Abstract
Human neutrophils constitutively express a unique combination of FcγRs, namely FcγRIIa and FcγRIIIb. Numerous lines of evidence support the concept that these FcγRs generate only partially characterized intracellular signals. However, despite the fact that both receptors are likely to be engaged simultaneously in a physiological setting, no recent publications have investigated the distinct, although partially convergent, results of their joint activation in IgG-dependent responses. To examine the significance of the co-expression of FcγRIIa and FcγRIIIb on human neutrophils, we analyzed the neutrophil responses to stimuli that engage these FcγRs, namely the phagocytosis of human IgG-opsonized zymosan and the responses to heat-aggregated IgGs. Blocking antibodies to either FcγR significantly decreased the phagocytic index and the stimulated production of superoxide anions. Both receptors are required for optimal IgG-dependent responses by human neutrophils. On the other hand, only blocking antibodies to FcγRIIIb, but not to FcγRIIa, inhibited the mobilization of calcium in response to heat-aggregated IgGs. Furthermore, phagocytosis of IgG-opsonized zymosan by human neutrophils required an extracellular influx of calcium that was blocked only by antibodies against FcγRIIIb. We also observed that this calcium influx as well as the IgG-dependent phagocytosis were dependent on the integrity of the plasma membrane detergent-resistant microdomains to which both isoforms were recruited following stimulation by heat-aggregated IgGs. These data clarify the mechanisms that regulate the FcγRs constitutively expressed on human neutrophils, describe a specific contribution of FcγRIIIb at the level of the mobilization of calcium, and provide evidence for a crucial role of detergent-resistant microdomains in this process.
Keywords: Calcium, Lipid Raft, Neutrophil, Phagocytosis, Superoxide Ion, Fcγ Receptors, Calcium Influx, Calcium Mobilization
Introduction
Fcγ receptors (FcγRs)3 are important key activators of the immune system. They play major roles in host resistance linking humoral and cellular responses, in particular in the contexts of phagocytosis, antibody-dependent cell cytotoxicity, enhanced antigen presentation and clearance of immune complexes (1–3). More and more studies suggest also a role for FcγRs in systemic auto-immune diseases such as, among others, rheumatoid arthritis, vasculititis, and lupus erythematosus (4). Immune cells express different members of this receptor family (2). Human neutrophils are unique in that they constitutively express two types of FcγRs (5, 6): FcγRIIa (CD32a), a transmembrane protein that possesses a noncanonical immunoreceptor tyrosine-based activation motif in its intracellular portion, and FcγRIIIb (CD16b), a GPI-anchored protein whose surface expression is 10-fold higher than that of FcγRIIa (135,000 versus 10,000 receptors/cell, respectively) (7). FcγRIIIb was thought to be exclusively expressed by human neutrophils (8), but a recent publication demonstrated that this receptor is also expressed at a low level by human basophils (9). The expression of these two FcγRs represents a combination that is a signature of human neutrophils. Under resting conditions, the affinities of these two receptors for the Fc portion of human monomeric IgG are similarly low. Despite the fact that numerous lines of evidence indicate that the engagement of each of these two FcγRs stimulates signaling pathways, it is more than likely that, under patho-physiological conditions (phagocytosis, clearance of immune complexes), they are both simultaneously engaged and activated.
Phagocytosis is an essential function of neutrophils. This mechanism of clearance of pathogens or immune complexes allows this leukocyte to make an important contribution to the innate immune response. Opsonization of microbial pathogens by antibodies or complement fragments favors the engulfment of the targets. Phagocytosis of IgG-opsonized pathogens or IgG-containing immune complexes is mediated in great part by the ligation of FcγRs. Several studies indicate that FcγRIIa is directly involved in the phagocytic process (10–13), and the results of different studies indicate that the expression of FcγRIIa (14), but not that of FcγRIIIb (15), is sufficient to confer phagocytic ability to transfected fibroblasts. These observations explain why FcγRIIa was considered as the major, if not the unique, FcγR isoform involved in the IgG-dependent phagocytosis in human neutrophils. However, a synergistic enhancement of phagocytosis is observed when these two receptors are present and triggered (16), and recent publications report decreased phagocytic activity in neutrophils from FcγRIIIb-deficient donors, despite the presence of functional FcγRIIa (17, 18). These data illustrate the complexity of the poorly understood roles of the FcγRIIIb in FcγR-dependent phagocytosis in human neutrophils. Most of the previous studies were performed using stimulation with FcγR isoform-specific monoclonal antibodies, which makes it difficult to clearly delineate the specific contributions of FcγRIIa-dependent versus FcγRIIIb-dependent signals to the functional responses of the neutrophils as well as providing little information about potential cooperative between these two receptors.
Several immunoreceptors, including Fc receptors, are thought to initiate their signaling cascades in detergent-insoluble glycolipid-enriched domains named DRMs (19). These lipid domains, often called rafts, represent signaling platforms where adaptor and signaling proteins are regrouped and interact to generate the appropriate signals inside the cell. We and others (20–24) have shown that signaling through FcγRIIa in different cell types including human neutrophils involves receptor aggregation, resulting in the translocation to high density DRMs. In our previous study, disruption of these microdomains modulated FcγRIIa-dependent signaling events, indicating that DRMs contained functional FcγRIIa signaling units (20). GPI-anchored proteins are also thought to preferentially reside in these cholesterol- and sphingolipid-enriched microdomains (25). In human neutrophils, our previous data demonstrated that FcγRIIIb also associates with high density DRMs, and DRM disrupting agents altered cellular responses to FcγRIIIb receptor ligation (26). Altogether, these results provide evidence that DRMs are involved in the signaling pathways of both FcγRs in human neutrophils. However, the specifics of the involvement of DRMs in phagocytosis remain unclear. Phagocytosis of nonopsonized mycobacteria or Bordetella pertussis by neutrophils was inhibited by depletion of cholesterol in contrast to that of serum-opsonized zymosan or bacteria (27, 28).
Most of the studies examining the specific roles and signaling pathways of the neutrophil FcγRs were restricted to myeloid/neutrophil-like cells that do not express the same combination of FcγRs. For this reason, and because mice neutrophils do not express either FcγRIIa or FcγRIIIb, in the present study, we focused exclusively on freshly isolated human neutrophils, the only human phagocyte that does not possess the inhibitory FcγR isoform (FcγRIIb) and that expresses this unique combination of FcγRs. No recent data are presently available about the mechanisms underlying the potential cooperation between the two receptors in the control of the responsiveness of human neutrophils. We attempted to dissect the respective roles of FcγRIIa and FcγRIIIb when they are simultaneously engaged by human IgGs-opsonized zymosan or heat-aggregated human IgGs. We observed that both FcγRs are required for an optimal phagocytosis of opsonized zymosan. They were similarly essential for the stimulation of tyrosine phosphorylation and degradation of FcγRIIa (29). Phagocytosis of IgG-opsonized zymosan by human neutrophils required an extracellular influx of calcium that was exclusively dependent on FcγRIIIb engagement. We also observed that the FcγRIIIb-dependent calcium influx and the regulation of phagocytosis were dependent on the integrity of the plasma membrane detergent-resistant microdomains where both FcγRs are recruited following heat-aggregated IgGs stimulation. Taken together, these data clarify how these isoforms work together and the regulation process between the FcγRs constitutively expressed on human neutrophils.
EXPERIMENTAL PROCEDURES
Antibodies
Two different antibodies against FcγRIIa were used in this study: (i) Monoclonal antibody IV.3 was purified from ascites of mice inoculated with hybridoma HB-217 obtained from the American Type Culture Collection (Manassas, VA). This antibody recognizes a native extracellular epitope of FcγRIIa and could be cross-linked to specifically engage this isoform. F(ab′)2 fragments of antibody IV.3 were used for blocking experiments. (ii) CT10 is an IgG fraction of a polyclonal rabbit serum raised against a synthetic peptide whose sequence was chosen in the cytoplasmic domain of FcγRIIa (30). It was used for immunoblotting.
