Abstract
The long pentraxin 3 (PTX3), serum amyloid P component (SAP), and C-reactive protein belong to the pentraxin family of pattern recognition molecules involved in tissue homeostasis and innate immunity. They interact with C1q from the classical complement pathway. Whether this also occurs via the analogous mannose-binding lectin (MBL) from the lectin complement pathway is unknown. Thus, we investigated the possible interaction between MBL and the pentraxins. We report that MBL bound PTX3 and SAP partly via its collagen-like domain but not C-reactive protein. MBL-PTX3 complex formation resulted in recruitment of C1q, but this was not seen for the MBL-SAP complex. However, both MBL-PTX3 and MBL-SAP complexes enhanced C4 and C3 deposition and opsonophagocytosis of Candida albicans by polymorphonuclear leukocytes. Interaction between MBL and PTX3 led to communication between the lectin and classical complement pathways via recruitment of C1q, whereas SAP-enhanced complement activation occurs via a hitherto unknown mechanism. Taken together, MBL-pentraxin heterocomplexes trigger cross-activation of the complement system.
Keywords: Complement, Inflammation, Innate Immunity, Lectin, Phagocytosis, C1q, CRP, PTX3, SAP, Mannose-binding Lectin
Introduction
Mannose-binding lectin (MBL)2 is a multimeric collagen-like serum protein consisting of an N-terminal cysteine-rich domain, a collagen-like domain, and a carbohydrate-recognition domain. MBL originates from the MBL2 gene located on chromosome 10q11.2-q21 and is primarily synthesized by hepatocytes (1). It is found in the blood with a highly variable inter-individual serum concentration in healthy individuals ranging from less than 20 μg/liter to more than 5000 μg/liter. This variation is genetically determined and regulated by polymorphisms in the promoter and coding regions of the MBL2 gene (2). MBL in serum is partly found associated with three different serine proteases (MASP-1, -2, and -3). MASP-2 is the main activator of the lectin pathway of complement (3), whereas MASP-1 may enhance lectin pathway activation (4, 5). However, it has recently been shown at least in mice that MASP-1 is crucial for alternative pathway activation by mediating cleavage of pro factor D to active factor D (6). No conclusive serine protease activity has so far been attributed to MASP-3. In addition, MBL is associated with two non-enzymatic molecules, sMAP and MAP-1, where the latter has been shown to inhibit complement activation by competition with MASP-2 (7). The binding site of the MASPs and the MAPs are located in the collagen-like domain of the MBL molecule (8).
MBL induces specific innate immune responses via recognizing infectious pathogen-associated molecular pattern, such as peptidoglycan (9), lipoteichoic acid (10), lipopolysaccharide (11), and 1,3-β-d-glucan (12). It has been shown that MBL binds to a range of clinically important microorganisms (13, 14).
The pentraxins constitute a protein superfamily characterized by a cyclic multimeric structure (15). Based on the primary structure of the subunit, the pentraxins are defined as short pentraxins or long pentraxins. C-reactive protein (CRP) and serum amyloid P-component (SAP) comprise the classical short pentraxins, whereas pentraxin 3 (PTX3) was the first long pentraxin to be described.
CRP and SAP share substantial sequence similarity of about 50% on the amino acid level (16). However, notable differences include basal serum levels, changes in expression during acute phase responses, and binding specificities. Under normal conditions the serum concentration of CRP in humans is less than 3 mg/liter but may increase 100–1000-fold after an acute phase stimulus (17). By contrast, the concentration of SAP in human serum is rather constant and varies only between 30 and 50 mg/liter under normal and inflammatory conditions. Both CRP and SAP are produced by hepatocytes. Unlike CRP and SAP, the major sources of PTX3 are different cell types of extrahepatic origin including myeloid, endothelial, and epithelial cells. In response to inflammatory and infectious stimuli, PTX3 synthesis is rapidly up-regulated and released into surrounding tissues and the blood stream. Under normal conditions PTX3 is hardly detectable in human serum (<2 ng/ml), whereas it may be found in concentrations of 200–800 μg/liter in response to inflammation (18).
Both CRP and SAP are molecules originating from genes present on chromosome 1q23 (15). CRP and SAP are non-covalently associated to form an oligomer composed of 5 identical 23-kDa protomers (16, 19). PTX3 is a molecule originating from a gene situated on chromosome 3q25, which assembles into an octameric structure composed of 45-kDa identical protomers linked by disulfide bonds (20). In contrast to CRP, SAP and PTX3 are both glycoproteins. All three molecules share C-terminal structural similarity, whereas the N-terminal sequence of CRP and SAP differs from PTX3 (18).
The pentraxins recognize different classes of molecular patterns present on microorganisms but also endogenous extracellular matrix proteins as well as structures exposed on dying host cells (21). A common theme for CRP, SAP, and PTX3 is that they all interact with C1q from the classical pathway of complement and may upon binding to a ligand mediate complement activation (22–24). Both CRP and PTX3 have also been shown to interact with Ficolin-1 and Ficolin-2, which are recognition molecules in the lectin complement pathway (25–27).
Invasive Candida infections have increased in most population-based surveys and is associated with an overall mortality of ∼40% (28, 29). Major risk groups are patients at intensive care units (∼50%) and patients experiencing severe or complicated abdominal surgery, but other risk factors are well described and include immunoincompetence, intravenous drug users, malignant diseases, broad spectrum antibiotics, and steroids (30). C. albicans is still the most common species and is involved in 50–75% of candidemia cases (28). It has been demonstrated that MBL plays an important role in the protection against C. albicans by enhancing complement activation and uptake in polymorphonuclear leukocytes (PMNs) (31). However, whether this also involves accessory assistance from e.g. the pentraxins is unknown.
Based on the knowledge of the structural and functional similarities between C1q, the ficolins, and MBL, we hypothesized that MBL and the pentraxins interact and modulate host defense. To have a pathophysiological readout, we used C. albicans as a model of infection.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Mouse monoclonal antibody (Ab) against MBL (HYB 131-01 and 131-11) was purchased from BioPorto (Gentofte, Denmark), and biotinylated HYB 131-01 was produced using the Immunoprobe biotinylation kit (Sigma) according to the manufacturer's instruction. Biotinylated anti-PTX3 monoclonal and polyclonal Ab were produced at the Mario Negri Institute for Pharmacological Research (Milan, Italy), and anti-MASP-3 monoclonal Ab were characterized in our laboratory (32). Both rabbit polyclonal Abs against SAP and CRP were purchased from Calbiochem. Mouse monoclonal antibodies against human Ficolin-1 (clone FCN115), Ficolin-2 (clone FCN216, clone FCN219, and biotinylated FCN219), and Ficolin-3 (clone FCN334) were characterized in our laboratory (33, 34). Horseradish peroxidase (HRP) conjugated donkey anti-rabbit IgG and streptavidin-HRP were purchased from Amersham Biosciences. Streptavidin-PE, allophycocyanin (APC) mouse anti-human CD15, and APC mouse anti-human IgG were purchased from BD Pharmingen. A rabbit polyclonal anti-C1q Ab, HRP-conjugated rabbit anti-mouse IgG, goat anti-mouse IgG-FITC conjugate, swine anti-rabbit IgG-FITC conjugate, rabbit polyclonal anti-C3 Ab, and rabbit polyclonal anti-C4 fluorescein isothiocyanate (FITC) conjugate were obtained from Dako (Glostrup, Denmark).
Hybond ECL nitrocellulose membrane was from Amersham Biosciences. Precision prestained protein standard was from Bio-Rad. NuPAGE 3∼8% Tris acetate gel, 4∼12% Bis-tris gel, Tris acetate SDS running buffer, MOPS SDS running buffer, NuPAGE transfer buffer, and NuPAGE lithium dodecyl sulfate sample buffer were all from Invitrogen. SuperSignal West Femto Maximum Sensitivity Substrate was from Pierce, Bie, and Berntsen A/S (Roedovre, Denmark). Microtiter plates (MaxiSorp) were from Nalge Nunc (Roskilde, Denmark). Ortho-phenylene diamine dihydrochloride tablets were from Dako (Glostrup, Denmark). Phosphate-buffered saline (PBS) buffer (10 mm Na2HPO4, 1.5 mm KH2PO4, 137 mm NaCl, 2.7 mm KCl, pH 7.4) was obtained from Bie and Berntsen). HEPES buffer (25 mm HEPES, 155 mm NaCl, 5 mm CaCl2, pH 7.4) was obtained from the hospital pharmacy at Rigshospitalet, Copenhagen. SAP and CRP were purchased from Calbiochem. The C. albicans strain was obtained from a clinical isolate (H29929) (Statens Serum Institut, Denmark). Sabouraud dextrose agar was purchased from Difco. PolymorphprepTM was purchased from Axis-Shield Poc. Red cell lysis buffer was obtained from Roche Diagnostics. Human serum albumin was obtained from ZLB Bioplasma. Other than the reagents described above, all chemicals were from Sigma.
