Abstract
The ability of Legionella pneumophila to cause pneumonia is determined by its capability to evade the immune system and grow within human monocytes and their derived macrophages. Human monocytes efficiently activate caspase-1 in response to Salmonella but not to L. pneumophila. The molecular mechanism for the lack of inflammasome activation during L. pneumophila infection is unknown. Evaluation of the expression of several inflammasome components in human monocytes during L. pneumophila infection revealed that the expression of the apoptosis-associated speck-like protein (ASC) and the NOD-like receptor NLRC4 are significantly down-regulated in human monocytes. Exogenous expression of ASC maintained the protein level constant during L. pneumophila infection and conveyed caspase-1 activation and restricted the growth of the pathogen. Further depletion of ASC with siRNA was accompanied with improved NF-κB activation and enhanced L. pneumophila growth. Therefore, our data demonstrate that L. pneumophila manipulates ASC levels to evade inflammasome activation and grow in human monocytes. By targeting ASC, L. pneumophila modulates the inflammasome, the apoptosome, and NF-κB pathway simultaneously.
Keywords: Caspase, Cellular Immune Response, Immunology, Inflammation, Innate Immunity, ASC, Legionella, NOD-like Receptors, Human Monocytes, Inflammasome
Introduction
Legionella pneumophila is an intracellular bacterium and the causative agent of Legionnaire pneumonia, which is a severe form of pneumonia that affects the immune-compromised and the elderly (1–4). The ability of L. pneumophila to cause pneumonia in humans is dependent on its ability to evade the immune system and multiply within human monocytes and derived macrophages (5–9). In murine macrophages, L. pneumophila activates the Nlrc4 inflammasome leading to the production of active caspase-1 (10–15). Then Naip5 cooperates with Nlrc4 to mediate caspase-7 activation downstream of caspase-1, which restricts the intracellular survival of the organism (16–19). For reasons that are still not understood, human monocytes and their derived macrophages do not activate caspase-1 in response to L. pneumophila and permit intracellular replication of the pathogen (17).
The apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC)4 is an adaptor molecule that mediates inflammatory and apoptotic signals and is predominantly expressed in monocytes and mucosal epithelial cells (20). ASC is an integral component of the inflammasome, which is a large protein complex responsible for caspase-1 activation (21–23). Within the inflammasome, ASC links caspase-1 and NOD-like receptors (NLR) leading to the activation of caspase-1 (16–19). Many Gram-negative bacteria, such as Salmonella typhimurium, Pseudomonas aeroginosa, Shigella flexneri, and L. pneumophila, are recognized in murine macrophages by the NLR Ipaf/Nlrc4 leading to caspase-1 activation through the inflammasome (24, 25).
ASC has been reported to cooperate with NLRP3 (cryopyrin) and NLRP12 (PYPAF7) in inducing NF-κB activity in an overexpression system (26, 27). However, other reports show that ASC uses its caspase recruitment domain for interaction to down-regulate NF-κB signaling (20, 28). Therefore, ASC can be an inducer or an inhibitor of NF-κB depending on expression level, cell type, and location within the cell (29).
ASC has also been suggested to induce apoptosis by recruiting caspase-8 and/or Bax (30). Thus, ASC can regulate the inflammasome and other signaling pathways depending on the stoichiometry of its expression and on whether other pyrin domain family proteins are expressed upon activation (26, 31). Moreover, emerging reports show novel roles for ASC in disease conditions such as arthritis (32).
Here we demonstrate that L. pneumophila down-regulates human ASC in primary human monocytes. Remarkably, exogenous expression of ASC in primary human monocytes promoted caspase-1 activation in response to L. pneumophila and hindered bacterial intracellular growth. Moreover, our data revealed that ASC controls L. pneumophila infection in human cells by modulating host cell survival through limiting NF-κB activation leading to early host cell death. Taken together, our data demonstrate that ASC is a central molecule that modulates the fate of L. pneumophila within human monocytes by different mechanisms. Therefore, designated down-regulation of ASC by L. pneumophila established the necessary environment for its replication within human monocytes.
