Abstract
RBX1 (RING box protein 1), also known as ROC1 (Regulator of Cullin 1), is an essential component of SCF (Skp1/Cullins/F-box) E3 ubiquitin ligases, which target diverse proteins for proteasome-mediated degradation. Our recent study showed that RBX1 silencing triggered a DNA damage response (DDR) leading to G2-M arrest, senescence, and apoptosis, with the mechanism remaining elusive. Here, we show that, in human cancer cells, RBX1 silencing causes the accumulation of DNA replication licensing proteins CDT1 and ORC1, leading to DNA double-strand breaks, DDR, G2 arrest, and, eventually, aneuploidy. Whereas CHK1 activation by RBX1 silencing is responsible for the G2 arrest, enhanced DNA damage renders cancer cells more sensitive to radiation. In Caenorhabditis elegans, RBX-1 silencing causes CDT-1 accumulation, triggering DDR in intestinal cells, which is largely abrogated by simultaneous CDT-1 silencing. RBX-1 silencing also induces lethality during development of embryos and in adulthood. Thus, RBX1 E3 ligase is essential for the maintenance of mammalian genome integrity and the proper development and viability in C. elegans.
Keywords: Development, DNA Damage, E3 Ubiquitin Ligase, Genome Structure, siRNA, Ubiquitination, C. elegans, Genomic Instability
Introduction
SCF (Skp1/Cullins/F-box; also known as CRL (Cullin-RING Ligase) E3 ubiquitin ligases are the largest multiunit E3 ligases that promote the degradation of numerous short-lived cellular proteins, including cell cycle regulators, transcription factors, signal transducers, and oncogene/tumor suppressors (1, 2). A recent global protein stability profiling analysis identified ∼350 potential SCF substrates, the majority of which were previously unidentified (3). Most recently, a study of SCF inactivation by a small molecule inhibitor of cullin neddylation suggested that up to 20% of ubiquitinated cellular proteins are mediated by SCF E3 for proteasome degradation (4). Thus, SCF E3 ubiquitin ligases regulate many aspects of cellular functions and biological processes under physiological conditions. Dysfunction of SCF is involved in the pathogenesis of a variety of diseases, including cancer (2, 5).
The core of SCF ubiquitin ligases is a complex of RBX1-cullins (6). RBX1 consists of 108 amino acids with a C-terminal RING-H2 finger domain required for zinc ion binding and ligase activity (7–9). Crystal structure studies revealed that RBX1 complexes with cullin/F-box proteins form functional SCF E3 ligases that transfer ubiquitin from E2 to specific substrates for proteasome-targeted degradation (10). Previous studies have shown that RBX1 interacts with all seven cullin family members to activate E3 ubiquitin ligases and regulate numerous biological processes by promoting timely degradation of cellular substrates (9, 11).
As an essential component of SCF E3 ligase, RBX1 plays a critical role in development. In yeast, deletion of Hrt1, the yeast homolog of RBX1, causes lethality, which can be rescued by human RBX1 or RBX2/SAG (Sensitive to Apoptosis gene) (9, 12, 13). In Caenorhabditis elegans, RBX-1 is crucial for cell cycle progression and chromosome metabolism, and RBX-1 silencing results in embryonic death (14, 15). In Drosophila, Roc1a, the Drosophila homolog of RBX1, is required for cell proliferation and embryonic development, and deletion of Roc1a results in animal death (16). Recently, we reported that mouse Rbx1 disruption causes early embryonic lethality due to significant accumulation of p27 to suppress proliferation, which can be partially rescued by a simultaneous deletion of p27 (17). These findings suggest that the in vivo physiological function of Rbx1 is to ensure cell proliferation during embryonic development.
Consistently, RBX1 was found to be essential for cancer cell proliferation (18) and survival (19). It appears that cancer cells are “addicted” to RBX1-overexpressed environments. Upon RBX1 siRNA silencing, cancer cells sequentially undergo G2-M arrest, senescence, and apoptosis, which are associated with a DNA damage response (DDR)4 (19). In this study, we demonstrate that RBX1 silencing actually causes DNA double-strand breaks (DSB), leading to chromosome aneuploidy, which is associated with the accumulation of DNA replication licensing proteins CDT1 and ORC1. We further demonstrate that RBX-1 silencing in C. elegans triggers similar DDR in intestinal cells, which can be completely rescued by simultaneous silencing of CDT-1. Thus, RBX1-SCF E3 ubiquitin ligases play an essential role in genomic stability in both in vitro cultured cells and in vivo animals.
EXPERIMENTAL PROCEDURES
Cell Culture
H1299 human lung cancer cells and U87 human glioblastoma cells were purchased from American Type Culture Collection and grown at 37 °C in 5% CO2 in DMEM supplemented with 10% FBS.
siRNA Silencing
U87 and H1299 cells were transfected with siRNA oligonucleotides (made by Dharmacon) using Lipofectamine 2000. The siRNA sequences are as follows: for RBX1, 5′-GACTTTCCCTGCTGTTACCTAA-3′; for CDT1, 5′-CGTGGATGAAGTACCC GAC-3′; for ORC1, 5′-CTGCACTACCAAACCTATA-3′; for CHK1, SMARTpool M-003255-04; for CHK2, SMARTpool M-003256-06; and for scrambled control siRNA, 5′-ATTGTATGCGATCGCA-GACTT-3′.