Recombinant Proteins and Human Sera
Recombinant MBL, PTX3, and MASP-3 expressed in CHO cells were purified as previously described (22, 32, 35). Purity of recombinant proteins was analyzed by SDS-PAGE followed by Coomassie Blue staining. Human serum-derived SAP and ascites-derived CRP were purchased from Calbiochem.
Serum samples were obtained from healthy volunteer donors with informed consent. MBL-defect serum (MBL−serum) was obtained from an MBL-defect individual with normal levels of C1q and SAP. C1q-defect serum (C1q−serum) was obtained from a C1q-defect individual with normal levels of MBL and SAP. MBL-defect serum depleted of SAP (MBL−/SAPdepleted serum) was generated by incubation with DNA-cellulose (36). For C1q-defect serum depleted of MBL (C1q−/MBLdepleted serum) and SAP (C1q−/MBL, SAPdepleted serum) MBL was depleted by agitating the C1q-defect serum with mannan-agarose beads, and SAP was depleted as described above. The quality of the depletion procedure was determined by Western blot and FACS analysis. Furthermore, the existence of Ab against C. albicans in the sera used above was also determined to be negative in FACS analysis by APC-conjugated mouse anti-human IgG Ab.
SDS-PAGE and Western Blots
Proteins were separated by NuPAGE 3∼8% Tris acetate gels or 4∼12% Bis-tris gels under non- reducing or reducing conditions according to the method of Laemmli (37) and stained with Coomassie Brilliant Blue. The separated proteins were transferred to nitrocellulose using the Xcell II mini-Cell blot apparatus in NuPAGE transfer buffer. For detection of MBL, PTX3, CRP, and SAP, biotinylated HYB 131-01, biotinylated anti-PTX3 polyclonal Ab, and rabbit polyclonal Ab against CRP and SAP were used as primary antibody, respectively. In the subsequent procedure, blots were stained with HRP-conjugated donkey anti-rabbit IgG Ab or streptavidin-HRP. Development was performed with SuperSignal West Femto Maximum Sensitivity Substrate on auto-radiographic films. Precision prestained protein standard was utilized as a molecular weight standard.
Culture and FITC Labeling of C. albicans
The C. albicans strain was maintained on Sabouraud dextrose agar by agar streak at 37 °C every 4 weeks. C. albicans was cultured for 12.5 h in yeast extract/peptone/dextrose medium, pH 4.0, at 30 °C with orbital agitation at 200 rpm before use. Under these conditions, C. albicans grew as a >95% pure yeast-phase population (38). After washing with PBS, harvested C. albicans was suspended to an appropriate concentration in PBS. For fluorescence labeling, C. albicans was labeled with 5 μg/ml FITC for 5 min at room temperature in the dark followed by washing with PBS five times and then resuspended in PBS to the desired concentration.
Solid Phase ELISA
To determine whether MBL interacts with PTX3 or CRP, ELISA microtiter plates were coated with or without mannan (10 μg/ml). For SAP, the plates were coated with MBL or BSA in 2-fold serial dilutions starting at 0.2 μg/ml to reduce nonspecific background. All reaction volumes were 100 μl, and microtiter plates were washed after each step in TBS-T (20 mm Tris, 150 mm NaCl, 2.5 mm CaCl2, 0.05% v/v Tween 20, pH 7.4). Exposed microtiter plates were blocked with 1% BSA/TBS-T. For PTX3 and CRP, binding serial dilutions of MBL (0.0625, 0.125, 0.25, 0.5, 1 μg/ml) were allowed to react with mannan in the wells before the addition of PTX3 or CRP in 2-fold serial dilutions starting at a concentration of 2 μg/ml. For SAP binding SAP was added to the wells in 2-fold serial dilutions starting at concentration of 1 μg/ml. Bound PTX3, SAP, CRP, or MBL were detected using biotinylated anti-PTX3 polyclonal Ab, anti-SAP polyclonal Ab, and anti-CRP polyclonal or biotinylated monoclonal anti-MBL clone HYB 131-01 followed by incubation with streptavidin-HRP and HRP-conjugated F(ab)2 from anti-rabbit donkey IgG, respectively. Final peroxidase reaction was performed by using ortho-phenylenediamine/hydrogen peroxidase solution as substrate. The reactions were terminated using 1 m H2SO4, and optical density was read at 490 nm.
Alternatively, microtiter plates were coated with or without mannan (10 μg/ml) before incubation with a mixture of MBL (1 μg/ml) and PTX3 (2 μg/ml) or CRP (2 μg/ml). In parallel experiments the effect of the single proteins added alone was also determined. To determine MBL-SAP interaction, plates were coated with MBL (1 μg/ml) or BSA (1 μg/ml) directly and then incubated with SAP (2 μg/ml). Bound MBL, PTX3, or SAP was detected by ELISA as depicted above.
In some experiments microtiter plates were coated with HYB 131-01 instead of mannan before the addition of MBL (1 μg/ml) for PTX3 binding or coated directly with MBL (0.2 μg/ml) for SAP binding followed by incubation of PTX3 (1 μg/ml) or SAP (1 μg/ml) in the presence or absence of EDTA (10 mm), GlcNAc (0.1 m), or mannose (0.1 m). Bound PTX3, SAP, or MBL was detected as described above.
Interaction between MBL and PTX3 or SAP on C. albicans
C. albicans cells (0.2 × 106) were washed and resuspended in HEPES buffer containing 1% heat-inactivated FCS (HEPES/HI-FCS) followed by incubation with PTX3 (10 μg/ml), SAP (20 μg/ml), or CRP (20 μg/ml) in the absence or presence of MBL (5 μg/ml) at 37 °C for 1 h. All reaction volumes were 100 μl, and C. albicans were washed after each step in cold HEPES/HI-FCS. Bound proteins were detected with biotinylated anti-PTX3 monoclonal Ab, anti-CRP, SAP polyclonal Ab, or HYB 131-11 by incubation at 4 °C for 30 min followed by 15 min of incubation at 4 °C with streptavidin-PE, FITC-conjugated anti-rabbit IgG from swine, or FITC-conjugated anti-mouse IgG from goat, respectively. Finally, the C. albicans were washed and resuspended in 200 μl of cold HEPES/HI-FCS. Flow cytometry was carried out on a BD FACSCalibur (BD Biosciences), and data were analyzed by BD CellQuest Pro software.
In some experiments C. albicans was incubated with MBL and PTX3 or SAP in the presence of MASP-3 (5 μg/ml). PTX3, SAP, or MBL were detected as described above. To detect MASP-3 binding to MBL, an anti-MASP-3 monoclonal Ab was used (32). Furthermore, to determine dose-dependent competitive inhibition of MASP-3 to interaction between MBL and PTX3 or SAP various concentrations of MASP-3 (0∼2500 ng/ml) were applied as above.
To confirm the formation and presence of MBL-SAP complexes in normal serum, C. albicans were incubated with various concentrations of SAP (5∼20 μg/ml) or normal serum (5∼10%). In some experiments, C. albicans was incubated with 10% MBL-defect serum or normal serum. Alternatively, MBL (1 μg/ml) was preincubated with C. albicans before the addition of 10% MBL-defect serum. SAP or MBL binding was detected as described above.
Binding of C1q to MBL-PTX3 or SAP Complexes on C. albicans
The procedure of PTX3 or SAP binding to C. albicans was the same as described above. C. albicans was incubated with or without MBL (5 μg/ml), PTX3 (10 μg/ml) (or SAP, 20 μg/ml) or a combination of both. C1q (30 μg/ml) was incubated with C. albicans immediately after the formation of MBL-PTX3 or SAP complexes. In alternative experiments different concentrations of C1q (5∼40 μg/ml) were applied. Bound C1q was detected using anti-C1q polyclonal Ab and anti-rabbit IgG-FITC from swine and analyzed by FACS as above.
