Abstract
Acid sphingomyelinase (aSMase) catalyzes the hydrolysis of sphingomyelin (SM) to form the bioactive lipid ceramide (Cer). Notably, aSMase exists in two forms: a zinc (Zn2+)-independent lysosomal aSMase (L-SMase) and a Zn2+-dependent secreted aSMase (S-SMase) that arise from alternative trafficking of a single protein precursor. Despite extensive investigation into the maturation and trafficking of aSMase, the exact identity of mature L-SMase has remained unclear. Here, we describe a novel mechanism of aSMase maturation involving C-terminal proteolytic processing within, or in close proximity to, endolysosomes. Using two different C-terminal-tagged constructs of aSMase (V5, DsRed), we demonstrate that aSMase is processed from a 75-kDa, Zn2+-activated proenzyme to a mature 65 kDa, Zn2+-independent L-SMase. L-SMase is recognized by a polyclonal Ab to aSMase, but not by anti-V5 or anti-DsRed antibodies, suggesting that the C-terminal tag is lost during maturation. Furthermore, indirect immunofluorescence staining demonstrated that mature L-SMase colocalized with the lysosomal marker LAMP1, whereas V5-aSMase localized to the Golgi secretory pathway. Moreover, V5-aSMase possessed Zn2+-dependent activity suggesting it may represent the common protein precursor of S-SMase and L-SMase. Importantly, the 65-kDa L-SMase, but not V5-aSMase, was sensitive to the lysosomotropic inhibitor desipramine, co-fractionated with lysosomes, and migrated at the same Mr as partially purified human aSMase. Finally, three aSMase mutants containing C-terminal Niemann-Pick mutations (R600H, R600P, ΔR608) exhibited defective proteolytic maturation. Taken together, these results demonstrate that mature L-SMase arises from C-terminal proteolytic processing of pro-aSMase and suggest that impaired C-terminal proteolysis may lead to severe defects in L-SMase function.
Keywords: Golgi, Intracellular Trafficking, Lysosomal Glycoproteins, Lysosomes, Sphingolipid, Acid Sphingomyelinase, Desipramine
Introduction
Acid sphingomyelinase (aSMase)2 (EC 3.1.4.12) is a soluble lysosomal hydrolase that plays a prominent role in the catabolism of sphingomyelin (SM) to ceramide (Cer). Interestingly, aSMase exists as two enzymatic forms, one that is targeted to the endolysosomal compartment, whereas the other is released extracellularly (1). Lysosomal aSMase (L-SMase) arises from mannose 6-phosphorylation of N-glycans, which targets pro-aSMase to the endolysosomal compartment where it encounters and tightly coordinates Zn2+, thus becoming “Zn2+-independent,” i.e. not requiring addition of zinc for activity (1). Acid SMase precursors that are not mannose 6-phosphorylated get directed to the Golgi secretory pathway and released extracellularly giving rise to secretory aSMase (S-SMase) (1, 2). Cells from patients with inherited defects in the mannose 6-phosphorylation pathway (i.e. I-cell disease), secrete large amounts of aSMase (3) and this form of aSMase is activated by Zn2+ (4).
Acid SMase is first synthesized as a 75-kDa prepro-enzyme representing the full-length, N-glycosylated protein (3). The prepro-aSMase is rapidly processed to pro-aSMase (72 kDa), and within the acidic compartment matures to a 70-kDa form, and last is processed to a 52-kDa polypeptide (3). Mature L-SMase is believed to represent the 70-kDa and/or the 52-kDa forms of aSMase, however, given the lack of investigation into the Zn2+ requirement of these different forms of aSMase, the precise molecular identity of the mature, Zn2+-independent L-SMase remains unclear. Hurwitz et al. (5) demonstrated that the in vivo aSMase inhibitor desipramine, induced the loss of the 70-kDa form of aSMase concomitant with the loss of L-SMase activity in treated cells. However, isoelectric focusing studies have described two forms of aSMase: a 70-kDa form and a 57-kDa form, which correlated with peaks of activity (6). The former was found in fractions with the highest level of L-SMase activity and was assigned a pI of 6.8–7.2. Importantly, aSMase protein was found in almost all of the fractions, whereas aSMase activity was concentrated in only 25% of the fractions. Therefore, despite extensive investigation, the true identity of mature, Zn2+-independent L-SMase remains unknown.
By virtue of its distinct cellular itinerary, S-SMase exhibits several defining characteristics that have been used to distinguish it from L-SMase. First, S-SMase does not encounter Zn2+ during its trafficking and maturation, and thus remains Zn2+-dependent (2). Second, S-SMase is trafficked through the distal Golgi pathway where it undergoes additional processing of N-glycans to become of the “complex” type, rendering the enzyme partially insensitive to digestion with endoglycosidase H (2, 3, 6). Third, it appears that L-SMase undergoes additional N-terminal proteolytic processing as purified S-SMase begins with His60 and L-SMase begins with Gly66 (2). Mature L-SMase may undergo additional N-terminal processing as microsequencing of purified placental L-SMase indicated the N terminus began with Gly83 (7).
Although N-terminal modifications to the different forms of aSMase have been documented, evidence for the C-terminal modification is lacking. It has previously been suggested that carboxyl-terminal modification of aSMase might serve as a mechanism to regulate enzyme activity. Qiu et al. (8) described a mechanism whereby oxidation, mutation, and/or deletion of the C-terminal Cys629 resulted in activation of the enzyme. Based on these results the authors postulated that loss of the C-terminal Cys629 might serve as a “cysteine switch,” as has been described for matrix metalloproteinases (9), whereby loss of C-terminal Cys residues favors hydration of Zn2+ thereby promoting enzyme activation (8). Also discussed was the possible relevance of this mechanism of enzyme activation to in vivo regulation. Given that C-terminal processing has been described for several lysosomal hydrolases that follow a similar path of trafficking and maturation, such as cathepsin D (10), it is conceivable that aSMase undergoes similar proteolytic processing to generate mature L-SMase.
To determine whether C-terminal processing was required for the formation of mature L-SMase in situ we utilized cells stably overexpressing aSMase with C-terminal V5/His or DsRed fusion tags. Here, we demonstrate that C-terminal processing of aSMase occurs within or near the endolysosomal compartment, giving rise to mature Zn2+-independent L-SMase. Mature L-SMase is recognized by an antibody directed to aSMase, is sensitive to the lysosomotropic inhibitor desipramine, and co-fractionates with lysosomes. Furthermore, we provide evidence that C-terminal-tagged aSMase represent pro-aSMase, is Zn2+-dependent, and localizes to the Golgi secretory pathway, but not the endolysosomal compartment. Last, three C-terminal Niemann-Pick mutants exhibit defective C-terminal processing with loss of L-SMase activity. These results indicate that C-terminal processing is essential for formation of mature L-SMase.
EXPERIMENTAL PROCEDURES
Materials
MCF7 and HEK 293 cells were obtained from ATCC (Manassas, VA). RPMI and MEM culture medium, fetal bovine serum, blasticidin S-HCl, and geneticin (G418) were obtained from Invitrogen. Anti-V5 mouse monoclonal antibody was from Invitrogen. DsRed rabbit polyclonal antibody was obtained from Clontech (Mountain View, CA). Rabbit polyclonal LAMP-1 antibody was obtained from Abcam (Cambridge, MA). Mouse monoclonal LAMP1 antibody and DsRed goat polyclonal antibody, and HRP-labeled secondary antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Calreticulin rabbit polyclonal antibody was from Sigma. Rabbit anti-α-mannosidase II polyclonal antibody was from Chemicon International (Temecula, CA). Rabbit anti-TGN46 antibody was from Novus (Littleton, CO). Enhanced chemiluminescence kit was from ThermoScientific (Rockford, IL). Leupeptin, pepstatin, and aprotinin were obtained from Roche Applied Science. Desipramine, peptide N-glycanase F (PNGaseF), and all other chemicals were obtained from Sigma. Partially purified placental acid sphingomyelinase was a gift from Dr. Gary Smith (GlaxoSmithKline), and has previously been described (11). Rabbit polyclonal antisera to aSMase (number 1598) (12) was a generous gift from Drs. Edward Schuchman and Richard Kolesnick.