The wild-type C. elegans (Bristol N2) strain was maintained at 20 °C and fed double-stranded RNA expressed in bacteria from the Ahringer RNAi Library (MRC Geneservice) beginning at the L1 larval stage (20) to silence rbx-1 and cdt-1. To enhance RNAi efficacy, the liquid bacterial cultures were treated with 2 mm isopropyl 1-thio-β-d-galactopyranoside for 2 h at 37 °C prior to seeding on the RNAi-agar plates. The cdt-1;rbx-1 double RNAi experiments were performed by seeding the RNAi plates with a 1:1 mixture of the cdt-1 and rbx-1 RNAi bacterial cultures. L1 larvae were maintained on the bacterium-seeded RNAi plates through adulthood and examined for mutant phenotypes following 3–12 days of feeding RNAi treatment and embryonic lethality in the offspring of the adults fed for 4 days. Adult lethality was assessed by immobility, non-responsiveness to touch, and absence of pharyngeal pumping. The effectiveness of RNAi silencing was determined by RT-PCR (21) using total RNA extracted from 100 worms per test condition, reverse-transcribed with oligo(dT), and analyzed by PCR using the following gene-specific primers: rbx-1, 5′-TGGCCCAAGCAAGCGACAG-3′ and 5′-AGTGTCCGTACTTTTGGAATTCCC-3′; cdt-1, 5′-CAGACTGCTGTAACCGAC-3′ and 5′-GTGGTCTTTGTGATGCTTTC-3′; and act-1, 5′-AAGGAAATCACCGCTCTTGC-3′ and 5′-TGCTTGGAGATCCACATCTG-3′.
Western Blot Analysis
Cell lysates were prepared for Western blot analysis using antibodies against CDT1, CDC6, c-JUN, c-MYC, H-RAS, and cyclin D1 (Santa Cruz Biotechnology); ORC1 (BD Transduction Laboratories); β-actin (Sigma); phospho-γH2A (Ser-139) (Millipore); phospho-CHK1 (Ser-345), phospho-CHK2 (Thr-68), CHK1, and CHK2 (Cell Signaling); and RBX1 (17). C. elegans lysates were prepared from 100 adult worms by boiling in 30 μl of SDS loading buffer containing 3.75 m urea. Immunoblotting was performed with antibodies to CDT-1 (22), α-tubulin (DM1alpha, Sigma), and RBX1 (17), which cross-react with the C. elegans RBX-1 protein.
Immunofluorescence Staining
Cells were fixed with 10% formalin, blocked with 3% horse serum, and incubated with primary antibody against γH2AX, 53BP1, or RAD51 at 1:100, followed by incubation with FITC-labeled anti-mouse IgG (for γH2AX) or Rhodamine Red-labeled anti-rabbit IgG (for 53BP1 or RAD51) at 1:250. Cellular nuclei were stained with DAPI. The stained cells were observed under a fluorescence microscope.
Adult worms were dissected, freeze-cracked, fixed with paraformaldehyde, and incubated with antibodies as described (23). Rabbit antibodies to C. elegans RAD-51 (Strategic Diagnostics, Inc.) at 1:5000 and Alexa 555-labeled secondary antibodies (Invitrogen) at 1:500 were used. The C. elegans fluorescence images were captured on an Olympus BX61 epifluorescence microscope with a Hamamatsu Orca ER camera, deconvolved with Huygens Essential (Scientific Volume Imaging), and processed with ImageJ and Photoshop CS2.
Neutral Comet Assays
Single-cell gel electrophoretic comet assays were done under neutral conditions. Briefly, H1299 and U87 cells transfected with RBX1 siRNA (siRBX1) or control siRNA (siCONT) for 24 h were split. 96 h later, cells were harvested and coasted on the slide. For cellular lysis, slides were immersed in N1 neutral lysis solution (2% Sarkosyl, 0.5 m EDTA, and 0.5 mg/ml proteinase K, pH 8.0) overnight at 37 °C, followed by electrophoresis at 15 V for 25 min (0.6 V/cm), staining for 20 min with 10 μg/ml propidium iodide, and image acquisition with a fluorescence microscope. The comet tail moment was analyzed by CometScore software with 100 cells analyzed for each condition.
Phosphohistone 3/Propidium Iodide Double Staining and FACS Analysis
Cells were harvested and fixed in 70% EtOH at −20 °C for 4 h. Cells were incubated with phosphohistone H3-specific antibody (Millipore), followed by a FITC-conjugated anti-rabbit secondary antibody (Sigma). Samples were then stained with propidium iodide and analyzed on a FACScan flow cytometer (BD Biosciences) with FlowJo software (Tree Star).
Irradiation and Radiosensitization Assay
48 h following siRNA silencing, cells were seeded into 6-well plates at three different cell densities in duplicate. The next day, cells were exposed to different doses of radiation, followed by incubation at 37 °C for 9 days. The colonies formed were fixed, and the surviving fraction was determined by the proportion of seeded cells following irradiation to form colonies relative to untreated cells as described (24).
