Abstract
In protein conformational disorders ranging from Alzheimer to Parkinson disease, proteins of unrelated sequence misfold into a similar array of aggregated conformers ranging from small oligomers to large amyloid fibrils. Substantial evidence suggests that small, prefibrillar oligomers are the most toxic species, yet to what extent they can be selectively targeted and remodeled into non-toxic conformers using small molecules is poorly understood. We have evaluated the conformational specificity and remodeling pathways of a diverse panel of aromatic small molecules against mature soluble oligomers of the Aβ42 peptide associated with Alzheimer disease. We find that small molecule antagonists can be grouped into three classes, which we herein define as Class I, II, and III molecules, based on the distinct pathways they utilize to remodel soluble oligomers into multiple conformers with reduced toxicity. Class I molecules remodel soluble oligomers into large, off-pathway aggregates that are non-toxic. Moreover, Class IA molecules also remodel amyloid fibrils into the same off-pathway structures, whereas Class IB molecules fail to remodel fibrils but accelerate aggregation of freshly disaggregated Aβ. In contrast, a Class II molecule converts soluble Aβ oligomers into fibrils, but is inactive against disaggregated and fibrillar Aβ. Class III molecules disassemble soluble oligomers (as well as fibrils) into low molecular weight species that are non-toxic. Strikingly, Aβ non-toxic oligomers (which are morphologically indistinguishable from toxic soluble oligomers) are significantly more resistant to being remodeled than Aβ soluble oligomers or amyloid fibrils. Our findings reveal that relatively subtle differences in small molecule structure encipher surprisingly large differences in the pathways they employ to remodel Aβ soluble oligomers and related aggregated conformers.
Keywords: Amyloid, Peptide Conformation, Protein Conformation, Protein Folding, Protein Self-assembly, Protein Aggregation, Protein Misfolding
Introduction
A central tenet of protein folding is that a given amino acid sequence encodes a single folded structure (1). By analogy, one would expect that a given protein sequence would encode a single misfolded structure (e.g. a single amyloid fibril conformation). Instead, each protein sequence encodes numerous aggregated isoforms that possess unique secondary and tertiary structures (2–12). Previous work has firmly established that small, prefibrillar conformers (herein referred to as soluble oligomers) of diverse polypeptides are the most toxic aggregates both in vitro and in vivo (11, 13–17). However, elucidating the structural attributes of such toxic conformers that differentiate them from their non-toxic counterparts has proven difficult (see Refs. 11 and 18–22 for recent progress).
Significant evidence linking protein misfolding to cellular toxicity in numerous aggregation disorders has motivated the search for small molecules that prevent aggregation (see Refs. 23–25, and references therein). A general conclusion of these studies is that many small molecules redirect the aggregation cascade rather than inhibiting it completely (26). In hindsight, this finding is logical based on the large amount of buried surface area within protein aggregates compared with the small size of inhibitor molecules (27, 28). Therefore, using small molecules to alter the nucleation pathway by disrupting specific intermolecular contacts or promoting atypical ones appears to be a more feasible approach to preventing formation of toxic aggregates than antagonizing all possible intermolecular contacts.
Much less is known about the capacity of small molecules to remodel mature protein aggregates (see Refs. 12 and 29–31 for recent progress), despite the therapeutic importance of abrogating toxic aggregates. This is surprising because it is more complex to understand how small molecules alter the aggregation of monomers where proteins necessarily undergo conformational change (unless prevented by small molecules) than it is in the reverse direction where mature aggregated conformers can be isolated that do not change structurally during experimentally relevant time scales. Nevertheless, difficulties in forming homogeneous populations of different aggregated conformers and discriminating between them have hampered mechanistic studies of protein disaggregation. The development of several conformation-specific antibodies capable of selectively detecting aggregated conformers ranging from intermediates (e.g. soluble oligomers (32–34), fibrillar oligomers (21), and annular protofibrils (35)) to end products (i.e. fibrils (36, 37)) of amyloid assembly have been critical to overcoming such challenges.
Indeed, such conformation-specific antibodies and related biochemical assays are beginning to illuminate pathways employed by aromatic small molecules to remodel mature soluble oligomers of Aβ and other disease-associated proteins (29–31, 38). Multiple polyphenols have been found recently to convert mature soluble oligomers of Aβ and Tau into off-pathway, SDS-resistant aggregates that are non-toxic (12, 31, 39). In fact, these and related studies suggest that conversion of soluble oligomers into high molecular weight aggregates may be a common remodeling pathway employed by other small molecules. Nevertheless, small molecules may neutralize the toxicity of mature Aβ soluble oligomers via other mechanisms as well (38, 40). Herein, we demonstrate that diverse aromatic small molecules utilize three independent pathways to remodel mature Aβ soluble oligomers into benign conformers with highly dissimilar biochemical properties.
EXPERIMENTAL PROCEDURES
Preparation of Aβ Conformers
Aβ42 (American Peptide) was dissolved in an aqueous, 50% acetonitrile solution (1 mg/ml), aliquoted, dried under vacuum and lyophilized, and then stored at −20 °C. The preparation of Aβ soluble oligomers, non-toxic oligomers, and fibrils is described elsewhere (12). Briefly, Aβ soluble oligomers and non-toxic oligomers were prepared by dissolving the peptide in 100% hexafluoroisopropanol (Fluka). After the hexafluoroisopropanol was evaporated, the dried peptide was reconstituted in 50 mm NaOH (1 mg/ml Aβ), sonicated (30 s), and diluted in PBS (25 μm Aβ). The peptide was then centrifuged (22,000 × g for 30 min), and the pelleted fraction (5% of starting volume) was discarded. The supernatant was incubated at 25 °C for 0–6 days without agitation. For preparing amyloid fibrils, aliquoted Aβ was solubilized as described above (12), diluted into PBS (25 μm), and mixed with pre-existing fibrils (10–20 wt % seed) without mixing for 24 h at 25 °C.
Thioflavin T (ThT)2 Assay
Aβ (25 μm) was diluted with ThT (44 μm) at a ratio of 1:19 by volume. The fluorescence was measured in 384-well microtiter plates (Microfluor 1, Thermo Fisher Scientific) using a Tecan Safire2 plate reader (450/482 nm excitation/emission, 15 nm bandwidth). Seeding experiments were conducted using Aβ monomers (25 μm) and various aggregated Aβ conformers (20 wt % seed) without agitation.
AFM
Aβ samples (25 μm) were spotted on cut mica mounted on glass slides. The samples were adsorbed (30 min), and then washed with water and dried overnight. Images were taken using an Asylum Research MFP three-dimensional AFM system with Olympus AC240TS cantilevers.
