Abstract
Soluble oligomers of the amyloid-β peptide (AβOs) accumulate in the brains of Alzheimer disease (AD) patients and are implicated in synapse failure and early memory loss in AD. AβOs have been shown to impact synapse function by inhibiting long term potentiation, facilitating the induction of long term depression and inducing internalization of both AMPA and NMDA glutamate receptors, critical players in plasticity mechanisms. Because activation of dopamine D1/D5 receptors plays important roles in memory circuits by increasing the insertion of AMPA and NMDA receptors at synapses, we hypothesized that selective activation of D1/D5 receptors could protect synapses from the deleterious action of AβOs. We show that SKF81297, a selective D1/D5 receptor agonist, prevented the reduction in surface levels of AMPA and NMDA receptors induced by AβOs in hippocampal neurons in culture. Protection by SKF81297 was abrogated by the specific D1/D5 antagonist, SCH23390. Levels of AMPA receptor subunit GluR1 phosphorylated at Ser845, which regulates AMPA receptor association with the plasma membrane, were reduced in a calcineurin-dependent manner in the presence of AβOs, and treatment with SKF81297 prevented this reduction. Establishing the functional relevance of these findings, SKF81297 blocked the impairment of long term potentiation induced by AβOs in hippocampal slices. Results suggest that D1/D5 receptors may be relevant targets for development of novel pharmacological approaches to prevent synapse failure in AD.
Keywords: Alzheimer Disease; Amyloid; Glutamate Receptors Ionotropic (AMPA, NMDA); Neurotransmitter Receptors; Synapses; Dopamine D1/D5 Receptors; Long Term Potentiation; Oligomers
Introduction
Alzheimer disease (AD)2 is the main cause of dementia among the elderly, and current estimates indicate that it affects around 25 million people worldwide (1, 2). Although much is known about the pathophysiology of AD, there is still no cure or effective treatment capable of slowing the progression of the disease. For this reason, development of novel pharmacological strategies for treatment is of critical importance.
Considerable evidence indicates that soluble oligomers of the amyloid-β peptide (AβOs) accumulate in the brains of AD patients and are responsible for synapse dysfunction and memory loss in AD (3–5). Among other deleterious actions, AβOs impair synaptic plasticity, likely leading to memory loss at early stages of the disease. AβOs have been shown to inhibit long term potentiation (LTP) (4, 6, 7), facilitate the induction of long term depression (LTD) (8, 9), induce internalization of AMPA and NMDA receptors (10–14), and increase activation of protein phosphatases, such as calcineurin and protein phosphatase-1 (9, 10, 15, 16), finally leading to spine loss (14).
Dopamine receptors have been grouped into two families: D1-type and D2-type (17). The D1 family comprises D1 and D5 receptor subtypes, which are mostly coupled to Gαs and stimulate production of the second messenger cyclic AMP, leading to activation of protein kinase A (PKA) (17). D1/D5 receptors play important roles in cognition, mediating plasticity and specific aspects of cognitive function, including working and spatial learning and memory processes (18). Stimulation of D1/D5 receptors promotes the insertion of AMPA receptors into the plasma membrane through phosphorylation of AMPA receptor subunit GluR1 at the PKA target site Ser845 (19, 20). Because recruitment of new AMPA receptors into the synapse is critical to the establishment of LTP (21), this likely accounts for the molecular basis of the effects of dopaminergic neurotransmission on learning and memory.
In the present study, we show that SKF81297, a selective D1/D5 receptor agonist, prevents AβO-induced removal of AMPA receptors and NMDA receptors from the dendrites of hippocampal neurons in culture. Results further show that AβOs reduce the levels of GluR1 phosphorylated at Ser845 (pS845-GluR1), which regulates membrane association of AMPA receptors and that this effect can be prevented by SKF81297 and by the calcineurin inhibitor, FK-506. Finally, we show that treatment with SKF81297 prevents AβO-induced impairment of LTP in hippocampal slices, providing functional evidence for the protection of synapse function by D1/D5 receptor activation.
EXPERIMENTAL PROCEDURES
Materials
Aβ1–42 was purchased from Bachem Inc. (Torrance, CA). SKF81297 and SCH23390 were from Tocris (Ellisville, MO). Prolong Gold Antifade, normal goat serum, anti-rabbit or anti-mouse Alexa Fluor 555- or Alexa Fluor 488-conjugated secondary antibodies, Neurobasal, B27, Glutamax, C-terminal anti-NR1 and anti-pS845-GluR1 antibodies were from Invitrogen. Antibodies against microtubule-associated protein 2 and extracellular domains of GluR1 and NR1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Anhydrous dimethyl sulfoxide, paraformaldehyde, and cytosine arabinoside were from Sigma. SuperSignal West Femto maximum sensitivity substrate, protease and phosphatase inhibitor mixture, and BCA protein assay kit were from Pierce. Cyclophilin B antibody was from Affinity Bioreagents (Golden, CO).