RESULTS AND DISCUSSION
RBX1 Silencing Induces DDR as a Result of DNA DSB
We recently reported that RBX1 silencing induces growth arrest at the G2-M phase and cell death via senescence and apoptosis, which are coupled with the phosphorylation of H2AX, an indicator of DDR (19). To further characterize the DDR upon RBX1 silencing, we first determined the levels of CHK1 and CHK2 phosphorylation, two classical DDR markers, and found that both were significantly induced upon RBX1 silencing in H1299 and U87 cancer cell lines (Fig. 1, A and B). We next performed immunofluorescence staining of DNA damage markers, including 53BP1, RAD51, and γH2AX, and detected an increase in 53BP1, RAD51, and γH2AX foci in response to RBX1 silencing in U87 cells (Fig. 1C) and H1299 cells (supplemental Fig. S1), but not in response to scrambled control siRNA. Finally, we performed a neutral comet assay to determine whether RBX1 silencing causes DNA DSB that may trigger the observed DDR and found that comet tail moment (a measurement of DSB) was significantly greater in RBX1 siRNA-transfected cells than in control siRNA-transfected cells (Fig. 1D). Taken together, these results clearly demonstrate that RBX1 silencing induces DNA DSB in the genome to trigger DDR.
FIGURE 1.
RBX1 silencing induces DDR as a result of DNA DSB. H1299 cells (A) and U87 cells (B) were transfected with siRBX1 or siCONT. Cells were split 24 h later and harvested at the indicated time points for Western blot analysis (A and B) as well as for immunofluorescence staining in U87 cells using the indicated antibodies (C, at 72–120 h), with the formation of DNA damage foci clearly visualized upon RBX1 silencing (C, right panels), or comet assay in both H1299 and U87 cells (D, at 96 h).
RBX1 Silencing Activates G2 Checkpoint Controls, Induces Aneuploidy, and Sensitizes Cancer Cells to Irradiation
To determine at which phase of the cell cycle (G2 versus M) cancer cells were arrested upon RBX1 silencing, we performed two-parameter flow cytometry with propidium iodide and phosphohistone H3 (25) and found that RBX1 silencing induced G2 but not M phase arrest in both U87 and H1299 cells, starting at 48 h or 72 h post-transfection, respectively (Fig. 2A and supplemental Fig. S2). Most strikingly, RBX1 silencing also caused a time-dependent increase in the aneuploid cell population with DNA content greater than 4N in both U87 cells (Fig. 2A) and H1299 cells (supplemental Fig. S2), indicating that RBX1 is required for proper cell cycle progression and the maintenance of genomic stability.
FIGURE 2.
RBX1 silencing triggers DNA re-replication, activates the DNA damage G2 checkpoint, and sensitizes cells to irradiation. A, RBX1 silencing induces G2 arrest and aneuploidy. U87 cells were transfected with siRBX1 or siCONT and split 24 h later. At the indicated time points post-splitting, cells were subjected to phosphohistone 3/propidium iodide double staining and FACS analysis. The percentage of cell populations at G2 and M and those with aneuploidy (4N+) is indicated. B and C, RBX1 silencing-induced G2 arrest is abrogated by CHK1 inhibition. U87 cells transfected with the indicated siRNAs for 24 h were split. At 72 h post-splitting, one portion of cells was subjected to propidium iodide staining and FACS analysis (B, upper panel), whereas the other portion was subjected to Western blot analysis (lower panel). U87 cells transfected with indicated siCONT or siRBX1 for 24 h were split. At 72 h post-splitting, cells were treated with PF-00477736 (PF) at the indicated concentrations for 24 h and subjected to propidium iodide staining and FACS analysis (C). D, RBX1 silencing sensitizes cancer cells to irradiation. U87 and H1299 cells after siRNA silencing for 48 h were subjected to standard clonogenic assay after exposure to different doses of radiation as described (24). Shown is x ± S.E. from four independent experiments: SiRBX1 versus siCONT, p < 0.05 for both H1299 and U87 cells with Student's t test. Gy, gray.
Because DDR triggers G2 checkpoint machinery to block cells at the G2 phase with CHK1 as a key player (26), we next determined if CHK1 abrogation via siRNA silencing or a small molecule inhibitor would rescue cells from G2 arrest. As shown in Fig. 2B, simultaneous silencing of CHK1 but not CHK2 abrogated RBX1 silencing-induced G2 arrest, suggesting that the CHK1-mediated DNA damage checkpoint plays a critical role in the induction of G2 arrest in response to RBX1 silencing. Similarly, a dose-dependent abrogation of RBX1 silencing-induced G2 arrest was observed by treatment with PF-00477736 (Fig. 2C), a small molecule inhibitor of CHK1 (27). Taken together, these findings suggest that RBX1 silencing-induced G2 arrest is triggered by a CHK1-mediated DNA damage checkpoint response.
It is well established that radiation induces DNA damage and G2 arrest as its major mechanism of action (28). Because RBX1 silencing triggers the G2 arrest as a result of DSB and DDR and because G2 is a relatively radiosensitive phase of the cell cycle, we reasoned that it could sensitize otherwise resistant cancer cells to irradiation. Indeed, as shown in Fig. 2D, RBX1 silencing significantly sensitized radioresistant U87 and H1299 cells to radiation with sensitivity enhancement ratios of 1.5 and 1.3, respectively (p < 0.05). Our results suggest that RBX1 could serve as an attractive radiosensitizing target and that small molecule RBX1 inhibitors may increase the efficacy of radiotherapy.