Cell Toxicity Assay
The procedure for assaying the toxicity of Aβ conformers is reported elsewhere (12). Briefly, rat adrenal medulla cells (PC12, ATCC) were cultured in Dulbecco's modified Eagle's medium (5% fetal bovine serum, 10% horse serum, and 1% penicillin-streptomycin). The cell suspension was aliquoted (90 μl) into 96-well microtiter plates (CellBIND, Corning) and allowed to adhere for 24 h. Afterward, Aβ or control samples (10 μl) were added to microtiter plates, and the cells were further incubated for 48 h at 37 °C. The medium was then removed, and fresh medium (200 μl) and thiazolyl blue tetrazolium bromide (Sigma; 50 μl of 2.5 mg/ml) were added to each well for 3 h at 37 °C. Finally, these solutions were discarded, 250 μl of DMSO was added, and the absorbance was measured at 562 nm. The toxicity values were normalized to the PBS control without Aβ.
Gel Electrophoresis and Silver Staining
Aβ samples (25 μm) were diluted into sample buffer (Novex LDS, Invitrogen), sonicated, analyzed using 10% BisTris gels (Invitrogen), and silver stained (SilverXpress kit, Invitrogen).
Antibody Dot Blot Analysis
Dot blot analysis of Aβ conformers (25 μm) was conducted as reported elsewhere (12). Briefly, each Aβ sample was spotted (1 μl) on nitrocellulose membranes (Hybond ECL, GE Healthcare). After the blots were dried, they were blocked overnight at 4 °C (10% nonfat dry milk in PBST) and washed in PBST. The blots were then incubated with A11 (Invitrogen), OC or 6E10 (Millipore) antibodies. The blots were washed and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody. After washing, the blots were exposed to substrate (ECL Western blotting Substrate, Thermo Fisher) and developed.
Circular Dichroism Spectroscopy
The secondary structures of Aβ conformers (25 μm in water; 300 μl) were evaluated using a Jasco 815 Spectrometer (1 mm path length cuvette) at 25 °C. Each sample spectra is the average of at least 10 readings.
ANS Fluorescence Analysis
8-Anilino-1-naphthalene sulfonate (ANS, Sigma) was used at 7.5–10 μm to assay the conformation of Aβ (2.5 μm). The ANS fluorescence spectra (λex = 380 nm) was measured using a Tecan Safire2 plate reader.
RESULTS
Aromatic Small Molecules Rapidly Remodel Mature Aβ Soluble Prefibrillar Oligomers
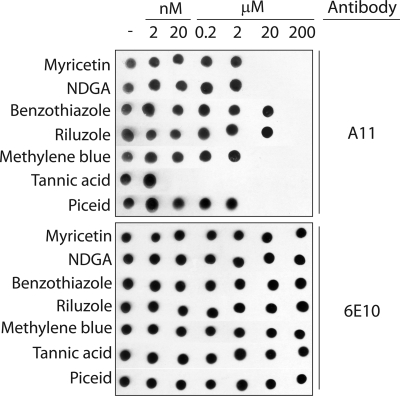
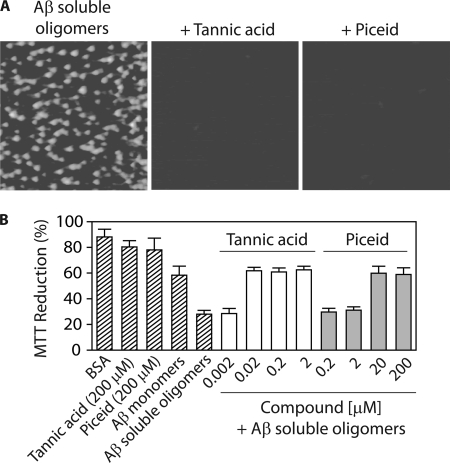
The goal of this work is to elucidate pathways employed by aromatic small molecules to remodel Aβ42 toxic soluble oligomers into alternative conformers with low toxicity. To accomplish this, we utilized in vitro assembly conditions that we have reported elsewhere (12) to form homogeneous preparations of Aβ soluble oligomers (25 μm). Such soluble oligomers possess a combination of biochemical properties and toxicities distinct from freshly disaggregated and fibrillar Aβ conformers, including immunoreactivity with the conformation-specific antibody A11 that selectively recognizes soluble prefibrillar oligomers (32). We first employed this antibody to identify small molecules that rapidly eliminate the A11 epitope of mature soluble oligomers. We excluded small molecules that were inactive during a short time interval (4 h) relative to the time required for soluble oligomers to convert into alternative structures (2 days; data not shown). Based on our preliminary studies and previous work (38–41), we identified seven aromatic molecules that rapidly remodel Aβ prefibrillar oligomers (Fig. 1; structures of small molecules are shown in supplemental Fig. S1). Four of these molecules are polyphenols or derivatives thereof (myricetin, tannic acid, piceid, and nordihydroguaiaretic acid or NDGA), one is a phenothiazine (methylene blue), and two are benzothiazoles (riluzole and benzothiazole). All small molecules except benzothiazole and riluzole neutralize the oligomer-specific conformational epitope at substoichiometric concentrations, with tannic acid being the most effective (Fig. 1).
FIGURE 1.
Analysis of the remodeling activity of aromatic small molecules for Aβ soluble oligomers. Aβ soluble oligomers (25 μm) were assembled for 1 day (without agitation), and then incubated with and without compounds for 4 h (25 °C). Each sample was spotted on nitrocellulose, and probed with the conformation-specific antibody A11 (specific for soluble prefibrillar oligomers) and the sequence-specific antibody 6E10 (specific for the N terminus of Aβ).
Small Molecule Antagonists Utilize Unique Remodeling Pathways
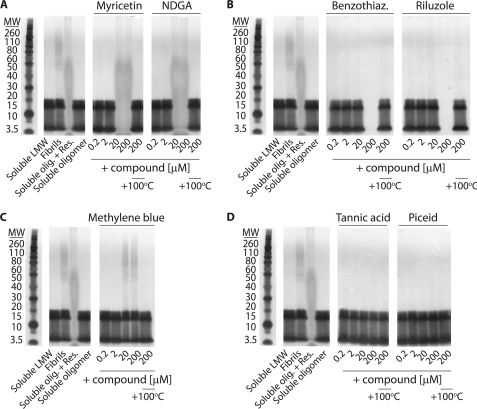
To elucidate whether these molecular antagonists employ common or unique remodeling mechanisms to neutralize toxic Aβ soluble oligomers, we first performed SDS-PAGE analysis of Aβ soluble oligomers dosed with either the vehicle (1% DMSO) or a range of concentrations of each antagonist (Fig. 2). We have included four Aβ conformational controls for comparison. Two Aβ controls, soluble oligomers and freshly disaggregated Aβ peptide that we herein refer to as soluble low molecular weight species, migrate on an SDS-PAGE gel as monomers, trimers, and tetramers. The other two Aβ conformational controls, fibrils and resveratrol-remodeled soluble oligomers, display different amounts of high molecular weight aggregate. We previously found that resveratrol-remodeled soluble oligomers are large off-pathway aggregates that are non-toxic (12).