Preparation and Characterization of Aβ Oligomers
AβOs were prepared exactly as described previously (4). The preparation was centrifuged at 14,000 × g for 10 min at 4 °C to remove insoluble aggregates, and the supernatant containing soluble AβOs was transferred to clean tubes and stored at 4 °C. Oligomer solutions were used within 24 h of preparation. Routine characterization of oligomer preparations was performed by size-exclusion chromatography, and a representative Western blot shows the presence of dimers, trimers, tetramers, and higher molecular mass oligomers (supplemental Fig. S1). Protein concentration was determined using the BCA assay, and the final AβO concentration used in all experiments was 400 nm.
Neuronal Cultures
Primary cultures of hippocampal neurons from day 18 rat embryos were prepared as described previously (22, 23) and maintained in Neurobasal medium supplemented with B27, Glutamax, and antibiotics. Cytosine arabinoside (200 nm) was added between 1 and 3 days in vitro to prevent glial cell proliferation, and cells were used after 19–20 days in vitro.
Immunocytochemistry
Cells were fixed for 10 min with ice-cold freshly prepared 4% paraformaldehyde containing 4% sucrose in PBS and blocked overnight with 10% normal goat serum in PBS. This fixation protocol minimizes permeabilization artifacts that might be induced by paraformaldehyde (24). To distinguish between receptors inserted in the plasma membrane and those that are in intracellular compartments, we skipped the permeabilization step so that the antibodies against GluR1 and NR1 (1:50 dilution, incubated at 4 °C overnight), which recognize extracellular epitopes of those proteins, could bind only to receptors inserted in the cell membrane. Furthermore, the possibility of artifactual permeabilization was routinely controlled for by concomitantly adding an antibody against microtubule-associated protein 2 (1:500), a dendritic intracellular protein, along with anti-GluR1 and anti-NR1. As suggested previously (25), any dendrite segments that were immunolabeled for microtubule-associated protein 2 were considered to be permeabilized and were excluded from further analysis for quantification of surface GluR1 and NR1 levels.
When probed for anti-pS845-GluR1 (1:100), cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min before blocking. After incubation with anti-rabbit or anti-mouse Alexa Fluor 555- or Alexa Fluor 488-conjugated secondary antibodies (1:2,000), coverslips were mounted using Prolong Gold Antifade.
Image Acquisition and Analysis
Cells were imaged on a Zeiss Axio Observer Z1 microscope with an EC-Plan-Neofluar 63×/1.25 NA oil immersion objective, equipped with the ApoTome structured illumination system and AxioVision Release 4.7.1 software. Axial and lateral resolutions were ∼1.05 and 0.22 μm, respectively, at 540 nm. Quantitative analysis of immunofluorescence data was carried out using National Institutes of Health ImageJ (Windows version) (26) as described (27). Appropriate thresholding was employed to eliminate background signal before histogram analysis. Cell bodies were digitally removed from the images so that only dendrite immunofluorescence was analyzed. In each experiment, 30 images were analyzed per experimental condition to allow quantitative determination of changes in GluR1, NR1, or pS845-GluR1 immunofluorescence levels. At least three experiments using independent cultures were performed, and results were normalized as percentage of control (vehicle-treated) cultures.
Western Blotting
Cultures were washed with ice-cold PBS and harvested with radioimmune precipitation assay buffer plus protease and phosphatase inhibitor mixture. Lysates were incubated on ice for 30 min with 5-s vortexing every 5 min and centrifuged at 10,000 × g for 5 min at 4 °C. Protein concentration in the supernatant was determined by the BCA assay. Sixty micrograms of protein (from soluble lysates) was applied to 10% SDS-PAGE and analyzed by Western blotting using anti-pS845-GluR1 (1:1,000), N-terminal anti-GluR1 (1:500), or C-terminal anti-NR1 (1:1,000) antibodies. Cyclophilin B (1:10,000) was used as a loading control.
Hippocampal Slices and Electrophysiological Recordings
Hippocampal slices from 3-month-old Wistar male rat were prepared as described (28). Complete horizontal slices (400 μm) were allowed to recover in artificial cerebrospinal fluid (129.0 mm NaCl, 3.0 mm KCl, 1.25 mm NaH2PO4, 10.0 mm glucose, 2 mm MgSO4, 1.6 mm CaCl2, 21.0 mm NaHCO3, pH 7.4; osmolality 302 ± 1 mOsm/kg) preheated to 34.5 ± 0.5 °C and fully oxygenated, circulated at a speed of 1.7 ml/min, for at least 90 min before recordings. Field extracellular recordings were performed by stimulating Schaeffer collateral fibers through a bipolar iridium-platinum microelectrode and recording in apical dendrites of CA1 stratum radiatum with a glass electrode (resistance of 3–5 megohms) filled with 1 m NaCl (28). A 15-min base line was recorded every 20 s at a stimulus intensity adjusted to cause ∼50% of the maximum evoked response. LTP was induced using a θ-burst stimulation (2 trains of 100 Hz each with a 20-s interval between them). Responses were recorded for 60 min after tetanization, measured as field EPSP (fEPSP) slopes and normalized by base-line response.