C4 and C3 Deposition on C. albicans
C4 and C3 deposition on C. albicans were assessed by FACS analysis. As source of complement component, MBL or C1q-defect serum was used in subsequent experiments. 10% MBL−/SAPdepleted serum and C1q−/MBL, SAPdepleted serum were prepared by dilution in HEPES/HI-FCS. For C4 and C3 deposition, C. albicans (0.2 × 106) were incubated with 10% MBL−/SAPdepleted serum or C1q−/MBL, SAPdepleted serum. Alternatively, both sera were restored by exogenous MBL (5 μg/ml) or C1q (30 μg/ml), respectively. All reaction volumes were 100 μl, and C. albicans were washed after each step in HEPES/HI-FCS. C4 and C3 deposition were detected with a rabbit anti-human C4 FITC-conjugated Ab and a rabbit anti-human C3 Ab followed by incubation with swine anti-rabbit FITC-conjugated IgG, respectively. Finally, C4 and C3 deposition were assessed by flow cytometry and assessed by the BD CellQuest Pro software.
In some experiments C. albicans was preincubated with MBL (5 μg/ml), PTX3 (10 μg/ml), or SAP (20 μg/ml) or a combination of both before assessment of C4 and C3 deposition. As a control, MBL levels in MBL or C1q-defect serum and C1q−/MBL/SAPdepleted serum was determined by assessing MBL binding to C. albicans by both FACS analysis and Western blot. C. albicans were incubated with 10% sera described above at 37 °C for 1 h. Bound MBL was detected with HYB 131-11 and goat anti-mouse IgG FITC conjugate. Furthermore, SAP levels in MBL-defect serum and MBL−/SAPdepleted serum were determined by Western blot. The sera described above before and after depletion (5 μg of total proteins) were analyzed by SDS-PAGE under reducing-conditions followed by detection of rabbit polyclonal anti-SAP Ab- and HRP-conjugated donkey anti-rabbit IgG. Furthermore, whether the ficolins in the sera described above bound to C. albicans and whether these sera mediated complement activation were also determined by anti-ficolin mAbs, anti-C4, or C3 Ab by flow cytometry.
Isolation of PMNs
Freshly isolated human PMNs were applied in a phagocytosis assay as described below. PMNs were purified from the blood of healthy volunteers using a density gradient separation method with heparin as anticoagulant as described previously (39). In brief, heparinized blood was carefully layered over polymorphoprepTM and centrifuged at 500 relative centrifugal force for 35 min at 25 °C. Neutrophils were removed and washed in Hanks' balanced salt solution without Ca2+/Mg2+. After lysis of erythrocytes, PMNs were washed in Hanks' balanced salt solution without Ca2+/Mg2+ and resuspended in RPMI 1640/0.05% (w/v) human serum albumin.
Opsonization
FITC-labeled C. albicans (0.1 × 106) were opsonized in 100 μl of 10% MBL−/SAPdepleted serum or 10% C1q−/MBL, SAPdepleted serum prepared by dilution in HEPES/HI-FCS for 30 min of incubation at 37 °C. After washing with HEPES/HI-FCS, opsonized FITC-labeled C. albicans were resuspended in RPMI 1640 for further use in the phagocytosis assay.
In some experiments, FITC-labeled C. albicans were preincubated with MBL (10 μg/ml), PTX3 (10 μg/ml), SAP (20 μg/ml), or a combination of MBL and PTX3 (or SAP) for 1 h at 37 °C before the addition of serum above. Alternatively, C1q (40 μg/ml) was incubated after MBL, PTX3, SAP, or a combination of those was anchored on FITC-labeled C. albicans.
Phagocytosis Assay
Opsonized FITC-labeled C. albicans were incubated with freshly isolated PMNs (0.1 × 106) in RPMI 1640 at 37 °C for 15 min with agitation. Phagocytosis was stopped by the addition of 500 μl of ice-cold RPMI 1640. APC anti-mouse CD15 was utilized as a marker to human PMN for the phagocytosis of C. albicans. The harvested cell pellet was stained with APC anti-mouse CD15 for 15 min at 4 °C and analyzed by FACS analysis. Red fluorescence of APC (FL-4) was plotted against green fluorescence of FITC (FL-1). Phagocytosis (phagocytic index) of the FITC-labeled C. albicans was evaluated by determining the mean fluorescence intensity (MFI) multiplied by the percentage of the PMNs positive for FITC-labeled C. albicans (FITC-positive PMNs) and expressed as results.
In some experiments phagocytosis of the FITC-labeled C. albicans opsonized with only MBL, PTX3, SAP or a combination of MBL and PTX3 or SAP without the addition of serum was determined as above. All data were measured at three different days (n = 6) with freshly isolated PMNs from different healthy donors.
Statistical Analysis
Data represent the mean ± S.E. of at least three independent experiments. Statistical analysis was performed using Student's t test and GraphPad Prism, Version 5.0 (GraphPad Software, San Diego, CA). p < 0.05 and p < 0.01 were considered to represent a statistical significant or very significant difference between two sample means, respectively.
RESULTS
Characterization of Recombinant MBL
Recombinant MBL was expressed in CHO cells and purified as previously described (35). We analyzed the purity of the purified recombinant MBL preparation by using SDS-PAGE under reducing conditions. Protein bands were visualized by Coomassie Blue stain (supplemental Fig. S1A). No additional bands except for the expected MBL band at 32 kDa were observed. To determine the oligomerization state of recombinant MBL, recombinant MBL was separated by SDS-PAGE and analyzed by Western blot under non-reducing or reducing conditions (supplemental Fig. S1B). Under non-reducing conditions, MBL presented higher oligomer forms but disassembled as a single band of ∼32 kDa under reducing conditions. This result showed that recombinant MBL produced in the CHO expression system were able to create highly oligomerized MBL.
Binding of the Pentraxins to MBL in Solid Phase ELISA
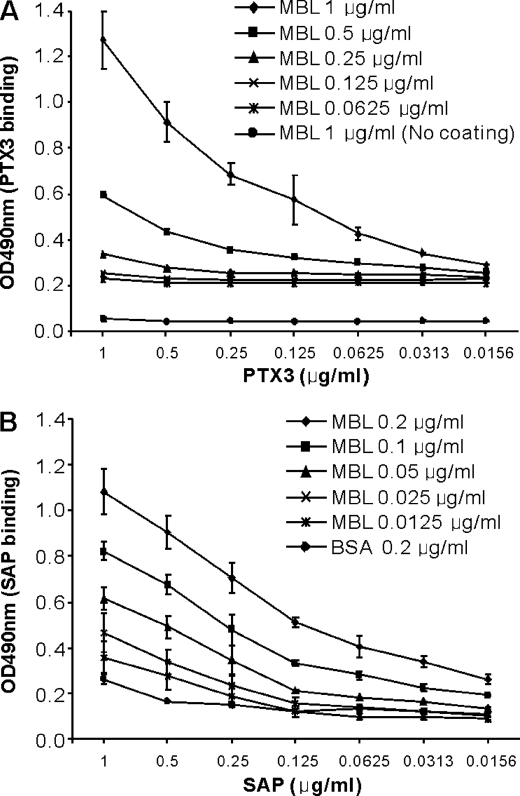
To investigate a possible interaction between MBL and the pentraxins, we used different ELISA set ups. Microtiter wells coated with mannan were incubated with various concentrations of MBL before the addition of PTX3 in equal concentrations in 2-fold serial dilutions. PTX3 bound to MBL immobilized on mannan in a dose-dependent manner (Fig. 1A). To reduce nonspecific background in this system, we choose to directly coat recombinant MBL to polystyrene wells in different concentrations and then incubate SAP in 2-fold serial dilutions. As shown in Fig. 1B, SAP bound to immobilized MBL in a dose-dependent manner. No dose-dependent binding of CRP to MBL was observed when MBL was bound to mannan followed by the addition of CRP (data not shown). When PTX3, SAP, and CRP were coated directly to the microtiter wells, we detected all proteins with the corresponding anti-pentraxin antibodies (data not shown). In all experimental setups MBL was shown to bind to the microtiter wells (data not shown).