FIGURE 2.
Characterization of SDS resistance of remodeled Aβ soluble oligomers. Aβ soluble oligomers (25 μm; formed after 1–2 days without agitation) were dosed with (A) myricetin and NDGA, (B) benzothiazole and riluzole, (C) methylene blue, and (D) tannic acid and piceid, and analyzed using SDS-PAGE. The Aβ conformational controls are freshly disaggregated Aβ (soluble low molecular weight (LMW)), fibrils, soluble oligomers remodeled by resveratrol (20 μm, 4 h), and soluble oligomers.
We find that myricetin and NDGA remodel Aβ soluble oligomers into large, SDS-resistant aggregates that are indistinguishable from resveratrol-remodeled soluble oligomers at concentrations ≥20 μm (Fig. 2A). We confirmed that these aggregates are non-covalently associated by dissociating them with heat (Fig. 2A). Benzothiazole and riluzole (200 μm) also convert Aβ soluble oligomers into large SDS-resistant aggregates similar to resveratrol-remodeled soluble oligomers (Fig. 2B). In contrast, methylene blue (≥20 μm) remodels soluble oligomers into SDS-resistant aggregates that are indistinguishable from Aβ fibrils (Fig. 2C). For each aromatic molecule in Fig. 2, A–C, the concentration required to generate SDS-resistant aggregates (Fig. 2, A–C) is identical to that required for eliminating the A11 epitope (Fig. 1). Finally, tannic acid and piceid do not convert Aβ soluble oligomers into SDS-resistant aggregates (Fig. 2D). Based on these preliminary findings, herein we refer to these molecules as Class I (myricetin, NDGA, benzothiazole, and riluzole), II (methylene blue), and III (tannic acid and piceid) antagonists, and we investigate the pathways they employ to eradicate mature Aβ soluble oligomers.
Class I Molecules Convert Aβ Soluble Oligomers into Large, Non-toxic Conformers
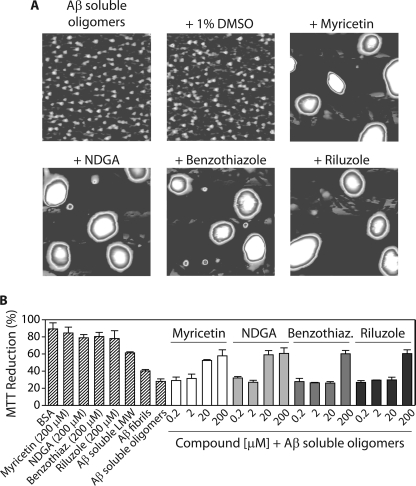
Our SDS-PAGE analysis in Fig. 2 revealed that Class I molecules utilize a pathway in which soluble oligomers are converted into large, SDS-resistant aggregates that appear similar to resveratrol-remodeled soluble oligomers (12). Because SDS may alter the size of the remodeled Aβ soluble oligomers, we first performed AFM imaging analysis of Aβ soluble oligomers before and after addition of Class I molecules to evaluate if Aβ oligomers are remodeled into large aggregates (Fig. 3A). Indeed, we find that all four Class I molecules convert Aβ soluble oligomers into large aggregates of similar morphology, whereas the vehicle does not. Moreover, circular dichroism analysis revealed that each Class I molecule remodels soluble oligomers into unstructured conformers that are similar to the secondary structures of soluble oligomers prior to remodeling and significantly different from β-sheet-rich fibrils (supplemental Fig. S2). Finally, soluble oligomers display negligible ThT fluorescence before and after being remodeled by Class I molecules (supplemental Fig. S2). Collectively, our findings reveal that Class I molecules convert soluble oligomers into off-pathway aggregates that are unstructured.
FIGURE 3.
Analysis of the pathway utilized by Class I molecules to remodel Aβ soluble oligomers. Aβ soluble oligomers (25 μm; formed after 1 day without agitation) were incubated with each small molecule (4 h) and analyzed using (A) AFM imaging, and (B) cell toxicity analysis (n = 3). In A, mature soluble oligomers were incubated with the vehicle (1% DMSO) or 200 μm compound, and each image is 3 × 3 μm.
We posited that the remodeled soluble oligomers would possess low toxicity given their large size and lack of the A11 epitope. Indeed, relative to freshly disaggregated Aβ (supplemental Fig. S3), we find that all four Class I molecules eliminate the toxicity of soluble oligomers in a dose-dependent manner (Fig. 3B) that is in exact concordance with loss of the A11 epitope (Fig. 1) and gain of SDS-resistant aggregates (Fig. 2, A and B). Thus, Class I molecules convert toxic soluble oligomers into large aggregates that are non-toxic.
The similar morphologies, biochemical properties, and toxicities of the remodeled soluble oligomers suggests that Class I molecules utilize a similar remodeling pathway. However, it is possible that Class I molecules remodel soluble oligomers at different rates (or generate unique intermediates) during the remodeling process. Thus, we sought additional insights into the kinetics of loss of the A11 epitope and whether β-sheet-rich intermediates are transiently populated during the remodeling process. We performed kinetic dot blot analysis using conformation-specific antibodies specific for prefibrillar oligomers (A11) and fibrillar conformers (OC; supplemental Fig. S2). We find that the four aromatic molecules eliminate the A11 epitope after 3–4 h without generating OC-positive conformers for at least 24 h (longer times not evaluated). The similarity in the kinetics of remodeling and the absence of OC-positive intermediates further suggests that Class I molecules employ a similar remodeling pathway to convert soluble oligomers into unstructured, off-pathway aggregates.
Despite the similar remodeling activity of Class I molecules, polyphenols (myricetin and NDGA) and benzothiazoles (riluzole and benzothiazole) differ significantly in structure and display unique dose dependence for remodeling soluble oligomers (Figs. 1 and 2). Because benzothiazoles are more hydrophobic than polyphenols, we suspected the former molecules utilize hydrophobic interactions to antagonize toxic soluble oligomers that could be attenuated by addition of surfactant. Thus, we evaluated the remodeling activity of Class I molecules in the presence of 0.1% Triton X-100 using A11 dot blot analysis (supplemental Fig. S2). Indeed, we find that both benzothiazoles are inactive in the presence of surfactant, whereas the remodeling activity of polyphenols is unchanged. Moreover, we confirmed that both benzothiazoles did not form colloidal aggregates that sequester polypeptides in a nonspecific manner (supplemental Fig. S2) (42). Thus, polyphenols (herein referred to as Class IA molecules) and benzothiazoles (herein referred to as Class IB molecules) employ similar remodeling pathways, yet they utilize unique interactions to convert soluble oligomers into high molecular weight aggregates.