Statistical Analysis
Results were expressed as mean ± S.E. Statistical significance was assessed by ANOVA followed by a post hoc Bonferroni test using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Activation of D1/D5 Receptors Prevents AβO-induced Loss of AMPA and NMDA Receptors
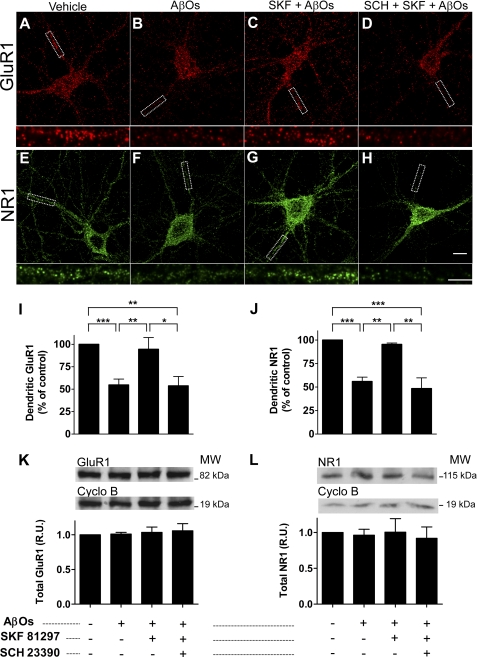
Treatment of hippocampal neurons in culture with AβOs (400 nm) for 4 h induced marked (∼45%) reductions in dendritic surface GluR1 (Fig. 1, A, B, and I) and NR1 immunoreactivities (Fig. 1, E, F, and J). However, when cells were preincubated for 15 min with SKF81297 (3 μm; a selective D1/D5 agonist) and then exposed to AβOs for 4 h, immunoreactivities for both GluR1 and NR1 remained close to control levels (Fig. 1, C, G, I, and J). In initial experiments, we tested the effect of 1 μm SKF81297, based on the fact that Gao and co-workers (19) showed that this concentration promoted the insertion of GluR1 into the plasma membrane of hippocampal neurons after 5 min. At 1 μm, however, SKF81297 showed only a slight tendency (which did not reach statistical significance) to protect against the effect of AβOs (not shown). We thus increased the concentration of SKF81297 to 3 μm, and this proved to be quite effective, as described above. Confirming the specific involvement of D1/D5 receptors, SCH23390 (10 μm), a selective D1/D5 receptor antagonist, abolished the protection by SKF81297 against AβO-induced loss of GluR1 and NR1 (Fig. 1, D and H). Under identical conditions, total cellular levels of GluR1 and NR1 were not affected (Fig. 1, K and L), indicating that receptors were internalized but not readily degraded upon treatment of neurons with AβOs for 4 h. Control experiments showed that, under our experimental conditions, SKF81297 and SCH23390 by themselves had no effect on total or surface levels of GluR1 and NR1 (data not shown).
FIGURE 1.
Activation of D1/D5 receptors prevents loss of dendritic AMPA and NMDA receptors induced by AβOs. Representative images of cultured hippocampal neurons (19–20 days in vitro) immunolabeled against surface GluR1 (red) or NR1 (green) are shown. Insets below each panel represent digital enlargements of the dendrite segments indicated by white dashed boxes in the main panels (scale bar, 5 μm) from each experimental condition. A and E, vehicle-treated neurons; B and F, AβO-treated neurons (400 nm, 4 h); C and G, neurons pretreated for 15 min with SKF81297 (3 μm) and then exposed to AβOs; D and H, neurons pretreated for 15 min with SCH23390 (10 μm) followed by 15 min with SKF81297 and 4 h with AβOs. Scale bar, 10 μm. I and J, quantitative immunofluorescence analysis of dendritic GluR1 (I) and NR1 (J). Bars represent means ± S.E. (error bars) from at least three independent experiments with 30 cells imaged per experimental condition in each experiment. Asterisks denote statistical significance levels (***, p < 0.001; **, p < 0.01; *, p < 0.05) in the indicated comparisons (ANOVA followed by Bonferroni's test). K and L, immunoblots for total GluR1 (K) and NR1 (L) showing that total protein levels are not altered by treatments. Graphs show results from four independent experiments normalized by cyclophilin B, used as a loading control. Bars represent means ± S.E.