MATERIALS AND METHODS
Quantitative PCR
Total RNA was extracted from primary human monocytes and lysed in TRIzol (Invitrogen), and 1–2 μg of the RNA was converted to cDNA by ThermoScript RNase H− reverse transcriptase (Invitrogen). 20–60 ng of the converted cDNA was used for quantitative PCR with SYBR Green I PCR master mix in the StepOne Plus real time PCR system (Applied Biosystems). The target gene Ct values were normalized to the Ct values of two housekeeping genes (human GAPDH and CAP-1, accordingly to the cell origin) and expressed as relative copy number, as described previously (33). Primers used in the study are presented in supplemental Table 1.
Bacterial Strains
L. pneumophila strain Lp02 is a thymine auxotrophic derivative of Philadelphia-1 (3). L. pneumophila was cultured as described previously (17). All experiments were performed at a low multiplicity of infection of 0.5 or 1, followed by centrifugation and rinsing of the wells after 30 min except when otherwise indicated (34). All experiments were performed in the absence of ferric nitrate and l-cysteine from the monocytes or macrophage culture medium to allow L. pneumophila multiplication only intracellularly. Viable bacteria per 5 × 105 cells per well were quantified as described previously (8). The quantification of the colony-forming units (CFU) in vitro was performed more than four independent times as described previously (8).
Primary Human Monocytes
Human buffy coats were obtained from the local Red Cross, and monocytes were isolated by CD14-positive selection as described previously (35).
Generation of THP1 Cell Line Stably Expressing YFP-ASC
To make a fusion protein, ASC was amplified from cDNA by PCR and inserted at the C terminus of yellow fluorescent protein (YFP) on the basis of pLenti6/V5 plasmid (Invitrogen), as described previously (36). After plasmid verification by sequencing, we generated lentivirus and transduced THP-1 cells (ATCC, lot 385653) with 10–15% efficiency, as described in detail previously (36). Stably transduced cells were selected with blasticidin (Invitrogen) for 10 days followed by two rounds of flow sorting using FACS ARIA (BD Biosciences), resulting in nearly 100% yield of stably transduced THP-1 cells.
Intracellular Growth of L. pneumophila
Monocytes were infected as described previously (8). At the designated time points, monocytes were lysed and plated for colony-forming units (CFUs).
Immunoblotting
Cell lysates or supernatants were prepared and immunoblotted with an antibody that recognizes phospho-p65, phospho-IKKαβ, and IKKα (Cell Signaling), actin (Abcam), NLRC4 and ASC (Alexis Biochemicals), and human caspase-1 and pro-IL-1β (Dr. Mark Wewers). The blots were then treated with appropriate secondary antibody as described previously (8).
Cytotoxicity Assays
In vitro quantification of cytoplasmic (apoptosis) histone-associated DNA fragments was performed using the cell death detection ELISAPlus photometric enzyme immunoassay kit from Roche Applied Science to the specifications of the manufacturer.
Transfection of Primary Human Monocytes with Small Interfering RNA
Small interfering RNA (siControl and siASC) was purchased from Dharmacon and nucleofected into the cells with the Lonza apparatus, as described previously (17, 36). A plasmid coding ASC tagged with YFP was nucleofected into the cells with the Lonza apparatus according to the manufacturer's protocol.
Statistical Analysis
All experiments were done at least three independent times and yielded similar results. Comparisons of groups for statistical differences were done using Student's two-tailed t test. p value ≤0.05 was considered significant.