A Class II Molecule Converts Prefibrillar Aβ Oligomers into Fibrils
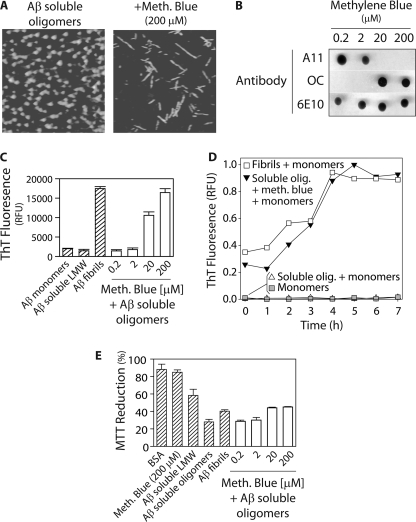
The SDS-PAGE analysis in Fig. 2C suggests that the Class II molecule methylene blue converts soluble oligomers into fibrils in a rapid manner. Indeed, we confirmed such a conformational transition using AFM, and found that Aβ soluble oligomers convert into clumped fibrils (supplemental Fig. S4) that can be readily dissociated with surfactant (2% SDS; Fig. 4A). We also performed dot blot analysis to probe the conformers that form after methylene blue remodeling (Fig. 4B). Strikingly, concentrations of methylene blue sufficient to eliminate the A11 epitope (≥20 μm) correspond to those that generate OC-positive conformers (Fig. 4B). These dose dependent findings are in precise accordance with the concentration dependence of methylene blue-mediated conversion of soluble oligomers into SDS-resistant aggregates (Fig. 2C). Moreover, we find that the A11 epitope is eliminated (and the OC epitope is generated) after 2 h (supplemental Fig. S4). These results strongly suggest that methylene blue rapidly remodels Aβ soluble oligomers into fibrils.
FIGURE 4.
Analysis of the pathway employed by the Class II molecule methylene blue to remodel Aβ soluble oligomers. Aβ soluble oligomers (25 μm; formed after 1 day without agitation) were incubated with methylene blue (4 h), and analyzed using (A) AFM, (B) dot blots probed with conformation-specific (A11, soluble oligomer and OC, fibrillar conformer) and sequence-specific (6E10) antibodies, (C) ThT fluorescence (n = 5), (D) templating activity (20% seed), and (E) cell toxicity analysis (n = 3). The soluble oligomers remodeled with methylene blue in A were incubated with 2% SDS to dissociate fibril clumps.
Nevertheless, we further characterized the biochemical properties of these remodeled oligomers. We first evaluated the ThT fluorescence of the methylene blue-remodeled oligomers, and found that the same concentrations of methylene blue that promote OC immunoreactivity also promote significant ThT fluorescence (Fig. 4C). Moreover, the highest concentration of methylene blue (200 μm) generates Aβ conformers that display ThT fluorescence indistinguishable to fibrils (Fig. 4C). Moreover, we find that the remodeled structures possess β-sheet secondary structures similar to fibrils (supplemental Fig. S4).
We also evaluated the templating activity of methylene blue-remodeled soluble oligomers because Aβ fibrils efficiently seed Aβ monomers into ThT-positive conformers, whereas many other fibrillar intermediates do not (7, 12, 21). Importantly, we find that methylene blue-remodeled oligomers template Aβ monomers in a manner that is similar to mature fibrils (Fig. 4D). In contrast, soluble oligomers (which were not treated with methylene blue) fail to seed Aβ monomers (Fig. 4D).
These findings provide strong evidence that methylene blue converts soluble oligomers into fibrils. Because fibrils are more toxic than Aβ monomers and off-pathway aggregates but less toxic than soluble oligomers (14, 15, 43), we assayed the toxicity of the remodeled soluble oligomers (Fig. 4E). We find that methylene blue concentrations ≥20 μm convert Aβ soluble oligomers into conformers that possess toxicities indistinguishable from mature Aβ fibrils and significantly lower than soluble oligomers. In summary, the Class II molecule methylene blue rapidly remodels Aβ toxic soluble oligomers into β-sheet-rich fibrils.
Class III Molecules Disaggregate Aβ Soluble Oligomers
Unlike Class I and II molecules that promote high molecular weight aggregation, the SDS-PAGE analysis in Fig. 2D suggests that Class III molecules tannic acid and piceid remodel soluble oligomers into either SDS-soluble aggregates or low molecular weight species. To differentiate between these possibilities, we used AFM analysis (Fig. 5A). Strikingly, we find that both small molecules disaggregate Aβ soluble oligomers. Moreover, we find that the remodeled oligomers are largely unstructured and ThT-negative (supplemental Fig. S5). These remodeled oligomers also possess toxicities indistinguishable to freshly disaggregated Aβ, and both Class III molecules eliminate the toxicity of soluble oligomers in a dose-dependent manner (Fig. 5B) identical to that observed for eliminating the A11 epitope (Fig. 1). Finally, the kinetics of elimination of the A11 epitope are similar for both molecules (A11 epitope is lost after 3–4 h; supplemental Fig. S5), and this remodeling activity is insensitive to addition of non-ionic surfactant (supplemental Fig. S5). Collectively, these results strongly argue that Class III molecules remodel soluble oligomers into low molecular weight species that possess a combination of biochemical properties and toxicities characteristic of disaggregated Aβ.
FIGURE 5.
Analysis of the pathway utilized by Class III molecules to disaggregate Aβ soluble oligomers. Aβ soluble oligomers (25 μm; formed after 1 day without agitation) were incubated with each small molecule (4 h) and analyzed using (A) AFM imaging, and (B) cell toxicity analysis (n = 3). In A, mature soluble oligomers were incubated with 2 μm tannic acid or 200 μm piceid, and each image is 3 × 3 μm.
