. We also sincerely thank the reviewers [SC3]for their very constructive comments and corrections that have helped to improve this manuscript.
Glossary
- Carbohydrate recognition domain (CRD)
a conserved 130 amino acid domain which binds the carbohydrate structures of different proteins. The presence of a CRD is a characteristic feature of all galectin family proteins.
- Epidermal growth factor (EGF)-like domain
a 30-40 residue long, and evolutionarily wellconserved, protein domain. Named for a repeat domain in EGF, it contains six cysteine residues, which are involved in forming three different disulfide bonds.
- ERM proteins
named for three closely-related proteins, ezrin, radixin and moesin. The ERM proteins contain three domains, an NH2-terminal globular domain, an extended alpha-helical domain and a charged COOH-terminal domain. These proteins play a crucial role in regulated cross-linking between membrane proteins and the actin cytoskeleton; they also contribute to signal transduction.
- Membrane-bound mucins
type I membrane-anchored proteins. To date, ten different membrane-bound mucins have been identified. Due to the presence of the cytoplasmic tail as well as structures such EGF and SEA domains in the extracellular regions, they are thought to be involved in different signal transduction pathways.
- PDZ domain
a common structural domain of 80-90 amino acids found in various signaling proteins. PDZ is an acronym derived from the first letters of three proteins – post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg) and zonula occludens-1 protein (ZO-1) in which the domain was first identified. PDZ-domain-binding proteins mediate cellular signaling pathways via binding of PDZ-domain containing proteins.
- Secretory mucins
these proteins lack a transmembrane domain, and are secreted directly into the extra-cellular space. To date, seven different secreted mucins have been identified. Of the known functions for secreted mucins, their roles in the maintenance of epithelial protection are the best characterized.
- SEA domain
also known as SEA module, it was first identified in sea urchin sperm protein, enterokinase, and agrin. Most SEA-domain bearing proteins are transmembrane in nature, and usually have only one SEA domain in the extracellular region. MUC16, however, contains multiple SEA domains. Although the exact physiological function of the SEA domain is not clear, its involvement in the cleavage of proteins such as MUC1 has been well studied.
- Thomsen-Friedenreich antigen/T antigen
the core disaccharide of O-glycosylated carbohydrate structures. It acts as an onco-fetal antigen.
- Type I proteins
transmembrane proteins with a single-pass through the plasma membrane. Their NH2-terminal portions remain exposed to the extra-cellular region, and the COOH-terminal portions remain inside the cell.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 2.Senapati S, et al. The MUC gene family: their role in the diagnosis and prognosis of gastric cancer. Histol Histopathol. 2008;23:1541–1552. doi: 10.14670/HH-23.1541. [DOI] [PubMed] [Google Scholar]
- 3.Singh AP, et al. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008;9:1076–1085. doi: 10.1016/S1470-2045(08)70277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Zalewska A, et al. Structure and biosynthesis of human salivary mucins. Acta Biochim Pol. 2000;47:1067–1079. [PubMed] [Google Scholar]
- 6.Mehrotra R, et al. Isolation and physical characterization of the MUC7 (MG2) mucin from saliva: evidence for self-association. Biochem J. 1998;334:415–422. doi: 10.1042/bj3340415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iontcheva I, et al. Molecular mapping of statherin- and histatin-binding domains in human salivary mucin MG1 (MUC5B) by the yeast two-hybrid system. J Dent Res. 2000;79:732–739. doi: 10.1177/00220345000790020601. [DOI] [PubMed] [Google Scholar]
- 8.Iontcheva I, et al. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich proteins, statherin, and histatins. J Dent Res. 1997;76:734–743. doi: 10.1177/00220345970760030501. [DOI] [PubMed] [Google Scholar]
- 9.Bruno LS, et al. Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim Biophys Acta. 2005;1746:65–72. doi: 10.1016/j.bbamcr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Soares RV, et al. MG2 and lactoferrin form a heterotypic complex in salivary secretions. J Dent Res. 2003;82:471–475. doi: 10.1177/154405910308200613. [DOI] [PubMed] [Google Scholar]
- 11.Lamkin MS, Oppenheim FG. Structural features of salivary function. Crit Rev Oral Biol Med. 1993;4:251–259. doi: 10.1177/10454411930040030101. [DOI] [PubMed] [Google Scholar]
- 12.Wu AM, et al. Structure, biosynthesis, and function of salivary mucins. Mol Cell Biochem. 1994;137:39–55. doi: 10.1007/BF00926038. [DOI] [PubMed] [Google Scholar]
- 13.Seelenmeyer C, et al. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J Cell Sci. 2003;116:1305–1318. doi: 10.1242/jcs.00312. [DOI] [PubMed] [Google Scholar]
- 14.Argueso P, et al. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belisle JA, et al. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125) Immunology. 2007;122:418–429. doi: 10.1111/j.1365-2567.2007.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu LG, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 17.Ramasamy S, et al. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carraway KL, III, et al. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi P, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder JA, et al. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;20(276):13057–13064. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 21.Pochampalli MR, et al. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693–1701. doi: 10.1038/sj.onc.1209976. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi T, et al. MUC20 suppresses the hepatocyte growth factor-induced Grb2-Ras pathway by binding to a multifunctional docking site of met. Mol Cell Biol. 2004;24:7456–7468. doi: 10.1128/MCB.24.17.7456-7468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh PK, et al. Phosphorylation of MUC1 by Met modulates interaction with p53 and MMP1 expression. J Biol Chem. 2008;283:26985–26995. doi: 10.1074/jbc.M805036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh PK, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67:5201–5210. doi: 10.1158/0008-5472.CAN-06-4647. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, et al. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res. 2006;4:873–883. doi: 10.1158/1541-7786.MCR-06-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahn JJ, et al. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis. 2005;22:475–483. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 27.Regimbald LH, et al. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res. 1996;56:4244–4249. [PubMed] [Google Scholar]