Cell Culture and Generation of Stable Cell Lines
MCF7 cells were grown in RPMI 1640, supplemented with L-glutamine and 10% (v/v) fetal bovine serum. HEK 293 were grown in 10% FBS/MEM. MCF7 stably expressing V5-aSMaseWT were previously described (13). To generate HEK 293 cells stably expressing DsRed plasmids, cells were plated in 6-well dishes (200,000 cells/well) and transfected with 1.0 μg of DNA using Lipofectamine 2000 according to the manufacturer's instructions. The following day, transfected cells were trypsinized, pelleted, and re-plated (1:5) in 10% FBS/MEM supplemented with 0.75 mg/ml of G418 (Invitrogen). After 2–3 weeks in selective medium, transfection efficiency was evaluated by confocal microscopy for DsRed fluorescence.
Plasmids and Site-directed Mutagenesis
pEF6.V5/His.aSMaseWT and pEF6.V5/His.aSMaseS508A were previously described (11). pEF6.V5/His.LacZ is the control plasmid pEF6/V5-His-TOPO cloning kit (Invitrogen). VSVG3-GFP was kindly provided by Dr. Chiara Luberto (Medical University of South Carolina), and was generated and described by the laboratory of Dr. Kai Simons (14). pDsRed-N1-Monomer-aSMaseWT was previously described (15). DsRed-aSMaseS508A mutant was generated via site-directed mutagenesis using the QuikChange kit from Stratagene, as previously described (11). DsRed-aSMaseR600P, DsRed-aSMaseR600H, and DsRed-aSMaseΔR608 were generated using the following mutagenesis primers: underlined nucleotides represent the changed codon, “/” represents site of deleted codon, R600H Fwd, 5′-GCTCTCTGCCCATGCTGACAGCC-3′, R600H Rev, 5′-GGCTGTCAGCATGGGCAGAGAGC-3′; R600P Fwd, 5′-GCTCTCTGCCCCTGCTGACAGCC-3′, R600H Rev, 5′-GGCTGTCAGCAGGGGCAGAGAGC-3′; ΔR608 Fwd, 5′-CTGCTCTGTGC/CACCTGATGCC-3′, ΔR608 Rev, 5′-GGCATCAGGTG/GCACAGAGCAG-3′. For all constructs, plasmid DNA was sequenced (GeneWiz) to confirm mutations and ensure that the C-terminal DsRed tag was in-frame with the aSMase cDNA.
Immunoprecipitation
Immunoprecipitation of V5-tagged proteins was performed as previously described (15). Briefly, V5-S-SMase was immunoprecipitated directly from conditioned medium with 1 μg/ml of anti-V5 mAb, unless otherwise indicated, and 1:20 protein A/G-agarose beads (Santa Cruz) in the presence of 1:500 protease inhibitor mixture (Sigma) by rocking overnight at 4 °C. For intracellular V5-aSMase, cells were washed two times and then scraped in ice-cold PBS. Cells were pelleted by centrifugation (5 min, 800 × g, 4 °C) and then lysed in IP buffer (20 mm Tris-HCl, pH 7.4, 100 mm NaCl, 1% Nonidet P-40, 1 mm EDTA, 1 mm EGTA, 1:200), each protease inhibitor mixture (Sigma), and phosphatase inhibitor mixtures 1 and 2 (Sigma). Lysates were clarified by centrifugation (10 min at 10,000 × g at 4 °C), normalized to the protein concentration, and incubated with Protein A/G-agarose for 1 h to remove nonspecific interacting proteins. Cleared lystates were then incubated overnight at 4 °C with 1 μg/ml of primary antibody (V5) and 1:10 protein A/G-agarose using a circular rotator. Bound protein was isolated by centrifugation (1 min, 12,000 × g), washed three times with lysis buffer, and the immunoprecipitates were prepared by addition of 60 μl of 2× Laemmli buffer. Equal volumes were subjected to SDS-PAGE electrophoresis and Western blotting. For immunotitration experiments, conditions were similar to those described above except that the concentration of V5 mAb was adjusted (0, 1, 2, and 4 μg/ml).
Glycosidase Digestion
For PNGaseF digestion, 37.5 μl of ddH2O was added to placental L-SMase (20 μl) or V5-aSMase immune complexes (20 μl), followed by 10 μl of 5× reaction buffer (250 mm sodium phosphate, pH 7.5) and 2.5 μl of denaturation solution (2% SDS + 1 mm β-ME) to each tube. Samples were boiled for 5 min and then cooled on ice. Two μl (0.01 units) of enzyme were then added to each sample along with Triton X-100 (0.75% final), mixed by gentle pipeting, and incubated overnight (∼ 18 h) at 37 °C. The following day, samples were retrieved, 50 μl of 2× sample buffer was added to 50 μl reaction volume, and samples were boiled again for 5 min. Cleavage was monitored by Western blotting following SDS-PAGE on a 7.5% acrylamide gel.
SDS-PAGE and Immunoblotting
SDS-PAGE and immunoblotting were performed as described previously (15). Primary antibody dilutions were V5, 1:5,000; aSMase, 1:500–1,000; DsRed, 1:500; and β-actin, 1:10,000.
In Vitro aSMase Activity Assay
S-SMase and L-SMase were measured as described previously (15).
Differential Centrifugation
2 × 106 DsRed-aSMaseWT HEK were seeded in 10-cm dishes in 10% FBS/MEM and allowed to grow for 2 days. Cells were then shifted to serum-free medium (10.0 ml of 0.1% BSA/MEM per dish) for 18 h. Adherent cells were washed, scraped, and pelleted (5 min, 1100 rpm, 4 °C) in ice-cold PBS. Following aspiration of the PBS wash, cell pellets were resuspended in 1.0 ml of Buffer L/F, 10 mm Tris-HCl (pH 7.5), 300 mm sucrose, 5 mm EDTA, 1:200 protease inhibitor mixture (Sigma), 1:200 phosphatase inhibitor mixture 1 (Sigma), 1:200 phosphatase inhibitor mixture 2 (Sigma), and incubated on ice for 1 h. Samples were then homogenized by passage through a 28½-gauge syringe 10–15 times. Samples were then centrifuged for 5 min at 1,000 × g (4 °C) to obtain the 1,000 × g (nuclear, unbroken cell) pellet. The supernatant from the 1,000 × g spin was then spun for 10 min at 10,000 × g (4 °C) to obtain the 10,000 × g (heavy membrane) pellet. The supernatant from the 10,000 × g spin was then spun for 1 h at 100,000 × g (4 °C) to obtain the 100,000 × g (light membrane) pellet. The pellets from each spin were resuspended in 0.1 ml of buffer L/F for subsequent analysis. To resuspend the 100,000 × g pellet buffer L/F was supplemented with Triton X-100 (1% final concentration). Aliquots of supernatants and pellets were snap frozen in CO2(s)/MeOH bath and stored at −80 °C prior to aSMase activity assay.
Silver Stain for Total Protein
Silver staining of polyacrylamide gels was performed using the SilverSNAP® Stain Kit II from Pierce, according to the manufacturer's instructions.
Statistical Analysis
Data are represented as mean ± S.E., unless otherwise indicated. Unpaired Student's t test, one-way ANOVA with Dunnett's post-test, and two-way ANOVA with Bonferroni post-test statistical analyses were performed using Prism/GraphPad software with additional post-test analysis using the Prism/Graphpad website.
RESULTS
Carboxyl-terminal V5-tagged aSMase Localizes to the Golgi Secretory Pathway
It is well established that aSMase localizes to the endolysosomal compartment (16, 17). However, in ongoing studies utilizing MCF7 stably expressing a C-terminal-tagged V5-aSMase, we observed that V5-aSMase exhibited minimal colocalization with the lysosomal marker LAMP1 (Fig. 1A), but instead co-localized more strongly with VSVG3-GFP (Fig. 1B), which marks all compartments of the Golgi secretory pathway (18). Counterstaining with markers of the endoplasmic reticulum (Fig. 1C, calreticulin) and Golgi (Fig. 1, D and E, α-mannosidase II and TGN46) also demonstrated strong colocalization with markers of Golgi secretory pathway, but not the ER. To further explore this, immunofluorescence staining was performed using aSMase-specific antisera (number 1598) (12). As expected, the aSMase antibody showed strong colocalization with V5-aSMase (Fig. 1F). However, the aSMase Ab recognized structures not detected by the V5 Ab. As partial colocalization of aSMase with LAMP1 was also evident (Fig. 1G), this indicated that the aSMase Ab recognized both mature lysosomal aSMase and V5-tagged aSMase. These results demonstrate that V5-aSMase resides primarily in the Golgi secretory pathway, and suggest that lysosomal aSMase had lost its C-terminal V5 tag upon entry into the endolysosomal compartment.