Three different antibodies against FcγRIIIb were used: (i) Monoclonal antibody 3G8 F(ab′)2 (catalogue number 165-520) obtained from Ancell (Bayport, MN) was used to block the recognition of the Fc portion of IgG. (ii) PeliCluster (catalogue number M1389) from Sanquin (Amsterdam, The Netherlands) is a complete monoclonal antibody used for all of the cross-linking experiments. (iii) DJ130c (catalogue number M7006) from Dako (Glostrup, Denmark) was used for immunoblotting.
The anti-phosphotyrosine (4G10, catalogue number 05-321) antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Anti-c-Cbl (catalogue number SC-170) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-mouse F(ab′)2 (anti-Fc, catalogue number 115-006-071 or anti-F(ab′)2 catalogue number 115-006-072), FITC-conjugated F(ab′)2 goat anti-human F(ab′)2 fragment specific (catalogue number 109-096-097) and horseradish peroxidase-labeled donkey anti-rabbit IgGs (catalogue number 711-035-152) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Horseradish peroxidase-labeled sheep anti-mouse IgGs (catalogue number NXA931) were obtained from GE Healthcare.
Reagents
Na3VO4, soybean trypsin inhibitor, phenylmethylsulfonyl fluoride, EGTA, methyl-β-cyclodextrin, cholesterol (water soluble), OptiPrep density gradient medium, and IgGs from human serum were obtained from Sigma-Aldrich Canada. Dextran T-500 was purchased from U.S. Biological (Swampscott, MA). Percoll was purchased from GE Healthcare. Aprotinin and leupeptin were purchased from Roche Applied Science. Western lightning chemiluminescence plus was obtained from PerkinElmer Life Sciences. Ficoll-Paque and Hepes were obtained from Wisent (St-Bruno, Canada). Di-isopropyl fluoro-phosphate (DFP) was purchased from Serva Electrophoresis (Heidelberg, Germany). Gelatin was obtained from Fisher. Zymosan A488 Bioparticles and Fura-2-AM were obtained from Invitrogen. Cytochrome c was purchased from VWR (Montreal, Canada).
Isolation of Neutrophils
Neutrophils were collected from healthy adult volunteers and sterilely isolated as described previously (31). They were resuspended in Mg2+-free HBSS containing 1.6 mm of CaCl2.
Cell Stimulation
To prepare heat-aggregated IgGs (HA-IgGs), human IgGs were resuspended at 25 mg/ml in HBSS and incubated at 63 °C for 1 h. Freshly purified human neutrophils were resuspended in HBSS at 20 × 106 cells/ml (except when indicated). The cells were then stimulated with HA-IgG at 1 mg/ml (final concentration) for the times indicated in the figure legends. To measure HA-IgG internalization, HA-IgG were labeled with FITC-conjugated F(ab′)2 goat anti-human F(ab′)2 fragment specific. For the cross-linking experiments using monoclonal antibodies, the cells were incubated with IV.3 (1 μg/ml) or PeliCluster (4 μg/ml) for 5 min at 37 °C and goat anti-mouse F(ab′)2 anti-F(ab′)2 (25 or 100 μg/ml) (or HBSS for negative controls) was used to cross-link FcγRs. For blocking experiments, the cells were incubated with IV.3 F(ab′)2 or 3G8 F(ab′)2 antibodies for 5 min at 37 °C before stimulation. For the immunoblotting experiments, the stimulations were stopped at the indicated times by transferring aliquots of the cell suspensions directly in the same volume of 2× boiling modified Laemmli's sample buffer (62.5 mm Tris-HCl, pH 6.8, 4% (w/v) SDS, 5% (v/v) β-mercaptoethanol, 8.5% (v/v) glycerol, 2.5 mm orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 0.025% bromphenol blue) and boiled for 7 min.
Phagocytosis Assay
Alexa 488-conjugated zymosan A bioparticles were opsonized by incubation with 10 mg/ml human IgGs at 37 °C for 1 h and washed twice in HBSS as described (29). A ratio of 10 particles/cell was added at 25 μl of neutrophils (10 × 106 cells/ml) preincubated as described in the corresponding legend to initiate phagocytosis. To synchronize the interaction between the particles and the cells, the mixture was centrifuged at 400 × g for 15 s before incubation at 37 °C. To quench the fluorescence of the noningested Alexa 488-conjugated zymosan, 0.4 mg/ml trypan blue in citrate buffer (pH 4.4) was added 2 min before flow cytometry analysis with BD FACSCanto II flow cytometer from BD Biosciences (San Jose, CA). Where indicated, the cells were incubated with methyl-β-cyclodextrin (10 mm) for 15 min at 37 °C and washed before the addition of opsonized zymosan. For the cholesterol repletion controls, 4 mm of cholesterol was added after the methyl-β-cyclodextrin for 30 min at 37 °C and washed before the addition of opsonized zymosan. A phagocytic index was calculated by multiplying the number of cells having internalized particles by the mean fluorescence intensity, which corresponds to the mean number of particles inside each positive cell (calibration: 10 units/zymosan particles).
Superoxide Anion Measurement
Superoxide production was measured using the reduction of cytochrome c assay. Human neutrophils (20 × 106 cells/ml) were incubated with HA-IgG as described in the legends and incubated for 10 min at 37 °C in the presence of 125 μm cytochrome c. The cells were removed by centrifugation, and OD readings were monitored on the supernatants. The differences between the results at 550 and 540 nm were multiplied by the millimolar extinction coefficient 47.4 to obtain nmol O2−/5 × 106 cells/ml (32).
Intracellular Calcium Mobilization Measurement
Neutrophils (10 × 106 cells/ml) were preincubated with 1 μm Fura-2-AM for 30 min at 37 °C. The extracellular probe was removed by washing in HBSS, and the cells were resuspended at 5 × 106 cells/ml. The cells were stimulated as described in the corresponding legends. Fluorescence was monitored in a fluorescence spectrophotometer (Fluorolog-SPEX from Jobin Yvon Inc., Edison, NJ) using two excitation wavelengths at 340 and 380 nm and an emission wavelength of 510 nm. The ratios of the fluorescence values obtained at 340 and 380 nm provided a measure of the intracellular levels of free cytoplasmic calcium.
Extracellular Calcium Influx Measurement
Neutrophils (10 × 106 cells/ml) were preincubated with 1 μm Fura-2-AM for 30 min at 37 °C. The extracellular probe was removed by washing in HBSS without calcium and magnesium, and the cells were resuspended at 5 × 106 cells/ml. 200 μm MnCl2 was added to the cell suspensions for 100 s before stimulating the cells as indicated in the legends. The fluorescence was monitored in a fluorescence spectrophotometer (Fluorolog-SPEX from Jobin Yvon Inc.) using an excitation wavelength at 360 nm and an emission wavelength of 505 nm. Fura-2 quenching, a result of manganese influx, is reported in arbitrary fluorescence units.
Electrophoresis and Immunoblotting
The proteins were separated by SDS-PAGE on 10% acrylamide gels or on 7.5–20% gradient acrylamide gels. The proteins were then transferred to PVDF membranes. Blocking agents and antibodies were diluted in a TBS-Tween solution (25 mm Tris-HCl, pH 7.8, 190 mm NaCl, 0.15% v/v Tween 20). Blotto solution (5% w/v) was used to block nonspecific sites before anti-FcγRIIa (CT10 antibody) and anti-FcγRIIIb (DJ130c antibody) immunoblotting. Gelatin solution (2% w/v) was used to block nonspecific sites before anti-phosphotyrosine (4G10) and anti-c-Cbl immunoblotting. 4G10 antibody was diluted 1:4000; CT10 and anti-c-Cbl antibodies were diluted 1:1000; and DJ130c antibody was diluted 1:300. Horseradish peroxidase-labeled donkey anti-rabbit IgGs and horseradish peroxidase-labeled sheep anti-mouse IgGs were diluted 1:20,000 in TBS-Tween solution. Chemiluminescence reagents were used to detect antibodies, with a maximal exposure time of 5 min. All of the immunoblots presented were controlled for equal protein loading with immunoblots against c-Cbl.