RBX1 Silencing Induces the Accumulation of CDT1 and ORC1, Two DNA Replication Licensing Proteins
Previous studies have shown that DNA replication aberrance, followed by activation of the DDR, could be triggered by the activation of oncogenes or the dysregulation of DNA replication licensing proteins (29–31). Because many oncoproteins (such as c-MYC, c-JUN, cyclin D, and H-RAS) are known substrates of RBX1-SCF E3 ligases (2, 32) and may accumulate upon RBX1 silencing to trigger DDR, we measured the level of these oncoproteins in U87 cells. We found none of them to be accumulated in response to RBX1 siRNA (Fig. 3A), thus excluding the potential involvement of oncogene activation in RBX1 silencing-induced DSB and DDR.
FIGURE 3.
RBX1 silencing induces the accumulation of CDT1 and ORC1 but not oncoproteins. U87 cells transfected with siRBX1 or siCONT for 24 h were split. At 120 h post-splitting, cells were subjected to Western blot analysis for the indicated proteins with β-actin as the loading control (A). U87 (B) or H1299 (C) cells were transfected with siRBX1 or siCONT for 24 h, followed by cell splitting. At the indicated time points post-splitting, cells were harvested and subjected to Western blot analysis with β-actin as the loading control.
We next determined the potential accumulation of DNA replication licensing proteins CDT1 and ORC1, two previously documented substrates of RBX1-SCF E3 ligases (33–36). As shown in Fig. 3, CDT1 was substantially accumulated upon RBX1 silencing in both U87 cells (Fig. 3B) and H1299 cells (Fig. 3C). ORC1 was accumulated, but in a cell line-dependent manner, with a moderate induction in U87 cells (Fig. 3B) and a greater induction in H1299 cells (Fig. 3C). No accumulation was found in CDC6 (Fig. 3, B and C), another key DNA replication licensing protein (37) previously shown to be a substrate of SCF E3 ligase in yeast (38, 39). Thus, RBX1 silencing abrogates SCF E3 ligase activity and causes CDT1/ORC1 accumulation, which likely triggers DSB and DDR as demonstrated by a recent report that CDT1 overexpression causes DSB to trigger DDR in human cancer cells (31).
We then determined if accumulation of CDT1 or ORC1 contributes to RBX1 silencing-induced G2 arrest by simultaneous silencing of RBX1/CDT1 or RBX1/ORC1 in H1299 cells. Because of the high level of CDT1 accumulation upon RBX1 silencing, only a partial depletion of CDT1 (∼50%) was achieved in RBX1-silenced cells, with the remaining level being much higher than the undetectable level in control cells (supplemental Fig. S3A, lane 5 versus lane 1). On the other hand, ORC1 siRNA completely abrogated ORC1 accumulation by RBX1 silencing, with the ORC1 level comparable with that in control cells (supplemental Fig. S3A, lane 6 versus lane 1). Consequently and expectedly, RBX1 silencing-induced G2 arrest was partially rescued by ORC1 siRNA but not by CDT1 siRNA (supplemental Fig. S3B). The results suggest that ORC1 (possibly CDT1), accumulated upon RBX1 silencing, contributes to observed G2 arrest. Other G2 regulators, such as 14-3-3σ (accumulated) and CDC2/cyclin B1 (depleted) in response to RBX1 silencing (19), are likely cooperating with ORC1/CDT1 to cause the G2 arrest, followed by genomic aneuploidy.
RBX-1 Silencing Induces DDR and Lethal Phenotypes in C. elegans
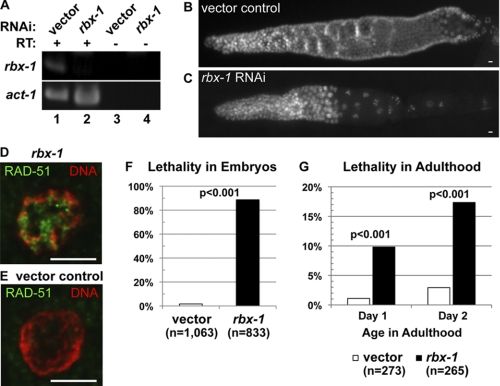
We then extended our in vitro cell culture observations to the in vivo C. elegans model. We first confirmed that C. elegans rbx-1 was indeed silenced by feeding with bacteria expressing double-stranded RNA targeting rbx-1 (Fig. 4A). We then performed RAD51 immunofluorescence staining in the gonad and intestine of C. elegans as the readout of induction of DDR. The gonad is a well established model for DDR in C. elegans (40). The knockdown of rbx-1 resulted in fewer rows of germ cells in the gonad compared with the vector control (Figs. 4, B and C, and 5E). Surprisingly, we did not observe any increase in RAD-51 staining in the mitotic germ cells (supplemental Fig. S4, A and B). Although the germ line of the rbx-1 RNAi worms had twice as many apoptotic corpses compared with the vector control, an observation consistent with a recent study (41), the cellular defect did not correlate with egl-1 transactivation (data not shown), which is normally required for apoptosis associated with DDR in the germ line (42, 43). This finding suggests that the apoptotic response may not be related to DDR. We did observe an apparent reduction in the density of mitotic germ cells in the gonads of the rbx-1 RNAi worms (supplemental Fig. S4B). This latter observation is consistent with reports that an RBX1-SCF complex is required for cell cycle progression of germ cells through G1 (44, 45). We hypothesize that rbx-1 silencing impaired entry into the S phase, which would in turn suppress replication-induced DDR in the mitotic germ line. We next reasoned that the intestine may be an excellent model to examine replication stress-induced DDR because the genome of the intestinal cells is endoreduplicated extensively during development to 32-ploid in adulthood, with the last cycle of endoreduplication occurring prior to adulthood (46). As shown in Figs. 4 (D and E) and 5C, whereas vector control cells showed RAD-51 staining in <5% of intestinal cells, rbx-1 silencing caused a striking induction of positive nuclear RAD-51 staining in ∼50% of intestinal cells, clearly demonstrating that rbx-1 silencing indeed triggered DDR in C. elegans.