RESULTS
Primary Human Monocytes Do Not Activate Caspase-1 in Response to L. pneumophila
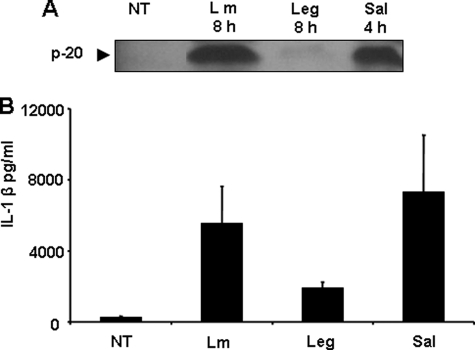
Because recognition of L. pneumophila by the murine Nlrc4 inflammasome is accompanied by caspase-1 activation and restriction of infection (8, 13, 17, 37), we examined if L. pneumophila infection activates the inflammasome in human monocytes. Primary human monocytes were infected with L. pneumophila, and the cleavage of caspase-1 was examined. Given that cleaved caspase-1 and IL-1β are released from monocytes once activated, we examined their amount in culture supernatants. Caspase-1 was not cleaved in response to L. pneumophila after 8 h of infection (Fig. 1A). Consequently, IL-1β production was minimal after a similar incubation time (Fig. 1B). It is possible that L. pneumophila deliberately avoids its own detection by the NLRC4 inflammasome by an unknown mechanism. It is also possible that the NLRC4 inflammasome is poorly functional in primary human monocytes. To distinguish between these possibilities and because Salmonella is detected by NLRC4, primary human monocytes were infected with Salmonella, and the release of active caspase-1 and IL-1β in culture supernatants was examined. Within 4 h of Salmonella infection caspase-1 was efficiently cleaved as observed by the detection of the p20 in culture supernatants (Fig. 1A). Accordingly, significant amounts of IL-1β were detected in culture supernatants of primary human monocytes infected with Salmonella (Fig. 1B). Furthermore, human monocytes were able to detect Gram-positive organisms such as Listeria monocytogenes, which was accompanied by caspase-1 activation (Fig. 1A). Therefore, the human inflammasome complex in general and the NLRC4 inflammasome in particular are perfectly functional in primary human monocytes, yet it is not activated in response to L. pneumophila.
FIGURE 1.
Human monocytes do not activate caspase-1 or IL-1β in response to L. pneumophila infection. Primary human monocytes were infected or not (NT) with L. pneumophila (Leg), L. monocytogenes (Lm) for 8 h, or S. typhimurium (Sal) for 4 h. A, cell culture supernatants were collected and analyzed by Western blot with anti-caspase-1 antibody. B, culture supernatants of primary human monocytes infected with L. pneumophila or S. typhimurium were analyzed for active IL-1β by ELISA.
L. pneumophila Reduces ASC and NLRC4 mRNA and Their Expression in Primary Human Monocytes
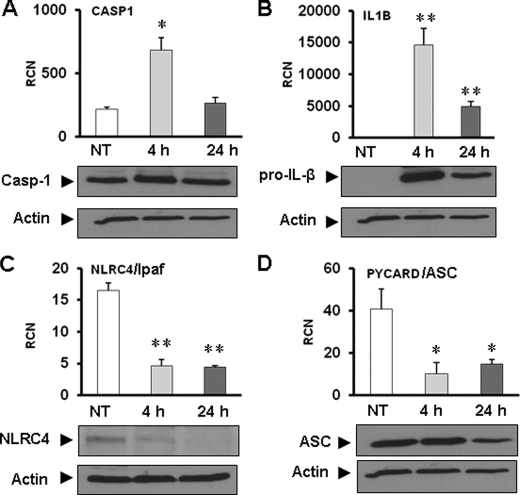
To understand the role of the inflammasome during L. pneumophila infection of human monocytes, primary human monocytes were infected with L. pneumophila for 4 and 24 h. Caspase-1, pro-IL-1β, ASC, and NLRC4 expressions were examined at the mRNA and protein levels using quantitative RT-PCR and Western blot, respectively (Fig. 2). Our results demonstrate that the expression of caspase-1 and pro-IL-1β was significantly increased at 4 h and then decreased after 24 h of L. pneumophila infection (Fig. 2, A and B). Western blot analysis showed that the levels of caspase-1 and IL-1β proteins correlated with mRNA levels revealed by RT-PCR (Fig. 2, A and B, lower panel).
FIGURE 2.
Effect of L. pneumophila infection on the expression of caspase-1, IL-1β, NLRC4, and ASC in human monocytes. Primary human monocytes were infected or not (NT) with L. pneumophila for 4 and 24 h at a multiplicity of infection of 1. The expression of caspase-1 (A), IL-1β (B), NLRC4 (C), and PYCARD/ASC (D), mRNA (upper panels), and protein levels (lower panels) were assessed using quantitative RT-PCR and Western blotting, respectively. The results are displayed as the mean of three independent experiments ± S.D. (*, p ≤ 0.05; **, p ≤ 0.01). Actin was used as a loading control in all panels.