Plasma Membrane Isolation
One ml of neutrophil suspension (40 × 106 cells/ml) was preincubated with 1 mm DFP for 10 min at room temperature before the addition of HA-IgG (1 mg/ml) or stimulation with monoclonal antibodies as described previously (29). For FcγRIIIb cross-linking, Pelicluster (4 μg/ml) was cross-linked with 120 μg/ml F(ab′)2 anti-Fc for 30 s. The cells were transferred to an ice bath to stop the stimulations. The cells were then quickly centrifuged (10 s at 15,000 × g) and resuspended in modified relaxation buffer (100 mm KCl, 3 mm NaCl, 10 mm Hepes, pH 7.4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm sodium orthovanadate, 250 μg/ml soybean trypsin inhibitor, 1 mm phenylmethylsulfonyl fluoride, 3 mm DFP). The cells were sonicated on ice for 22 s at power level 1 in a Branson Sonifier 450 sonicator, and the lysates were then centrifuged at 400 × g for 2 min. The supernatants (900 μl) were applied on top of a two-step Percoll gradient composed of 1.4 ml of a 1.12 g/ml Percoll solution layered beneath 1.4 ml of a 1.05 g/ml Percoll solution, as described previously by Kjeldsen et al. (33). The Percoll gradients were centrifuged for 30 min at 37,000 × g at 4 °C in a fixed angle rotor (Beckman TLA100.4). The plasma membranes were found on top of the gradients, under clear cytosol fractions. An aliquot of the cytosolic fractions was collected and boiled for 7 min in Laemmli's buffer. The plasma membranes were collected and centrifuged at 100,000 × g for 45 min at 4 °C to remove Percoll. Plasma membranes formed a visible disc above the Percoll pellet. They were collected, resuspended in relaxation buffer, and stored at −80 °C. An aliquot was boiled for 3 min in Laemmli's buffer.
Isolation of DRMs
Plasma membranes (40 × 106 cell equivalent/ml) were solubilized in 1% Nonidet P-40-containing isotonic buffer (137 mm NaCl, 20 mm Hepes, pH 7.4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm sodium orthovanadate, 250 μg/ml soybean trypsin inhibitor, 1 mm phenylmethylsulfonyl fluoride, 3 mm DFP) for 20 min on ice. To remove soluble proteins, the solubilized membranes were placed on the top of a 48% Optiprep cushion prepared from a stock solution (59.4% OptiPrep, 10 mm Hepes, pH 7.4) in the same buffer as above. They were centrifuged at 100,000 × g for 1 h at 4 °C in a TLA 100.4 rotor. The supernatants were removed, and two Optiprep pellets from the same donors were pooled to concentrate the insoluble membrane fractions. An aliquot was kept in Laemmli's buffer (insoluble fraction), and the remainder was adjusted to 40% (v/v) OptiPrep with 1% Nonidet P-40-containing isotonic buffer. Then 700 μl were transferred to 4-ml centrifuge tubes and overlaid with 700 μl of ice-cold solutions of 35, 30, 25, and 20% (v/v) OptiPrep and 300 μl of isotonic buffer without Nonidet P-40. The gradients were centrifuged at 380,000 × g for 3 h at 4 °C in a TLA 100.4 rotor, and subsequently, 13 fractions of 300 μl were collected from the top of the gradients. Proteins of fractions 3–10 were chloroform/methanol-precipitated as described previously (34). The precipitates were resuspended in 60 μl of Laemmli's buffer and boiled for 7 min.
Statistical Analysis
Statistical analyses were performed using Student's paired t test (two-tailed) with GraphPad Prism4 Software. Significance was considered to be attained at a value of p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
RESULTS
Requirement of FcγRIIa and FcγRIIIb in IgG-dependent Responses
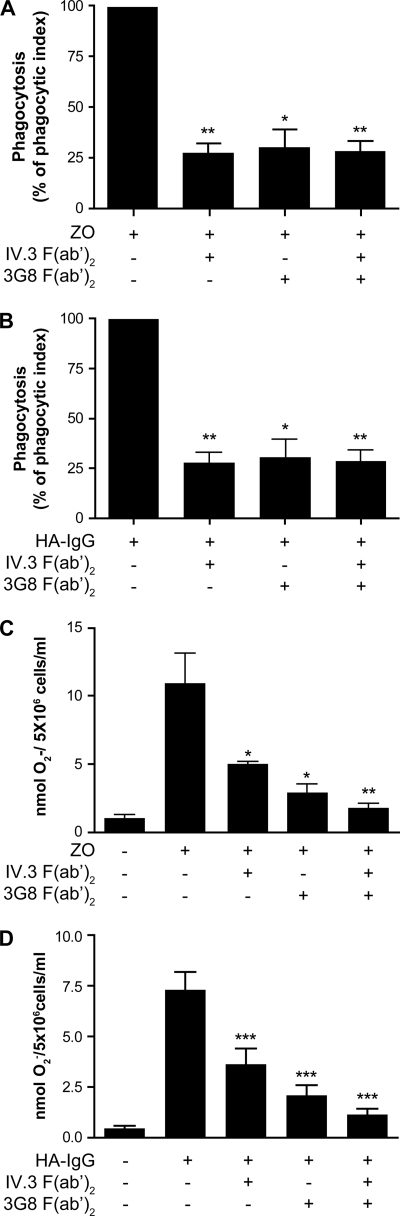
Because FcγRIIa and FcγRIIIb both recognize the Fc portion of IgGs, these receptors are likely to be simultaneously engaged when neutrophils meet opsonized pathogens or IgG-containing immune complexes. We investigated the respective involvement of each of these two receptors in FcγRs-dependent responses in neutrophils. Freshly isolated neutrophils were incubated with IgG-opsonized zymosan, and the phagocytic index (a composite of the percentage of positive cells and the number of internalized zymosan particles by cell) was measured. The results are presented in Fig. 1A. Blocking antibodies against FcγRIIa (IV.3 F(ab′)2) or against FcγRIIIb (3G8 F(ab′)2) significantly decreased the phagocytic capacity of these cells to a similar extent. The addition of both blocking antibodies results in a similar decrease of phagocytic index. No effects of blocking antibodies were observed when nonopsonized zymosan was used to measure phagocytosis (data not shown). The internalization capacity of FcγRs was also examined when neutrophils were challenged with HA-IgGs (Fig. 1B). Again, both receptors appear to be involved because preincubation with IV.3 F(ab′)2 or 3G8 F(ab′)2 results in a decrease of the internalization index. However, the involvement of FcγRIIIb appears to be more preponderant in internalization than in phagocytosis where both isoforms are equally involved. In these two tests, control isotypes of both antibodies had no effect.
FIGURE 1.
Both FcγRs isoforms are required in IgG-dependent phagocytosis and superoxide production. A and B, neutrophils were preincubated with the blocking monoclonal antibodies IV.3 F(ab′)2 or 3G8 F(ab′)2 for 5 min at 37 °C and incubated with fluorescent IgG-opsonized zymosan (ZO, A) or 25 μg of fluorescent HA-IgG (B) as described under “Experimental Procedures.” Fluorescence was measured by flow cytometry. These graphs are quantifications of the means of at least three independent phagocytosis experiments. C and D, neutrophils were preincubated with the blocking monoclonal antibodies IV.3 F(ab′)2 or 3G8 F(ab′)2 for 5 min at 37 °C, and cytochrome c (125 μm) was added. Superoxide production measurement was then initiated by the addition of IgG-opsonized zymosan (C) or HA-IgG (1 mg/ml) (D), and the reactions were stopped on ice after 10 min. The data shown are compiled from six independent experiments.