AβOs Reduce Levels of pS845-GluR1
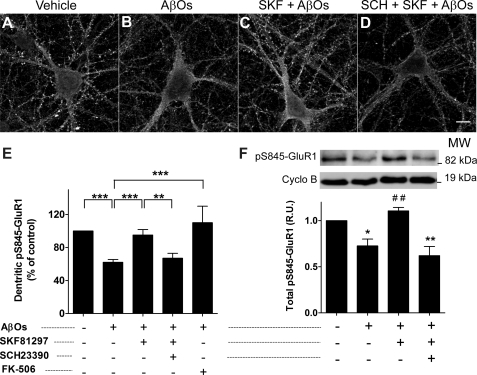
To investigate the mechanism underlying internalization of AMPA receptors induced by AβOs, we examined the levels of GluR1 phosphorylation at Ser845 (pS845-GluR1), a site known to be involved in regulation of membrane association of AMPA receptors. Treatment with 400 nm AβOs for 4 h induced a significant (∼40%) decrease in the level of dendritic pS845-GluR1 immunofluorescence (Fig. 2, A, B, and E). Total cellular levels of pS845-GluR1 were analyzed by Western blotting of cell lysates and were also found to be significantly reduced (∼30%) by treatment with AβOs compared with control, vehicle-treated cultures (Fig. 2F). The excellent agreement between the magnitudes of reduction in dendritic GluR1 (Fig. 1) and pS845-GluR1 levels suggests that reduced GluR1 phosphorylation plays a key role in the mechanism by which AβOs induce loss of surface AMPA receptors.
FIGURE 2.
Activation of D1/D5 receptors prevents calcineurin-dependent decrease in pS845-GluR1 levels induced by AβOs. Representative images of hippocampal neurons immunolabeled against pS845-GluR1 are shown. A, vehicle-treated neurons. B, AβO-treated neurons (400 nm, 4 h). C, neurons pretreated for 15 min with SKF81297 (3 μm) and then exposed to AβOs. D, neurons pretreated for 15 min with SCH23390 (10 μm) followed by 15 min with SKF81297 and 4 h with AβOs. Scale bar, 10 μm. E, quantitative analysis of pS845-GluR1 immunofluorescence in neuronal dendrites (excluding cell bodies). Bars represent means ± S.E. (error bars) from at least three independent experiments with 30 cells imaged per experimental condition in each experiment. F, immunoblot analysis of cell lysates showing total levels of pS845-GluR1 normalized by cyclophilin B (loading control). Asterisks denote statistical significance (***, p < 0.001; **, p < 0.01; and *, p < 0.01 compared with control; ##, p < 0.01 compared with AβO-treated group) (ANOVA followed by Bonferroni's test). Bars represent means ± S.E. of four independent experiments.
Aβ has been reported to inhibit PKA (29) and to activate protein phosphatases calcineurin and protein phosphatase-1 (10, 15), suggesting that it disrupts the balance between cellular kinases and phosphatases. In line with a recent report that Aβ-induced endocytosis of AMPA receptors is mediated by calcineurin (10), we found that calcineurin inhibitor FK-506 (20 nm) fully blocked the reduction in pS845-GluR1 levels induced by AβOs (Fig. 2E). FK-506 by itself had no effect on pS845-GluR1 levels (data not shown).
D1/D5 Receptor Activation Prevents AβO-induced Decrease in pS845-GluR1 Levels
We next asked whether the mechanism by which activation of D1/D5 receptors prevents loss of AMPA receptors involves preservation of GluR1 phosphorylation at Ser845. When hippocampal neurons were preincubated for 15 min with SKF81297 followed by a 4-h exposure to AβOs, there was no decrease in dendritic pS845-GluR1 levels relative to control cells (Fig. 2, A, C, and E). Similarly, treatment with SKF81297 prevented the reduction in total cellular levels of pS845-GluR1 (Fig. 2F). Specific involvement of D1/D5 receptors in preservation of dendritic and total pS845-GluR1 levels was confirmed by the fact that SCH23390 completely abolished protection by SKF81297 (Fig. 2, D–F).
SKF81297 Blocks AβO-induced Impairment of LTP
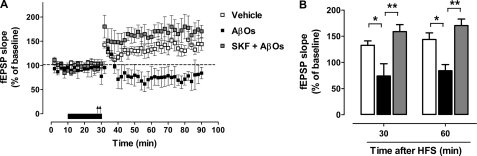
As expected, hippocampal slices subjected to high frequency stimulation displayed statistically significant increases in fEPSP slope over time, lasting >60 min (Fig. 3A). In line with previous reports (4), perfusion of AβOs (400 nm) for 20 min completely blocked LTP induction, with significant reductions in slope ratios (74 ± 24% and 84 ± 12% compared with base line at 30 and 60 min after high frequency stimulation, respectively) compared with control, vehicle-treated slices (133 ± 8% and 144 ± 13% over base line at 30 and 60 min after high frequency stimulation, respectively) (Fig. 3B). Interestingly, when SKF81297 was co-perfused with AβOs, full potentiation of fEPSP was preserved (159 ± 13% and 170 ± 13% over base line at 30 and 60 min after high frequency stimulation, respectively). In fact, there was a tendency (which, however, did not reach statistical significance) for fEPSP slopes to be even higher in the presence of SKF81297 than in control slices (Fig. 3A). Results thus demonstrate that activation of D1/D5 receptors completely prevents LTP impairment induced by AβOs.