On the other hand, the NLRC4 message was significantly suppressed at 4 h and remained low after 24 h of infection by L. pneumophila (Fig. 2C). Western blot analysis showed that NLRC4 protein levels decreased after the decrease in mRNA (Fig. 2C). Next, we explored the expression of PYCARD/ASC mRNA in primary human monocytes because it is a general adaptor molecule in the inflammasome. We found that L. pneumophila infection diminished PYCARD mRNA expression within 4 h of infection and remained low at 24 h (Fig. 2D). The ASC protein level was decreased within 24 h after infection in the same samples (Fig. 2D). As an independent control, mRNA levels of other molecules such as annexin1 and pyrin were not altered during L. pneumophila infection (supplemental Fig. 1). To further confirm our observation, we examined the expression of the same inflammasome components in the human monocytic cell line THP-1. The supplemental Fig. 2 shows that both NLRC4 and ASC were down-regulated in response to L. pneumophila infection in THP-1 cells that are also permissive to L. pneumophila growth. Therefore, our results reveal that L. pneumophila infection decreases the expression of essential inflammasome components such as NLRC4 and ASC in permissive primary human monocytes and in human monocytic cell lines.
Exogenous Expression of ASC in Human Monocytes Restores Caspase-1 Activation in Response to L. pneumophila
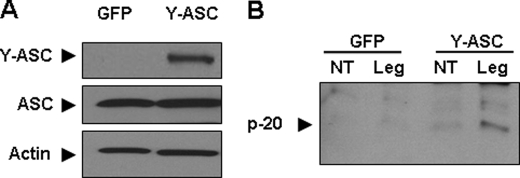
Despite its up-regulation, caspase-1 was not activated or released in culture supernatants of L. pneumophila-infected human monocytes (Fig. 1). Because mRNAs for ASC and NLRC4 were down-regulated in response to L. pneumophila, we postulated that their down-regulation contributes to the absence of caspase-1 activation during L. pneumophila infection. To test this hypothesis, we transfected primary human monocytes with a plasmid coding ASC tagged with YFP (Fig. 3A). GFP plasmid was used as control. Exogenous expression of ASC was calibrated so as to maintain moderate ASC levels during L. pneumophila. After transfection, cells were infected with L. pneumophila, and the cleavage of caspase-1 was examined by Western blot. Notably, endogenous ASC was decreased during L. pneumophila infection, yet exogenous ASC levels remained unchanged (supplemental Fig. 3). Active caspase-1 (p-20) was detected only in the supernatants of human monocytes expressing Y-ASC infected with L. pneumophila but not in supernatants derived from human monocytes transfected with the GFP plasmid prior to L. pneumophila infection (Fig. 3B). This finding supports the idea that the down-regulation of ASC mRNA by L. pneumophila may contribute to the lack of caspase-1 activation in human monocytes.
FIGURE 3.
Exogenous expression of ASC in human monocytes restores caspase-1 activation. A, primary human monocytes were nucleofected with a plasmid coding ASC tagged with YFP (Y-ASC). A plasmid encoding the green florescent protein (GFP) was used as a control. Twenty four hours after nucleofection, cells were lysed, and Y-ASC (52 kDa) and normal ASC (25 kDa) were assessed using Western blotting. B, human monocytes were nucleofected with a plasmid encoding GFP or Y-ASC and then they were either left untreated (NT) or infected with L. pneumophila (Leg) for 24 h at a multiplicity of infection of 1. Supernatant was collected, and cleaved caspase-1 (p-20) was assessed via immunoblotting.
Exogenous Expression of ASC Restricts L. pneumophila Infection and Diminishes NF-κB Activation
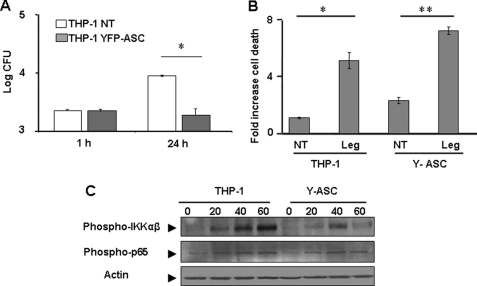
To further investigate the role of ASC during L. pneumophila infection, both THP-1 cells stably overexpressing Y-ASC and normal THP-1 cells were infected with L. pneumophila, and the ability of L. pneumophila to grow intracellularly was then examined by scoring CFUs within 24 h. THP-1 cells allowed L. pneumophila replication, whereas those expressing Y-ASC did not (Fig. 4A). To understand the mechanism by which ASC may regulate L. pneumophila infection, apoptosis was evaluated in THP-1 cells after 24 h of infection (Fig. 4B). Exogenous expression of ASC in THP-1 cells increased cell death induced in response to L. pneumophila infection (Fig. 4B). L. pneumophila activates NF-κB to extend host cell survival until it replicates intracellularly. Given that ASC may activate or inhibit NF-κB activation depending on the circumstances, we examined the effect of ASC on the phosphorylation of IKKαβ and p65 (Fig. 4C). Exogenous expression of ASC decreased the phosphorylation of IKKαβ and p65. Therefore, ASC restricts L. pneumophila growth by hindering NF-κB activation.
