Abstract
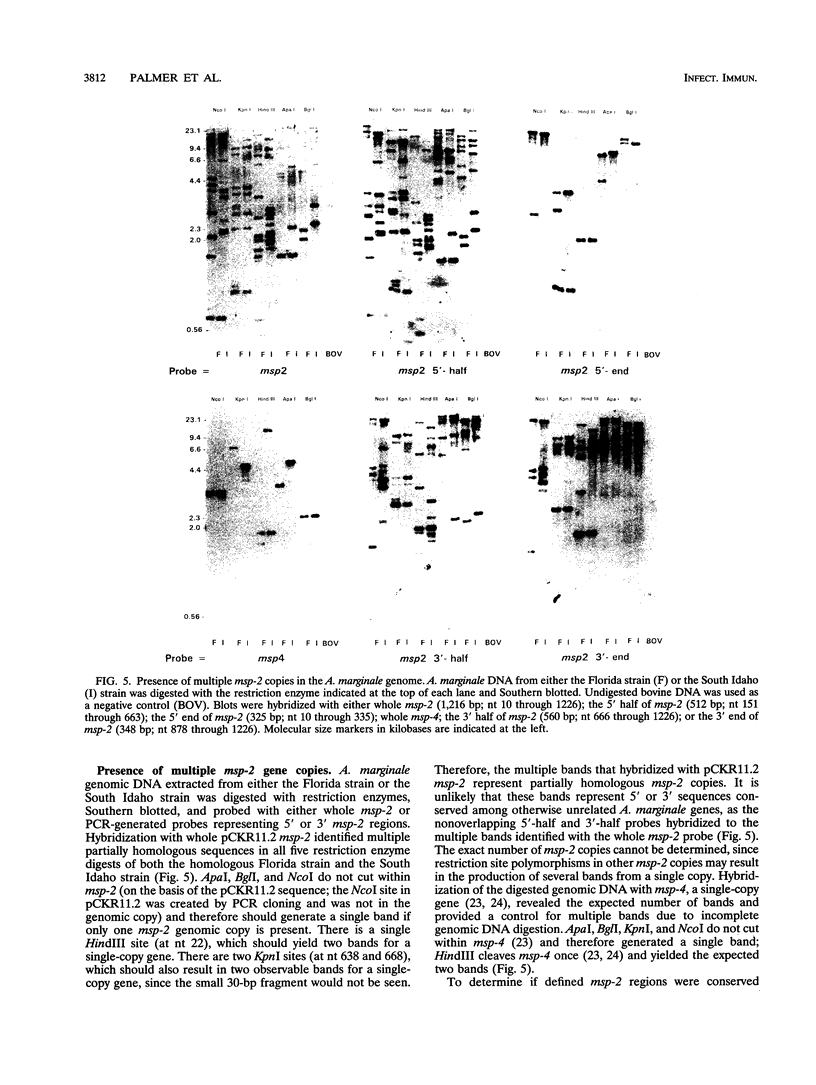
An Anaplasma marginale Florida msp-2 gene was cloned and expressed in Escherichia coli. Pulsed-field gel electrophoresis and Southern blot analysis revealed the presence of multiple msp-2 gene copies that were widely distributed throughout the chromosomes of all three strains examined. Genomic polymorphism among copies was greatest in the 5' end of msp-2 but also occurred in 3' regions. The presence of gene-copy-specific epitopes was indicated by the reactivity of the cloned msp-2 copy with some, but not all, monoclonal antibodies that bound native MSP-2. Multiple antigenically distinct MSP-2 molecules were expressed within strains and were coexpressed by individual A. marginale organisms. These results suggest that expression of polymorphic msp-2 gene copies is responsible for the significant percentages of A. marginale organisms within strains that do not react with individual anti-MSP-2 monoclonal antibodies. Sequence analysis revealed highly significant MSP-2 homology with two rickettsial surface proteins, A. marginale MSP-4 and Cowdria ruminantium MAP-1. Immunization with MSP-4 has been shown to induce protective immunity in a manner similar to that of immunization with MSP-2. These findings support the hypothesis that A. marginale surface proteins are targets of protective immune responses but are antigenically polymorphic.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. H., Smith R. D. Differential extraction of antigens of Anaplasma marginale. Am J Vet Res. 1988 Feb;49(2):257–260. [PubMed] [Google Scholar]
- Alleman A. R., Kamper S. M., Viseshakul N., Barbet A. F. Analysis of the Anaplasma marginale genome by pulsed-field electrophoresis. J Gen Microbiol. 1993 Oct;139(10):2439–2444. doi: 10.1099/00221287-139-10-2439. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Barbet A. F., Allred D. R. The msp1 beta multigene family of Anaplasma marginale: nucleotide sequence analysis of an expressed copy. Infect Immun. 1991 Mar;59(3):971–976. doi: 10.1128/iai.59.3.971-976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriks I. S., Palmer G. H., McGuire T. C., Allred D. R., Barbet A. F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989 Feb;27(2):279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriks I. S., Stiller D., Palmer G. H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J Clin Microbiol. 1993 Aug;31(8):2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kieser S. T., Eriks I. S., Palmer G. H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990 Apr;58(4):1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J. N. Cryogenic preservation of Anaplasma marginale with Dimethyl sulfoxide. Am J Vet Res. 1972 Dec;33(12):2557–2560. [PubMed] [Google Scholar]
