Abstract
Peptidoglycan from a Staphylococcus epidermidis strain, isolated from a patient with septicemia, was preincubated with human serum. This mixture was then investigated for its potency to induce tumor necrosis factor (TNF) secretion by human blood monocytes. TNF was measured in the supernatants by using a bioassay and/or an enzyme-linked immunosorbent assay specific for TNF alpha (TNF-alpha). Although earlier studies indicated that staphylococcal peptidoglycan alone is a relatively poor stimulator of TNF-alpha production, the present study shows that human serum highly potentiates peptidoglycan-induced TNF-alpha release by human monocytes. In the presence of serum and in the low-dose range, peptidoglycan was almost as potent as endotoxin. At high peptidoglycan concentrations, monocytes showed an extremely high TNF-alpha response, but again only in the presence of serum. At low peptidoglycan doses, the stimulatory effect of serum was abrogated by heat treatment or depleting serum of complement components C1 and C3/C4, which suggests a role for the classical complement pathway. At high doses of peptidoglycan, the serum stimulatory effect depended mainly on immunoglobulin G.
Full text
PDF



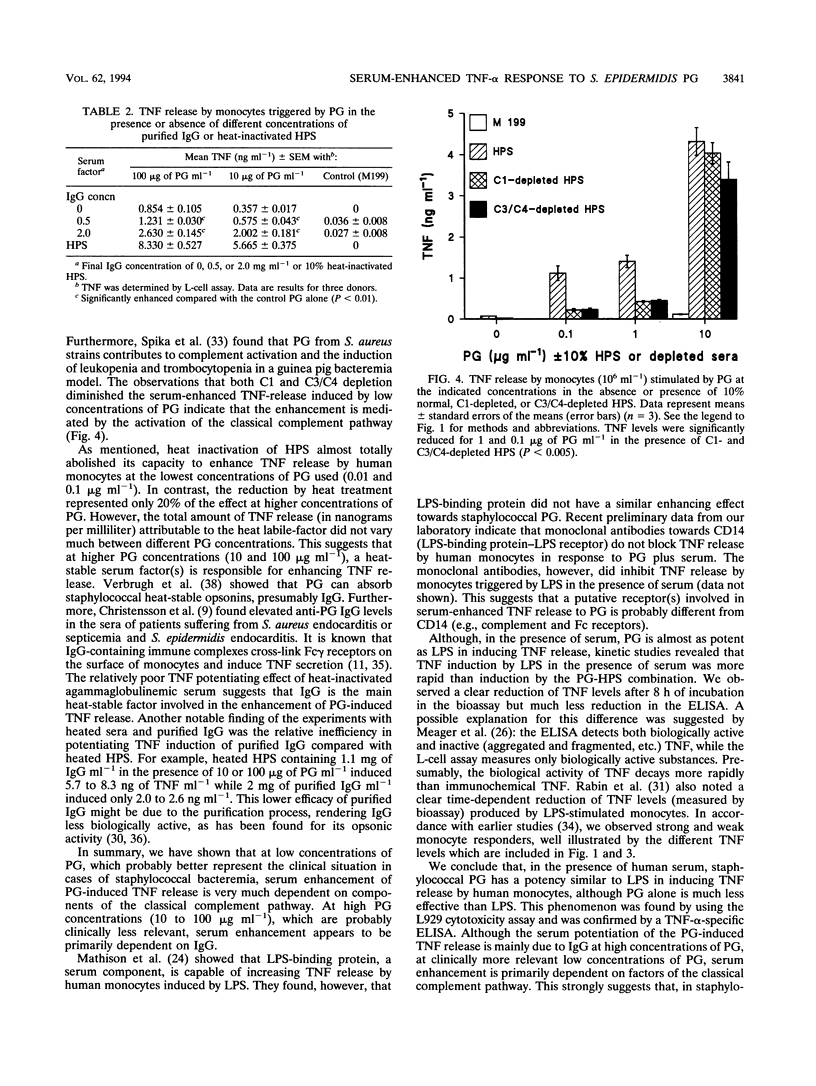


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. J., Kruse J. A., Haupt M. T., Chandrasekar P. H., Carlson R. W. Hemodynamic responses to gram-positive versus gram-negative sepsis in critically ill patients with and without circulatory shock. Crit Care Med. 1991 Dec;19(12):1520–1525. doi: 10.1097/00003246-199112000-00014. [DOI] [PubMed] [Google Scholar]
- Barton P. A., Warren J. S. Complement component C5 modulates the systemic tumor necrosis factor response in murine endotoxic shock. Infect Immun. 1993 Apr;61(4):1474–1481. doi: 10.1128/iai.61.4.1474-1481.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Cadranel J., Offenstadt G., Luguel L., Guidet B., Amstutz P. Tumor necrosis factor and septic shock. Crit Care Med. 1990 Mar;18(3):349–350. doi: 10.1097/00003246-199003000-00034. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Calandra T., Baumgartner J. D., Grau G. E., Wu M. M., Lambert P. H., Schellekens J., Verhoef J., Glauser M. P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990 May;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Tompkins R. G., Gelfand J. A., Michie H. R., Stanford G. G., van der Meer J. W., Endres S., Lonnemann G., Corsetti J., Chernow B. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990 Jan;161(1):79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- Cerami A., Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988 Jan;9(1):28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- Christensson B., Espersen F., Hedström S. A., Kronvall G. Serological assays against Staphylococcus aureus peptidoglycan, crude staphylococcal antigen and staphylolysin in the diagnosis of serious S. aureus infections. Scand J Infect Dis. 1985;17(1):47–53. doi: 10.3109/00365548509070419. [DOI] [PubMed] [Google Scholar]