FIGURE 1.
Subcellular localization of V5-tagged aSMase. Subcellular localization of V5-aSMaseWT was assessed by confocal microscopy. 2 × 105 cells were seeded in 35-mm glass-bottom plated dishes (MatTek). Where indicated, cells were transfected with VSVG-GFP for 18 h prior to immunofluorescence staining. The following day, cells were shifted to serum-free medium (0.1% BSA/RPMI) overnight. Cells were rinsed with PBS, fixed with 3.7% formaldehyde, and then processed for indirect immunofluorescence staining as described under “Experimental Procedures.” A, green, V5 (1:500); red, LAMP-1 (1:100); B, green, VSVG3-GFP; red, V5 (1:500); C, green, V5 (1:500); red, calreticulin (1:500); D, green, V5 (1:500); red, α-mannosidase II (1:100); E, green, V5 (1:500); red, TGN46 (1:100); F, green, V5 (1:500); red, aSMase (1:100); G, green, LAMP-1 (1:100); red, aSMase (1:100).
65-kDa aSMase Develops from 75-kDa V5-aSMase but Lacks a V5 Tag
The loss of the ability to detect the C-terminal V5 tag of the lysosomally localized aSMase suggested proteolytic processing of aSMase within the endolysosomal compartment. Consequently, such processing would be expected to yield lower molecular weight forms of aSMase. To investigate this, cellular extracts from vector control (V5-LacZ) and V5-aSMaseWT MCF7 were assayed for L-SMase activity to confirm L-SMase activity, and were subjected to Western blot analysis using both anti-V5 and anti-aSMase antibodies. L-SMase activity was markedly increased (∼15-fold) in V5-aSMase MCF7 compared with vector control MCF7, indicating that overexpressed V5-aSMase acquired Zn2+-independent aSMase activity (Fig. 2A). Western blot analysis with V5 mAb revealed a single band in V5-aSMaseWT MCF7 (75 kDa), whereas two distinct bands were evident with the aSMase Ab (75 kDa, 65 kDa) (Fig. 2B). These results indicate that a 65-kDa form of aSMase is generated from 75-kDa V5-aSMase, but is lacking the C-terminal tag. Moreover, given that Zn2+-independent L-SMase activity develops from V5-aSMase, this 65-kDa aSMase likely represents lysosomal aSMase observed above (Fig. 1D).
FIGURE 2.
Activity and expression of aSMase in V5-LacZ and V5-aSMaseWT MCF7. 5 × 105 cells V5-LacZ or V5-aSMaseWT MCF7 were seeded in 60-mm dishes. Cells were serum-starved overnight and then collected for Western blot analysis and L-SMase activity as described under “Experimental Procedures.” A, L-SMase activity was determined as described under “Experimental Procedures” using 20 μg of cleared cell lysate; n = 3; ***, p < 0.001 (unpaired Student's t test). B, levels of V5-aSMase and aSMase were determined by immunoblotting using V5 mAb (1:5000) and aSMase rabbit polyclonal Ab (1:1000), respectively. β-Actin (1:10,000) was used as a loading control.
Selective loss of 65-kDa aSMase following Desipramine Treatment
To further investigate which of the 75-kDa V5-aSMase or 65-kDa aSMases corresponds to mature L-SMase, the aSMase inhibitor desipramine was utilized (19). Desipramine is a cationic amphiphile that accumulates in the acidic compartment and induces proteolysis of mature L-SMase activity (5, 20). Indeed, desipramine (25 μm) induced a time-dependent loss of L-SMase activity, with complete loss of activity by ∼60 min (Fig. 3A). Desipramine also induced a time-dependent loss of 65-kDa aSMase, with a concomitant appearance of a 52–55-kDa form of aSMase, presumably representing a breakdown product. In contrast, no change in V5-aSMase protein levels was apparent (Fig. 3B). Similarly, levels of the 75-kDa form detected with the aSMase Ab were also unchanged. Taken together, these results indicate that the desipramine-sensitive form of aSMase corresponds to 65-kDa aSMase, and not to the 75-kDa V5-aSMase. This strongly suggests that mature L-SMase has lost its C-terminal tag.
FIGURE 3.
Effect of desipramine on L-SMase activity and protein levels. A, 5 × 105 V5-aSMaseWT MCF7 were seeded in 60-mm dishes in 10% FBS/RPMI. The following day, cells were changed to serum-free medium (3.0 ml, 0.1% BSA/RPMI) for 18 h, before replacing medium (2.0 ml of 0.1% BSA/RPMI) 1 h prior to addition of desipramine (25 μm in PBS) for the indicated time points. Cells were collected and processed for L-SMase activity as described under “Experimental Procedures.” n = 3; *, p < 0.01 (one-way ANOVA, Dunnett's post-test). B, aliquots of the cellular extracts from V5-aSMaseWT MCF7 treated with desipramine were analyzed for protein levels of V5-aSMase (SE, short exposure; LE, long exposure), aSMase, with β-actin as a loading control. Single asterisk indicates the 65-kDa aSMase and the double asterisk denotes the 52-kDa aSMase, which becomes more prominent as 65 kDa is degraded following desipramine treatment. The nonspecific band is denoted by ns.
V5 mAb Fails to Immunoprecipitate L-SMase Activity
As mature L-SMase acquires Zn2+ en route to the lysosome, cellular L-SMase activity is Zn2+-independent when measured in vitro (2). Therefore, to further confirm that V5-aSMase does not account for the Zn2+-independent L-SMase activity, an immunotitration experiment was performed to deplete V5-aSMase from cell extracts prior to analysis of L-SMase activity. As expected, increasing amounts of V5 mAb effectively immunoprecipitated intracellular V5-aSMase (Fig. 4A). Notably, despite the effective removal of V5-aSMase from cell lysates, there was no effect on cellular L-SMase activity (Fig. 4B). In contrast to L-SMase, secreted S-SMase requires Zn2+ for full activity. As above, V5-S-SMase was effectively immunoprecipitated from conditioned medium (Fig. 4A). Importantly, this significantly depleted the Zn2+-dependent S-SMase activity (Fig. 4B). Taken together, this indicates that intracellular V5-aSMase does not account for intracellular L-SMase activity, supporting the hypothesis that L-SMase has lost its C-terminal V5 tag. Moreover, unlike its lysosomal counterpart, extracellular Zn2+-dependent S-SMase has an intact C terminus.
FIGURE 4.
Immunotitration of S-SMase and L-SMase. 2 × 106 V5-aSMaseWT MCF7 were seeded in 10-cm dishes in 10% FBS/RPMI. The following day cells were changed to serum-free medium (10.0 ml, 0.1% BSA/RPMI) for 18 h, before replacing medium (6.0 ml of 0.1% BSA/RPMI) 18 h prior to collection. Conditioned medium and adherent cells were collected and processed for immunoprecipitation as described under “Experimental Procedures.” Cleared medium and cellular extracts were immunoprecipitated with the indicated amounts of V5 mAb, and the following day immune complexes were pelleted. A, immune complexes were analyzed by immunoblot for levels of V5-aSMase, whereas in B, supernatants were analyzed for residual S-SMase and L-SMase activity. (n = 3, **, p < 0.01; one-way ANOVA, Dunnett's post-test). Control levels of L-SMase = 108.6 ± 4.6 nmol/mg/h, and S-SMase = 29.6 ± 5.2 nmol/ml/h.