FIGURE 4.
rbx-1 silencing induces DDR and lethal phenotypes in C. elegans. Wild-type C. elegans worms were fed bacteria expressing rbx-1-targeting double-stranded RNA or the blank expression vector as described under “Experimental Procedures.” A, total RNA extracted from 100 worms fed for 5 days was either mock (RT−) or reverse-transcribed (RT+) and then analyzed by PCR for the presence of the rbx-1 cDNA. Actin (act-1) served as a loading control. Isolated gonad (B and C) and intestine (D and E) from worms on the 3rd day of adulthood were examined by microscopy. Shown are micrographs of germ cells in intact gonads visualized by DAPI DNA fluorescence (B and C) and intestinal nuclei with RAD-51 immunostaining and DAPI DNA fluorescence (D and E). Scale bars = 5 μm. Bar graphs represent the percentages of offspring embryonic lethality (F) and adult lethality (G) in vector and rbx-1 RNAi worms. Offspring (eggs) were collected from RNAi-treated worms, and embryonic lethality was assessed as unhatched eggs at 48 h after egg collection (F). Adult worms were examined for lethality based on the absence of movement, pharyngeal pumping, and responsiveness to touch at Days 1 and 2 of adulthood (G). The vector and rbx-1 worms were compared by two-tailed Fisher's exact test.
FIGURE 5.
RNAi depletion of CDT-1 suppresses the rbx-1 silencing-induced DDR but not death in C. elegans. A, RT-PCR detection of rbx-1 and actin (act-1) mRNAs in 100 worms fed for 5 days on vector control (lane 1), rbx-1 (lane 2), and cdt-1;rbx-1 (lane 3) RNAi bacterial vectors. B, Western blot of lysates from 100 worms prepared by boiling in 30 μl of SDS-PAGE loading buffer with 3.75 m urea, freeze-thawed, and reboiled in the presence of β-mercaptoethanol prior to gel electrophoresis. Each set of lysates was loaded into three lanes at 2-fold serial dilutions. The blot was probed for CDT-1 (upper panel) and RBX-1 (middle panel) proteins and α-tubulin (lower panel) as a loading control. C, micrographs of intestinal nuclei immunostained for RAD-51 and DAPI DNA fluorescence. Scale bars = 5 μm. D, the graph represents the average fraction of intestinal nuclei with RAD51 staining. Error bars represent mean ± S.D. from a total of four independent experiments (D and F). p < 0.001 for statistical comparisons for vector versus rbx-1 (*) and rbx-1 versus cdt-1;rbx-1 (**) using two-tailed Fisher's exact test. E, the graph represents the average length of the syncytial gonad from the distal tip to the end of pachytene in meiotic prophase. p < 0.001 for statistical comparisons for vector versus rbx-1 (*) and rbx-1 versus cdt-1;rbx-1 (**) using two-tailed Student's t test. Error bars represent mean ± S.E. F, the graph represents the average lethality in the RNAi-treated worms at the indicated age. Twenty worms were examined for each set of RNAi vector(s) per experiment for a total of four independent experiments.
Previous studies showed that RBX1 is crucial for cell cycle progression and chromosome metabolism in C. elegans and that rbx-1 RNAi knockdown causes embryonic lethality and larval developmental arrest (14, 15). For the aforementioned studies, the effects of rbx-1 RNAi were examined in the offspring of the treated worms. In this study, we designed the RNAi experiments to circumvent the early developmental requirements for rbx-1 and to focus on rbx-1 function later in development and in adulthood. The feeding RNAi regiment was initiated with L1 larvae that had already completed embryonic development. Under our RNAi conditions, the L1 larvae developed into morphologically wild-type and fertile adults when fed both the vector control and the rbx-1 RNAi bacteria, which contrasted with the depletion of rbx-1 during early development that led to larval arrest and defects in body morphology (15). By Day 4 on the feeding plates or ∼2 days into adulthood, the rbx-1 silencing caused embryonic lethality in >80% of the offspring (Fig. 4F), confirming the reduction in rbx-1 activity. Intriguingly, we made the novel observation that the adults depleted for rbx-1, which had wild-type body morphology, began to die prematurely by Day 1 into adulthood (Fig. 4G). This time-dependent lethality was significantly more prevalent in the rbx-1 RNAi worms, appearing in 18% of the population at Day 2 (versus 3% in controls) (Fig. 4G) and up to 100% of the population at Day 8 (versus 20% in controls) (Fig. 5F). Thus, RBX-1 is essential for viability or life span of adult C. elegans.