- 28.Rahn JJ, et al. MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J Biol Chem. 2004;279:29386–29390. doi: 10.1074/jbc.C400010200. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi T, et al. MUC1 mucin core protein binds to the domain 1 of ICAM-1. Digestion. 2001;63(Suppl 1):87–92. 87–92. doi: 10.1159/000051917. [DOI] [PubMed] [Google Scholar]
- 30.Glinsky VV, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- 31.Gubbels JA, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rump A, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko O, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergan L, et al. Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett. 2007;255:263–274. doi: 10.1016/j.canlet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Carson DD. The cytoplasmic tail of MUC1: a very busy place. Sci Signal. 2008;1:e35. doi: 10.1126/scisignal.127pe35. [DOI] [PubMed] [Google Scholar]
- 36.Li Q, et al. Interaction of human MUC1 and beta-catenin is regulated by Lck and ZAP-70 in activated Jurkat T cells. Biochem Biophys Res Commun. 2004;315:471–476. doi: 10.1016/j.bbrc.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, et al. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J Biol Chem. 2001;276:6061–6064. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, et al. DF3/MUC1 signaling in multiple myeloma cells is regulated by interleukin-7. Cancer Biol Ther. 2003;2:187–193. doi: 10.4161/cbt.2.2.282. [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee P, et al. MUC1 (CD227) interacts with lck tyrosine kinase in Jurkat lymphoma cells and normal T cells. J Leukoc Biol. 2005;77:90–99. doi: 10.1189/jlb.0604333. [DOI] [PubMed] [Google Scholar]
- 40.Al MA, Gendler SJ. Muc1 affects c-Src signaling in PyV MT-induced mammary tumorigenesis. Oncogene. 2005;24:5799–5808. doi: 10.1038/sj.onc.1208738. [DOI] [PubMed] [Google Scholar]
- 41.Raina D, et al. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. EMBO J. 2006;25:3774–3783. doi: 10.1038/sj.emboj.7601263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey P, et al. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 1995;55:4000–4003. [PubMed] [Google Scholar]
- 43.Meerzaman D, et al. Involvement of the MAP kinase ERK2 in MUC1 mucin signaling. Am J Physiol Lung Cell Mol Physiol. 2001;281:L86–L91. doi: 10.1152/ajplung.2001.281.1.L86. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad R, et al. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat Cell Biol. 2007;9:1419–1427. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren J, et al. MUC1 oncoprotein is targeted to mitochondria by heregulininduced activation of c-Src and the molecular chaperone HSP90. Oncogene. 2006;25:20–31. doi: 10.1038/sj.onc.1209012. [DOI] [PubMed] [Google Scholar]
- 46.Ren J, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto M, et al. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 48.Schroeder JA, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23:5739–5747. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 49.Schroeder JA, et al. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, et al. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18:7216–7224. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hattrup CL, et al. MUC1 can interact with adenomatous polyposis coli in breast cancer. Biochem Biophys Res Commun. 2004;316:364–369. doi: 10.1016/j.bbrc.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 52.Leng Y, et al. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 53.Wei X, et al. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–178. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Wei X, et al. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 55.Blalock TD, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 56.Malmberg EK, et al. The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem J. 2008;410:283–289. doi: 10.1042/BJ20071068. [DOI] [PubMed] [Google Scholar]
- 57.Bitler BG, et al. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res. 2009;15:100–109. doi: 10.1158/1078-0432.CCR-08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kam JL, et al. MUC1 synthetic peptide inhibition of intercellular adhesion molecule-1 and MUC1 binding requires six tandem repeats. Cancer Res. 1998;58:5577–5581. [PubMed] [Google Scholar]
- 59.Raina D, et al. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang T, et al. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci U S A. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gendler SJ, et al. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987;84:6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pochampalli MR, et al. Transforming growth factor alpha dependent cancer progression is modulated by Muc1. Cancer Res. 2007;67:6591–6598. doi: 10.1158/0008-5472.CAN-06-4518. [DOI] [PubMed] [Google Scholar]
- 63.Al MA, Gendler SJ. Muc1 affects c-Src signaling in PyV MT-induced mammary tumorigenesis. Oncogene. 2005;24:5799–5808. doi: 10.1038/sj.onc.1208738. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, et al. Heregulin targets gamma-catenin to the nucleolus by a mechanism dependent on the DF3/MUC1 oncoprotein. Mol Cancer Res. 2003;1:765–775. [PubMed] [Google Scholar]