FIGURE 4.
Exogenous expression of ASC in THP-1 cells restricts L. pneumophila infection, increases apoptosis, and deters NF-κB activation. A, THP-1 cells overexpressing Y-ASC and normal THP-1 were infected with L. pneumophila, and bacterial growth was assessed at 1 and 24 h after infection by counting the CFUs. The bacterial count is displayed as log CFU/ml. The results are displayed as the mean of three independent experiments ± S.D. B, THP-1 cells overexpressing Y-ASC and normal THP-1 were infected or not (NT) with L. pneumophila (Leg), and apoptosis was evaluated after 24 h of infection. The results are displayed as the mean of three independent experiments ±S.D. (*, p ≤ 0.05; **, p ≤ 0.01.) C, THP-1 overexpressing Y-ASC and normal THP-1 were infected or not (0) with L. pneumophila for 20, 40, or 60 min. Levels of phosphorylation of IKKαβ and p65 were detected using immunoblots with corresponding antibodies. Actin was used as loading control.
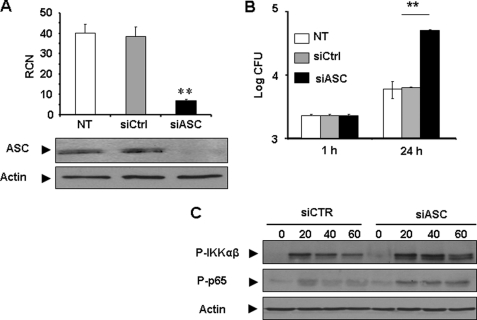
Down-regulation of ASC in Human Monocytes by siRNA Allows More L. pneumophila Growth and More NF-κB Activation
ASC expression in human monocytes is decreased during L. pneumophila infection and that is accompanied by permissiveness to infection. To further confirm the contribution of ASC in restriction of L. pneumophila infection, primary human monocytes were transfected with siRNA specific for ASC or control siRNA (Fig. 5A). Intracellular growth of L. pneumophila was evaluated by scoring CFU within 24 h of infection. Notably, depletion of ASC allowed more L. pneumophila growth when compared with human monocytes harboring scrambled siRNA as a control (Fig. 5B). Therefore, ASC also controls L. pneumophila infection in human monocytes independently of caspase-1. To discern the mechanism by which depletion of ASC may allow more L. pneumophila growth, NF-κB activation was examined in human monocytes harboring siRNA specific for ASC or control siRNA after L. pneumophila infection for 20, 40, or 60 min. Our data show that human monocytes treated with siRNA specific for ASC permitted more phosphorylation of IKKαβ and p65 than cells treated with control siRNA (Fig. 5C). Thus, in the context of L. pneumophila infection, ASC hinders NF-κB activation. Taken together, ASC controls L. pneumophila infection in a caspase-1-dependent and -independent manner.
FIGURE 5.
Depletion of ASC in primary human monocytes with specific siRNA allows more L. pneumophila growth and improves NF-κB activation. A, human monocytes were transfected with ASC-specific or control siRNA and then the expression of ASC was examined by quantitative PCR and by Western blot. B, transfected cells described in A were infected with L. pneumophila, and bacterial growth was evaluated by scoring CFU. The results are displayed as log CFU/ml, the mean of three independent experiments ± S.D. (**, p ≤ 0.01). Primary human monocytes transfected with siRNA targeting ASC (siASC) or control siRNA (siCTR) were infected or not (0) with L. pneumophila for 20, 40, or 60 min. The phosphorylation of IKKαβ and p65 was examined by Western blots using corresponding antibodies. Actin was used as loading control.