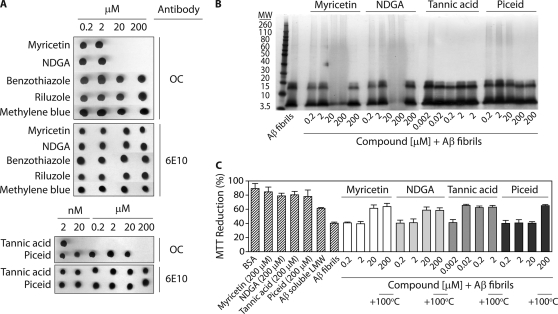
Class I, II, and III Molecules Display Unique Remodeling Activities for Fibrillar and Disaggregated Aβ
Our findings that aromatic small molecules utilize three unique remodeling pathways led us to investigate if they employ similar pathways to remodel other Aβ conformers or if these pathways are specific to Aβ soluble oligomers. Thus, we first evaluated whether each small molecule could rapidly remodel mature fibrils (Fig. 6). We find that Class IA (myricetin and NDGA) and III (tannic acid and piceid) molecules eliminate the OC epitope specific for fibrillar conformers, whereas Class IB (benzothiazole and riluzole) and II (methylene blue) molecules do not (Fig. 6A). Kinetic analysis of the fibril remodeling process revealed that each active molecule required 3–4 h to eliminate the OC epitope, and A11-positive intermediates were not generated (supplemental Fig. S6). In each case, the dose-dependent loss of the OC epitope is mirrored precisely by the dose-dependent loss of ThT fluorescence (supplemental Fig. S6). In contrast, the Class IB and III molecules that fail to eliminate the OC epitope also fail to eliminate the ThT fluorescence of Aβ fibrils (supplemental Fig. S6).
FIGURE 6.
Impact of Class I, II, and III molecules on mature Aβ fibrils. Aβ fibrils (25 μm; formed via seeding for 1 day without agitation) were incubated with each small molecule (4 h) and analyzed using (A) dot blots probed with conformation-specific (OC, fibrillar conformer) and sequence-specific (6E10) antibodies, (B) SDS-PAGE, and (C) cell toxicity analysis (n = 3).
Given that soluble oligomers and fibrils possess significantly different biochemical properties, we posited that Class IA and III molecules would utilize different pathways to remodel fibrils relative to the pathways they employ to remodel soluble oligomers. To test this hypothesis, we first performed SDS-PAGE analysis of fibrils before and after incubation with aromatic small molecules (Fig. 6B). We find that Class IA molecules convert fibrils into SDS-resistant aggregates, whereas Class III molecules disassemble fibrils into species that are indistinguishable from freshly disaggregated Aβ. We confirmed these findings using AFM (supplemental Fig. S6). Class IA molecules remodel fibrils into large aggregates (supplemental Fig. S6) that are indistinguishable from soluble oligomers after being remodeled by the same molecules (Fig. 3A). In contrast, Class III molecules disaggregate fibrils into low molecular weight species not detectable by AFM (supplemental Fig. S6), as observed for soluble oligomers disaggregated by the same molecules (Fig. 5A). Finally, we confirmed that each active small molecule eliminated the toxicity of Aβ fibrils in a dose-dependent manner identical to that observed for elimination of the OC- and ThT-epitopes (Fig. 6C). In contrast, the inactive molecules did not prevent fibril-mediated toxicity (supplemental Fig. S6). Taken collectively, our findings strongly argue that Class IA and III molecules remodel Aβ fibrils through the same pathways they employ to remodel soluble oligomers, and Class IB and II molecules are selective for remodeling Aβ soluble oligomers.
Several previous reports suggest that aromatic small molecules can alter the aggregation pathway of freshly disaggregated Aβ, yet many such experiments were conducted over long times or at high concentrations where Aβ matures structurally even in the absence of small molecules (30, 38, 44, 45). Thus, we evaluated if the small molecules investigated in this work would rapidly accelerate aggregation of freshly disaggregated Aβ over a time interval (4 h) in which Aβ otherwise fails to aggregate when not agitated (12). Strikingly, we find that Class IB molecules (benzothiazole and riluzole) rapidly convert soluble Aβ into large aggregates that are similar to soluble oligomers remodeled with the same molecules (Fig. 3), whereas the other aromatic small molecules were inactive (supplemental Fig. S7). Moreover, we find that Class IB molecules do not accelerate soluble Aβ aggregation in the presence of non-ionic surfactant (supplemental Fig. S7). Thus, Class IB molecules remodel freshly disaggregated and oligomeric Aβ through a common mechanism that is distinct from the other aromatic molecules investigated in this work.
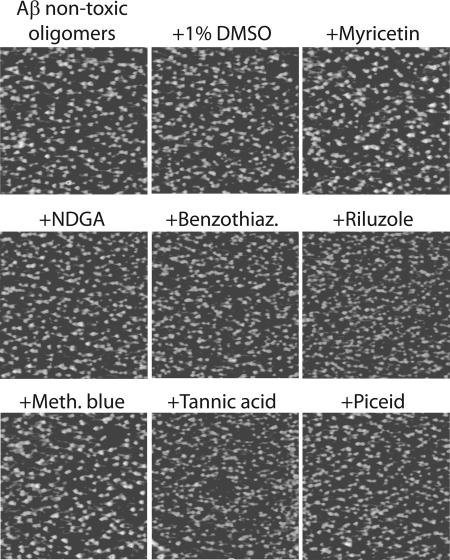
Aβ Non-toxic Oligomers Are More Resistant to Remodeling Than Soluble Oligomers or Fibrils
We recently identified an oligomeric form of Aβ that is morphologically indistinguishable from Aβ soluble oligomers, yet these oligomers are non-toxic and possess a collection of biochemical properties not found in other Aβ conformers (12). Interestingly, these non-toxic oligomers are negative for ThT and multiple conformation-specific antibodies (A11 and OC), yet are SDS-resistant. Thus, we employed AFM and SDS-PAGE analysis to evaluate the impact of each small molecule antagonist on these conformers (Fig. 7 and supplemental Fig. S8). Strikingly, we find that all aromatic small molecules were inactive against these oligomers, including those that are active against Aβ fibrils. Nevertheless, over longer time intervals (8–24 h), we find that Class I molecules remodel non-toxic oligomers into large SDS-resistant aggregates, whereas Class II molecules are inactive and Class III molecules disaggregate non-toxic oligomers into low molecular weight species (supplemental Fig. S8). Thus, non-toxic oligomers are more resistant to being remodeled by diverse aromatic small molecules, which confirms that they possess structural features absent in other Aβ conformers.
FIGURE 7.
Impact of Class I, II, and III molecules on mature Aβ non-toxic oligomers. Aβ non-toxic oligomers (25 μm; formed after 5 days without agitation) were incubated with each small molecule (200 μm except for 2 μm tannic acid; 4 h) and analyzed using AFM imaging (each image is 3 × 3 μm).