FIGURE 3.
D1/D5 receptor activation prevents LTP impairment induced by AβOs. A, rat hippocampal slices stimulated at Schaffer collaterals with two consecutive 100-Hz pulses (arrows) exhibiting an increase in CA1 fEPSP slope lasting at least 60 min (open symbols). The same stimulus failed to induce stable LTP (filled symbols) in slices perfused with AβOs (400 nm) for 20 min prior to tetanic stimulation (black horizontal bar). Concomitant perfusion of SKF81297 (3 μm) with AβOs prevented the inhibition of LTP (gray symbols). B, analysis of normalized fEPSP responses at 30 or 60 min after tetanus. White (vehicle), black (AβOs), and gray (SKF + AβOs) bars represent means ± S.E. (error bars) from four independent experiments. Asterisks denote statistical significance of differences in the indicated comparisons (**, p < 0.01; *, p < 0,01; ANOVA followed by Bonferroni's test).
DISCUSSION
Driven by previous reports showing that Aβ oligomers impair synapse function (30, 31) and that activation of dopamine D1/D5 receptors enhances excitatory transmission and facilitates synaptic plasticity (18, 32), the present study was designed to investigate whether pharmacological manipulation of D1/D5 receptors could represent a novel mechanism to protect synapses from damage instigated by Aβ oligomers.
Among several aspects of synapse function reported to be affected by AβOs is the internalization of AMPA and NMDA receptors. Both receptors exhibit reduced surface levels upon in vitro exposure of neurons to different AβO preparations and reduced total levels in transgenic mouse models of AD and in AD brains (10–14, 33–35). AMPA and NMDA receptors are essential for glutamatergic synaptic transmission and play key roles in LTP and LTD, cellular mechanisms of plasticity thought to underlie learning, memory, and cognition (36). Current results show that activation of D1/D5 receptors by SKF81297 prevents AβO-induced loss of GluR1 and NR1 from dendrites. The fact that total levels of GluR1 and NR1 were unaffected by exposure to AβOs suggests that changes in their surface levels are a consequence of altered regulation of trafficking rather than changes in expression or stability of these receptors.
It is well established that NMDA receptors and D1/D5 receptors interact physically and functionally in the brain (37, 38). This interaction accounts for several aspects of dopaminergic regulation of cognitive function, including reward, addiction, and working memory (37). Activation of D1/D5 receptors enhances PKA-mediated phosphorylation of NR1 at Ser897, which signals synaptic insertion of NMDA receptors (39, 40), resulting in increased NMDA currents and synaptic potentiation (41). Preventing the removal of NMDA receptors from synaptic membranes, as accomplished by D1/D5 receptor activation, is thus critical for the integrity of synaptic plasticity.
AMPA receptors are the major excitatory neurotransmitter receptors in the brain, and their levels at synapses are highly dynamic and regulated (42). AMPA receptor trafficking is critically engaged in modulating synaptic strength; for example, recruitment of new AMPA receptors occurs during LTP (43), whereas they are removed by endocytosis from synaptic membranes upon induction of LTD (42). From its insertion into the synaptic membrane to receptor endocytosis, AMPA receptor subunits are regulated mainly by two mechanisms: phosphorylation at specific sites and interaction with scaffolding proteins, which confer stability to the receptor at the postsynaptic membrane (44). Among the four distinct subunits that may form AMPA receptors, GluR1 is the one whose trafficking depends directly on neuronal activity, whereas GluR2 is more prone to undergo constitutive, activity-independent recycling (45). Membrane insertion of GluR1 is regulated by two phosphorylation sites in the intracellular C-terminal tail: Ser845 and Ser831, targets of PKA and calcium/calmodulin-dependent kinase II, respectively (46). When phosphorylated, Ser845 signals AMPA receptor insertion at extrasynaptic sites, and Ser831 signals subsequent translocation to synaptic sites (21, 47, 48). Phosphorylation of GluR1 Ser845 is required for the induction of LTP (49), whereas its dephosphorylation is essential for NMDA receptor-dependent LTD (48).