Loss of C Terminus Defines Zinc-independent aSMase
Although V5-aSMase was effectively immunoprecipitated from cellular extracts, it did not contribute to mature L-SMase activity. Thus, we reasoned that the immunoprecipitated V5-aSMase would exhibit similar properties to V5-S-SMase that was immunoprecipitated from conditioned medium (i.e. S-SMase). To test this, V5-aSMase was immunoprecipiated from conditioned medium and from cellular extracts, and activity was tested both in the IP pellet and in the supernatant, both in the presence or absence of zinc (Fig. 5A). As expected and observed above, Zn2+-independent activity was unaffected by the loss of V5-aSMase from cell lysates, whereas Zn2+-dependent V5-S-SMase activity was completely removed from conditioned medium and showed no activity in the presence of EDTA. Importantly, whereas V5-aSMase derived from cellular extracts showed minimal activity in the presence of EDTA, it was strongly activated by Zn2+ (Fig. 5A). Thus, the cellular V5-aSMase represents a Zn2+-dependent form of aSMase.
FIGURE 5.
Zn2+ dependence of aSMase activity in V5-aSMase immunoprecipitates from cellular extracts and conditioned medium. 5 × 105 V5-aSMaseWT MCF7 were seeded in 60-mm dishes in 10% FBS/RPMI. The following day cells were changed to serum-free medium (3.0 ml, 0.1% BSA/RPMI) overnight, before replacing medium (2.0 ml 0.1% BSA/RPMI) 18 h prior to collection. V5-aSMase was immunoprecipitated from conditioned medium and cleared cellular extracts as described under “Experimental Procedures,” and supernatants and pellets were analyzed for aSMase activity (A) in the presence of 1.0 mm EDTA or 0.1 mm ZnCl2, and levels of V5-aSMase, aSMase, and β-actin (B) were determined by immunoblot (IB) on supernatant (SUP) and immune complexes (n = 5, ***, p < 0.001; two-way ANOVA, Bonferroni post-test). Single asterisk indicates the 65-kDa aSMase. The nonspecific band is denoted by ns. The nonspecific band conditioned medium supernatant results from nonspecific binding of the aSMase Ab to BSA present in the culture medium (data not shown).
Removal of V5-aSMase from cellular extracts had no effect on Zn2+-independent L-SMase activity. Accordingly, to determine whether this activity corresponded to the 65-kDa form, immunoblot analysis was performed. As above, V5-aSMase was present in the pellet from the IP (using the V5 antibody for IP) from both cellular extracts and conditioned medium (Fig. 5B). However, both 70–75- and 65-kDa bands remained in the cell extract supernatant after immunoblotting with the α-aSMase Ab. Importantly, the 65-kDa aSMase was neither immunoprecipitated by nor immunoreactive to the V5 mAb. Taken together, these results demonstrate that both L-SMase activity and the 65-kDa aSMase are insensitive to V5 immunoprecipitation, thus further suggesting that the 65-kDa aSMase represents mature L-SMase. In contrast, the 75-kDa cellular V5-aSMase exhibits Zn2+-dependent activity, and therefore does not contribute to mature (Zn2+-independent) L-SMase activity.
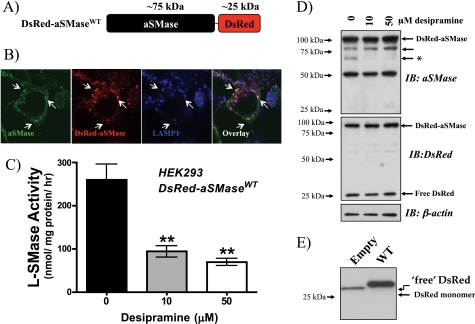
Processing and Localization of DsRed-tagged aSMase
Thus far, our data suggest that mature L-SMase has lost its C-terminal tag. To corroborate thus, it became necessary to track the C-terminal tag. However, as V5 comprises 14 amino acids (21), it is very difficult to follow by conventional methods. In contrast, DsRed, a 28-kDa red fluorescent protein (22, 23), is easily tracked by immunoblot or immunofluorescence; accordingly, an aSMase-DsRed construct was generated (Fig. 6A) and this was used to generate stably expressing HEK cells. Initially, localization of DsRed fluorescence was determined by confocal microscopy (Fig. 6B). Notably, unlike V5 staining in MCF7 cells, DsRed strongly colocalized with LAMP1. To further confirm that DsRed-aSMase was in fact processed to mature L-SMase, the susceptibility of DsRed-aSMase to desipramine-induced proteolysis was determined. As before, desipramine induced a significant loss of L-SMase activity at 10 and 50 μm (Fig. 6C). Importantly, the action of desipramine resulted in a selective loss of the 65-kDa aSMase form (Fig. 6D). Moreover, immunoblotting for DsRed revealed not only the expected fusion protein of ∼100 kDa, but also a smaller 25–30-kDa fragment that likely represents a “liberated” C-terminal fragment of the fusion protein. Consistent with this, the DsRed fragment from the HEK-asMase-DsRed cells migrated slower than native DsRed from HEK cells expressing empty DsRed vector (Fig. 6E), suggesting that this larger fragment may contain elements of the C terminus of aSMase. Taken together, these data indicate a C-terminal processing of DsRed-aSMase producing a 65-kDa aSMase and a ∼30-kDa DsRed fragment.
FIGURE 6.
Subcellular localization and protein processing of DsRed-tagged aSMase in HEK 293. A, schematic representation of the aSMase-DsRed fusion protein. B, 2 × 105 DsRed-aSMaseWT HEK were seeded in 35-mm glass-bottom plated dishes (MatTek) in 10% FBS/MEM. The following day cells were changed to serum-free medium (2.0 ml, 0.1% BSA/MEM) overnight, before being processed for indirect immunofluorescence staining. Cells were fixed, permeabilized, blocked as described under “Experimental Procedures,” and stained for aSMase (1:100, green, λ488) and LAMP1 (1:100, blue, λ647). DsRed fluorescence was detected using the λ555 channel. Arrowheads denote triple-positive structures. C, 5 × 105 DsRed-aSMaseWT HEK were seeded in 60-mm dishes in 10% FBS/MEM. The following day, cells were changed to serum-free medium (3.0 ml of 0.1% BSA/MEM) for 18 h, before replacing medium (2.0 ml of 0.1% BSA/MEM) 1 h prior to addition of desipramine (10 and 50 μm in PBS) for 2 h. Cells were collected and processed for L-SMase activity as described under “Experimental Procedures” (n = 3; **, p < .01; one-way ANOVA, Dunnett's post-test). D, levels of aSMase and DsRed-aSMase in cellular extracts following desipramine treatment were determined by immunoblot (IB) with β-actin as a loading control. Single asterisk indicates 65-kDa aSMase. E, molecular weight of the liberated DsRed fragment from DsRed-aSMaseWT HEK (WT) compared with that of native, unfused DsRed (28 kDa) from HEK stably expressing pDsRed-N1-Monomer (EMPTY). Equal protein was loaded and resolved by SDS-PAGE (4–20%). Membranes were probed with α-DsRed Ab (1:500).
Proteolytic Processing of aSMase Occurs in the Acidic Compartment
The strong colocalization of DsRed and LAMP1 (Fig. 6B) but not V5-aSMase and LAMP1 suggests that proteolytic processing of aSMase occurs within endolysosomes. Thus, the DsRed observed in the lysosomes would represent a 30-kDa liberated fragment from the 100-kDa precursor. To confirm this biochemically, a differential centrifugation approach was taken utilizing the DsRed-aSMaseWT HEK 293 cells (Fig. 7). Results showed that the heavy membrane fraction (10,000 × g pellet) was enriched in all forms of aSMase (100, 75, 65, and 52 kDa) including the full-length aSMase-DsRed (100 kDa) and the ∼30-kDa DsRed fragment. Importantly, this fraction and to a lesser extent the 1,000 × g pellet contained a strong LAMP1 signal suggesting enrichment of lysosomes; notably, this fraction contained the majority of the free DsRed. In contrast, the DsRed-aSMase precursor was also found in the light membrane fraction (100,000 × g), but both the 65-kDa aSMase and the free DsRed fragment were absent. Taken together, these data confirm the confocal observations above (Fig. 6B) and suggest that DsRed is removed from the aSMase-DsRed precursor within the endolysosomal compartment.