CDT-1 RNAi Silencing Abrogates RBX-1 Silencing-induced DDR but Not Premature Death in Adulthood
We next determined whether rbx-1 silencing in C. elegans also causes accumulation of the C. elegans CDT-1 ortholog and, if so, whether simultaneous silencing of CDT-1 abrogates DDR and rescues the germ cell reduction and death phenotypes. We did not pursue ORC1 because C. elegans has at least two ORC1 homologs, clones Y39A1A.12 and CDC-6, and the true ortholog of human ORC1 has not been well defined. Rbx-1 silencing completely eliminated the accumulation of rbx-1 mRNA (Fig. 5A) and the C. elegans RBX-1 protein (Fig. 5B, lanes 4–6 and 10–12), whereas cdt-1 RNAi completely eliminated CDT-1 protein (lanes 7–9). Consistent with our observations made in human cancer cells, rbx-1 silencing induced remarkable accumulation of CDT1 in C. elegans (Fig. 5B, lanes 4–6). Simultaneous silencing of rbx-1 and cdt-1 reduced CDT-1 to a level comparable with the vector control (Fig. 5B, lanes 10–12 versus lanes 1–3). Consequently, rbx-1 silencing-induced RAD-51 nuclear staining was nearly completely abrogated (Fig. 5C, with quantification data shown in D), indicating that CDT-1 accumulation triggered by rbx-1 silencing is causally related to DDR. The reduced germ cell phenotype in the rbx-1 RNAi worms was potently suppressed by the knockdown of cdt-1 (Fig. 5E). On the other hand, simultaneous cdt-1 silencing accelerated worm death, partially because cdt-1 silencing alone could cause animal death (Fig. 5F). Taken together, these results indicate that CDT-1 accumulation is responsible for rbx-1 silencing-induced DDR and germ cell reduction phenotype, whereas the death phenotype is likely contributable to abnormal accumulations of multiple substrates of RBX1-SCF E3 ligases.
In this study, we mechanistically followed up our recent observations that RBX1 silencing triggers DDR and causes G2 arrest and cell death (19). We have shown here that RBX1 silencing actually caused DNA DSB, likely because of accumulation of DNA replication licensing proteins CDT1 and ORC1. Our study is consistent with a previous observation that depletion of CDT2, a DDBI-CUL4A-associated protein required for CDT1 degradation, causes DNA re-replication and checkpoint activation (47). It is well established that precise duplication of the genome at the S phase of each cell cycle requires initiation of DNA replication from thousands of origins. Initiation of too few origins would cause collapse of replication forks, leading to DNA damage and incomplete replication of the genome, whereas more than one initiation of DNA replication per cell cycle would cause DNA “hyper-replication” or “re-replication” and subsequent DNA damage (48), leading to genomic instability and cancer (49). CDT1 is a key licensing factor that, along with the protein CDC6, functions to license DNA by forming the pre-replicative complex (50), whereas ORC1 is a central component for eukaryotic DNA replication (51). The binding of ORC1 at replication origins serves as the foundation for assembly of the pre-replicative complex, including CDT1 and CDC6 (37). In normal cells, CDT1 activity is precisely controlled by its negative regulator Geminin, which ensures the firing of each replication origin to occur once per cell cycle. CDT1 accumulation, as a result of RBX1 silencing, would disrupt this fine balance, thus causing aberrant replication to trigger DNA damage checkpoints (52). Indeed, a recent study showed that CDT-1 overexpression in human cancer cells causes DSB to trigger DDR (31), whereas overexpression of Double-arked (Dup), the Drosophila ortholog of CDT1, causes DNA re-replication and DNA damage (53). We have shown here that CDT-1 accumulation, as a result of rbx-1 silencing, triggered DDR in the intestinal nuclei of C. elegans. Thus, CDT1 is likely responsible for DSB and subsequent DDR upon RBX1 silencing. This notion is further supported by a recent finding that MLN4924, a small molecule inhibitor of SCF E3 ligases via cullin deneddylation, causes accumulation of CDT1 to trigger DNA re-replication and genomic instability (4).
We also elucidated the mechanism by which RBX1 silencing induces G2 arrest. Using both a molecular biology approach (siRNA silencing) and a pharmacological approach (small molecule inhibitor), we determined that CHK1, activated in response to DNA damage, is responsible for DDR-induced G2 arrest. We further found that RBX1 could be a promising radiosensitizing target whose silencing, by introducing DSB and triggering DDR, renders cancer cells more sensitive to radiation. This provides a sound rationale for future development of an RBX1-targeting agent such as MLN4924 (4) as a novel class of radiosensitizers.
Finally, we demonstrated that RBX-1 is essential for the development of embryos, germ cell abundance, and adult viability or life span in C. elegans. Because RBX1-SCF E3 controls the turnover of many short-lived regulatory proteins in a cell content-dependent and spatially dependent manner, it is very likely that biological phenotype changes resulting from RBX1 inactivation are attributable to the accumulation of a number of its substrates. It is therefore conceivable that the sole silencing of accumulated ORC1/CDT1 is not sufficient to rescue G2 arrest in cancer cells and adult death in C. elegans. Nevertheless, our study provides solid experimental evidence demonstrating an essential role of RBX1 in the maintenance of mammalian genomic stability and in C. elegans development and adulthood.
Supplementary Material
Acknowledgment
We thank Dr. E. Kipreos for providing antibodies to the C. elegans CDT-1 protein.