FIGURE 1.
Dose-dependent binding of PTX3 and SAP to MBL in solid phase ELISA. A, binding of PTX3 to MBL is shown. Microtiter plates coated with or without mannan (10 μg/ml) were incubated with the indicated concentrations of MBL before the addition of PTX3 in 2-fold serial dilutions. B, binding of SAP to MBL is shown. To reduce nonspecific background, MBL or BSA (control) was coated directly to microtiter plates in various concentrations and incubated with SAP in 2-fold serial dilutions. Binding of the pentraxins to the microtiter plates was assessed by anti-PTX3 and anti-SAP antibodies. Results are represented as the mean ± S.E. from three independent experiments with duplicates.
MBL-PTX3 interaction also occurred when both MBL and PTX3 were applied together at the same time (supplemental Fig. S2A). MBLSAP interaction was also confirmed (supplemental Fig. S2B). No reaction was seen when single proteins were applied alone. Again no reaction was observed for CRP (supplemental Fig. S2C).
Binding of Pentraxins to C. albicans in the Presence of MBL Detected by Flow Cytometry
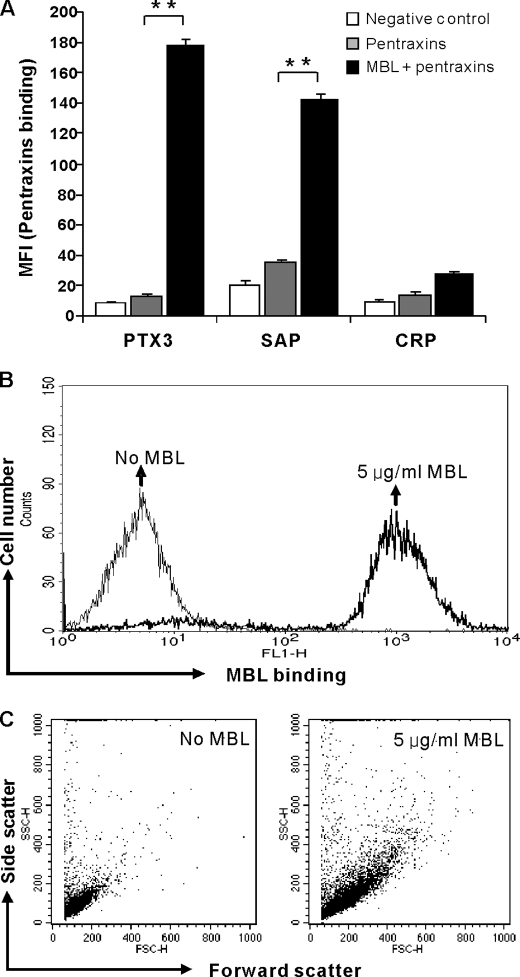
Assessed by flow cytometry, a significant binding of PTX3 and SAP to C. albicans was observed only in the presence of MBL, whereas no significant binding was observed for CRP (Fig. 2A). MBL binding (Fig. 2B) and MBL-dependent agglutination (Fig. 2C) to C. albicans were confirmed.
FIGURE 2.
Binding of pentraxins to C. albicans in the presence of MBL. A, C. albicans was incubated with PTX3 (10 μg/ml), SAP (20 μg/ml), or CRP (20 μg/ml) in the absence or presence of MBL (5 μg/ml) and detected by FACS analysis. As controls, MBL binding (B) and MBL-dependent agglutination (C) were assessed. The MFI was used to assess PTX3, SAP, CRP, and MBL binding. Agglutination was assessed by the change in forward and scatter morphology. Data are expressed as the mean ± S.E. from triplicate experiments. Results are representative of at least six independent experiments. The asterisks indicate the statistical significance versus controls: **, p < 0.01.
Formation of MBL-SAP Complexes in Normal Serum
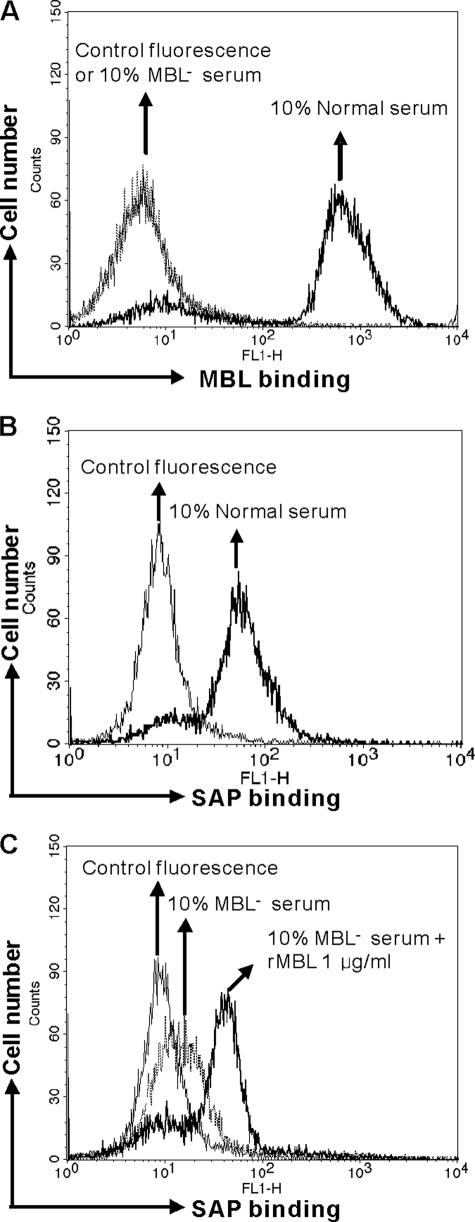
SAP is a normal serum protein; thus, we investigated whether serum-derived MBL-SAP complexes may be formed on C. albicans. We could observe MBL binding to C. albicans in 10% normal serum but not in MBL-defect serum assessed by FACS analysis (Fig. 3A). SAP binding was only observed in normal serum (Fig. 3B), but virtually no binding of SAP could be observed in MBL-defect serum (MBL−) (Fig. 3C). However, when we spiked MBL− serum with recombinant MBL, SAP binding to C. albicans could easily be detected (Fig. 3C).
FIGURE 3.
Formation and presence of MBL-SAP complexes in normal serum. A, detection of serum MBL on C. albicans is shown. B, detection of serum SAP binding to C. albicans is shown. C, detection of serum SAP binding in MBL-defect (MBL−) serum with or without exogenous MBL is shown. The MFI was used to assess SAP or MBL binding. Results are representative of three independent experiments.
PTX3 and SAP Bind to MBL in a Calcium-dependent Manner
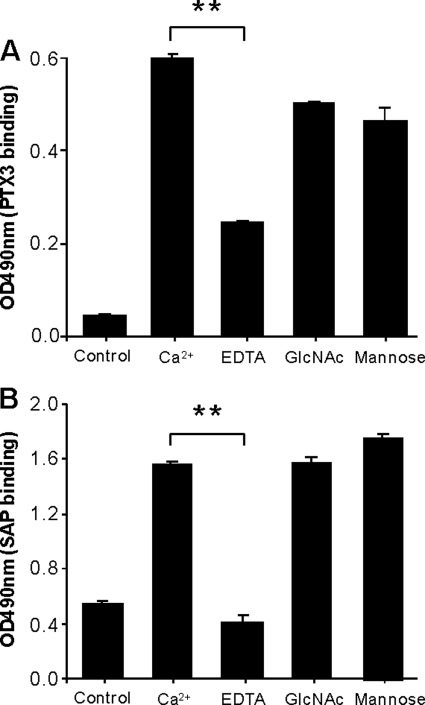
To clarify whether the binding of PTX3 and SAP to MBL was dependent on calcium, we assessed their binding to MBL in microtiter plates. In the case of PTX3 we immobilized MBL to an anti-MBL monoclonal antibody coated in microtiter plates. In the case of SAP we coated MBL directly in microtiter plates due to nonspecific interaction with the anti-MBL antibody. In the presence of TBS with Ca2+ both PTX and SAP bound (Fig. 4, A and B). The addition of EDTA significantly reduced the binding. Inclusion of either GlcNAc or mannose did not influence the binding of PTX3 or SAP to immobilized MBL. As a control, the MBL binding to the microtiter plates was also determined and revealed no reduction in any of the experimental set ups (data not shown).