DISCUSSION
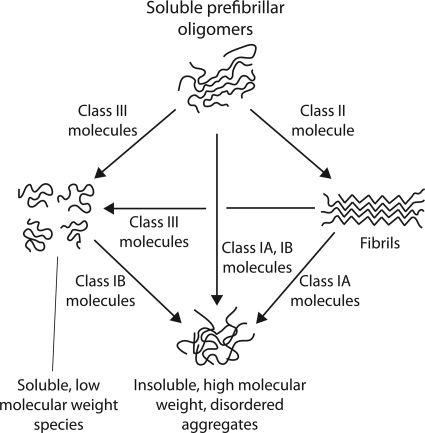
Our findings illuminate multiple pathways employed by small molecules to remodel soluble oligomers of Aβ42 (Fig. 8). Indeed, even in the absence of small molecule antagonists, we have reported previously that Aβ soluble oligomers are not committed to a single nucleation pathway (12). Instead, soluble oligomers can mature into multiple Aβ conformers (fibrillar intermediates and non-toxic oligomers) that possess highly dissimilar biochemical properties, and each conformer can be formed in a specific manner via simple changes in agitation. This multifaceted capacity of Aβ soluble oligomers to mature into different isoforms is consistent with our findings that small molecules can remodel soluble oligomers into conformers with significantly different sizes and structures. That only four of the small molecules investigated in this work remodel mature Aβ fibrils (whereas all seven remodel soluble oligomers) highlights the conformational plasticity of soluble oligomers to evolve into diverse conformers.
FIGURE 8.
Summary of pathways utilized by aromatic small molecules to remodel Aβ soluble oligomers. Class I molecules remodel Aβ soluble oligomers (ThT- and OC-negative, unstructured as judged by circular dichroism, highly toxic, SDS-soluble) into large aggregates that are SDS-resistant, negative for multiple conformational probes (OC and A11 antibodies, and ThT), unstructured, incompetent for templating Aβ monomers, and non-toxic relative to freshly disaggregated Aβ. In contrast, a Class II molecule selectively converts soluble oligomers into fibrils (which are OC- and ThT-positive, β-sheet rich, SDS-insoluble, A11-negative and mildly toxic). Class III molecules convert soluble oligomers into low molecular species (which are OC-, A11-, and ThT-negative, unstructured, disaggregated as judged by AFM, SDS-soluble and non-toxic relative to freshly disaggregated Aβ). Class IA molecules also convert mature fibrils into large off-pathway structures, and these molecules are inactive against freshly disaggregated Aβ. Class IB molecules convert Aβ monomers into large off-pathway aggregates, and are inactive against fibrils. Class II molecules are inactive against Aβ monomers and fibrils. Class III molecules remodel fibrils into low molecular weight species, and they are inactive against freshly disaggregated Aβ.
Our results for Class I molecules confirm that converting soluble oligomers into large, unstructured aggregates is a common pathway for remodeling toxic oligomers. We reason that it is simpler to promote nonspecific intermolecular interactions between Aβ peptides within soluble oligomers than it is to promote specific ones necessary for fibril formation or to prevent all possible intermolecular interactions. Studies of small molecule inhibitors of protein aggregation also support our findings because many such antagonists promote alternative, unstructured aggregates rather than preventing condensation of protein monomers (30, 44, 45).
Nevertheless, we found that a Class II molecule (methylene blue) selectively converts soluble oligomers into fibrils, which is consistent with previous work (38). Importantly, methylene blue fails to remodel amyloid fibrils within 24 h or accelerate aggregation of freshly disaggregated Aβ within 4 h (longer times not tested). Thus, the primary activity of methylene blue is to accelerate the conversion of Aβ oligomers into fibrillar conformers. Because a key structural difference between Aβ soluble oligomers and fibrils is the presence of β-sheets, it is possible that Class II molecules facilitate mature self-stacking interactions between residues necessary for β-sheet formation. Alternatively, Class II molecules may accelerate conversion of soluble oligomers into fibrils by inhibiting nonspecific interactions sampled within Aβ soluble oligomers that govern assembly of non-toxic oligomers.
The conformational specificity of methylene blue to remodel Aβ soluble oligomers into fibrils makes it and related variants potential drug candidates to evaluate the hypothesis that Aβ soluble oligomers are the primary cellular insult in Alzheimer disease. The therapeutic activity of many small molecules for remodeling toxic Aβ oligomers is limited because they do not cross the blood-brain barrier. In contrast, methylene blue crosses the blood-brain barrier, and possesses many other desirable drug properties, including high solubility and low toxicity (46–48). Indeed, methylene blue and derivatives of it are currently being evaluated as therapeutic candidates to treat Alzheimer disease (49).
Given that many small molecule antagonists fail to disaggregate mature Aβ soluble oligomers and fibrils into low molecular weight species (including the Class I and II molecules studied here), the activity of Class III molecules is intriguing. Tannic acid was the most active small molecule we studied (which is consistent with previous work (41, 50, 51)), and it appears that its large size enciphers this activity. However, the remarkable disaggregation activity of piceid is more difficult to explain. Piceid was found previously to partially disaggregate oligomers and amyloids of a more soluble Aβ peptide fragment (residues 25–35; neither Aβ conformer was confirmed with A11 or OC antibodies) (40). However, its disaggregation activity for A11-positive soluble oligomers and OC-positive fibrils of Aβ42 was unexpected not only because the full-length peptide is significantly more aggregation-prone, but also because piceid is closely related to another polyphenol (resveratrol) that converts Aβ oligomers and fibrils into large off-pathway aggregates (12). Because piceid is identical to resveratrol except for the 3-O-β-glucosidic moiety, it appears that the sugar moiety is an important determinant of its disaggregation activity. Future structure-activity analysis is necessary to resolve whether the stilbenoid backbone is important for the disaggregation activity of piceid and whether other phenolic glycosides possess similar disaggregation activity.
Are there additional pathways by which aromatic small molecules remodel toxic soluble oligomers into non-toxic assemblies? Indeed, we expect that several other pathways are possible. For example, soluble oligomers could be converted into various types of fibrillar conformers other than fibrils or precipitated into large aggregates without structural changes (thereby reducing toxicity due to increased size). Moreover, some aromatic small molecules modify polypeptides covalently (52, 53) and are likely to remodel mature soluble oligomers through unique pathways not considered here.
A puzzling finding of our work is that Class IA polyphenols selectivity remodel Aβ soluble oligomers and fibrils into indistinguishable, unstructured aggregates with identical dose dependence. A simple explanation would be that soluble oligomers and fibrils possess common structural features recognized by Class IA molecules. However, we were unable to demonstrate a single biochemical property shared by these two conformers. We find that Aβ soluble oligomers are A11-positive, OC-negative, ThT-negative, SDS-soluble, highly toxic and largely unstructured, whereas fibrils possess the “opposite” properties (i.e. they are A11-negative, OC-positive, ThT-positive, SDS-insoluble, mildly toxic and β-sheet rich). Because polyphenols are suspected to antagonize π-stacking interactions between aromatic side chains in amyloids (54), it may be that such side chains stack in soluble oligomers (despite that they lack mature β-sheets detectable by circular dichroism) as they do in Aβ fibrillar oligomers and fibrils (21, 55). Such stacking interactions between aromatic side chains of Aβ may render soluble oligomers and fibrils sensitive to being remodeled by polyphenols. It is also possible that Class IA molecules recognize other structural motifs that may be common to soluble oligomers and fibrils, such as sheet-like structures in both prefibrillar oligomers (α-pleated (56), anti-parallel (57), or disordered (58, 59) sheets) and fibrils (parallel (55) or anti-parallel (60) β-sheets), although additional analysis is needed to test these speculative hypotheses.