DISCUSSION
Pathogens have developed different mechanisms to avoid the engagement of the inflammasome and its activation (38). Viruses utilize proteins to bind ASC and prevent inflammasome assembly or disrupt the proteolytic activity of caspase-1. Yersinia enterocolitica express proteins that prevent caspase-1 oligomerization. P. aeruginosa and Mycobacterium tuberculosis generate molecules that block the activation of the NLRC4 and NLRP3 inflammasomes, respectively (38). In this study, we demonstrate that L. pneumophila suppresses the expression of ASC, thus preventing the activation of the NLRC4 inflammasome. This finding explains the lack of caspase-1 activation and IL-1β release during infection of human monocytes. This also provides mechanistic insight for the permissiveness of human monocytes to L. pneumophila infection.
Caspase-1 and IL-1β were up-regulated in human monocytes during L. pneumophila infection, yet they were not activated or released in culture supernatants of infected monocytes. Caspase-1 activation in response to L. pneumophila was re-instated when ASC was exogenously expressed under a different promoter impervious to L. pneumophila. Concurrently, the activation of caspase-1 was accompanied with restriction of infection. Thus, the down-regulation of ASC during L. pneumophila infection dampened caspase-1 activation and its bactericidal consequences. However, we believe that the down-regulation of ASC impinges on several pathways not only on caspase-1 activation.
Because L. pneumophila resides in macrophages for 6–8 h before replication starts, it must ensure the survival of the host cell for an extended duration (10, 14, 15). To achieve this, L. pneumophila activates NF-κB in a TLR5-dependent manner at early stages and in a TLR5-independent manner at later stages of infection for up to 30 h (10, 15). The mechanism for the prolonged activation of NF-κB for several hours is still not clear. Here we demonstrate that ASC prevents NF-κB activation induced by L. pneumophila, thus its down-regulation could be an elegant virulence strategy employed by L. pneumophila to extend host cell survival allowing more intracellular growth (10, 15).
Optiz and co-workers (39) demonstrated that exogenous expression of human NLRC4 and human NAIP restricts L. pneumophila. Yet their study did not offer a mechanism for restriction and did not examine caspase-1 activation. Anders and co-workers (40) recently showed that NLRs tend to be down-regulated in human cells and up-regulated in murine cells in response to LPS. Thus, it is possible that, similar to NLR expression patterns, the down-regulation of ASC during L. pneumophila infection is an inherent response of human monocytes to infection exploited by the pathogen to avoid caspase-1 activation and to establish infection. Alternatively, it is plausible that L. pneumophila intentionally down-regulates ASC in human monocytes to avoid the detrimental consequences of caspase-1 activation. We favor the second possibility because Salmonella infection of human monocytes was not accompanied by down-regulation of ASC, and caspase-1 was efficiently activated (data not shown). This prospect is supported by a study in clinically ill patients showing that the ASC gene expression levels are elevated irrespective of the infectious agent (41). This is also supported by several studies demonstrating that ASC is up-regulated in human monocytes and neutrophils during inflammation (30, 42). Taken together, it is likely that L. pneumophila uses many of its genes that encode eukaryote-like proteins or motifs to modulate human ASC functions to its advantage (43, 44).
Therefore, by down-regulating ASC, L. pneumophila achieves several aims; it avoids caspase-1 activation, evades bacterial clearance, and extends host cell survival. This is an elegant mechanism by which L. pneumophila exploits an adaptor molecule to establish infection in human monocytes.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants R01HL094586 and R21AI083871. This work was also supported by Grant GRT00013604 from the American Lung Association.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–3.
- ASC
- apoptosis-associated speck-like protein containing a caspase recruitment domain
- NLR
- NOD-like receptor
- IKK
- IκB kinase
- CFU
- colony-forming unit.