FIGURE 4.
Effect of EDTA, GlcNAc, and mannose on the binding of PTX3 or SAP to MBL. A, microtiter plates coated with or without (control) anti-MBL monoclonal Ab (HYB 131-01) were incubated with MBL (1 μg/ml) before the addition of PTX3 (1 μg/ml) alone in TBS-T buffer containing Ca2+ (2.5 mm) or the buffer containing EDTA (10 mm), GlcNAc (0.1 m), or mannose (0.1 m), respectively. Bound PTX3 was detected as depicted above. B, microtiter plates coated with MBL (0.2 μg/ml) or control BSA (0.2 μg/ml) followed by incubation of SAP (1 μg/ml) in TBS-T buffer containing Ca2+ (2.5 mm) or the buffer containing EDTA (10 mm), GlcNAc (0.1 m), and mannose (0.1 m), respectively are shown. Data are expressed as the mean ± S.E. from triplicate experiments. Results are representative of three independent experiments. The asterisks indicate the statistical significance versus controls: **, p < 0.01.
MBL Interacts with PTX3 and SAP Partly via Its Collagen-like Domain
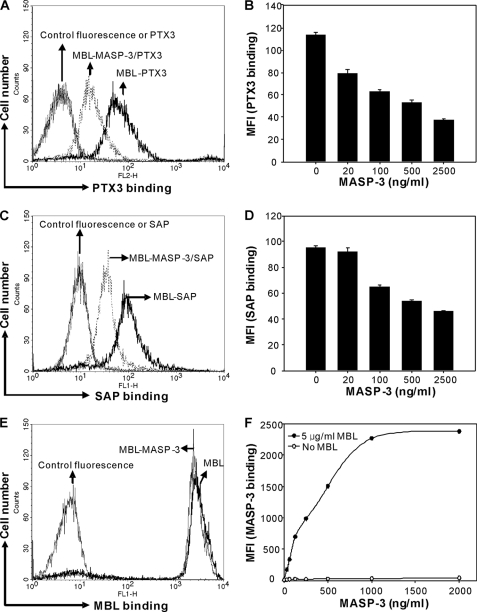
To further explore which domain on the MBL molecule PTX3 and SAP interact with, we performed competition experiments using MASP-3, as MASP-3 is known to interact with collagen-like domain of MBL (8). When MBL was bound to C. albicans in the presence of MASP-3, a markedly reduced binding of PTX3 and SAP was observed (Fig. 5, A and C). The inhibitory effect of MASP-3 was shown to be dose-dependent (Fig. 5, B and D). In parallel experiments, we showed that the binding of MASP-3 to MBL indeed took place (Fig. 5, E and F).
FIGURE 5.
Involvement of collagen-like domain of MBL in MBL-PTX3 or MBL-SAP complex formation. C. albicans was incubated with or without MBL (5 μg/ml) followed by incubation with PTX3 (10 μg/ml) (A) or SAP (20 μg/ml) (C) in the absence or presence of MASP-3 (5000 ng/ml). Dose-dependent inhibitory effect of MASP-3 was determined by incubating PTX3 (10 μg/ml) (B) or SAP (20 μg/ml) (D) in the presence of MBL (5 μg/ml) with increasing concentrations of MASP-3. E, control for MBL binding was determined using MBL (5 μg/ml) in the absence or presence of MASP-3 (5000 ng/ml). F, control for MASP-3 binding was determined with or without MBL in increasing concentrations of MASP-3. The MFI was used to assess PTX3, SAP, MBL, or MASP-3 binding. Results (A, C, E, and F) are representative of three independent experiments. B and D are expressed as the mean ± S.E. from triplicate experiments and are representative of two independent experiments that yield similar results.
C1q Interacts with MBL-PTX3 Complexes but Not MBL-SAP Complexes
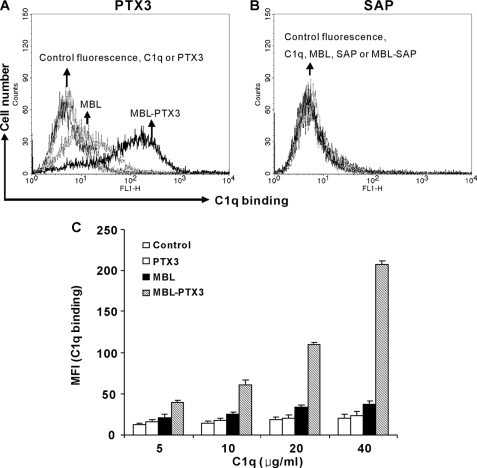
The interaction between the pentraxins and C1q is well characterized. Hence, we envisaged that the interaction between MBL and PTX3 or SAP might invoke C1q recruitment. To substantiate this hypothesis, we established MBL-PTX3 or SAP complexes on C. albicans as described above and then determined by flow cytometry whether a physiological concentration of C1q could bind to the complexes. We found that C1q bound to PTX3 complexed with MBL on C. albicans in a concentration-dependent manner (Fig. 6, A and C). By contrast, C1q did not bind to SAP complexed with MBL (Fig. 6B).
FIGURE 6.
C1q binding to MBL-PTX3 complexes but not MBL-SAP complexes. C. albicans treated with MBL (5 μg/ml), PTX3 (10 μg/ml) (A), SAP (20 μg/ml) (B), or mixtures of both were incubated with C1q (30 μg/ml). Bound C1q was detected. As a control, C. albicans was incubated with C1q alone. C, in some experiments, increasing concentrations of C1q were applied to determine dose dependence. The MFI was used to assess C1q binding. Results A and B are representative of three independent experiments. Data C are expressed as the mean ± S.E. from triplicate experiments. Results are representative of two independent experiments that yield similar results.
MBL-PTX3 and MBL-SAP Complexes Trigger Amplification of Complement Activation
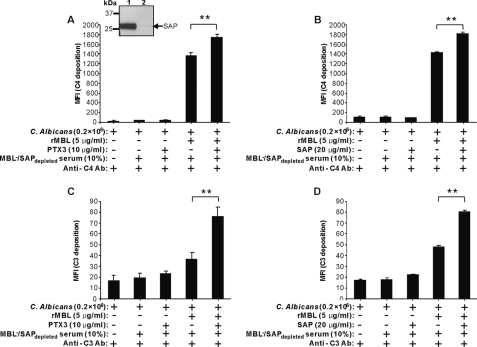
To characterize the physiological relevance of the MBL-PTX3 and MBL-SAP complexes on C. albicans, we determined their influence on complement activation using MBL− serum depleted for SAP (MBL−/SAPdepleted serum). SAP depletion was assessed by Western blot, compared with the level before depletion (inset, Fig. 7A). Furthermore, anchorage of MBL or MBL-PTX3 or SAP complexes on C. albicans was also confirmed before induction of complement activation for each experiment. Then C4 and C3 deposition were assessed by using 10% MBL−/SAPdepleted serum as a complement source incubated with C. albicans in the presence of MBL, PTX3, or SAP. We observed C4 and C3 deposition only when MBL was present (Fig. 7). However, deposition of C4 and C3 was significantly enhanced by PTX3 or SAP in the presence of MBL, whereas this was not apparent when PTX3 or SAP were added alone (Fig. 7), showing that both MBL-PTX3 and MBL-SAP complexes amplify complement activation.
FIGURE 7.
Enhancement of C4 and C3 deposition by MBL-PTX3 or SAP complex formation on C. albicans. MBL-PTX3 or MBL-SAP complexes were first established on C. albicans as described above before the addition of 10% MBL−/SAPdepleted serum. C4 and C3 deposition were assessed. As controls, the addition of MBL and PTX3 alone was applied. The inset shows depletion of SAP from MBL− serum using DNA-cellulose. Lane 1, before depletion; lane 2, after depletion. The MFI was used to assess C4 and C3 deposition. Results are presented as the mean ± S.E. of samples analyzed in triplicate. Results are representative of three independent experiments. The asterisks indicate the statistical significance versus controls: **, p < 0.01.