- Damas P., Reuter A., Gysen P., Demonty J., Lamy M., Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med. 1989 Oct;17(10):975–978. doi: 10.1097/00003246-198910000-00001. [DOI] [PubMed] [Google Scholar]
- Debets J. M., Van der Linden C. J., Dieteren I. E., Leeuwenberg J. F., Buurman W. A. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol. 1988 Aug 15;141(4):1197–1201. [PubMed] [Google Scholar]
- Eykyn S. J. Staphylococcal sepsis. The changing pattern of disease and therapy. Lancet. 1988 Jan 16;1(8577):100–104. doi: 10.1016/s0140-6736(88)90294-2. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser M. P., Zanetti G., Baumgartner J. D., Cohen J. Septic shock: pathogenesis. Lancet. 1991 Sep 21;338(8769):732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- Gunn B. A. Comparative virulence of human isolates of coagulase-negative staphylococci tested in an infant mouse weight retardation model. J Clin Microbiol. 1989 Mar;27(3):507–511. doi: 10.1128/jcm.27.3.507-511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., van der Meer J. W., Dinarello C. A. Induction by toxic-shock-syndrome toxin-1 of a circulating tumor necrosis factor-like substance in rabbits and of immunoreactive tumor necrosis factor and interleukin-1 from human mononuclear cells. J Infect Dis. 1988 Nov;158(5):1017–1025. doi: 10.1093/infdis/158.5.1017. [DOI] [PubMed] [Google Scholar]
- Jessen T. E., Barkholt V., Welinder K. G. A simple alternative pathway for hemolytic assay of human complement component C3 using methylamine-treated plasma. J Immunol Methods. 1983 May 27;60(1-2):89–100. doi: 10.1016/0022-1759(83)90338-1. [DOI] [PubMed] [Google Scholar]
- Klein J. O. From harmless commensal to invasive pathogen--coagulase-negative staphylococci. N Engl J Med. 1990 Aug 2;323(5):339–340. doi: 10.1056/NEJM199008023230511. [DOI] [PubMed] [Google Scholar]
- Klerx J. P., Beukelman C. J., Van Dijk H., Willers J. M. Microassay for colorimetric estimation of complement activity in guinea pig, human and mouse serum. J Immunol Methods. 1983 Oct 14;63(2):215–220. doi: 10.1016/0022-1759(83)90425-8. [DOI] [PubMed] [Google Scholar]
- Kotb M., Ohnishi H., Majumdar G., Hackett S., Bryant A., Higgins G., Stevens D. Temporal relationship of cytokine release by peripheral blood mononuclear cells stimulated by the streptococcal superantigen pep M5. Infect Immun. 1993 Apr;61(4):1194–1201. doi: 10.1128/iai.61.4.1194-1201.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. D., Marks C. B., Luce J. M., Montgomery A. B., Turner J., Metz C. A., Murray J. F. Plasma tumor necrosis factor in patients with septic shock. Mortality rate, incidence of adult respiratory distress syndrome, and effects of methylprednisolone administration. Am Rev Respir Dis. 1990 Jan;141(1):94–97. doi: 10.1164/ajrccm/141.1.94. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Pfaller M. A., Wenzel R. P. Coagulase-negative staphylococcal bacteremia. Mortality and hospital stay. Ann Intern Med. 1989 Jan 1;110(1):9–16. doi: 10.7326/0003-4819-110-1-9. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Tobias P. S., Wolfson E., Ulevitch R. J. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J Immunol. 1992 Jul 1;149(1):200–206. [PubMed] [Google Scholar]