The engagement of FcγRs on human neutrophils has previously been reported to stimulate the production of superoxide anions (35, 36). As shown in Fig. 1 (C and D), stimulation of neutrophils with IgG-opsonized zymosan or HA-IgG did result in the detection of measurable amounts of superoxide anions with the former being more efficient than the latter in this respect. The addition of opsonized zymosan to neutrophils led to a substantial production of superoxide anions. This response was partially reduced by antibodies IV.3 and 3G8 and essentially abrogated when both were utilized (Fig. 1C). Similar inhibitory effects of the isoform-specific antibodies were obtained in response to HA-IgG, which also activated the oxidative burst in human neutrophils (Fig. 1D). This response was significantly reduced by both 3G8 and IV.3 with the former being more potent (Fig. 1D). Thus, as observed for phagocytosis (Fig. 1, A and B), both receptors are important for an optimal production of superoxide anions in response to the engagement of FcγRs.
Both FcγR Isoforms Are Essential for HA-IgG-mediated Signaling
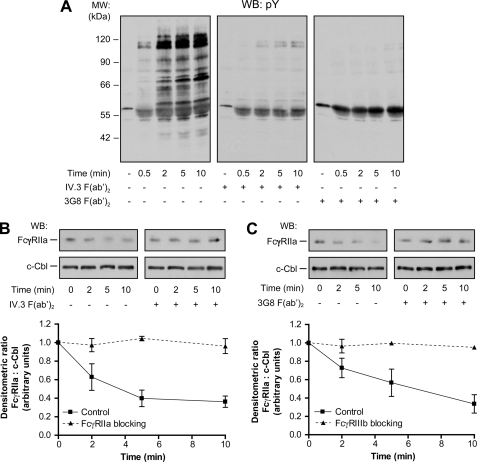
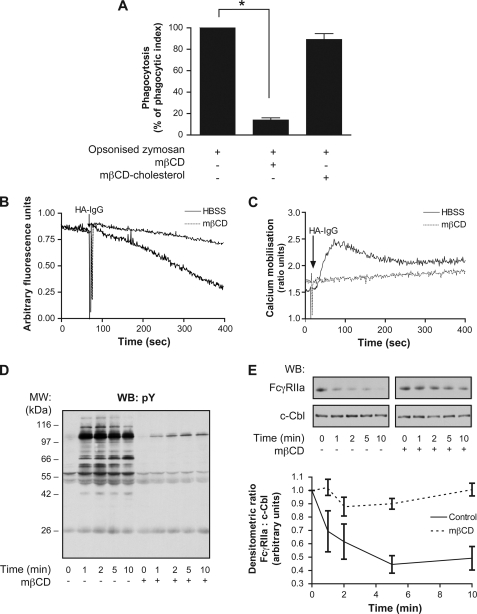
The above results suggest the existence of a complementary process between FcγRIIa and FcγRIIIb during IgG-dependent responses. Previous studies have shown that following the cross-linking of FcγRIIa or FcγRIIIb, rapid and transient patterns of tyrosine phosphorylation were induced (20, 37). The effect of the simultaneous stimulation of both FcγRs using HA-IgGs was tested on human neutrophils and analyzed by immunoblotting using the anti-phosphotyrosine antibody 4G10. The results of these experiments, illustrated in Fig. 2A, indicate that an extensive increase in the level of tyrosine phosphorylation is observed upon the addition of HA-IgG to human neutrophils. The maximum level of tyrosine phosphorylation was observed 2 min after stimulation with HA-IgG and was maintained for 5–10 min. Prominent tyrosine phosphorylation bands at 60–66, 70, 90, and 120 kDa were consistently observed. Somewhat unexpectedly, this pattern of phosphorylation was completely inhibited when neutrophils were preincubated with either 1 μg/ml IV.3 F(ab′)2 or 4 μg/ml 3G8 F(ab′)2 blocking monoclonal antibodies (Fig. 2A) and loaded on the same gel for immunoblotting. The residual bands at 55–60 kDa correspond to HA-IgG. When the same samples were immunoblotted with CT10 (an antibody directed against the cytoplasmic tail of FcγRIIa), a decrease of the amount of FcγRIIa (a decreased immunoreactivity) was observed as soon as 2 min after HA-IgG addition (c-Cbl was used as a loading control). Blocking antibodies against FcγRIIa (Fig. 2B) or FcγRIIIb (Fig. 2C) completely inhibited the decreased immunoreactivity of FcγRIIa. These results indicate that both receptors are required for tyrosine phosphorylation in response to HA-IgG, although they both have been shown to trigger this type of signal when individually engaged with monoclonal antibodies (20, 26, 29).
FIGURE 2.
Both FcγR isoforms are required in IgG-mediated signaling. Neutrophils were preincubated with monoclonal antibodies IV.3 F(ab′)2 (1 μg/ml) or 3G8 F(ab′)2 (4 μg/ml) for 5 min at 37 °C and stimulated with HA-IgG (1 mg/ml) for the indicated times. Whole cell lysates were loaded on the same gel and probed by immunoblotting for tyrosine phosphorylated residues (pY) (A) and FcγRIIa (CT10 antibody) (B and C). Ubiquitin ligase c-Cbl was probed as loading control. Compilations of densitometric ratios of four independent experiments are represented on the graphs. WB, Western blot; MW, molecular mass.
Generation of an Essential FcγRIIIb-dependent Calcium Influx
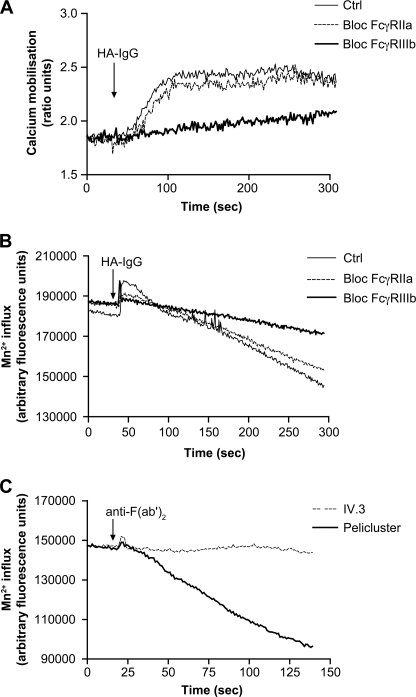
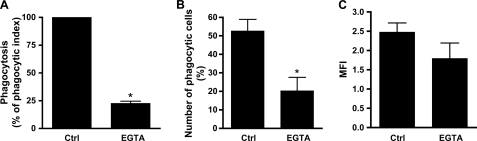
An increase of the concentration of cytoplasmic free calcium is another type of signal generated after the cross-linking of FcγRIIa or FcγRIIIb (37, 38). We next monitored the mobilization of calcium following stimulation of human neutrophils with HA-IgG (Fig. 3A). Immediately following the addition of HA-IgG, an increase in the concentration of free cytoplasmic calcium was observed. This augmentation was maintained for up to 200–300 s before a return to the base-line level (not shown). Preincubation with F(ab′)2 fragment of 3G8 completely blocked this calcium mobilization. On the other hand, the F(ab′)2 fragment of antibody IV.3 did not have a significant effect on this response. The results of a previous study have documented the stimulation of an extracellular influx of calcium in human neutrophils upon stimulation with HA-IgG (39). We went one step further and evaluated the involvement of each of the two FcγRs in this event. Calcium influx was monitored by following the quenching of intracellular Fura-2 that results from the entry of Mn2+ through the calcium channels (40, 41). As expected, the addition of HA-IgG to a suspension of human neutrophils loaded with Fura-2 increased after 50 s the rate of quenching of the calcium probe (Fig. 3B). This effect corresponds to the increased permeability to divalent cations, i.e. increased calcium influx. A significant inhibition of the opening of calcium channels was observed in the presence of blocking FcγRIIIb antibodies (3G8 F(ab′)2) but not with blocking FcγRIIa antibodies (IV.3 F(ab′)2). Consistent with this result, cross-linking of FcγRIIIb with Pelicluster led to a generation of an influx of extracellular calcium, whereas no evidence of an influx of extracellular calcium was observed following cross-linking of FcγRIIa (Fig. 3C). Opening of calcium channels could be necessary for the generation of an appropriate signal leading to the optimization of the functions of the opsonin receptors. We therefore sought to determine whether the stimulated influx of calcium was necessary for the quintessential FcγR-dependent function, namely the phagocytosis of IgG opsonized particles (Fig. 1). As illustrated in Fig. 4A, chelation of extracellular calcium by EGTA led to a 75% reduction of the phagocytosis of IgG-opsonized zymosan by human neutrophils. The decrease in the phagocytic index in presence of EGTA was due in major part to the reduction of the number of cells able to internalize particles (Fig. 4B) rather than to the number of the fluorescent opsonized zymosan by cell as measured by mean fluorescent intensity (Fig. 4C). However, because the number of internalized particles was low (1–3 particles/cell), it may be difficult to observe a down-modulation of mean fluorescence intensity.