FIGURE 7.
Differential centrifugation of DsRed-tagged aSMase in HEK 293. 2 × 106 DsRed-empty or DsRed-aSMaseWT HEK were seeded in 10-cm dishes in 10% FBS/MEM and allowed to grow for 2 days. Cells were then shifted to serum-free medium (10.0 ml of 0.1% BSA/MEM per dish) for 18 h before collection. Cells were collected and prepared for differential centrifugation as described under “Experimental Procedures,” and equal volumes were loaded on a 4–20% gel and resolved by SDS-PAGE. Membranes were blotted for aSMase, DsRed, and LAMP1, as described (n = 3). Single asterisk indicates the 65-kDa aSMase. Relative enrichment of L-SMase activity in the 10,000 × g pellet (compared with whole cell extracts) is at least 4–5-fold (data not shown). IB, immunoblot.
65-kDa aSMase Is Enriched in Lysosomal Fractions and Is Uniquely Sensitive to Desipramine
The localization of the 65-kDa aSMase to lysosome-enriched fractions further suggested that this form represents mature L-SMase. To further confirm this, HEK cells expressing empty-DsRed or DsRed-aSMase were treated with vehicle (PBS) or desipramine (50 μm, 2 h) and then processed for differential centrifugation. As can be seen, desipramine treatment caused a selective loss of the 65-kDa aSMase from the lysosome-rich, heavy membrane fraction (10,000 × g pellet) (Fig. 8A) with an associated loss of L-SMase activity from this fraction (Fig. 8B). Similarly, the 65-kDa aSMase seen in the 1,000 × g pellet, which was also positive for LAMP1 (Fig. 7) and associated L-SMase activity, was also desipramine sensitive. Taken together, these results identify 65 kDa as a lysosomal-enriched, desipramine-sensitive form of aSMase, further suggesting it represents the mature L-SMase.
FIGURE 8.
Effect of desipramine on aSMase protein levels in different subcellular fractions. A, 2 × 106 DsRed-empty or DsRed-aSMaseWT HEK were seeded in 10-cm dishes in 10% FBS/MEM and allowed to grow for 2 days. Cells were then shifted to serum-free medium (10.0 ml of 0.1% BSA/MEM per dish) for 18 h, before replacing medium (10.0 ml of 0.1% BSA/MEM per dish) 1 h prior to addition of vehicle (PBS) or desipramine (50 μm in PBS) for 2 h. Cells were collected and prepared for differential centrifugation as described under “Experimental Procedures,” and equal volumes of the pellets from the various fractions were loaded on a 4–20% gel and resolved by SDS-PAGE. Membranes were blotted for aSMase (1:1000) (n = 3). The single asterisk indicates 65-kDa aSMase. B, aliquots from the indicated pellets from control (ctl) and desipramine-treated (Des) cells were assessed for L-SMase activity. Equal volumes (5 μl from each fraction) were assayed at pH 5.0 in the presence of 1.0 mm EDTA (37 °C, 30 min) (n = 3; ***, p < 0.001 versus DsRed-empty; #, p < 0.05 versus control (ctl); two-way ANOVA, Bonferroni post-test).
Leupeptin Prevents Degradation of Mature L-SMase
Desipramine-induced proteolysis of L-SMase requires the action of lysosomal proteases (5). To determine the effect of protease inhibition on both the formation and degradation of 65-kDa aSMase, DsRed-aSMaseWT-expressing HEK cells were treated with various lysosomal protease inhibitors, and 10,000 × g pellets were analyzed for L-SMase activity (Fig. 9A) and aSMase protein levels (Fig. 9B). Of the inhibitors utilized, leupeptin caused a significant elevation in L-SMase activity (∼2.5-fold over vehicle), whereas pepstatin induced a modest but not significant increase in L-SMase activity. Immunoblot analysis again revealed four specific bands (100, 75, 65, and 52 kDa). Although pepstatin and aprotinin caused modest increases in the 65-kDa aSMase form, they also increased the 52-kDa form, which appears following degradation of the 65-kDa aSMase (see Fig. 2). In contrast, leupeptin treatment strongly increased the levels of the 100-, 75-, and 65-kDa aSMase forms, whereas the 52-kDa form was absent. These results suggest that leupeptin-sensitive protease(s) is important for degrading active L-SMase to an inactive 52-kDa form and further indicates that impaired degradation of 65-kDa aSMase results in enhanced L-SMase activity.
FIGURE 9.
Effect of protease inhibitors on aSMase activity and protein levels. A, 2 × 106 DsRed-aSMaseWT HEK were seeded in 10-cm dishes in 10% FBS/MEM and allowed to grow for 2 days. Cells were then shifted to serum-free medium (10.0 ml of 0.1% BSA/MEM per dish) for 18 h, before replacing medium (10.0 ml of 0.1% BSA/MEM per dish) 1 h prior to addition of vehicle (DMSO), pepstatin (10 μm), aprotinin (5 μm), or leupeptin (50 μm) for 20 h. Desipramine (50 μm) was added for 2 h. Cells were collected and prepared for differential centrifugation as described under “Experimental Procedures.” Aliquots from the 10,000 × g pellet (heavy membrane fraction) were assessed for L-SMase activity. Equal volumes (5 μl from 10,000 × g pellet) were assayed at pH 5.0 in the presence of 1.0 mm EDTA (37 °C, 30 min). Activity is expressed as % of the vehicle control (n = 3; *, p < 0.05; **, p < 0.01; one-way ANOVA, Dunnett's post-test). B, equal volumes of the 10,000 × g pellet (heavy membrane fraction) were loaded on a 4–20% gel and resolved by SDS-PAGE. Membranes were blotted for aSMase (1:1000) (n = 3). The single asterisk indicates the 65-kDa aSMase. As a negative control, samples from cells treated with desipramine were included to indicate the selective loss of the 65-kDa aSMase with associated elevations in both the 100- and 75-kDa bands. IB, immunoblot.
Characterization of Partially Purified Lysosomal aSMase
Many of the results presented above suggested that the 65-kDa aSMase corresponds to mature L-SMase. To definitively confirm this, a partially purified placental aSMase preparation was utilized as a reference for studies on cellular L-SMase. Analysis of the preparation by silver stain revealed a number of contaminants; nevertheless, only a single immunoreactive band for aSMase was evident at 65 kDa (Fig. 10A). This corresponded strongly to the lower 65-kDa band found in the supernatant of V5-aSMase cells following V5 immunoprecipitation (Fig. 10B, cf. Fig. 4, A and B). Importantly, the placental aSMase preparation also exhibited Zn2+-independent aSMase activity (Fig. 10C). Variations in N-glycan modifications can also influence migration of aSMase on SDS-PAGE. Consequently, V5-aSMase immune complexes and purified L-SMase were digested with PNGaseF to assess the native protein size (Fig. 10D). Fully deglycosylated V5-aSMase migrated at ∼62–64 kDa, consistent with previous reports for the native full-length protein precursor, whereas native L-SMase migrated at 55–60 kDa, again suggesting loss of a portion of the polypeptide backbone in mature L-SMase versus pro-aSMase. Taken together, these results confirm that mature, Zn2+-independent L-SMase corresponds to the glycosylated 65-kDa aSMase.
FIGURE 10.
Characterization of partially purified human placental aSMase. A, equal volumes of purified L-SMase were loaded on a 4–20% gel and resolved by SDS-PAGE. One gel was processed for silver staining (see “Experimental Procedures”) and the other was processed for immunoblotting with α-aSMase Ab. B, an aliquot of the supernatant (SUP) from cellular extracts following IP:V5-aSMase (see Fig. 4) was loaded on a 10% gel with an aliquot of purified L-SMase and the membrane was probed with anti-aSMase antibody. The single asterisk denotes the 65-kDa aSMase. C, acid SMase activity of partially purified L-SMase was assessed with 1.0 mm EDTA (0 mm Zn2+), or 50 μm, 500 μm, or 5 mm ZnCl2 (n = 3; *, p < 0.05; one-way ANOVA, Dunnett's post-test). D, V5-aSMase immunoprecipitated from cellular extracts and an aliquot of purified L-SMase were either boiled directly in Laemmli buffer (control) or digested with PNGase F (2 units/ml, 18 h, 37 °C). Equal volumes were loaded on a 7.5% gel and resolved by SDS-PAGE. Membranes were blotted for aSMase (1:1000) (n = 3). WCL, whole cell lysate.