- Mattsson E., Verhage L., Rollof J., Fleer A., Verhoef J., van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol Med Microbiol. 1993 Oct;7(3):281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Meager A., Leung H., Woolley J. Assays for tumour necrosis factor and related cytokines. J Immunol Methods. 1989 Jan 6;116(1):1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- Miethke T., Wahl C., Heeg K., Echtenacher B., Krammer P. H., Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992 Jan 1;175(1):91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Verbrugh H. A., Verhoef J. Suppression of phagocytosis and chemotaxis by cell wall components of Staphylococcus aureus. J Immunol. 1981 Jul;127(1):84–88. [PubMed] [Google Scholar]
- Neoh S. H., Gordon T. P., Roberts-Thomson P. J. A simple one-step procedure for preparation of C1-deficient human serum. J Immunol Methods. 1984 Apr 27;69(2):277–280. doi: 10.1016/0022-1759(84)90325-9. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin R. L., Bieber M. M., Teng N. N. Lipopolysaccharide and peptidoglycan share binding sites on human peripheral blood monocytes. J Infect Dis. 1993 Jul;168(1):135–142. doi: 10.1093/infdis/168.1.135. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Spika J. S., Peterson P. K., Wilkinson B. J., Hammerschmidt D. E., Verbrugh H. A., Verhoef J., Quie P. G. Role of peptidoglycan from Staphylococcus aureus in leukopenia, thrombocytopenia, and complement activation associated with bacteremia. J Infect Dis. 1982 Aug;146(2):227–234. doi: 10.1093/infdis/146.2.227. [DOI] [PubMed] [Google Scholar]
- Timmerman C. P., Mattsson E., Martinez-Martinez L., De Graaf L., Van Strijp J. A., Verbrugh H. A., Verhoef J., Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993 Oct;61(10):4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Van Dijk W. C., Peters R., Van Der Tol M. E., Verhoef J. The role of Staphylococcus aureus cell-wall peptidoglycan, teichoic acid and protein A in the processes of complement activation and opsonization. Immunology. 1979 Jul;37(3):615–621. [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., Peters R., van Erne M. E., Daha M. R., Peterson P. K., Verhoef J. Opsonic recognition of staphylococci mediated by cell wall peptidoglycan: antibody-independent activation of human complement and opsonic activity of peptidoglycan antibodies. J Immunol. 1980 Mar;124(3):1167–1173. [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Jung W. K., Connolly R. J., Burke J. F., Dinarello C. A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Invest. 1991 Jun;87(6):1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Leijh P. C., Klein F. Correlation between opsonic activity for various microorganisms and composition of gammaglobulin preparations for intravenous use. J Infect Dis. 1984 Apr;149(4):511–517. doi: 10.1093/infdis/149.4.511. [DOI] [PubMed] [Google Scholar]
- van de Winkel J. G., Capel P. J. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993 May;14(5):215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]