This work was supported, in whole or in part, by National Institutes of Health Grants CA111554 and CA118762 from NCI (to Y. S.). This work was also supported by Chinese National Nature Sciences Foundation Grant 31071204 and a grant from Fudan University in China (to L. J.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- DDR
- DNA damage response(s)
- DSB
- double-strand break(s)
- siRBX1
- RBX1 siRNA
- siCONT
- control siRNA.
REFERENCES
- 1. Deshaies R. J., Joazeiro C. A. (2009) Annu. Rev. Biochem. 78, 399–434 [DOI] [PubMed] [Google Scholar]
- 2. Nakayama K. I., Nakayama K. (2006) Nat. Rev. Cancer 6, 369–381 [DOI] [PubMed] [Google Scholar]
- 3. Yen H. C., Elledge S. J. (2008) Science 322, 923–929 [DOI] [PubMed] [Google Scholar]
- 4. Soucy T. A., Smith P. G., Milhollen M. A., Berger A. J., Gavin J. M., Adhikari S., Brownell J. E., Burke K. E., Cardin D. P., Critchley S., Cullis C. A., Doucette A., Garnsey J. J., Gaulin J. L., Gershman R. E., Lublinsky A. R., McDonald A., Mizutani H., Narayanan U., Olhava E. J., Peluso S., Rezaei M., Sintchak M. D., Talreja T., Thomas M. P., Traore T., Vyskocil S., Weatherhead G. S., Yu J., Zhang J., Dick L. R., Claiborne C. F., Rolfe M., Bolen J. B., Langston S. P. (2009) Nature 458, 732–736 [DOI] [PubMed] [Google Scholar]
- 5. Nalepa G., Rolfe M., Harper J. W. (2006) Nat. Rev. Drug Discov. 5, 596–613 [DOI] [PubMed] [Google Scholar]
- 6. Petroski M. D., Deshaies R. J. (2005) Nat. Rev. Mol. Cell Biol. 6, 9–20 [DOI] [PubMed] [Google Scholar]
- 7. Chen A., Wu K., Fuchs S. Y., Tan P., Gomez C., Pan Z. Q. (2000) J. Biol. Chem. 275, 15432–15439 [DOI] [PubMed] [Google Scholar]
- 8. Kamura T., Koepp D. M., Conrad M. N., Skowyra D., Moreland R. J., Iliopoulos O., Lane W. S., Kaelin W. G., Jr, Elledge S. J., Conaway R. C., Harper J. W., Conaway J. W. (1999) Science 284, 657–661 [DOI] [PubMed] [Google Scholar]
- 9. Ohta T., Michel J. J., Schottelius A. J., Xiong Y. (1999) Mol. Cell 3, 535–541 [DOI] [PubMed] [Google Scholar]
- 10. Zheng N., Schulman B. A., Song L., Miller J. J., Jeffrey P. D., Wang P., Chu C., Koepp D. M., Elledge S. J., Pagano M., Conaway R. C., Conaway J. W., Harper J. W., Pavletich N. P. (2002) Nature 416, 703–709 [DOI] [PubMed] [Google Scholar]
- 11. Ohta T., Michel J. J., Xiong Y. (1999) Oncogene 18, 6758–6766 [DOI] [PubMed] [Google Scholar]
- 12. Seol J. H., Feldman R. M., Zachariae W., Shevchenko A., Correll C. C., Lyapina S., Chi Y., Galova M., Claypool J., Sandmeyer S., Nasmyth K., Deshaies R. J., Shevchenko A., Deshaies R. J. (1999) Genes Dev. 13, 1614–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swaroop M., Wang Y., Miller P., Duan H., Jatkoe T., Madore S. J., Sun Y. (2000) Oncogene 19, 2855–2866 [DOI] [PubMed] [Google Scholar]
- 14. Moore R., Boyd L. (2004) Genesis 38, 1–12 [DOI] [PubMed] [Google Scholar]
- 15. Sasagawa Y., Urano T., Kohara Y., Takahashi H., Higashitani A. (2003) Genes Cells 8, 857–872 [DOI] [PubMed] [Google Scholar]
- 16. Noureddine M. A., Donaldson T. D., Thacker S. A., Duronio R. J. (2002) Dev. Cell 2, 757–770 [DOI] [PubMed] [Google Scholar]
- 17. Tan M., Davis S. W., Saunders T. L., Zhu Y., Sun Y. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6203–6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlabach M. R., Luo J., Solimini N. L., Hu G., Xu Q., Li M. Z., Zhao Z., Smogorzewska A., Sowa M. E., Ang X. L., Westbrook T. F., Liang A. C., Chang K., Hackett J. A., Harper J. W., Hannon G. J., Elledge S. J. (2008) Science 319, 620–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jia L., Soengas M. S., Sun Y. (2009) Cancer Res. 69, 4974–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamath R. S., Ahringer J. (2003) Methods 30, 313–321 [DOI] [PubMed] [Google Scholar]
- 21. Bickel J. S., Chen L., Hayward J., Yeap S. L., Alkers A. E., Chan R. C. (2010) PLoS Genet. 6, e1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhong W., Feng H., Santiago F. E., Kipreos E. T. (2003) Nature 423, 885–889 [DOI] [PubMed] [Google Scholar]