FIGURE 3.
FcγRIIIb mediates an influx of calcium in response to HA-IgG. Neutrophils were incubated with Fura-2-AM as described under “Experimental Procedures.” Monoclonal antibodies IV.3 F(ab′)2 (1 μg/ml) or 3G8 F(ab′)2 (4 μg/ml) were added for 5 min at 37 °C before stimulation. A, calcium mobilization was measured following the addition of HA-IgG (1 mg/ml). B and C, Mn2+ influx, monitored by the quenching of intracellular Fura-2, was measured following stimulation initiated with HA-IgG (1 mg/ml) (B) or anti-mouse F(ab′)2 (C) as described under “Experimental Procedures.” These data are representative of five independent determinations. Ctrl, control.
FIGURE 4.
FcγR-dependent phagocytosis requires an influx of calcium. Neutrophils, in the presence or absence of 2 mm EGTA, were incubated with fluorescent IgG-opsonized zymosan as described under “Experimental Procedures.” Fluorescence was measured by flow cytometry. Phagocytic index (A) was calculated by multiplying the number of phagocytic cells (B) by the number of internalized zymosan per cell (mean fluorescence intensity, MFI) (C). These graphs are a quantification of the means of six independent experiments. Ctrl, control.
DRM-dependent Regulation of FcγRs Signaling and Calcium Influx
The recognition of HA-IgG by FcγRIIa and FcγRIIIb takes place at the plasma membrane of human neutrophils. We previously showed that when individually cross-linked by isoform-specific antibodies, FcγRIIa and FcγRIIIb partition in a dynamic fashion between detergent-soluble and detergent-resistant domains (DRMs) of the plasma membrane (21, 26, 29). We next evaluated the role of DRMs in FcγRs-dependent neutrophil responses. Pretreating the neutrophils with 10 mm methyl-β-cyclodextrin (mβCD), a cholesterol-depleting agent, for 15 min at 37 °C had an important inhibitory effect on the phagocytic capacity of the cells (Fig. 5A). The decrease in phagocytosis index corresponds to a reduction in the number of opsonized zymosan by neutrophils as well as in the total number of cells having internalized zymosan particles (data not shown). The concentration of mβCD used (10 mm) did not affect cell viability under these experimental conditions as evidenced by trypan blue exclusion (data not shown and Ref. 38). Furthermore, the inhibitory effect of mβCD on phagocytosis was completely reversed by repleting the cells with cholesterol using cholesterol-cyclodextrin (4 mm for 30 min after mβCD pretreatment). We next examined the effects of cholesterol depletion on signaling events downstream of FcγRs engagement by immune complexes. Because IgG-dependent phagocytosis was found to be dependent on an enhancement of calcium influx (Fig. 4), we asked whether this influx was dependent on DRM integrity. We observed a significant reduction of calcium influx in mβCD-treated neutrophils in response to HA-IgG (Fig. 5B). These data highlight the functional role of FcγRIIIb in phagocytosis likely via its specific role in DRM-dependent calcium influx.
FIGURE 5.
DRMs integrity is essential for optimal FcγR-dependent responses. Neutrophils were preincubated or not with 10 mm mβCD for 15 min at 37 °C. A, neutrophils were incubated with fluorescent zymosan as described under “Experimental Procedures.” Fluorescence was measured by flow cytometry. For cholesterol repletion, neutrophils were washed after the mβCD incubation, and then 4 mm of cholesterol was added for 30 min at 37 °C and washed before the addition of opsonized zymosan. This graph is a quantification of the means of three independent experiments. B and C, neutrophils were incubated with Fura-2-AM as described under “Experimental Procedures,” and mβCD was added for the last 15 min. B and C, calcium influx (B) and calcium mobilization (C) were measured following HA-IgG (1 mg/ml) stimulation. These data are representative of four independent determinations. D and E, neutrophils were stimulated with HA-IgG (1 mg/ml) for the indicated times. Whole cell lysates were probed by immunoblotting for tyrosine phosphorylated residues (pY) (D) and FcγRIIa (E). Ubiquitin ligase c-Cbl was probed as loading control. Compilations of densitometric ratios of four independent experiments are represented on the graph. WB, Western blot; MW, molecular mass.
Previous studies have shown that following the cross-linking of FcγRIIa or FcγRIIIb, a rapid and transient mobilization of calcium and an enhanced pattern of tyrosine phosphorylation were induced (20, 37). Cholesterol depletion inhibited the mobilization of calcium stimulated by HA-IgG in human neutrophils (Fig. 5C). Calcium mobilization was totally restored by cholesterol repletion (data not shown). The time course of the increase in the level of tyrosine phosphorylation in response to HA-IgG is illustrated in Fig. 5D and is similar to that shown in Fig. 2A. This pattern was strongly inhibited by the above-described mβCD pretreatment (cholesterol depletion). When the same samples were immunoblotted with an antibody directed toward FcγRIIa (CT10), a decreased amount of FcγRIIa (decreased immunoreactivity) was observed (as reported previously (29) and Fig. 2, B and C) as soon as 1 min after cross-linking (Fig. 5E). We have previously shown that this effect represents a degradation of FcγRIIa through the proteasome (29). The degradation observed in response to HA-IgG stimulation was inhibited by cholesterol depletion. The recovery of function following cholesterol repletion further supports the argument that the inhibitory effects of mβCD are not due to nonspecific toxic effects.
Co-fractionation of FcγRIIa and FcγRIIIb in High Density DRMs
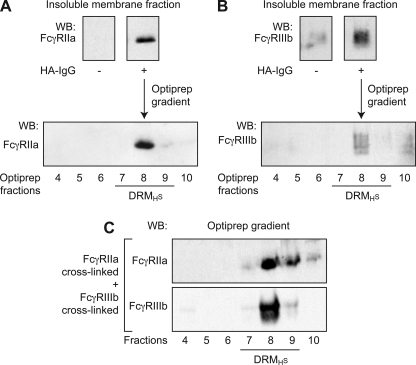
The distribution of the neutrophil FcγRs in the plane of the plasma membrane was previously analyzed in our laboratory, and we provided evidence that FcγRIIa was recruited to DRM of high density (DRMH) following its cross-linking with IV.3 antibody (21). Another study determined that, under resting conditions, FcγRIIIb was distributed equally inside and outside of the DRMH and that, after its cross-linking, FcγRIIIb was recruited in these microdomains (26). In the present study, HA-IgGs were used to engage both FcγR isoforms simultaneously, and their distribution in these membrane microdomains was monitored on the same population of cells. As an initial step toward the purification of DRMH, plasma membranes from resting and stimulated cells were solubilized in 1% Nonidet P-40, overlaid on an OptiPrep cushion, and centrifuged to isolate the total insoluble membrane fraction (Fig. 6, A and B, upper panels). This insoluble fraction was further analyzed on Optiprep gradients to isolate DRMs as described under “Experimental Procedures” (Fig. 6, A and B, lower panels). Following 30 s of incubation with HA-IgG, FcγRIIa and FcγRIIIb appear in DRMH centered on fraction 8. These results indicate that both isoforms of FcγRs co-fractionate in the same DRMH fractions. It should be noted that the quality of FcγRIIIb immunoblot is affected by the highly level of glycosylation of this receptor that results in a smear between 50 and 70 kDa as classically published (26). The distribution of flotillin-1, a structural raft component (42, 43), remained the same before and after stimulation by HA-IgG (data not shown and Ref. 21).