Our finding that AβOs induce loss of surface AMPA receptors and a parallel reduction in pS845-GluR1 levels is consistent with the fact that AβOs facilitate LTD and induce long lasting depression of synaptic activity (8, 50). This impact of AβOs may be part of the underlying mechanism by which AβOs facilitate/induce LTD, inhibit LTP, and cause synapse failure. Giving further support to this notion is the observation that reduction in pS845-GluR1 levels induced by AβOs was blocked by a calcineurin inhibitor, FK-506. It is well established that dephosphorylation of GluR1 by calcineurin and other phosphatases, such as protein phosphatases-1 and -2A, cause removal of AMPA receptors from the plasma membrane (51). Calcineurin can also act indirectly, through the relief of protein phosphatase-1 inhibition by DARPP-32 (52). Specifically, previous works have shown that calcineurin may be involved in the deleterious synaptic effects of AβOs, including LTP impairment, LTD facilitation, and induction of AMPA receptor endocytosis (8, 10, 16, 53). Based on the present data, we propose that AβOs cause a reduction in pS845-GluR1 levels mediated by activation of calcineurin, triggering AMPA receptor removal and synapse failure.
Current results show that activation of D1/D5 receptors prevents the reduction in pS845-GluR1 and loss of surface GluR1 induced by AβOs. D1/D5 receptor activation leads to increased cyclic AMP levels and increased PKA activity (17), favoring phosphorylation of GluR1 (54) and AMPA receptor insertion into extrasynaptic sites (19). This may explain why D1/D5-selective agonists prevent LTD (55) and facilitate the induction of LTP (41). PKA activity is known to be affected by Aβ (29), and this is likely the kinase involved in mediating the protective effect of SKF81297 on the surface levels of AMPA receptor. However, other kinases thought to be involved in AD pathogenesis, including GSK3β (56), have been implicated in Aβ effects on AMPA receptor trafficking (57). Therefore, the possible roles of GSK3β and other kinases in the regulation of AMPA receptor trafficking by D1/D5 receptors merits further investigation.
Finally, we found that SKF81297 prevented AβO-induced impairment of LTP in hippocampal slices, further substantiating the notion that activation of D1/D5 receptors prevents synapse dysfunction induced by AβOs. LTP is a central event in learning and memory processes, and it is known to be compromised in AD (30). It is generally accepted that synaptic recruitment of AMPA receptors is necessary for LTP (45). Phosphorylation of GluR1 at Ser845 is required for delivery of new AMPA receptors into perisynaptic sites (36), which precedes full expression of LTP (58). Thus, D1/D5 receptor-mediated extrasynaptic insertion of AMPA receptors (19) via Ser845 phosphorylation of GluR1 by PKA (59) is likely the mechanism involved in prevention of AβO-induced LTP impairment by SKF81297.
In conclusion, we have established a novel connection between dopaminergic signaling and AD by demonstrating that activation of D1/D5 receptors prevents pathological changes in synapse composition and function induced by AβOs. One of the implications of this connection is that dopamine D1/D5 receptors may constitute a target for the development of novel approaches to combat synapse failure in AD. Also of interest is that dopaminergic neurotransmission, which is reduced with aging (60), plays important roles in nonpharmacological strategies aimed to improve age-related cognitive decline, such as environmental enrichment and physical exercise (61). Previous works have suggested that such strategies could be beneficial to ameliorate AD symptoms (62). Thus, investigating the relationship between dopamine D1/D5 receptor activation and relief of AD symptoms achieved by either pharmacological tools or nonpharmacological approaches may be an interesting avenue for future investigations.
Supplementary Material
Acknowledgments
We thank Dr. Patricia T. Bozza (Fiocruz, Brazil) for providing FK-506 and Drs. Helena Decker, Ana Paula Wasilewska-Sampaio, and Paulina Achurra for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants 5SC1NS063950-04 and 3SC1NS063950-03S1 (to E. R. G.-S.). This work was also supported by grants from Conselho Nacional de Desenvolvimento Científico Tecnológico, Instituto Nacional de Neurociência Translacional, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (to S. T. F.); Fundação de Amparo à Pesquisa do Estado de São Paulo (to E. A. C.); and Human Frontier Science Program (to F. G. D. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- AD
- Alzheimer disease
- AβO
- amyloid-β peptide oligomer
- fEPSP
- field excitatory postsynaptic potential
- GluR1
- subunit of AMPA receptors
- LTD
- long term depression
- LTP
- long term potentiation
- PKA
- protein kinase A
- pS845-GluR1
- GluR1 AMPA receptor subunit phosphorylated at serine 845
- NR1
- subunit of NMDA receptors.