- 23. Bhalla N., Wynne D. J., Jantsch V., Dernburg A. F. (2008) PLoS Genet. 4, e1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng M., Morgan-Lappe S. E., Yang J., Bockbrader K. M., Pamarthy D., Thomas D., Fesik S. W., Sun Y. (2008) Cancer Res. 68, 7570–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu B., Kim St., Kastan M. B. (2001) Mol. Cell. Biol. 21, 3445–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai Y., Grant S. (2010) Clin. Cancer Res. 16, 376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blasina A., Hallin J., Chen E., Arango M. E., Kraynov E., Register J., Grant S., Ninkovic S., Chen P., Nichols T., O'Connor P., Anderes K. (2008) Mol. Cancer Ther. 7, 2394–2404 [DOI] [PubMed] [Google Scholar]
- 28. Wilson G. D. (2004) Cancer Metastasis Rev. 23, 209–225 [DOI] [PubMed] [Google Scholar]
- 29. Bartek J., Bartkova J., Lukas J. (2007) Oncogene 26, 7773–7779 [DOI] [PubMed] [Google Scholar]
- 30. Hook S. S., Lin J. J., Dutta A. (2007) Curr. Opin. Cell Biol. 19, 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liontos M., Koutsami M., Sideridou M., Evangelou K., Kletsas D., Levy B., Kotsinas A., Nahum O., Zoumpourlis V., Kouloukoussa M., Lygerou Z., Taraviras S., Kittas C., Bartkova J., Papavassiliou A. G., Bartek J., Halazonetis T. D., Gorgoulis V. G. (2007) Cancer Res. 67, 10899–10909 [DOI] [PubMed] [Google Scholar]
- 32. Skaar J. R., D'Angiolella V., Pagan J. K., Pagano M. (2009) Cell 137, 1358–1358e1 [DOI] [PubMed] [Google Scholar]
- 33. Li X., Zhao Q., Liao R., Sun P., Wu X. (2003) J. Biol. Chem. 278, 30854–30858 [DOI] [PubMed] [Google Scholar]
- 34. Méndez J., Zou-Yang X. H., Kim S. Y., Hidaka M., Tansey W. P., Stillman B. (2002) Mol. Cell 9, 481–491 [DOI] [PubMed] [Google Scholar]
- 35. Hu J., Xiong Y. (2006) J. Biol. Chem. 281, 3753–3756 [DOI] [PubMed] [Google Scholar]
- 36. Nishitani H., Sugimoto N., Roukos V., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K. I., Nakayama K., Fujita M., Lygerou Z., Nishimoto T. (2006) EMBO J. 25, 1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Speck C., Chen Z., Li H., Stillman B. (2005) Nat. Struct. Mol. Biol. 12, 965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drury L. S., Perkins G., Diffley J. F. (2000) Curr. Biol. 10, 231–240 [DOI] [PubMed] [Google Scholar]
- 39. Perkins G., Drury L. S., Diffley J. F. (2001) EMBO J. 20, 4836–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gartner A., MacQueen A. J., Villeneuve A. M. (2004) Methods Mol. Biol. 280, 257–274 [DOI] [PubMed] [Google Scholar]
- 41. Gao M. X., Liao E. H., Yu B., Wang Y., Zhen M., Derry W. B. (2008) Cell Death Differ. 15, 1054–1062 [DOI] [PubMed] [Google Scholar]
- 42. Gartner A., Milstein S., Ahmed S., Hodgkin J., Hengartner M. O. (2000) Mol. Cell 5, 435–443 [DOI] [PubMed] [Google Scholar]
- 43. Hofmann E. R., Milstein S., Boulton S. J., Ye M., Hofmann J. J., Stergiou L., Gartner A., Vidal M., Hengartner M. O. (2002) Curr. Biol. 12, 1908–1918 [DOI] [PubMed] [Google Scholar]
- 44. Merlet J., Burger J., Tavernier N., Richaudeau B., Gomes J. E., Pintard L. (2010) Development 137, 3857–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Starostina N. G., Simpliciano J. M., McGuirk M. A., Kipreos E. T. (2010) Dev. Cell 19, 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hedgecock E. M., White J. G. (1985) Dev. Biol. 107, 128–133 [DOI] [PubMed] [Google Scholar]
- 47. Jin J., Arias E. E., Chen J., Harper J. W., Walter J. C. (2006) Mol. Cell 23, 709–721 [DOI] [PubMed] [Google Scholar]
- 48. Arias E. E., Walter J. C. (2007) Genes Dev. 21, 497–518 [DOI] [PubMed] [Google Scholar]
- 49. Dutta A. (2007) Nat. Genet. 39, 10–11 [DOI] [PubMed] [Google Scholar]
- 50. Rialland M., Sola F., Santocanale C. (2002) J. Cell Sci. 115, 1435–1440 [DOI] [PubMed] [Google Scholar]
- 51. Bell S. P. (2002) Genes Dev. 16, 659–672 [DOI] [PubMed] [Google Scholar]
- 52. Petropoulou C., Kotantaki P., Karamitros D., Taraviras S. (2008) Front. Biosci. 13, 4485–4494 [DOI] [PubMed] [Google Scholar]
- 53. Mehrotra S., Maqbool S. B., Kolpakas A., Murnen K., Calvi B. R. (2008) Genes Dev. 22, 3158–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.