FIGURE 6.
FcγRIIa and FcγRIIIb co-fractionate in DRMH upon FcγRs engagement. Neutrophils (40 × 106 cells/ml) were preincubated with 1 mm DFP for 10 min at room temperature before stimulation at 37 °C for 30 s. Plasma membranes were prepared as described under “Experimental Procedures” and solubilized in 1% Nonidet P-40. A and B, solubilized plasma membranes from HA-IgG-stimulated neutrophils were subjected to ultracentrifugation at 100,000 × g on OptiPrep cushion. An aliquot of the pellet (insoluble membrane fraction) was probed for FcγRIIa (A, upper panels) and FcγRIIIb (B, upper panels). Insoluble membrane fraction from HA-IgG-stimulated neutrophils was fractionated on Optiprep gradient as described under “Experimental Procedures.” The gradient fractions were collected, and the proteins were precipitated and analyzed by immunoblotting for FcγRIIa (A, lower panel) and FcγRIIIb (B, lower panel). These data are representative of three independent experiments. C, FcγRIIa or FcγRIIIb were independently cross-linked on neutrophils using monoclonal antibodies as described under “Experimental Procedures.” Plasma membranes were isolated, solubilized, and ultracentrifuged. The two resulting independently obtained insoluble membrane fractions were then mixed before fractionation on Optiprep gradients. The fractions were analyzed by immunoblotting for FcγRIIa and FcγRIIIb. WB, Western blot.
To rule out the possibility that the co-fractionation of the two receptors is a physical consequence of their co-engagement by the HA-IgGs, we specifically stimulated each receptor in two independent experiments using isoform-specific monoclonal antibodies. The membranes were then isolated and solubilized. Following an ultracentrifugation, the two resulting insoluble fractions were mixed. DRM fractions were then isolated as above and immunoblotted for FcγRIIa and FcγRIIIb. As shown in Fig. 6C, the two FcγRs were found in the DRM fractions (fraction 8) irrespective of whether they were simultaneously stimulated by HA-IgGs or independently by cross-linking with specific monoclonal antibodies. These data indicate that the recruitment of both receptors in DRMHs in response to HA-IgG cannot be explained by physical cross-linking between the two isoforms.
DISCUSSION
Much recent data, including that supporting a role for DRMs in the control of the neutrophil signaling pathways and functions dependent on FcγRs (21, 26, 29), prompted us to re-examine the respective roles of the two constitutively expressed FcγRs on human neutrophils. In the present study, we focused on the underlying biochemical mechanisms that may explain how these receptors, which interact with similar ligands, coordinate their activation and functions.
Our results indicate that FcγRIIa and FcγRIIIb fulfill nonredundant roles in the control of the immune complex clearing functions of neutrophils and that FcγRIIIb provides a unique contribution at the level of the stimulation of calcium influx. This event is an essential element for an optimal IgG-dependent phagocytosis. FcγRIIa and FcγRIIIb are shown to co-fractionate in DRMHs following stimulation with IgG-containing immune complexes. Furthermore, the integrity of the DRMHs is essential for efficient signal transduction through the FcγRs, in particular for the generation of influx of calcium and for phagocytosis. Both isoforms are required for optimal IgG-dependent phagocytosis, superoxide production, tyrosine phosphorylation, calcium mobilization, and the regulation of the degradation of FcγRIIa.
The complementary roles of FcγRIIa and FcγRIIIb in the mediation of the stimulation of IgG-dependent functions in human neutrophils are also brought to light at the signal transduction level. Joint dependence on the two isoforms was observed at the tyrosine phosphorylation level, in the specifics of the mobilization of calcium as well as in the control of the stimulated proteasomal degradation of FcγRIIa. These data illustrate the necessity of the contribution of both FcγRs for the initiation of the proper signaling cascades in response to stimulation by these patho-physiological agonists. The HA-IgG-stimulated degradation of FcγRIIa is a novel observation that furthers that previously reported subsequent to the cross-linking of FcγRIIa (29). Indeed, we had demonstrated previously that FcγRIIa, when cross-linked with a specific monoclonal antibody, was degraded via the proteasome and that this serves to arrest signal and prevent hyperstimulation that could lead to autoimmune damages (29). This mechanism is not observed upon the cross-linking of FcγRIIIb alone (data not shown). Surprisingly, upon stimulation by IgG-containing immune complexes (HA-IgG), FcγRIIa was degraded, and blocking anti-FcγRIIIb antibody inhibited FcγRIIa degradation as efficiently as blocking anti-FcγRIIa antibodies. This observation graphically illustrates a major qualitative difference in the regulation of signaling initiated by cross-linking with monoclonal antibodies and by IgG-containing immune complexes. The participation of FcγRIIIb in the degradation of FcγRIIa cannot be seen using monoclonal antibodies for cross-linking but only upon stimulation by HA-IgG. Several previous studies in which the respective roles of FcγRIIa and FcγRIIIb were examined following their engagement by specific monoclonal antibodies (16, 17, 44, 45) proposed synergistic or modulatory interactions between FcγRIIa and FcγRIIIB. Here, we stimulated both FcγRs of human neutrophils using HA-IgG, a model of circulating immune complexes. This mode of stimulation approximates better a physiological environment in that, first, both receptors are simultaneously engaged and, second, the FcγRs interact with their natural ligands (the Fc fragment of IgG) rather than with the F(ab′)2 fragments of monoclonal antibodies. Because of their high affinity for their respective receptor, monoclonal antibodies represent an important and useful tool for stimulating each FcγR individually and deciphering downstream signaling events using biochemical techniques (21, 26, 29). However, more physiological ligands, such as HA-IgG, represent better tools for examining functional responses and especially the fine regulation of these responses.
The results of the present study provide direct evidence that the functional coordination step between FcγRIIa and FcγRIIIb occurs in plasma membrane domains resistant to solubilization in nonionic detergents (DRMs) and in particular in DRMHs. This conclusion is based on several lines of complementary evidence. We have previously shown (21, 26) that both FcγRIIa and FcγRIIIb migrate to DRMH upon cross-linking with monoclonal antibodies. We show here that both receptors are recruited into the same DRMH fractions in response to stimulation by HA-IgG. To the best of our knowledge, our results represent the first demonstration that both FcγRIIa and FcγRIIIb translocate to DRMH in response to HA-IgG. Furthermore, disruption of DRMs by pharmacological means (Ref. 46 and the present results) as well as transmembrane mutations that perturb the translocation of FcγRIIa into DRMs (47) have profound inhibitory effects on downstream signaling and functional responsiveness. The integrity of DRMs is thus required for a functional and regulated response not only when FcγRIIa but also when both receptors are engaged. We also provide novel evidence that disruption of these microdomains inhibits specific signaling steps and in particular the stimulation of calcium influx, an FcγRIIIb-dependent event that is required for optimal IgG-mediated phagocytosis. These results are consistent with our hypothesis that DRMs represent signaling platforms where FcγR-dependent signaling pathways and subsequent functions are regulated and coordinated. Additional studies are required to determine whether, in addition to partitioning in the same DRMH, FcγRIIa and FcγRIIIb directly interact physically with each other. This consideration is of particular relevance because FcγRIIIb is a GPI-anchored protein that does not possess a transmembrane domain and therefore is generally thought to require a partner for intracellular signaling. DRMs could facilitate these associations by lipid-lipid interactions as proposed for other GPI-anchored receptors (48) or as a membrane compartment allowing concentration of signaling partners.