REFERENCES
- 1. Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H. M. (2007) Alzheimers Dement 3, 186–191 [DOI] [PubMed] [Google Scholar]
- 2. Kalaria R. N., Maestre G. E., Arizaga R., Friedland R. P., Galasko D., Hall K., Luchsinger J. A., Ogunniyi A., Perry E. K., Potocnik F., Prince M., Stewart R., Wimo A., Zhang Z. X., Antuono P. (2008) Lancet Neurol. 7, 812–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haass C., Selkoe D. J. (2007) Nat. Rev. Mol. Cell Biol. 8, 101–112 [DOI] [PubMed] [Google Scholar]
- 4. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferreira S. T., Vieira M. N., De Felice F. G. (2007) IUBMB life 59, 332–345 [DOI] [PubMed] [Google Scholar]
- 6. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 7. Townsend M., Shankar G. M., Mehta T., Walsh D. M., Selkoe D. J. (2006) J. Physiol. 572, 477–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S., Hong S., Shepardson N. E., Walsh D. M., Shankar G. M., Selkoe D. (2009) Neuron 62, 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008) Nat. Med. 14, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao W. Q., Santini F., Breese R., Ross D., Zhang X. D., Stone D. J., Ferrer M., Townsend M., Wolfe A. L., Seager M. A., Kinney G. G., Shughrue P. J., Ray W. J. (2010) J. Biol. Chem. 285, 7619–7632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. (2006) Neuron 52, 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almeida C. G., Tampellini D., Takahashi R. H., Greengard P., Lin M. T., Snyder E. M., Gouras G. K. (2005) Neurobiol. Dis. 20, 187–198 [DOI] [PubMed] [Google Scholar]
- 13. Snyder E. M., Nong Y., Almeida C. G., Paul S., Moran T., Choi E. Y., Nairn A. C., Salter M. W., Lombroso P. J., Gouras G. K., Greengard P. (2005) Nat. Neurosci. 8, 1051–1058 [DOI] [PubMed] [Google Scholar]
- 14. Lacor P. N., Buniel M. C., Furlow P. W., Clemente A. S., Velasco P. T., Wood M., Viola K. L., Klein W. L. (2007) J. Neurosci. 27, 796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knobloch M., Farinelli M., Konietzko U., Nitsch R. M., Mansuy I. M. (2007) J. Neurosci. 27, 7648–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu H. Y., Hudry E., Hashimoto T., Kuchibhotla K., Rozkalne A., Fan Z., Spires-Jones T., Xie H., Arbel-Ornath M., Grosskreutz C. L., Bacskai B. J., Hyman B. T. (2010) J. Neurosci. 30, 2636–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Missale C., Nash S. R., Robinson S. W., Jaber M., Caron M. G. (1998) Physiol. Rev. 78, 189–225 [DOI] [PubMed] [Google Scholar]
- 18. Dalley J. W., Everitt B. J. (2009) Semin. Cell Dev. Biol. 20, 403–410 [DOI] [PubMed] [Google Scholar]
- 19. Gao C., Sun X., Wolf M. E. (2006) J. Neurochem. 98, 1664–1677 [DOI] [PubMed] [Google Scholar]
- 20. Smith W. B., Starck S. R., Roberts R. W., Schuman E. M. (2005) Neuron 45, 765–779 [DOI] [PubMed] [Google Scholar]
- 21. Shepherd J. D., Huganir R. L. (2007) Annu. Rev. Cell Dev. Biol. 23, 613–643 [DOI] [PubMed] [Google Scholar]
- 22. Brewer G. J., Torricelli J. R., Evege E. K., Price P. J. (1993) J. Neurosci. Res. 35, 567–576 [DOI] [PubMed] [Google Scholar]
- 23. Paula-Lima A. C., Tricerri M. A., Brito-Moreira J., Bomfim T. R., Oliveira F. F., Magdesian M. H., Grinberg L. T., Panizzutti R., Ferreira S. T. (2009) Int. J. Biochem. Cell Biol. 41, 1361–1370 [DOI] [PubMed] [Google Scholar]
- 24. Cuitino L., Godoy J. A., Farías G. G., Couve A., Bonansco C., Fuenzalida M., Inestrosa N. C. (2010) J. Neurosci. 30, 8411–8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glynn M. W., McAllister A. K. (2006) Nat. Protocols 1, 1287–1296 [DOI] [PubMed] [Google Scholar]
- 26. Abramoff M. D., Magalhaes P. J., Ram S. J. (2004) Biophoton. Int. 11, 36–42 [Google Scholar]
- 27. De Felice F. G., Velasco P. T., Lambert M. P., Viola K., Fernandez S. J., Ferreira S. T., Klein W. L. (2007) J. Biol. Chem. 282, 11590–11601 [DOI] [PubMed] [Google Scholar]
- 28. Sanabria E. R., Pereira M. F., Dolnikoff M. S., Andrade I. S., Ferreira A. T., Cavalheiro E. A., Fernandes M. J. (2002) Brain Res. Bull. 59, 47–51 [DOI] [PubMed] [Google Scholar]
- 29. Vitolo O. V., Sant'Angelo A., Costanzo V., Battaglia F., Arancio O., Shelanski M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13217–13221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamin G. (2009) J. Neurosci. Res. 87, 1729–1736 [DOI] [PubMed] [Google Scholar]