REFERENCES
- 1. Isberg R. R., O'Connor T. J., Heidtman M. (2009) Nat. Rev. Microbiol. 7, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cianciotto N. P. (2001) Int. J. Med. Microbiol. 291, 331–343 [DOI] [PubMed] [Google Scholar]
- 3. McDade J. E., Shepard C. C., Fraser D. W., Tsai T. R., Redus M. A., Dowdle W. R. (1977) N. Engl. J. Med. 297, 1197–1203 [DOI] [PubMed] [Google Scholar]
- 4. Cordes L. G., Wilkinson H. W., Gorman G. W., Fikes B. J., Fraser D. W. (1979) Lancet 2, 927–930 [DOI] [PubMed] [Google Scholar]
- 5. Horwitz M. A. (1983) J. Exp. Med. 158, 2108–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horwitz M. A. (1983) J. Exp. Med. 158, 1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kagan J. C., Roy C. R. (2002) Nat. Cell Biol. 4, 945–954 [DOI] [PubMed] [Google Scholar]
- 8. Amer A., Franchi L., Kanneganti T. D., Body-Malapel M., Ozören N., Brady G., Meshinchi S., Jagirdar R., Gewirtz A., Akira S., Núñez G. (2006) J. Biol. Chem. 281, 35217–35223 [DOI] [PubMed] [Google Scholar]
- 9. Tilney L. G., Harb O. S., Connelly P. S., Robinson C. G., Roy C. R. (2001) J. Cell Sci. 114, 4637–4650 [DOI] [PubMed] [Google Scholar]
- 10. Losick V. P., Isberg R. R. (2006) J. Exp. Med. 203, 2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin S., Roy C. R. (2008) Cell. Microbiol. 10, 1209–1220 [DOI] [PubMed] [Google Scholar]
- 12. Laguna R. K., Creasey E. A., Li Z., Valtz N., Isberg R. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18745–18750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zamboni D. S., Kobayashi K. S., Kohlsdorf T., Ogura Y., Long E. M., Vance R. E., Kuida K., Mariathasan S., Dixit V. M., Flavell R. A., Dietrich W. F., Roy C. R. (2006) Nat. Immunol. 7, 318–325 [DOI] [PubMed] [Google Scholar]
- 14. Abu-Zant A., Jones S., Asare R., Suttles J., Price C., Graham J., Kwaik Y. A. (2007) Cell. Microbiol. 9, 246–264 [DOI] [PubMed] [Google Scholar]
- 15. Bartfeld S., Engels C., Bauer B., Aurass P., Flieger A., Brüggemann H., Meyer T. F. (2009) Cell. Microbiol. 11, 1638–1651 [DOI] [PubMed] [Google Scholar]
- 16. Yu H. B., Finlay B. B. (2008) Cell Host Microbe 4, 198–208 [DOI] [PubMed] [Google Scholar]
- 17. Akhter A., Gavrilin M. A., Frantz L., Washington S., Ditty C., Limoli D., Day C., Sarkar A., Newland C., Butchar J., Marsh C. B., Wewers M. D., Tridandapani S., Kanneganti T. D., Amer A. O. (2009) PLoS Pathog. 5, e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamkanfi M., Kanneganti T. D., Van Damme P., Vanden Berghe T., Vanoverberghe I., Vandekerckhove J., Vandenabeele P., Gevaert K., Núñez G. (2008) Mol. Cell. Proteomics 7, 2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamkanfi M., Moreira L. O., Makena P., Spierings D. C., Boyd K., Murray P. J., Green D. R., Kanneganti T. D. (2009) Blood 113, 2742–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sarkar A., Duncan M., Hart J., Hertlein E., Guttridge D. C., Wewers M. D. (2006) J. Immunol. 176, 4979–4986 [DOI] [PubMed] [Google Scholar]
- 21. Petrilli V., Papin S., Tschopp J. (2005) Curr. Biol. 15, R581. [DOI] [PubMed] [Google Scholar]
- 22. Fernandes-Alnemri T., Wu J., Yu J. W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E. S. (2007) Cell Death Differ. 14, 1590–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srinivasula S. M., Poyet J. L., Razmara M., Datta P., Zhang Z., Alnemri E. S. (2002) J. Biol. Chem. 277, 21119–21122 [DOI] [PubMed] [Google Scholar]
- 24. Abdelaziz D. H., Amr K., Amer A. O. (2010) Int. J. Biochem. Cell Biol. 42, 789–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amer A. O. (2010) Cell. Microbiol. 12, 140–147 [DOI] [PubMed] [Google Scholar]