Our results also provide new biochemical insights on the role of DRMs on the control of calcium influx and phagocytosis. Calcium is a well known second messenger implicated in neutrophil activation (49). We show in this study that the IgG-dependent phagocytosis of zymosan particles is profoundly affected by the absence of extracellular calcium. FcγRIIa and FcγRIIIb cross-linking induces a mobilization of intracellular calcium (13, 37–39). Furthermore, heterotypic co-stimulation of these receptors induces a synergic calcium response higher than that obtained after separate FcγR cross-linking (50). FcγRIIa and FcγRIIIb appear to rely on specific and independent pathways of calcium mobilization because the responses to FcγRIIIb in contrast to those to FcγRIIa were not affected by genistein (51). HA-IgG-induced ligation of FcγRs also resulted in a mobilization of calcium. Somewhat unexpectedly, this response appears to rely nearly exclusively on FcγRIIIb because only blocking antibodies to the latter (and not to FcγRIIa) inhibited it. The specific contribution of FcγRIIIb to the mobilization of calcium observed in response to HA-IgG is further highlighted by our observations concerning the stimulation of the influx of extracellular calcium. HA-IgG increased the rate of influx of calcium in an exclusively FcγRIIIb-dependent manner. Furthermore, cross-linking of FcγRIIIb, but not of FcγRIIa, induced an influx of calcium. These results indicate that the contribution of FcγRIIIb to the response to HA-IgG or immune complexes is not only important but also distinct from that of FcγRIIa. In this context, a recent publication has shown that FcγRIIIb but not FcγRIIa activates a signaling pathway leading to ERK phosphorylation in nucleus (52).
The mobilization of calcium mediated by FcγRIIIb in response to HA-IgG is likely to represent an important element in the optimization of FcγR-dependent functions of neutrophils. On the other hand, the results presented on Fig. 3A indicate only a minor role of the FcγRIIa in calcium mobilization, illustrating again the distinctness of the responses of neutrophil depending on the mode of engagement of FcγRs (monoclonal antibodies versus Fc portion of IgG).
Interactions between FcγRs and other receptors have previously been reported in other cell types. An association between FcγRIIIa and TLR4 has been described in the context of stimulation of mice neutrophils and macrophages by immune complexes (53). In macrophages, a co-clustering of FcγRI and the leukotriene B4 receptor (BLT1) in lipid rafts has also been observed (54), strengthening our description of DRMs as signaling platforms that can coordinate responses to different receptors. Finally, exposure of human neutrophils to serum-opsonized bacteria leads to an up-regulation of the expression of PAR-2, an effect that is inhibited by blocking antibodies to either FcγRIIa or FcγRIIIb (55).
These data about the cross-talk and complementarity between FcγRs in human neutrophils are relevant to the pathogenesis of multiple diseases. Neutrophil activation through FcγRs is exacerbated in systemic autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, or anti-neutrophil cytoplasmic antibody-associated vasculitis (Wegener's granulomatosis) (56). In these pathologies, immune complexes are detected in blood where neutrophils carry the most abundant pool of FcγRs exposed to these potential ligands (1, 57). Moreover, methotrexate (58) and anti-TNF (59) treatments in rheumatoid arthritis are associated with a loss of function of FcγRIIa. This is in accord with the priming effect of TNF-α on FcγRs activation in human neutrophils (35, 36). FcγRs are also involved in superoxide production in systemic lupus erythematosus (60, 61). These and other considerations amply justify concerted future investigations about the respective roles of each FcγR that follows IgG-mediated stimulations. In the present study, we describe novel processes involving DRMs as signaling platforms that coordinate FcγRs signaling and where FcγRIIIb recruitment leads to a specific calcium influx required for an optimal IgG-mediated phagocytosis of the human neutrophil.
Acknowledgment
We thank Dr. Maurice Dufour for expert technical assistance with the flow cytometric analysis.
This work was supported in part by grants from the Canadian Institutes of Health Research.
- FcγR
- Fcγ receptor
- DRM
- detergent-resistant microdomain
- HA-IgG
- heat-aggregated IgG
- mβCD
- methyl-β-cyclodextrin
- DRMH
- detergent-resistant microdomain of high density
- GPI
- glycosylphosphatidylinositol
- HBSS
- Hanks' balanced salt solution
- DFP
- di-isopropyl fluoro-phosphate.
REFERENCES
- 1. Schmidt R. E., Gessner J. E. (2005) Immunol. Lett. 100, 56–67 [DOI] [PubMed] [Google Scholar]
- 2. Willcocks L. C., Smith K. G., Clatworthy M. R. (2009) Expert. Rev. Mol. Med. 11, e24. [DOI] [PubMed] [Google Scholar]
- 3. Selvaraj P., Fifadara N., Nagarajan S., Cimino A., Wang G. (2004) Immunol. Res. 29, 219–230 [DOI] [PubMed] [Google Scholar]
- 4. Hogarth P. M. (2002) Curr. Opin. Immunol. 14, 798–802 [DOI] [PubMed] [Google Scholar]
- 5. Hulett M. D., Hogarth P. M. (1994) Adv. Immunol. 57, 1–127 [DOI] [PubMed] [Google Scholar]
- 6. Kimberly R. P., Salmon J. E., Edberg J. C. (1995) Arthritis Rheum. 38, 306–314 [DOI] [PubMed] [Google Scholar]
- 7. Fleit H. B., Wright S. D., Unkeless J. C. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 3275–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li M., Wirthmueller U., Ravetch J. V. (1996) J. Exp. Med. 183, 1259–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meknache N., Jönsson F., Laurent J., Guinnepain M. T., Daëron M. (2009) J. Immunol. 182, 2542–2550 [DOI] [PubMed] [Google Scholar]
- 10. Indik Z. K., Park J. G., Hunter S., Schreiber A. D. (1995) Blood 86, 4389–4399 [PubMed] [Google Scholar]
- 11. Hunter S., Kamoun M., Schreiber A. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10232–10236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowry M. B., Duchemin A. M., Robinson J. M., Anderson C. L. (1998) J. Exp. Med. 187, 161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odin J. A., Edberg J. C., Painter C. J., Kimberly R. P., Unkeless J. C. (1991) Science 254, 1785–1788 [DOI] [PubMed] [Google Scholar]
- 14. Indik Z., Kelly C., Chien P., Levinson A. I., Schreiber A. D. (1991) J. Clin. Invest. 88, 1766–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagarajan S., Chesla S., Cobern L., Anderson P., Zhu C., Selvaraj P. (1995) J. Biol. Chem. 270, 25762–25770 [DOI] [PubMed] [Google Scholar]