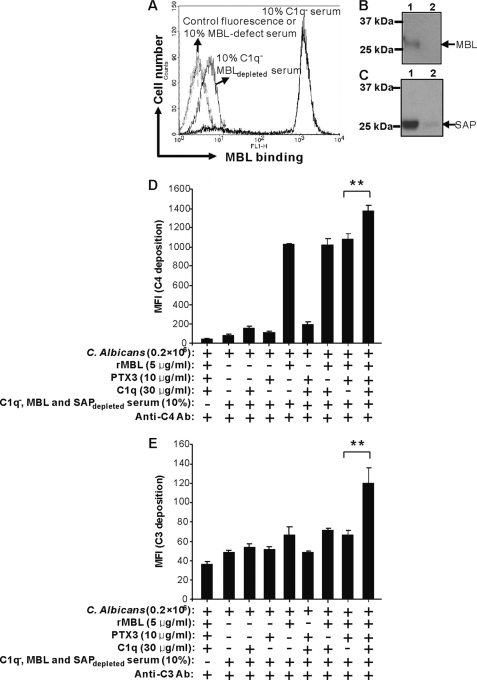
C1q Is Necessary for Amplification of Complement Activation Induced by MBL-PTX3 Complexes but Not by MBL-SAP Complexes
Based on our previous findings, we reasoned that PTX3-dependent enhancement of complement activation on C. albicans could be dependent on C1q. To substantiate this we used serum deficient of C1q that was depleted for MBL and SAP (C1q−/MBL, SAPdepleted serum). MBL and SAP depletion were assessed by both Western blot and FACS analysis (Fig. 8, A–C). Subsequently, C4 and C3 deposition were assessed in the presence of MBL, PTX3, C1q, or a combination of the proteins. We found that exogenous C1q significantly enhanced C4 and C3 deposition in this serum when added in conjunction with MBL and PTX3 (Fig. 8D). Virtually no complement deposition was detected in the presence of PTX3, C1q, or mixture of both in the absence of MBL (Fig. 8D). In control experiments it was revealed that the other lectin pathway recognition molecules ficolin-2 or ficolin-3 could not bind C. albicans. Moreover, we did not observe that any of the sera used contained detectable anti-C. albicans antibodies that could have activated the classical complement pathway and masked the results observed (data not shown).
FIGURE 8.
C1q-dependent enhancement of C4 and C3 deposition by MBL-PTX3 complexes on C. albicans. MBL was depleted from C1q− serum and assessed by FACS (A) and Western blot (B), then SAP was depleted and assessed by Western blot (C). Lane 1, before depletion; lane 2, after depletion. The MBL-PTX3 complex was first established on C. albicans as described above followed by induction of C4 and C3 deposition in 10% C1q−/MBL and SAPdepleted serum with or without exogenous C1q. D, and E, C4 and C3 deposition were assessed by FACS. As controls, the addition of MBL, PTX3 alone, or mixtures of both were applied. The MFI was used to assess C4 and C3 deposition. Results are presented as the mean ± S.E. of samples analyzed in triplicate. Results are representative of three independent experiments. The asterisks indicate the statistical significance versus controls: **, p < 0.01.
C1q Is Necessary for Amplification of Serum-dependent Opsonophagocytosis Induced by MBL-PTX3 Complexes but Not by MBL-SAP Complexes
To investigate whether the MBL-PTX3 complexes and MBL-SAP complexes could influence serum-dependent opsonophagocytosis of C. albicans by PMNs, we used FITC-labeled C. albicans that were preopsonized with 10% MBL−/SAPdepleted serum with or without MBL, PTX3, or SAP. Thereafter, phagocytosis of opsonized C. albicans was induced by freshly isolated PMNs, which was analyzed by flow cytometry. We observed that serum lacking MBL was not able to mediate phagocytosis of C. albicans (Fig. 9A). However, when MBL was added, the phagocytic activity was restored. Recombinant MBL alone could mediate ∼20% of the phagocytic index obtained when both recombinant MBL and serum was used together (data not shown).
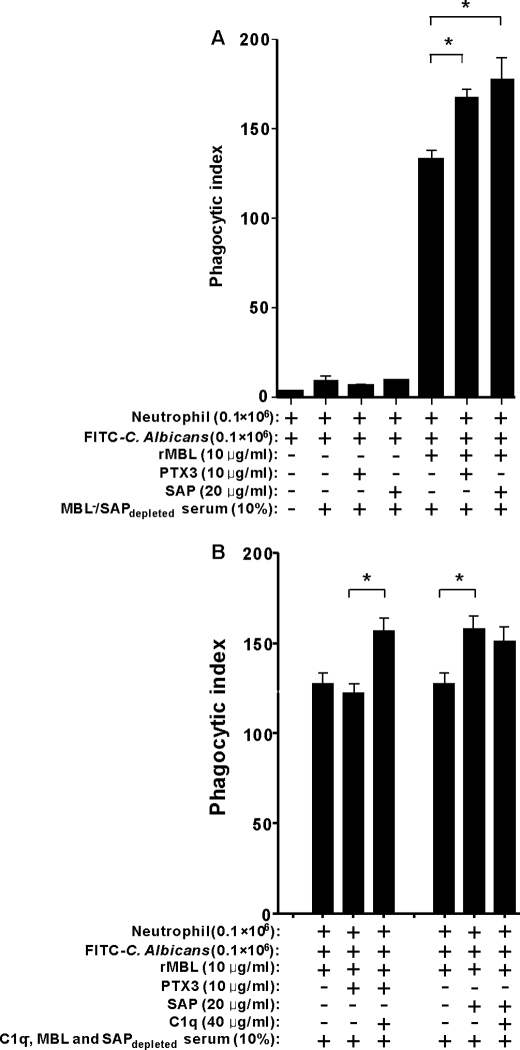
FIGURE 9.
C1q-dependent enhancement of phagocytosis by human neutrophils. A, complement dependent-phagocytosis of C. albicans by human neutrophils is shown. C. albicans were labeled with FITC and preincubated with or without MBL, PTX3, or SAP or a combination of the molecules to induce complement activation in MBL−/SAPdepleted serum. B, effect of C1q on phagocytosis of C. albicans induced by MBL-PTX3 complex is shown. C1q−/MBL and SAPdepleted serum was used as a complement source to opsonize FITC-labeled C. albicans after incubation with or without MBL, PTX3, SAP, C1q, or a combination of the molecules. Phagocytosis was analyzed by FACS. The bars represent values expressed as the average opsonophagocytosis ± S.E. of six donors (A) or three donors (B) measured on three different days. The asterisk indicates the statistical significance versus controls: *, p < 0.05.
We found that MBL-PTX3 and MBL-SAP complexes enhanced opsonophagocytosis in the presence of serum (Fig. 9A). To investigate the influence of C1q on this system, we used C1q-defect serum as the complement source that was depleted for MBL and SAP (C1q−/MBL, SAPdepleted). Consistent with our previous data on complement activation, in C1q−/MBL, SAPdepleted serum no obvious difference was observed in the opsonophagocytosis experiments when MBL-PTX3 complexes were compared with MBL alone (Fig. 9B). However, a marked enhancement of opsonophagocytosis was observed when exogenous C1q was added to the reaction mixture (Fig. 9B). By contrast, C1q did not affect the enhanced opsonophagocytosis induced by MBL-SAP complex compared with MBL alone (Fig. 9B).
DISCUSSION
The pentraxins, CRP, SAP, and PTX3, are multifunctional pattern-recognition proteins involved in inflammation and innate immunity (21). The interaction of pentraxins with C1q and its role in the activation of the classical complement pathway are well described (22–24). Recently, several studies have shown that Ficolin-1 and Ficolin-2 from the lectin complement pathway interact with CRP and PTX3 via their fibrinogen-like domain, expanding the pattern recognition capabilities of the ficolin molecules (25–27, 40). In this study we investigated whether MBL, which has structural and functional similarities with C1q and the ficolins, interacts with the pentraxins using C. albicans as a model pathogen.