C-terminal Niemann-Pick Mutants Exhibit Impaired Maturation of aSMase
Niemann-Pick disease (types A and B) result from mutations in the SMPD1 gene that encodes aSMase (24). Although some of these mutations affect catalysis directly, the cause of enzyme dysfunction for other aSMase mutants is incompletely understood (16, 17). To determine whether C-terminal processing is defective in certain aSMase mutants, the maturation of three C-terminal Niemann-Pick mutants, R600H, R600P, and ΔR608 (25, 26), was investigated. Following generation of stable HEK cells lines, the localization of these mutants was analyzed by confocal microscopy. Strikingly, all three mutants displayed altered localization with minimal colocalization observed for LAMP1 (Fig. 11A). Notably, this corresponded to the virtual absence of Zn2+-independent L-SMase activity (Fig. 11B), with residual activity because of the endogenous aSMase activity in HEK. Moreover, analysis of the heavy membrane fractions (10,000 × g pellet) revealed an impaired formation of mature L-SMase (65 kDa) and a drastic reduction in formation of the liberated 30-kDa DsRed fragment (Fig. 11C). Taken together, these results suggest that the C terminus of aSMase is essential for proper proteolytic maturation of L-SMase, and mutation of residues in this region is sufficient to prevent formation of mature, Zn2+-independent L-SMase.
FIGURE 11.
Effect of C-terminal Niemann-Pick mutations on aSMase maturation. A, 2 × 105 HEK stably expressing DsRed-aSMaseWT (WT), DsRed-aSMaseR600H (R600H), DsRed-aSMaseR600p (R600P), and DsRed-aSMaseΔR608 (ΔR608) were seeded in 35-mm glass-bottom plated dishes (MatTek) in 10% FBS/MEM. The following day cells were changed to serum-free medium (2.0 ml, 0.1% BSA/MEM) overnight, before being processed for indirect immunofluorescence staining. Cells were fixed, permeabilized, blocked as described under “Experimental Procedures,” and stained for LAMP1 (1:100, green, λ488). DsRed fluorescence was detected using the λ555 channel. B, 5 × 105 HEK stables (WT, R600H, R600P, Δand R608) were seeded in 60-mm dishes in 10% FBS/MEM. The following day cells were changed to serum-free medium (3.0 ml, 0.1% BSA/MEM) for 18 h. Cells were collected and processed for L-SMase activity as described under “Experimental Procedures” (n = 3; **, p < 0.01; one-way ANOVA, Dunnett's post-test). C, 2 × 106 HEK stables (WT, R600H, R600P, and ΔR608) were seeded in 10-cm dishes in 10% FBS/MEM and allowed to grow for 2 days. Cells were then shifted to serum-free medium (10.0 ml of 0.1% BSA/MEM per dish) for 18 h before collection. Cells were collected and prepared for differential centrifugation as described under “Experimental Procedures.” Equal volumes of the 10,000 × g pellets (heavy membrane fraction) were loaded on a 4–20% gel and resolved by SDS-PAGE. Membranes were blotted for aSMase and DsRed, as described (n = 3). A 75-kDa band indicated by an arrow indicates an alternative cleavage product that retains the DsRed tag. The single asterisk indicates the 65-kDa aSMase and the double asterisk denotes the 52-kDa aSMase.
DISCUSSION
In this study, we set out to determine the identity of the mature, lysosomal, Zn2+-independent form of aSMase (L-SMase). We were initially prompted to pursue this line of investigation when confocal microscopic analysis failed to demonstrate significant colocalization of V5-aSMaseWT with lysosomal markers, but instead indicated localization to the Golgi secretory pathway. This finding led to the hypothesis that mature L-SMase was formed following C-terminal proteolytic processing, which would result in the loss of the C-terminal V5-tag upon trafficking to the lysosome. Through multiple complementary approaches, we provide evidence that 1) aSMase undergoes C-terminal proteolytic processing, 2) this processing event occurs in (or in close proximity to) the endolysosomal compartment, 3) this processing yields a C-terminal truncated 65-kDa protein that exhibits Zn2+-independent aSMase activity, and 4) mutations in the C terminus prevent maturation of aSMase. Together, these results suggest that mature L-SMase arises from C-terminal proteolytic processing within the endolysosomal compartment (Fig. 12).
FIGURE 12.
Schematic representation of aSMase processing. A, pro-aSMase (72–75 kDa) arises from prepro-aSMase (75 kDa) within the Golgi and is either targeted to the lysosome via mannose 6-phosphorylation (M6P), or is released extracellularly through the default Golgi secretory pathway giving rise to S-SMase (75–80 kDa). Upon entry to the endolysosomal compartment pro-aSMase is cleaved by “protease X” giving rise to the 65-kDa aSMase, which upon encountering Zn2+ in the lysosome becomes L-SMase. L-SMase (65 kDa) is in turn degraded by “protease Y” to the 52-kDa inactive L-SMase. The action of protease Y is promoted by desipramine, and inhibited by leupeptin. B, both pro-aSMase and S-SMase have an intact C terminus, require exogenous Zn2+ for activity, and are insensitive to desipramine. Mature L-SMase has lost a portion of its C terminus, which may promote coordination of lysosomal Zn2+, thereby rendering the 65-kDa aSMase, Zn2+-independent, and is sensitive to desipramine.
Identity of Mature L-SMase
Acid SMase has been purified from a variety of different sources including human urine (27–29), human brain (30), human placenta (7, 31, 32), overexpression in CHO cells (33), and Sf21 insect cells (34). Importantly, aSMase purified from extracellular sources (e.g. urine, conditioned medium) likely represents forms of S-SMase, whereas aSMase purified from tissue is believed to represent L-SMase. Human placental aSMase, which presumably represents L-SMase, has been purified as a 89-kDa protein (31), a 75-kDa protein (7), a 70-kDa protein (35–37), and a 62-kDa protein (32).
Given the variability in the reported Mr of aSMase, we utilized cell lines stably expressing C-terminal-tagged aSMase fusion proteins to determine the identity of L-SMase. Using antibodies against aSMase as well as the C-terminal fusion peptide/protein, we were able to monitor both that status of the C terminus (V5, DsRed) as well as the core polypeptide (aSMase). Several lines of evidence demonstrate C-terminal proteolytic processing of pro-aSMase to form mature L-SMase. First, V5-aSMase failed to colocalize with markers of the endolysosomal compartment and instead colocalized with markers of the Golgi secretory pathway (i.e. VSVG3-GFP, TGN46). Second, desipramine treatment failed to affect levels of 75-kDa V5-aSMase, despite inducing a rapid loss of L-SMase activity. This loss of L-SMase activity correlated instead with the disappearance of a 65-kDa form of aSMase that was recognized by an aSMase Ab, but not a V5 Ab, indicating carboxyl terminus processing of the mature active form. Third, immunoprecipitation of V5-aSMase failed to precipitate Zn2+-independent aSMase (L-SMase) activity, but effectively depleted Zn2+-dependent S-SMase activity. Fourth, the generation of mature L-SMase in HEK expressing DsRed-aSMase occurs with the liberation of a 25–30-kDa DsRed-immunoreactive fragment (free DsRed) that is concentrated within lysosome-rich fractions, providing strong support of C-terminal processing within endolysosomal processing. Collectively, these findings indicate that upon trafficking to the lysosome, pro-aSMase (72 kDa) matures to L-SMase (65 kDa) via C-terminal proteolytic processing.
The findings on 75-kDa V5-aSMase protein have implications on previous reports of C-terminal-tagged aSMase analyzed in cell culture. Schissel et al. (2) purified FLAG-tagged aSMase from cellular homogenates and conditioned medium. C-terminal FLAG-tagged aSMase from cellular extracts migrated at ∼72 kDa and was activated nearly 5-fold by exogenous Zn2+, consistent with the current results. This sensitivity to Zn2+ was believed to result from incomplete saturation of Zn2+ binding sites. Based on our results with V5-aSMase immunoprecipitated from cellular extracts (Figs. 3 and 4), we propose that the 75-kDa aSMase with an intact C terminus is a pre-lysosomal form of aSMase. Consistent with this model, V5-aSMase colocalized with markers of the Golgi secretory pathway but not with lysosomes. Given that the acquisition of Zn2+ by aSMase is thought to occur within the acidic compartment, we reasoned that intracellular 75-kDa aSMase may represent the common protein precursor of S-SMase and L-SMase.