- 16. Edberg J. C., Kimberly R. P. (1994) J. Immunol. 152, 5826–5835 [PubMed] [Google Scholar]
- 17. Nagarajan S., Venkiteswaran K., Anderson M., Sayed U., Zhu C., Selvaraj P. (2000) Blood 95, 1069–1077 [PubMed] [Google Scholar]
- 18. Nagarajan S., Fifadara N. H., Selvaraj P. (2005) J. Immunol. 174, 5423–5432 [DOI] [PubMed] [Google Scholar]
- 19. Van Laethem F., Leo O. (2002) Curr. Mol. Med. 2, 557–570 [DOI] [PubMed] [Google Scholar]
- 20. Barabé F., Rollet-Labelle E., Gilbert C., Fernandes M. J., Naccache S. N., Naccache P. H. (2002) J. Immunol. 168, 4042–4049 [DOI] [PubMed] [Google Scholar]
- 21. Rollet-Labelle E., Marois S., Barbeau K., Malawista S. E., Naccache P. H. (2004) Biochem. J. 381, 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwiatkowska K., Sobota A. (2001) Eur. J. Immunol. 31, 989–998 [DOI] [PubMed] [Google Scholar]
- 23. Strzelecka-Kiliszek A., Korzeniowski M., Kwiatkowska K., Mrozińska K., Sobota A. (2004) Mol. Membr. Biol. 21, 101–108 [DOI] [PubMed] [Google Scholar]
- 24. Katsumata O., Hara-Yokoyama M., Sautès-Fridman C., Nagatsuka Y., Katada T., Hirabayashi Y., Shimizu K., Fujita-Yoshigaki J., Sugiya H., Furuyama S. (2001) J. Immunol. 167, 5814–5823 [DOI] [PubMed] [Google Scholar]
- 25. Sharom F. J., Lehto M. T. (2002) Biochem. Cell Biol. 80, 535–549 [DOI] [PubMed] [Google Scholar]
- 26. Fernandes M. J., Rollet-Labelle E., Paré G., Marois S., Tremblay M. L., Teillaud J. L., Naccache P. H. (2006) Biochem. J. 393, 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peyron P., Bordier C., N′Diaye E. N., Maridonneau-Parini I. (2000) J. Immunol. 165, 5186–5191 [DOI] [PubMed] [Google Scholar]
- 28. Lamberti Y., Perez Vidakovics M. L., van der Pol L. W., Rodríguez M. E. (2008) Microb. Pathog. 44, 501–511 [DOI] [PubMed] [Google Scholar]
- 29. Marois L., Vaillancourt M., Marois S., Proulx S., Paré G., Rollet-Labelle E., Naccache P. H. (2009) J. Immunol. 182, 2374–2384 [DOI] [PubMed] [Google Scholar]
- 30. Ibarrola I., Vossebeld P. J., Homburg C. H., Thelen M., Roos D., Verhoeven A. J. (1997) Biochim. Biophys. Acta 1357, 348–358 [DOI] [PubMed] [Google Scholar]
- 31. Rollet E., Caon A. C., Roberge C. J., Liao N. W., Malawista S. E., McColl S. R., Naccache P. H. (1994) J. Immunol. 153, 353–363 [PubMed] [Google Scholar]
- 32. Dahlgren C., Karlsson A. (1999) J. Immunol. Methods 232, 3–14 [DOI] [PubMed] [Google Scholar]
- 33. Kjeldsen L., Sengelov H., Borregaard N. (1999) J. Immunol. Methods 232, 131–143 [DOI] [PubMed] [Google Scholar]
- 34. Wessel D., Flügge U. I. (1984) Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 35. Fossati G., Moots R. J., Bucknall R. C., Edwards S. W. (2002) Arthritis Rheum. 46, 1351–1361 [DOI] [PubMed] [Google Scholar]
- 36. Fossati G., Bucknall R. C., Edwards S. W. (2002) Ann. Rheum. Dis. 61, 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandes M. J., Lachance G., Paré G., Rollet-Labelle E., Naccache P. H. (2005) J. Leukocyte Biol. 78, 524–532 [DOI] [PubMed] [Google Scholar]
- 38. Barabé F., Paré G., Fernandes M. J., Bourgoin S. G., Naccache P. H. (2002) J. Biol. Chem. 277, 13473–13478 [DOI] [PubMed] [Google Scholar]
- 39. Chuang F. Y., Sassaroli M., Unkeless J. C. (2000) J. Immunol. 164, 350–360 [DOI] [PubMed] [Google Scholar]
- 40. Bianchini L., Todderud G., Grinstein S. (1993) J. Biol. Chem. 268, 3357–3363 [PubMed] [Google Scholar]
- 41. Sage S. O., Pintado E., Mahaut-Smith M. P., Merritt J. E. (1990) Biochem. J. 265, 915–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slaughter N., Laux I., Tu X., Whitelegge J., Zhu X., Effros R., Bickel P., Nel A. (2003) Clin. Immunol. 108, 138–151 [DOI] [PubMed] [Google Scholar]
- 43. Shao D., Segal A. W., Dekker L. V. (2003) FEBS Lett. 550, 101–106 [DOI] [PubMed] [Google Scholar]
- 44. Naziruddin B., Duffy B. F., Tucker J., Mohanakumar T. (1992) J. Immunol. 149, 3702–3709 [PubMed] [Google Scholar]
- 45. Salmon J. E., Brogle N. L., Edberg J. C., Kimberly R. P. (1991) J. Immunol. 146, 997–1004 [PubMed] [Google Scholar]
- 46. Bournazos S., Hart S. P., Chamberlain L. H., Glennie M. J., Dransfield I. (2009) J. Immunol. 182, 8026–8036 [DOI] [PubMed] [Google Scholar]
- 47. García-García E., Brown E. J., Rosales C. (2007) J. Immunol. 178, 3048–3058 [DOI] [PubMed] [Google Scholar]
- 48. Gauthier L. R., Robbins S. M. (2003) Life Sci. 74, 207–216 [DOI] [PubMed] [Google Scholar]
- 49. Petroski R. J., Naccache P. H., Becker E. L., Sha'afi R. I. (1979) Am. J. Physiol. 237, C43–C49 [DOI] [PubMed] [Google Scholar]
- 50. Vossebeld P. J., Kessler J., von dem Borne A. E., Roos D., Verhoeven A. J. (1995) J. Biol. Chem. 270, 10671–10679 [DOI] [PubMed] [Google Scholar]
- 51. Edberg J. C., Moon J. J., Chang D. J., Kimberly R. P. (1998) J. Biol. Chem. 273, 8071–8079 [DOI] [PubMed] [Google Scholar]
- 52. Rivas-Fuentes S., García-García E., Nieto-Castañeda G., Rosales C. (2010) Cell. Immunol. 263, 114–121 [DOI] [PubMed] [Google Scholar]
- 53. Rittirsch D., Flierl M. A., Day D. E., Nadeau B. A., Zetoune F. S., Sarma J. V., Werner C. M., Wanner G. A., Simmen H. P., Huber-Lang M. S., Ward P. A. (2009) PLoS Pathog. 5, e1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Serezani C. H., Aronoff D. M., Sitrin R. G., Peters-Golden M. (2009) Blood 114, 3316–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. St-Onge M., Lagarde S., Laflamme C., Rollet-Labelle E., Marois L., Naccache P. H., Pouliot M. (2010) FASEB J. 24, 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Porges A. J., Redecha P. B., Kimberly W. T., Csernok E., Gross W. L., Kimberly R. P. (1994) J. Immunol. 153, 1271–1280 [PubMed] [Google Scholar]
- 57. Ravetch J. V., Bolland S. (2001) Annu. Rev. Immunol. 19, 275–290 [DOI] [PubMed] [Google Scholar]
- 58. Wijngaarden S., van Roon J. A., van de Winkel J. G., Bijlsma J. W., Lafeber F. P. (2005) Rheumatology 44, 729–734 [DOI] [PubMed] [Google Scholar]
- 59. Belostocki K., Park M. S., Redecha P. B., Masuda E., Salmon J. E., Pricop L. (2005) Clin. Immunol. 117, 78–86 [DOI] [PubMed] [Google Scholar]
- 60. Tan Sardjono C., Mottram P. L., Hogarth P. M. (2003) Immunol. Cell Biol. 81, 374–381 [DOI] [PubMed] [Google Scholar]
- 61. Alves C. M., Marzocchi-Machado C. M., Louzada-Junior P., Azzolini A. E., Polizello A. C., de Carvalho I. F., Lucisano-Valim Y. M. (2008) Clin. Rheumatol. 27, 701–708 [DOI] [PubMed] [Google Scholar]