- 26. Hasegawa M., Imamura R., Kinoshita T., Matsumoto N., Masumoto J., Inohara N., Suda T. (2005) J. Biol. Chem. 280, 15122–15130 [DOI] [PubMed] [Google Scholar]
- 27. Masumoto J., Dowds T. A., Schaner P., Chen F. F., Ogura Y., Li M., Zhu L., Katsuyama T., Sagara J., Taniguchi S., Gumucio D. L., Núñez G., Inohara N. (2003) Biochem. Biophys. Res. Commun. 303, 69–73 [DOI] [PubMed] [Google Scholar]
- 28. Bedoya F., Sandler L. L., Harton J. A. (2007) J. Immunol. 178, 3837–3845 [DOI] [PubMed] [Google Scholar]
- 29. Stehlik C., Fiorentino L., Dorfleutner A., Bruey J. M., Ariza E. M., Sagara J., Reed J. C. (2002) J. Exp. Med. 196, 1605–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taniguchi S., Sagara J. (2007) Semin. Immunopathol. 29, 231–238 [DOI] [PubMed] [Google Scholar]
- 31. Ohtsuka T., Ryu H., Minamishima Y. A., Macip S., Sagara J., Nakayama K. I., Aaronson S. A., Lee S. W. (2004) Nat. Cell Biol. 6, 121–128 [DOI] [PubMed] [Google Scholar]
- 32. Ippagunta S. K., Brand D. D., Luo J., Boyd K. L., Calabrese C., Stienstra R., Van de Veerdonk F. L., Netea M. G., Joosten L. A., Lamkanfi M., Kanneganti T. D. (2010) J. Biol. Chem. 285, 12454–12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gavrilin M. A., Bouakl I. J., Knatz N. L., Duncan M. D., Hall M. W., Gunn J. S., Wewers M. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Derré I., Isberg R. R. (2004) Infect. Immun. 72, 6221–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hall M. W., Gavrilin M. A., Knatz N. L., Duncan M. D., Fernandez S. A., Wewers M. D. (2007) Pediatr. Res. 62, 597–603 [DOI] [PubMed] [Google Scholar]
- 36. Gavrilin M. A., Mitra S., Seshadri S., Nateri J., Berhe F., Hall M. W., Wewers M. D. (2009) J. Immunol. 182, 7982–7989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ren T., Zamboni D. S., Roy C. R., Dietrich W. F., Vance R. E. (2006) PLoS Pathog. 2, e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taxman D. J., Huang M. T., Ting J. P. (2010) Cell Host Microbe 8, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vinzing M., Eitel J., Lippmann J., Hocke A. C., Zahlten J., Slevogt H., N′guessan P. D., Günther S., Schmeck B., Hippenstiel S., Flieger A., Suttorp N., Opitz B. (2008) J. Immunol. 180, 6808–6815 [DOI] [PubMed] [Google Scholar]
- 40. Lech M., Avila-Ferrufino A., Skuginna V., Susanti H. E., Anders H. J. (2010) Int. Immunol. 22, 717–728 [DOI] [PubMed] [Google Scholar]
- 41. Fahy R. J., Exline M. C., Gavrilin M. A., Bhatt N. Y., Besecker B. Y., Sarkar A., Hollyfield J. L., Duncan M. D., Nagaraja H. N., Knatz N. L., Hall M., Wewers M. D. (2008) Am J. Respir. Crit. Care Med. 177, 983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shiohara M., Taniguchi S., Masumoto J., Yasui K., Koike K., Komiyama A., Sagara J. (2002) Biochem. Biophys. Res. Commun. 293, 1314–1318 [DOI] [PubMed] [Google Scholar]
- 43. Chien M., Morozova I., Shi S., Sheng H., Chen J., Gomez S. M., Asamani G., Hill K., Nuara J., Feder M., Rineer J., Greenberg J. J., Steshenko V., Park S. H., Zhao B., Teplitskaya E., Edwards J. R., Pampou S., Georghiou A., Chou I. C., Iannuccilli W., Ulz M. E., Kim D. H., Geringer-Sameth A., Goldsberry C., Morozov P., Fischer S. G., Segal G., Qu X., Rzhetsky A., Zhang P., Cayanis E., De Jong P. J., Ju J., Kalachikov S., Shuman H. A., Russo J. J. (2004) Science 305, 1966–1968 [DOI] [PubMed] [Google Scholar]
- 44. Cazalet C., Rusniok C., Brüggemann H., Zidane N., Magnier A., Ma L., Tichit M., Jarraud S., Bouchier C., Vandenesch F., Kunst F., Etienne J., Glaser P., Buchrieser C. (2004) Nat. Genet. 36, 1165–1173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.