A striking finding of our work is that non-toxic oligomers are more resistant to being remodeled by diverse aromatic small molecules than all other Aβ conformers, including highly stable Aβ fibrils. A possible explanation is that non-toxic oligomers lack aromatic stacking interactions, which allows them to more effectively evade the remodeling activity of the small molecules studied in this work. This hypothesis would also predict that substituting aromatic amino acids for non-aromatic ones would desensitize soluble oligomers to the remodeling activity of polyphenols. However, it is also possible that Aβ non-toxic oligomers are simply more stable than other Aβ conformers (61), which would also explain their increased resistance to being remodeled by diverse aromatic molecules.
It is notable that two oligomeric forms of a non-disease associated protein (HypF) have been reported to also possess unique toxicities (11). Similar to Aβ soluble and non-toxic oligomers reported here, the N-terminal domain of HypF (denoted HypF-N) forms two oligomeric forms, one of which is toxic. Chiti and co-workers (11) demonstrate that the toxic conformer is less structured, which is generally consistent with our findings that soluble oligomers are less stable than non-toxic oligomers (as judged by SDS resistance). However, both HypF-N oligomers bind ThT and possess extensive β-sheets, confirming that they are fibrillar intermediates rather than prefibrillar oligomers. In contrast, we find that Aβ soluble and non-toxic oligomers possess random coil structures (as judged by circular dichroism) and, thus, the relevance of these and related (62) observations remains to be evaluated for Aβ soluble and non-toxic oligomers. We expect that future site-specific structural studies of Aβ toxic and non-toxic oligomers will illuminate the structural differences between these oligomers that confer their unique toxicities and susceptibilities to being remodeling by aromatic small molecules.
Supplementary Material
Acknowledgments
We greatly appreciate the gift of the OC antibody from Dr. Charles Glabe (University of California, Irvine), and assistance with the figures from Dr. Juan Mauricio Mora Pale.
This work was supported by the Alzheimer's Association Grant NIRG-08-90967 (to P. M. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- ThT
- thioflavin
- NDGA
- nordihydroguaiaretic acid
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- ANS
- 8-anilino-1-naphthalene sulfonate.
REFERENCES
- 1. Anfinsen C. B. (1973) Science 181, 223–230 [DOI] [PubMed] [Google Scholar]
- 2. Frost B., Ollesch J., Wille H., Diamond M. I. (2009) J. Biol. Chem. 284, 3546–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chien P., Weissman J. S. (2001) Nature 410, 223–227 [DOI] [PubMed] [Google Scholar]
- 4. Chien P., DePace A. H., Collins S. R., Weissman J. S. (2003) Nature 424, 948–951 [DOI] [PubMed] [Google Scholar]
- 5. Tanaka M., Chien P., Naber N., Cooke R., Weissman J. S. (2004) Nature 428, 323–328 [DOI] [PubMed] [Google Scholar]
- 6. Tessier P. M., Lindquist S. (2007) Nature 447, 556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldsbury C., Frey P., Olivieri V., Aebi U., Müller S. A. (2005) J. Mol. Biol. 352, 282–298 [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi K., Takahashi S., Kawai T., Naiki H., Goto Y. (2005) J. Mol. Biol. 352, 952–960 [DOI] [PubMed] [Google Scholar]
- 9. Petkova A. T., Leapman R. D., Guo Z., Yau W. M., Mattson M. P., Tycko R. (2005) Science 307, 262–265 [DOI] [PubMed] [Google Scholar]
- 10. Paravastu A. K., Leapman R. D., Yau W. M., Tycko R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18349–18354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campioni S., Mannini B., Zampagni M., Pensalfini A., Parrini C., Evangelisti E., Relini A., Stefani M., Dobson C. M., Cecchi C., Chiti F. (2010) Nat. Chem. Biol. 6, 140–147 [DOI] [PubMed] [Google Scholar]
- 12. Ladiwala A. R., Lin J. C., Bale S. S., Marcelino-Cruz A. M., Bhattacharya M., Dordick J. S., Tessier P. M. (2010) J. Biol. Chem. 285, 24228–24237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ono K., Condron M. M., Teplow D. B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14745–14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glabe C. G. (2008) J. Biol. Chem. 283, 29639–29643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiti F., Dobson C. M. (2006) Annu. Rev. Biochem. 75, 333–366 [DOI] [PubMed] [Google Scholar]
- 16. Cleary J. P., Walsh D. M., Hofmeister J. J., Shankar G. M., Kuskowski M. A., Selkoe D. J., Ashe K. H. (2005) Nat. Neurosci. 8, 79–84 [DOI] [PubMed] [Google Scholar]
- 17. Silveira J. R., Raymond G. J., Hughson A. G., Race R. E., Sim V. L., Hayes S. F., Caughey B. (2005) Nature 437, 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmed M., Davis J., Aucoin D., Sato T., Ahuja S., Aimoto S., Elliott J. I., Van Nostrand W. E., Smith S. O. (2010) Nat. Struct. Mol. Biol. 17, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu L., Edalji R., Harlan J. E., Holzman T. F., Lopez A. P., Labkovsky B., Hillen H., Barghorn S., Ebert U., Richardson P. L., Miesbauer L., Solomon L., Bartley D., Walter K., Johnson R. W., Hajduk P. J., Olejniczak E. T. (2009) Biochemistry 48, 1870–1877 [DOI] [PubMed] [Google Scholar]
- 20. Chimon S., Shaibat M. A., Jones C. R., Calero D. C., Aizezi B., Ishii Y. (2007) Nat. Struct. Mol. Biol. 14, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 21. Wu J. W., Breydo L., Isas J. M., Lee J., Kuznetsov Y. G., Langen R., Glabe C. (2010) J. Biol. Chem. 285, 6071–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang A., Qi W., Good T. A., Fernandez E. J. (2009) Biophys. J. 96, 1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bulic B., Pickhardt M., Schmidt B., Mandelkow E. M., Waldmann H., Mandelkow E. (2009) Angew. Chem. Int. Ed. Engl. 48, 1740–1752 [DOI] [PubMed] [Google Scholar]