When we examined the interaction between the pentraxins and MBL using the ELISA platform as the read-out, we could demonstrate a direct interaction between MBL and PTX3 or SAP but not with CRP. The pentraxins, in contrast to MBL, do not recognize C. albicans; thus, we used this organism as a model to further substantiate the MBL-pentraxin interaction using MBL as anchoring molecule. We could show that PTX3 and SAP could be detected on C. albicans only in the presence of MBL but not CRP when we used flow cytometry as the detection system. SAP is present in normal serum in relatively high concentrations; thus, we were able to investigate the interaction between endogenous serum MBL and SAP on C. albicans using normal and naturally occurring MBL-defect serum. This experiment showed that SAP could be detected on C. albicans in normal serum but not in MBL-defect serum. However, when recombinant MBL was added to MBL-defect serum, the binding of SAP to C. albicans could be restored, supporting our previous observation in a system with purified proteins. This finding indicate that MBL-SAP complexes may be a primary biological defense mechanism present in the circulation or created immediately after an antimicrobial challenge even before an inflammatory reaction has been elicited.
Inclusion of EDTA in the binding buffer attenuated the binding of PTX3 and SAP to MBL, whereas neither excess mannose nor GlcNAc blocked these interactions, showing that the interactions between MBL and the pentraxins are partly dependent on divalent cations. By contrast, this does not appear to be the case for the interaction between the ficolins and pentraxins, which is Ca+-independent (25–27). MASP-3 interacts with some specific residues in the collagen-like domains of MBL and the ficolins (8). Our experiments clearly showed that MASP-3 and the pentraxins competed for the same binding sites on MBL, suggesting that PTX3 and SAP appear to partly interact via the MASP binding motif of the collagen-like domain. This is different from the ficolins (ficolin-1 and -2), which primarily appear to interact with CRP and PTX3 via their globular fibrinogen-like domains (26, 27, 40).
Interaction of C1q with the pentraxins and its role in the activation of classical complement pathway have been extensively characterized (21). We speculated whether MBL-pentraxin complex formation indeed might recruit C1q. Our results clearly demonstrated that C1q formed complexes when both MBL and PTX3 were present on C. albicans. By contrast, MBL-SAP complex formation were not able to recruit C1q. We assume that this difference might be because interaction of C1q with PTX3 and SAP might be different, i.e. that the interaction between MBL and PTX3 allows binding of C1q, whereas there might be a steric hindrance not allowing C1q to interact with the MBL-SAP complexes. It has previously been reported that PTX3 interacts with the globular head region of C1q, whereas it is still controversial whether SAP interacts either with the globular head region or collagen-like region of C1q (23, 41, 42). This difference could explain our results.
We then investigated whether the MBL-pentraxins interaction could modulate complement activity assessed by C4 and C3 deposition on C. albicans. To control the different parameters, we depleted an MBL-defect serum without measurable antibodies against C. albicans for SAP and added sequentially MBL, PTX3, or SAP to the reaction mixture. The results demonstrated that MBL was crucial for complement factors C4 and C3 deposition on C. albicans but that the addition of PTX3 or SAP significantly enhanced complement deposition. We then went on to use a C1q-defect serum that was depleted for MBL and SAP and reconstituted with exogenous MBL, PTX3, and C1q. This experiment confirmed that C1q was a prerequisite in order for the MBL-PTX3 complex to amplify complement deposition. These data demonstrated that the classical pathway of complement may serve as a key link of amplification for lectin pathway activation under inflammatory conditions when MBL-PTX3 complexes are formed. Surprisingly, we found that MBL-SAP interaction also induced significant enhancement of complement deposition on C. albicans despite no C1q recruitment, suggesting that an unknown potential complement-related serum factor could collaborate with MBL-SAP complexes and amplify complement activation. However, the molecular mechanism behind the latter amplification is still an enigma that requires further studies. Previously the existence of an MBL-dependent C2 bypass mechanism that activates C3 and the alternative pathway has been described (43). Although not formally proven in this study, our results indicate that the initial complement activation steps mediated by the MASPs of the lectin pathway might be bypassed by several different mechanisms. This could be a very important compensatory mechanism in inflammatory situations where certain complement components might have been consumed or in various inherited or acquired complement deficiency states.
MBL has been shown to bind strongly to C. albicans through mannan, thus, resulting in complement activation and subsequent opsonophagocytosis by PMNs (31, 44). To further extend and elucidate the physiological relevance of MBL collaboration with PTX3 and SAP, we performed phagocytosis experiments of C. albicans by PMNs using either MBL-defect serum that was depleted for SAP or C1q-defect serum that was depleted for MBL and SAP. Our results demonstrated that MBL-defect serum hardly could induce opsonophagocytosis of C. albicans. When MBL was added to MBL-defect serum, opsonophagocytosis was markedly increased. MBL without the presence of serum did also enhance opsonophagocytosis but only to about 20% of that seen when MBL was added in the presence of serum (data not shown). MBL-PTX3 and MBL-SAP complexes without serum increased opsonophagocytosis only to the level as seen for MBL alone (data not shown). However, in the presence of MBL both PTX3 and SAP amplified serum-dependent opsonophagocytosis, which agrees with the complement activation results. Furthermore MBL-PTX3 amplification was again dependent on the presence of C1q, suggesting that the classical pathway could cross-talk with the lectin pathway. The MBL-SAP complex-induced amplification of opsonophagocytosis was independent of C1q compatible with the complement activation experiments.
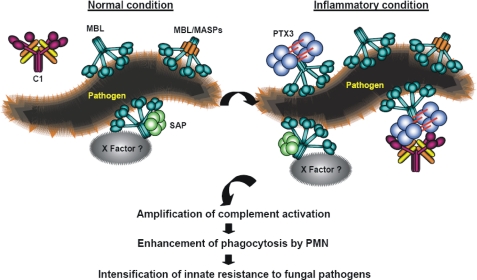
Based on our results, we suggest a model as outlined in Fig. 10 where the MBL-SAP complex operates in normal serum in the earliest phase of an infectious challenge when the body encounters an invading pathogen, where the possibility for the MBL-PTX3 complexes requires an inflammatory reaction stimulating synthesis of PTX3. However, both these types of complexes will work in parallel and contribute to enhance host defense.
FIGURE 10.
A proposed model of the novel complement amplification mechanisms created by MBL-PTX3 and MBL-SAP complexes. MBL-PTX3 interaction induces amplification of complement activation via C1q recruitment and the classical pathway, resulting in increased deposition of complement factors. MBL-SAP interaction induces amplification of complement activation via a hitherto unidentified X serum factor(s) without involvement of C1q.
In conclusion, this study demonstrates the existence of two novel complement amplification mechanisms. One of these mechanisms consists of complexes comprising MBL, PTX3, and C1q that amplify complement activation via the classical pathway, whereas the other involves MBL and SAP that amplify complement activation via a hitherto unknown mechanism.
Supplementary Material
This work was supported by grants from The Benzon Foundation, The Lundbeck foundation, The Carlsberg Foundation, Rigshospitalet, The Capital Region of Denmark, The Novo Nordisk Foundation, The European Commission (Project Tolerage), Telethon, and the Italian Ministry of Health.

The on-line version of this article (available at http://www.jbc.org) contains supplemental S1 and S2.
- MBL
- mannose-binding lectin
- rMBL
- recombinant MBL
- Ab
- antibody
- APC
- allophycocyanin
- CD
- collagen-like domain
- CRP
- C-reactive protein
- MASP
- mannose-binding lectin-associated serine protease
- MFI
- mean fluorescence intensity
- PE
- phycoerythrine
- PMN
- polymorphonuclear leukocyte
- PTX3
- the long penttraxin 3
- SAP
- serum amyloid P component
- Bis-tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- HI-FCS
- heat-inactivated FCS.