- Löhr K. F. Immunisierung gegen Babesiose und Anaplasmose von 40 nach Kenya importierten Charollais-Rindern und Bericht über Erscheinungen der Photosensibilität bei diesen Tieren. Zentralbl Veterinarmed B. 1969 Feb;16(1):40–46. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-James S., James M. A., Benitez M. T., Leon E., Baek B. K., Guillen A. T. Efficacy of purified Anaplasma marginale initial bodies as a vaccine against anaplasmosis. Parasitol Res. 1991;77(2):93–101. doi: 10.1007/BF00935421. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Kawazu S., Minami T. Analysis of protein compositions and surface protein epitopes of Anaplasma centrale and Anaplasma marginale. J Vet Med Sci. 1991 Feb;53(1):73–79. doi: 10.1292/jvms.53.73. [DOI] [PubMed] [Google Scholar]
- Oberle S. M., Barbet A. F. Derivation of the complete msp4 gene sequence of Anaplasma marginale without cloning. Gene. 1993 Dec 22;136(1-2):291–294. doi: 10.1016/0378-1119(93)90482-i. [DOI] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F. Expression and immune recognition of the conserved MSP4 outer membrane protein of Anaplasma marginale. Infect Immun. 1993 Dec;61(12):5245–5251. doi: 10.1128/iai.61.12.5245-5251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Musoke A. J., Katende J. M., Rurangirwa F., Shkap V., Pipano E., Davis W. C., McGuire T. C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988 Feb;18(1):33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Kocan K. M., Barron S. J., Hair J. A., Barbet A. F., Davis W. C., McGuire T. C. Presence of common antigens, including major surface protein epitopes, between the cattle (intraerythrocytic) and tick stages of Anaplasma marginale. Infect Immun. 1985 Dec;50(3):881–886. doi: 10.1128/iai.50.3.881-886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Palmer G. H., Oberle S. M., Barbet A. F., Goff W. L., Davis W. C., McGuire T. C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988 Jun;56(6):1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. J., Dimmock C. K., de Vos A. J., Rodwell B. J. Bovine leucosis virus contamination of a vaccine produced in vivo against bovine babesiosis and anaplasmosis. Aust Vet J. 1988 Sep;65(9):285–287. doi: 10.1111/j.1751-0813.1988.tb16144.x. [DOI] [PubMed] [Google Scholar]
- Shkap V., Pipano E., McGuire T. C., Palmer G. H. Identification of immunodominant polypeptides common between Anaplasma centrale and Anaplasma marginale. Vet Immunol Immunopathol. 1991 Aug;29(1-2):31–40. doi: 10.1016/0165-2427(91)90050-m. [DOI] [PubMed] [Google Scholar]
- Tebele N., McGuire T. C., Palmer G. H. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect Immun. 1991 Sep;59(9):3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet A. H., Jongejan F., van Kleef M., van der Zeijst B. A. Molecular cloning, sequence analysis, and expression of the gene encoding the immunodominant 32-kilodalton protein of Cowdria ruminantium. Infect Immun. 1994 Apr;62(4):1451–1456. doi: 10.1128/iai.62.4.1451-1456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]