- 24. Amijee H., Scopes D. I. (2009) J. Alzheimers Dis. 17, 33–47 [DOI] [PubMed] [Google Scholar]
- 25. Stains C. I., Mondal K., Ghosh I. (2007) Chem. Med. Chem. 2, 1674–1692 [DOI] [PubMed] [Google Scholar]
- 26. Kodali R., Wetzel R. (2007) Curr. Opin. Struct. Biol. 17, 48–57 [DOI] [PubMed] [Google Scholar]
- 27. Roberts B. E., Shorter J. (2008) Nat. Struct. Mol. Biol. 15, 544–546 [DOI] [PubMed] [Google Scholar]
- 28. Wells J. A., McClendon C. L. (2007) Nature 450, 1001–1009 [DOI] [PubMed] [Google Scholar]
- 29. Roberts B. E., Duennwald M. L., Wang H., Chung C., Lopreiato N. P., Sweeny E. A., Knight M. N., Shorter J. (2009) Nat. Chem. Biol. 5, 936–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H., Duennwald M. L., Roberts B. E., Rozeboom L. M., Zhang Y. L., Steele A. D., Krishnan R., Su L. J., Griffin D., Mukhopadhyay S., Hennessy E. J., Weigele P., Blanchard B. J., King J., Deniz A. A., Buchwald S. L., Ingram V. M., Lindquist S., Shorter J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7159–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bieschke J., Russ J., Friedrich R. P., Ehrnhoefer D. E., Wobst H., Neugebauer K., Wanker E. E. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 7710–7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003) Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 33. Lambert M. P., Viola K. L., Chromy B. A., Chang L., Morgan T. E., Yu J., Venton D. L., Krafft G. A., Finch C. E., Klein W. L. (2001) J. Neurochem. 79, 595–605 [DOI] [PubMed] [Google Scholar]
- 34. Zameer A., Kasturirangan S., Emadi S., Nimmagadda S. V., Sierks M. R. (2008) J. Mol. Biol. 384, 917–928 [DOI] [PubMed] [Google Scholar]
- 35. Kayed R., Pensalfini A., Margol L., Sokolov Y., Sarsoza F., Head E., Hall J., Glabe C. (2009) J. Biol. Chem. 284, 4230–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Nuallain B., Wetzel R. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1485–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kvam E., Nannenga B. L., Wang M. S., Jia Z., Sierks M. R., Messer A. (2009) PLoS One 4, e5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Necula M., Breydo L., Milton S., Kayed R., van der Veer W. E., Tone P., Glabe C. G. (2007) Biochemistry 46, 8850–8860 [DOI] [PubMed] [Google Scholar]
- 39. Taniguchi S., Suzuki N., Masuda M., Hisanaga S., Iwatsubo T., Goedert M., Hasegawa M. (2005) J. Biol. Chem. 280, 7614–7623 [DOI] [PubMed] [Google Scholar]
- 40. Rivière C., Delaunay J. C., Immel F., Cullin C., Monti J. P. (2009) Neurochem. Res. 34, 1120–1128 [DOI] [PubMed] [Google Scholar]
- 41. Ono K., Hasegawa K., Naiki H., Yamada M. (2004) Biochim. Biophys. Acta 1690, 193–202 [DOI] [PubMed] [Google Scholar]
- 42. Feng B. Y., Toyama B. H., Wille H., Colby D. W., Collins S. R., May B. C., Prusiner S. B., Weissman J., Shoichet B. K. (2008) Nat. Chem. Biol. 4, 197–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walsh D. M., Selkoe D. J. (2007) J. Neurochem. 101, 1172–1184 [DOI] [PubMed] [Google Scholar]
- 44. Necula M., Kayed R., Milton S., Glabe C. G. (2007) J. Biol. Chem. 282, 10311–10324 [DOI] [PubMed] [Google Scholar]
- 45. Ehrnhoefer D. E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E. E. (2008) Nat. Struct. Mol. Biol. 15, 558–566 [DOI] [PubMed] [Google Scholar]
- 46. Peter C., Hongwan D., Küpfer A., Lauterburg B. H. (2000) Eur. J. Clin. Pharmacol. 56, 247–250 [DOI] [PubMed] [Google Scholar]
- 47. Küpfer A., Aeschlimann C., Wermuth B., Cerny T. (1994) Lancet 343, 763–764 [DOI] [PubMed] [Google Scholar]
- 48. DiSanto A. R., Wagner J. G. (1972) J. Pharm. Sci. 61, 1086–1090 [DOI] [PubMed] [Google Scholar]
- 49. Gura T. (2008) Nat. Med. 14, 894. [DOI] [PubMed] [Google Scholar]
- 50. Boyé-Harnasch M., Cullin C. (2006) J. Biotechnol. 125, 222–230 [DOI] [PubMed] [Google Scholar]
- 51. Kocisko D. A., Baron G. S., Rubenstein R., Chen J., Kuizon S., Caughey B. (2003) J. Virol. 77, 10288–10294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu M., Rajamani S., Kaylor J., Han S., Zhou F., Fink A. L. (2004) J. Biol. Chem. 279, 26846–26857 [DOI] [PubMed] [Google Scholar]
- 53. Meng X., Munishkina L. A., Fink A. L., Uversky V. N. (2009) Biochemistry 48, 8206–8224 [DOI] [PubMed] [Google Scholar]
- 54. Gazit E. (2002) FASEB J. 16, 77–83 [DOI] [PubMed] [Google Scholar]
- 55. Margittai M., Langen R. (2008) Q. Rev. Biophys. 41, 265–297 [DOI] [PubMed] [Google Scholar]
- 56. Armen R. S., DeMarco M. L., Alonso D. O., Daggett V. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11622–11627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cerf E., Sarroukh R., Tamamizu-Kato S., Breydo L., Derclaye S., Dufrêne Y. F., Narayanaswami V., Goormaghtigh E., Ruysschaert J. M., Raussens V. (2009) Biochem. J. 421, 415–423 [DOI] [PubMed] [Google Scholar]
- 58. Manavalan P., Johnson W. C. (1983) Nature 305, 831–832 [Google Scholar]
- 59. Gursky O., Aleshkov S. (2000) Biochim. Biophys. Acta 1476, 93–102 [DOI] [PubMed] [Google Scholar]
- 60. Tycko R., Sciarretta K. L., Orgel J. P., Meredith S. C. (2009) Biochemistry 48, 6072–6084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee S., Fernandez E. J., Good T. A. (2007) Protein Sci. 16, 723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nekooki-Machida Y., Kurosawa M., Nukina N., Ito K., Oda T., Tanaka M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9679–9684 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.