REFERENCES
- 1. Takahashi K., Ip W. E., Michelow I. C., Ezekowitz R. A. (2006) Curr. Opin. Immunol. 18, 16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garred P., Larsen F., Seyfarth J., Fujita R., Madsen H. O. (2006) Genes Immun 7, 85–94 [DOI] [PubMed] [Google Scholar]
- 3. Thiel S., Vorup-Jensen T., Stover C. M., Schwaeble W., Laursen S. B., Poulsen K., Willis A. C., Eggleton P., Hansen S., Holmskov U., Reid K. B., Jensenius J. C. (1997) Nature 386, 506–510 [DOI] [PubMed] [Google Scholar]
- 4. Chen C. B., Wallis R. (2004) J. Biol. Chem. 279, 26058–26065 [DOI] [PubMed] [Google Scholar]
- 5. Takahashi M., Iwaki D., Kanno K., Ishida Y., Xiong J., Matsushita M., Endo Y., Miura S., Ishii N., Sugamura K., Fujita T. (2008) J. Immunol. 180, 6132–6138 [DOI] [PubMed] [Google Scholar]
- 6. Takahashi M., Ishida Y., Iwaki D., Kanno K., Suzuki T., Endo Y., Homma Y., Fujita T. (2010) J. Exp. Med. 207, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skjoedt M. O., Hummelshoj T., Palarasah Y., Honore C., Koch C., Skjodt K., Garred P. (2010) J. Biol. Chem. 285, 8234–8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teillet F., Lacroix M., Thiel S., Weilguny D., Agger T., Arlaud G. J., Thielens N. M. (2007) J. Immunol. 178, 5710–5716 [DOI] [PubMed] [Google Scholar]
- 9. Nadesalingam J., Dodds A. W., Reid K. B., Palaniyar N. (2005) J. Immunol. 175, 1785–1794 [DOI] [PubMed] [Google Scholar]
- 10. Polotsky V. Y., Fischer W., Ezekowitz R. A., Joiner K. A. (1996) Infect. Immun. 64, 380–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devyatyarova-Johnson M., Rees I. H., Robertson B. D., Turner M. W., Klein N. J., Jack D. L. (2000) Infect. Immun. 68, 3894–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koneti A., Linke M. J., Brummer E., Stevens D. A. (2008) Infect. Immun. 76, 994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack D. L., Klein N. J., Turner M. W. (2001) Immunol. Rev. 180, 86–99 [DOI] [PubMed] [Google Scholar]
- 14. Neth O., Jack D. L., Dodds A. W., Holzel H., Klein N. J., Turner M. W. (2000) Infect. Immun. 68, 688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garlanda C., Bottazzi B., Bastone A., Mantovani A. (2005) Annu. Rev. Immunol. 23, 337–366 [DOI] [PubMed] [Google Scholar]
- 16. Emsley J., White H. E., O'Hara B. P., Oliva G., Srinivasan N., Tickle I. J., Blundell T. L., Pepys M. B., Wood S. P. (1994) Nature 367, 338–345 [DOI] [PubMed] [Google Scholar]
- 17. Hirschfield G. M., Pepys M. B. (2003) QJM 96, 793–807 [DOI] [PubMed] [Google Scholar]
- 18. Mantovani A., Garlanda C., Doni A., Bottazzi B. (2008) J. Clin. Immunol. 28, 1–13 [DOI] [PubMed] [Google Scholar]
- 19. Shrive A. K., Cheetham G. M., Holden D., Myles D. A., Turnell W. G., Volanakis J. E., Pepys M. B., Bloomer A. C., Greenhough T. J. (1996) Nat. Struct. Biol. 3, 346–354 [DOI] [PubMed] [Google Scholar]
- 20. Inforzato A., Rivieccio V., Morreale A. P., Bastone A., Salustri A., Scarchilli L., Verdoliva A., Vincenti S., Gallo G., Chiapparino C., Pacello L., Nucera E., Serlupi-Crescenzi O., Day A. J., Bottazzi B., Mantovani A., De Santis R., Salvatori G. (2008) J. Biol. Chem. 283, 10147–10161 [DOI] [PubMed] [Google Scholar]
- 21. Bottazzi B., Doni A., Garlanda C., Mantovani A. (2010) Annu. Rev. Immunol. 28, 157–183 [DOI] [PubMed] [Google Scholar]
- 22. Bottazzi B., Vouret-Craviari V., Bastone A., De Gioia L., Matteucci C., Peri G., Spreafico F., Pausa M., D'Ettorre C., Gianazza E., Tagliabue A., Salmona M., Tedesco F., Introna M., Mantovani A. (1997) J. Biol. Chem. 272, 32817–32823 [DOI] [PubMed] [Google Scholar]
- 23. Nauta A. J., Bottazzi B., Mantovani A., Salvatori G., Kishore U., Schwaeble W. J., Gingras A. R., Tzima S., Vivanco F., Egido J., Tijsma O., Hack E. C., Daha M. R., Roos A. (2003) Eur. J. Immunol. 33, 465–473 [DOI] [PubMed] [Google Scholar]
- 24. Baruah P., Dumitriu I. E., Peri G., Russo V., Mantovani A., Manfredi A. A., Rovere-Querini P. (2006) J. Leukoc. Biol. 80, 87–95 [DOI] [PubMed] [Google Scholar]
- 25. Ng P. M., Le Saux A., Lee C. M., Tan N. S., Lu J., Thiel S., Ho B., Ding J. L. (2007) EMBO J. 26, 3431–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang J., Koh J., Lu J., Thiel S., Leong B. S., Sethi S., He C. Y., Ho B., Ding J. L. (2009) PLoS Pathog. 5, e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma Y. J., Doni A., Hummelshøj T., Honoré C., Bastone A., Mantovani A., Thielens N. M., Garred P. (2009) J. Biol. Chem. 284, 28263–28275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaller M. A., Diekema D. J. (2007) Clin. Microbiol. Rev. 20, 133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arendrup M. C., Fuursted K., Gahrn-Hansen B., Schønheyder H. C., Knudsen J. D., Jensen I. M., Bruun B., Christensen J. J., Johansen H. K. (2008) Clin. Microbiol. Infect. 14, 487–494 [DOI] [PubMed] [Google Scholar]
- 30. León C., Ruiz-Santana S., Saavedra P., Galván B., Blanco A., Castro C., Balasini C., Utande-Vázquez A., González, de Molina F. J., Blasco-Navalproto M. A., López M. J., Charles P. E., Martín E., Hernández-Viera M. A. (2009) Crit. Care Med. 37, 1624–1633 [DOI] [PubMed] [Google Scholar]
- 31. Brouwer N., Dolman K. M., van Houdt M., Sta M., Roos D., Kuijpers T. W. (2008) J. Immunol. 180, 4124–4132 [DOI] [PubMed] [Google Scholar]
- 32. Skjoedt M. O., Palarasah Y., Munthe-Fog L., Jie Ma Y., Weiss G., Skjodt K., Koch C., Garred P. (2010) Immunobiology 215, 921–931 [DOI] [PubMed] [Google Scholar]
- 33. Munthe-Fog L., Hummelshøj T., Hansen B. E., Koch C., Madsen H. O., Skjødt K., Garred P. (2007) Scand J. Immunol. 65, 383–392 [DOI] [PubMed] [Google Scholar]
- 34. Munthe-Fog L., Hummelshøj T., Ma Y. J., Hansen B. E., Koch C., Madsen H. O., Skjødt K., Garred P. (2008) Mol. Immunol. 45, 2660–2666 [DOI] [PubMed] [Google Scholar]
- 35. Larsen F., Madsen H. O., Sim R. B., Koch C., Garred P. (2004) J. Biol. Chem. 279, 21302–21311 [DOI] [PubMed] [Google Scholar]
- 36. de Haas C. J., van Leeuwen E. M., van Bommel T., Verhoef J., van Kessel K. P., van Strijp J. A. (2000) Infect. Immun. 68, 1753–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 38. Sudbery P., Gow N., Berman J. (2004) Trends Microbiol. 12, 317–324 [DOI] [PubMed] [Google Scholar]
- 39. Oh H., Siano B., Diamond S. (2008) J. Vis. Exp. 17, pii: 745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tanio M., Wakamatsu K., Kohno T. (2009) Mol. Immunol. 47, 215–221 [DOI] [PubMed] [Google Scholar]
- 41. Roumenina L. T., Ruseva M. M., Zlatarova A., Ghai R., Kolev M., Olova N., Gadjeva M., Agrawal A., Bottazzi B., Mantovani A., Reid K. B., Kishore U., Kojouharova M. S. (2006) Biochemistry 45, 4093–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ying S. C., Gewurz A. T., Jiang H., Gewurz H. (1993) J. Immunol. 150, 169–176 [PubMed] [Google Scholar]
- 43. Selander B., Mårtensson U., Weintraub A., Holmström E., Matsushita M., Thiel S., Jensenius J. C., Truedsson L., Sjöholm A. G. (2006) J. Clin. Invest. 116, 1425–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Asbeck E. C., Hoepelman A. I., Scharringa J., Herpers B. L., Verhoef J. (2008) BMC Microbiol. 8, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.