Proteolytic Processing of Acid Sphingomyelinase
Processing of aSMase has been previously described to proceed via sequential formation of prepro-aSMase (75 kDa), pro-aSMase (72 kDa), and then 70- and 52-kDa forms of aSMase (3). Generation of the 70- and 52-kDa forms is absent in cells with impaired trafficking to lysosomes (3), suggesting that either or both the 70- and 52-kDa forms represent mature L-SMase. Desipramine promotes degradation of mature L-SMase, which proceeds through a leupeptin-sensitive protease (5). The results provided here indicate that 52-kDa aSMase is formed from the degradation of mature L-SMase (65 kDa). Desipramine induced a time-dependent loss of L-SMase activity, concomitant with the loss of 65-kDa aSMase, and the appearance of 52-kDa aSMase (Fig. 3). On the other hand, leupeptin resulted in accumulation of mature 65-kDa aSMase (as well as all precursor forms) and diminished formation of 52-kDa aSMase, with an associated elevation in L-SMase activity (Fig. 9). Taken together, these results indicate that mature L-SMase is 65 kDa and that the 52-kDa form is an inactive form of aSMase generated via the action of a leupeptin-sensitive protease (Fig. 12). The identity of this thiol protease is currently not known, but given its capacity to regulate the levels of L-SMase it might represent in important regulator of sphingolipid metabolism and signaling in the endolysosomal compartment.
Significance of Removal of Carboxyl Terminus of aSMase
A role for C-terminal modification in the regulation of aSMase activity has previously been described whereby oxidation or deletion of the carboxyl-terminal Cys629 induced a 4–5-fold increase in aSMase activity (8). This activation was recapitulated by addition of carboxypeptidase Y suggesting that removal of C-terminal residues has a similar effect on enzyme activity. The authors suggested that C-terminal modification of aSMase might serve as a post-translational mechanism to increase enzyme activity (8) via a mechanism similar to the cysteine switch that has been described for matrix metalloproteinases (9). However, these studies focused on the in vitro modifications to recombinant human aSMase, which was derived from S-SMase. Thus, this mechanism may operate on the secreted S-SMase, which from our studies is shown to retain the entire C terminus. Moreover, the extracellular milieu is more oxidized relative to the intracellular environment and thus is expected to promote the proposed oxidation of the C-terminal Cys. On the other hand, the current results show that the mature lysosomal L-SMase has lost this Cys and thus is activated by removal of the Cys rather than its oxidation. Moreover, one could speculate that this regulatory cysteine functions to maintain the precursor non-lysosomal aSMase in an inactive (low activity) form while en route from the Golgi to the lysosomes, thus, preventing activity in the enzyme in the Golgi.
Processing of the C termini of proteins within the acidic compartment has been reported for other lysosomal hydrolases, including cathepsin D (10, 38), β-glucuronidase (38), acid α-glucosidase (39), and γ-interferon-inducible lysosomal thiol reductase (GILT) (40), and has been suggested for acid ceramidase (41). The current results demonstrate that carboxyl-terminal processing of the enzyme, which would remove the C-terminal Cys, results in formation of the active enzyme. The mechanism of this activation remains to be determined as removal of Cys629 during processing of pre-aSMase to mature L-SMase could increase Zn2+ affinity, substrate binding, and/or catalytic capacity.
Despite the multiple lines of evidence provided here that indicate C-terminal processing of aSMase, previous analysis of purified placental aSMase suggested an intact C terminus (42). However, preliminary LC-MS analysis of the partially purified L-SMase used in the current study (cf. Fig. 10) reveals that the carboxyl-terminal residue is Gln620, indicating that a minimum of 9 amino acids are lost from the C terminus in mature L-Smase.3 Consistent with this finding, the slower migration of the liberated DsRed fragment (∼30 kDa) from DsRed-aSMase HEK relative to native DsRed (∼28 kDa), suggests that only a few amino acids are removed from the C terminus of aSMase during processing (which become retained in the cleaved DsRed). The significant difference in the size of the core polypeptide between pre-aSMase (∼62–64 kDa) and L-SMase (∼55–60 kDa) may reflect the combined effects of N- (2, 7) and C-terminal processing. One possible explanation for this discrepancy is that the form analyzed by Lansmann et al. (7) may represent a Zn2+-dependent form of pro-aSMase that may have co-purified with the placental mature form. Further studies will be required to determine the precise molecular identity of mature L-SMase.
Impaired Processing of NPD aSMase Mutants
The subcellular localization of wild-type or some mutant aSMase has previously been investigated (16, 17). Lee et al. (17) described the localization of YFP-tagged wild-type and ΔR608 aSMase, and reported that WT aSMase localized properly to lysosomes, whereas the ΔR608 exhibited minimal colocalization with LAMP2-positive structures and instead appeared to be trapped in the ER. However, Jones et al. (16) reported that several NPD mutants reached the lysosome. Our results indicate that a portion of mutant pro-aSMase reaches the lysosome (partial colocalization with LAMP1, appearance of precursors in 10,000 × g pellet, minimal generating of free DsRed fragment), suggesting that these C-terminal NPD mutants are not exclusively trapped in the ER subsequent to protein misfolding. However, whereas it is evident that the localization of the majority of mutant DsRed-aSMase is distinct from wild-type, further investigation will be required to determine whether impaired trafficking leads to diminished proteolytic processing, or whether impaired processing results in altered localization. Given that the C-terminal region of aSMase harbors the second most number of mutations, 2nd to the catalytic domain, it is apparent that this domain is critical for formation of active L-SMase (26, 43). Based on these results, we propose a novel role for the C-terminal region in maturation of aSMase, and demonstrate that mutations in the C terminus may prevent proper trafficking and proteolytic maturation of aSMase.
Conclusions
Mature Zn2+-independent L-SMase is generated from Zn2+-dependent pro-aSMase by carboxyl-terminal proteolytic processing within, or in close proximity to, endolysosomes. This represents a novel mechanism of post-translational regulation of aSMase, which has implications for the regulation of L-SMase activity and function, and may provide new insight into the molecular basis of NPD.
Acknowledgments
We thank Dr. Leah Siskind (MUSC) for careful reading of this manuscript. We offer special thanks to Dr. Gary Smith (GlaxoSmithKline) for providing partially purified human aSMase, and Branka Stancevic, Dr. Richard Kolesnick (Memorial Sloan-Kettering Cancer Center), and Dr. Edward Schuchman (Mount Sinai School of Medicine) for providing aSMase number 1598 antisera. We also thank Dr. Konrad Sandhoff (LIMES, Bonn, Germany) for many helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant P01 CA097132 from the NCI (to Y. A. H.), National Institutes of Health MSTP Training Grant GM08716 (to R. W. J.), American Heart Association Pre-doctoral Fellowship AHA 081509E (to R. W. J.), a Medical University of South Carolina Hollings Cancer Center Abney Foundation scholarship (to R. W. J.), Ministerio de Educacion y Ciencia (Spain) Predoctoral Fellowship AP2006-02190 (to F. S.), and Administrative Supplements Providing Summer Research Experiences for Students and Science Educators Grant HL043707-19S1 (to C. D. R.). The confocal core is supported in part by National Institutes of Health NCI Medical University of South Carolina-Cancer Center Support Grant 1P30 CA138313-01.
R. W. Jenkins, unpublished results.
- aSMase
- acid sphingomyelinase
- S-SMase
- secretory sphingomyelinase
- L-SMase
- lysosomal sphingomyelinase
- SM
- sphingomyelin
- NPD
- Niemann-Pick disease
- PNGaseF
- peptide N-glycanase F
- MEM
- minimal essential medium
- ANOVA
- analysis of variance
- IP
- immunoprecipitation.