- 31. Parameshwaran K., Dhanasekaran M., Suppiramaniam V. (2008) Exp. Neurol. 210, 7–13 [DOI] [PubMed] [Google Scholar]
- 32. Wolf M. E., Mangiavacchi S., Sun X. (2003) Ann. N. Y. Acad. Sci. 1003, 241–249 [DOI] [PubMed] [Google Scholar]
- 33. Roselli F., Tirard M., Lu J., Hutzler P., Lamberti P., Livrea P., Morabito M., Almeida O. F. (2005) J. Neurosci. 25, 11061–11070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yasuda R. P., Ikonomovic M. D., Sheffield R., Rubin R. T., Wolfe B. B., Armstrong D. M. (1995) Brain Res. 678, 161–167 [DOI] [PubMed] [Google Scholar]
- 35. Ikonomovic M. D., Mizukami K., Warde D., Sheffield R., Hamilton R., Wenthold R. J., Armstrong D. M. (1999) Exp. Neurol. 160, 194–204 [DOI] [PubMed] [Google Scholar]
- 36. Malenka R. C., Bear M. F. (2004) Neuron 44, 5–21 [DOI] [PubMed] [Google Scholar]
- 37. Castner S. A., Williams G. V. (2007) Brain Cognition 63, 94–122 [DOI] [PubMed] [Google Scholar]
- 38. Missale C., Fiorentini C., Busi C., Collo G., Spano P. F. (2006) Curr. Top. Med. Chem. 6, 801–808 [DOI] [PubMed] [Google Scholar]
- 39. Chen B. S., Roche K. W. (2007) Neuropharmacology 53, 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snyder G. L., Fienberg A. A., Huganir R. L., Greengard P. (1998) J. Neurosci. 18, 10297–10303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Granado N., Ortiz O., Suárez L. M., Martín E. D., Ceña V., Solís J. M., Moratalla R. (2008) Cereb. Cortex 18, 1–12 [DOI] [PubMed] [Google Scholar]
- 42. Malenka R. C. (2003) Ann. N. Y. Acad. Sci. 1003, 1–11 [DOI] [PubMed] [Google Scholar]
- 43. Park M., Penick E. C., Edwards J. G., Kauer J. A., Ehlers M. D. (2004) Science 305, 1972–1975 [DOI] [PubMed] [Google Scholar]
- 44. Groc L., Choquet D. (2006) Cell Tiss. Res. 326, 423–438 [DOI] [PubMed] [Google Scholar]
- 45. Bredt D. S., Nicoll R. A. (2003) Neuron 40, 361–379 [DOI] [PubMed] [Google Scholar]
- 46. Song I., Huganir R. L. (2002) Trends Neurosci. 25, 578–588 [DOI] [PubMed] [Google Scholar]
- 47. He K., Song L., Cummings L. W., Goldman J., Huganir R. L., Lee H. K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20033–20038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee H. K., Takamiya K., He K., Song L., Huganir R. L. (2010) J. Neurophysiol. 103, 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee H. K., Takamiya K., Han J. S., Man H., Kim C. H., Rumbaugh G., Yu S., Ding L., He C., Petralia R. S., Wenthold R. J., Gallagher M., Huganir R. L. (2003) Cell 112, 631–643 [DOI] [PubMed] [Google Scholar]
- 50. Cerpa W., Farías G. G., Godoy J. A., Fuenzalida M., Bonansco C., Inestrosa N. C. (2010) Mol. Neurodegen. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee H. K. (2006) Pharmacol. Ther. 112, 810–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Greengard P., Allen P. B., Nairn A. C. (1999) Neuron 23, 435–447 [DOI] [PubMed] [Google Scholar]
- 53. Chen Q. S., Wei W. Z., Shimahara T., Xie C. W. (2002) Neurobiol. Learn. Mem. 77, 354–371 [DOI] [PubMed] [Google Scholar]
- 54. Chao S. Z., Lu W., Lee H. K., Huganir R. L., Wolf M. E. (2002) J. Neurochem. 81, 984–992 [DOI] [PubMed] [Google Scholar]
- 55. Mockett B. G., Guévremont D., Williams J. M., Abraham W. C. (2007) J. Neurosci. 27, 2918–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Decker H., Lo K. Y., Unger S. M., Ferreira S. T., Silverman M. A. (2010) J. Neurosci. 30, 9166–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rui Y., Gu J., Yu K., Hartzell H. C., Zheng J. Q. (2010) Mol. Brain 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang Y., Wang X. B., Frerking M., Zhou Q. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11388–11393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Man H. Y., Sekine-Aizawa Y., Huganir R. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 3579–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Keyser J., De Backer J. P., Vauquelin G., Ebinger G. (1990) Brain Res. 528, 308–310 [DOI] [PubMed] [Google Scholar]
- 61. van Praag H., Kempermann G., Gage F. H. (2000) Nat. Rev. Neurosci. 1, 191–198 [DOI] [PubMed] [Google Scholar]
- 62. Schaeffer E. L., Novaes B. A., da Silva E. R., Skaf H. D., Mendes-Neto A. G. (2009) Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1087–1102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.