REFERENCES
- 1. Schissel S. L., Schuchman E. H., Williams K. J., Tabas I. (1996) J. Biol. Chem. 271, 18431–18436 [DOI] [PubMed] [Google Scholar]
- 2. Schissel S. L., Keesler G. A., Schuchman E. H., Williams K. J., Tabas I. (1998) J. Biol. Chem. 273, 18250–18259 [DOI] [PubMed] [Google Scholar]
- 3. Hurwitz R., Ferlinz K., Vielhaber G., Moczall H., Sandhoff K. (1994) J. Biol. Chem. 269, 5440–5445 [PubMed] [Google Scholar]
- 4. Takahashi I., Takahashi T., Mikami T., Komatsu M., Ohura T., Schuchman E. H., Takada G. (2005) Tohoku J. Exp. Med. 206, 333–340 [DOI] [PubMed] [Google Scholar]
- 5. Hurwitz R., Ferlinz K., Sandhoff K. (1994) Biol. Chem. Hoppe Seyler 375, 447–450 [DOI] [PubMed] [Google Scholar]
- 6. Ferlinz K., Hurwitz R., Vielhaber G., Suzuki K., Sandhoff K. (1994) Biochem. J. 301, 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lansmann S., Ferlinz K., Hurwitz R., Bartelsen O., Glombitza G., Sandhoff K. (1996) FEBS Lett. 399, 227–231 [DOI] [PubMed] [Google Scholar]
- 8. Qiu H., Edmunds T., Baker-Malcolm J., Karey K. P., Estes S., Schwarz C., Hughes H., Van Patten S. M. (2003) J. Biol. Chem. 278, 32744–32752 [DOI] [PubMed] [Google Scholar]
- 9. Van Wart H. E., Birkedal-Hansen H. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 5578–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yonezawa S., Takahashi T., Wang X. J., Wong R. N., Hartsuck J. A., Tang J. (1988) J. Biol. Chem. 263, 16504–16511 [PubMed] [Google Scholar]
- 11. Zeidan Y. H., Hannun Y. A. (2007) J. Biol. Chem. 282, 11549–11561 [DOI] [PubMed] [Google Scholar]
- 12. Rotolo J. A., Zhang J., Donepudi M., Lee H., Fuks Z., Kolesnick R. (2005) J. Biol. Chem. 280, 26425–26434 [DOI] [PubMed] [Google Scholar]
- 13. Zeidan Y. H., Wu B. X., Jenkins R. W., Obeid L. M., Hannun Y. A. (2008) FASEB J. 22, 183–193 [DOI] [PubMed] [Google Scholar]
- 14. Toomre D., Keller P., White J., Olivo J. C., Simons K. (1999) J. Cell Sci. 112, 21–33 [DOI] [PubMed] [Google Scholar]
- 15. Jenkins R. W., Canals D., Idkowiak-Baldys J., Simbari F., Roddy P., Perry D. M., Kitatani K., Luberto C., Hannun Y. A. (2010) J. Biol. Chem. 285, 35706–35718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones I., He X., Katouzian F., Darroch P. I., Schuchman E. H. (2008) Mol. Genet. Metab. 95, 152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee C. Y., Tamura T., Rabah N., Lee D. Y., Ruel I., Hafiane A., Iatan I., Nyholt D., Laporte F., Lazure C., Wada I., Krimbou L., Genest J. (2007) Biochemistry 46, 14969–14978 [DOI] [PubMed] [Google Scholar]
- 18. Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. (1997) Nature 389, 81–85 [DOI] [PubMed] [Google Scholar]
- 19. Albouz S., Hauw J. J., Berwald-Netter Y., Boutry J. M., Bourdon R., Baumann N. (1981) Biomedicine 35, 218–220 [PubMed] [Google Scholar]
- 20. Kölzer M., Werth N., Sandhoff K. (2004) FEBS Lett. 559, 96–98 [DOI] [PubMed] [Google Scholar]
- 21. Southern J. A., Young D. F., Heaney F., Baumgärtner W. K., Randall R. E. (1991) J. Gen. Virol. 72, 1551–1557 [DOI] [PubMed] [Google Scholar]
- 22. Baird G. S., Zacharias D. A., Tsien R. Y. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11984–11989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matz M. V., Fradkov A. F., Labas Y. A., Savitsky A. P., Zaraisky A. G., Markelov M. L., Lukyanov S. A. (1999) Nat. Biotechnol. 17, 969–973 [DOI] [PubMed] [Google Scholar]
- 24. Schuchman E. H. (2007) J. Inherit. Metab. Dis. 30, 654–663 [DOI] [PubMed] [Google Scholar]
- 25. Lee C. Y., Lesimple A., Denis M., Vincent J., Larsen A., Mamer O., Krimbou L., Genest J., Marcil M. (2006) J. Lipid Res. 47, 622–632 [DOI] [PubMed] [Google Scholar]
- 26. Simonaro C. M., Desnick R. J., McGovern M. M., Wasserstein M. P., Schuchman E. H. (2002) Am. J. Hum. Genet. 71, 1413–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quintern L. E., Sandhoff K. (1991) Methods Enzymol. 197, 536–540 [DOI] [PubMed] [Google Scholar]
- 28. Quintern L. E., Zenk T. S., Sandhoff K. (1989) Biochim. Biophys. Acta 1003, 121–124 [DOI] [PubMed] [Google Scholar]
- 29. Quintern L. E., Weitz G., Nehrkorn H., Tager J. M., Schram A. W., Sandhoff K. (1987) Biochim. Biophys. Acta 922, 323–336 [DOI] [PubMed] [Google Scholar]
- 30. Yamanaka T., Suzuki K. (1982) J. Neurochem. 38, 1753–1764 [DOI] [PubMed] [Google Scholar]
- 31. Jones C. S., Shankaran P., Callahan J. W. (1981) Biochem. J. 195, 373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zou L., Kojima N., Kito M., Yagi K. (1989) Biotechnol. Appl Biochem. 11, 217–225 [PubMed] [Google Scholar]
- 33. He X., Miranda S. R., Xiong X., Dagan A., Gatt S., Schuchman E. H. (1999) Biochim. Biophys. Acta 1432, 251–264 [DOI] [PubMed] [Google Scholar]
- 34. Bartelsen O., Lansmann S., Nettersheim M., Lemm T., Ferlinz K., Sandhoff K. (1998) J. Biotechnol. 63, 29–40 [DOI] [PubMed] [Google Scholar]
- 35. Rousson R., Parvaz P., Bonnet J., Rodriguez-Lafrasse C., Louisot P., Vanier M. T. (1993) J. Immunol. Methods 160, 199–206 [DOI] [PubMed] [Google Scholar]
- 36. Kurth J., Stoffel W. (1991) Biol. Chem. Hoppe Seyler 372, 215–223 [DOI] [PubMed] [Google Scholar]
- 37. Sakuragawa N. (1982) J. Biochem. 92, 637–646 [DOI] [PubMed] [Google Scholar]
- 38. Erickson A. H., Blobel G. (1983) Biochemistry 22, 5201–5205 [DOI] [PubMed] [Google Scholar]
- 39. Wisselaar H. A., Kroos M. A., Hermans M. M., van Beeumen J., Reuser A. J. (1993) J. Biol. Chem. 268, 2223–2231 [PubMed] [Google Scholar]
- 40. Arunachalam B., Phan U. T., Geuze H. J., Cresswell P. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He X., Okino N., Dhami R., Dagan A., Gatt S., Schulze H., Sandhoff K., Schuchman E. H. (2003) J. Biol. Chem. 278, 32978–32986 [DOI] [PubMed] [Google Scholar]
- 42. Lansmann S., Schuette C. G., Bartelsen O., Hoernschemeyer J., Linke T., Weisgerber J., Sandhoff K. (2003) Eur. J. Biochem. 270, 1076–1088 [DOI] [PubMed] [Google Scholar]
- 43. Pittis M. G., Ricci V., Guerci V. I., Marcais C., Ciana G., Dardis A., Gerin F., Stroppiano M., Vanier M. T., Filocamo M., Bembi B. (2004) Hum. Mutat. 24, 186–187 [DOI] [PubMed] [Google Scholar]