Abstract
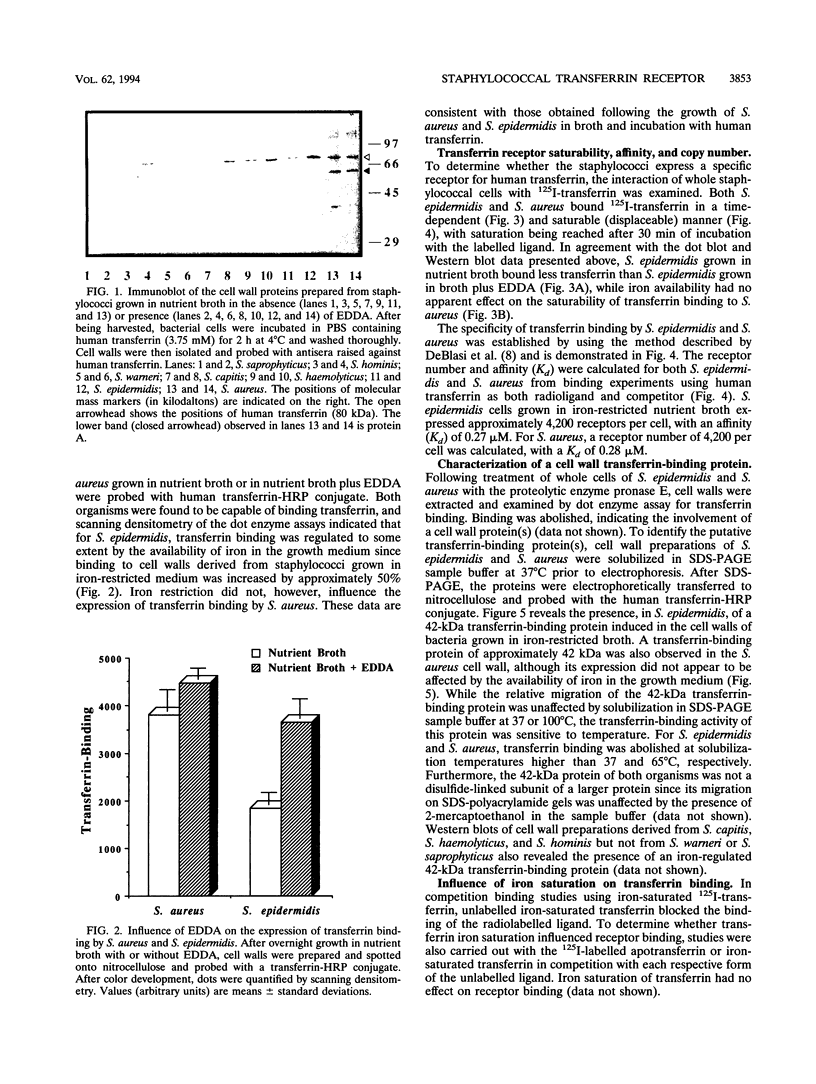
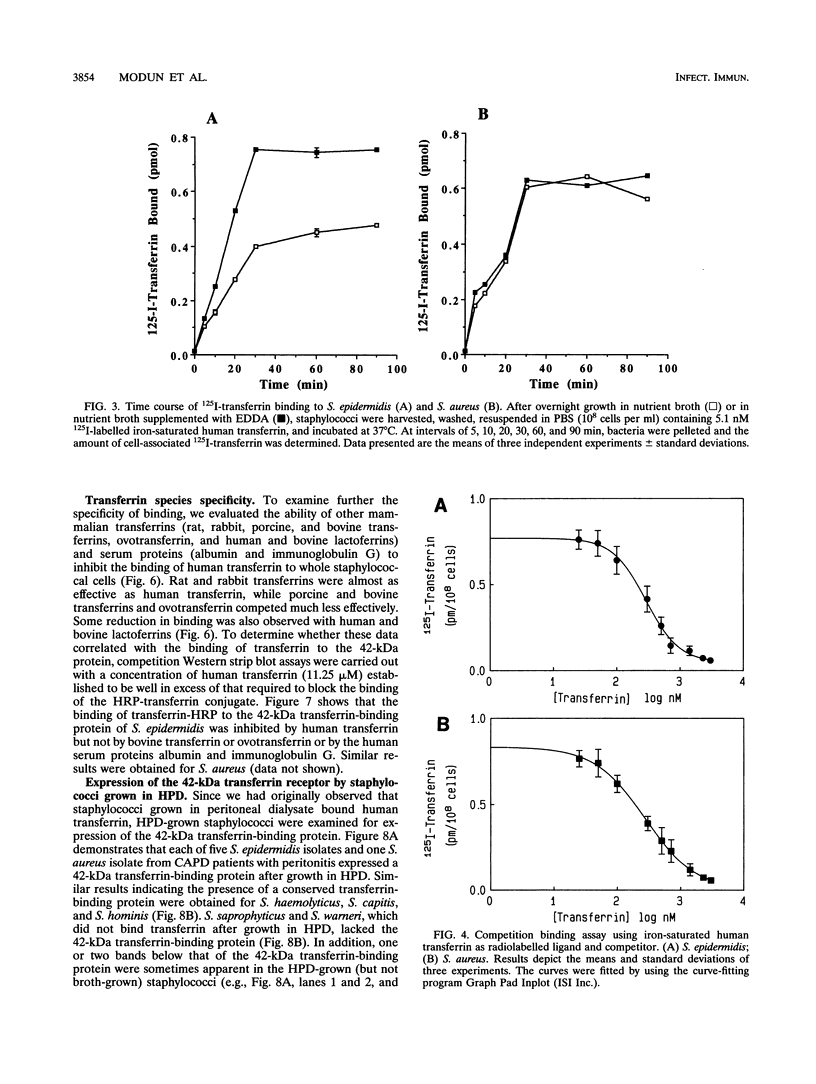
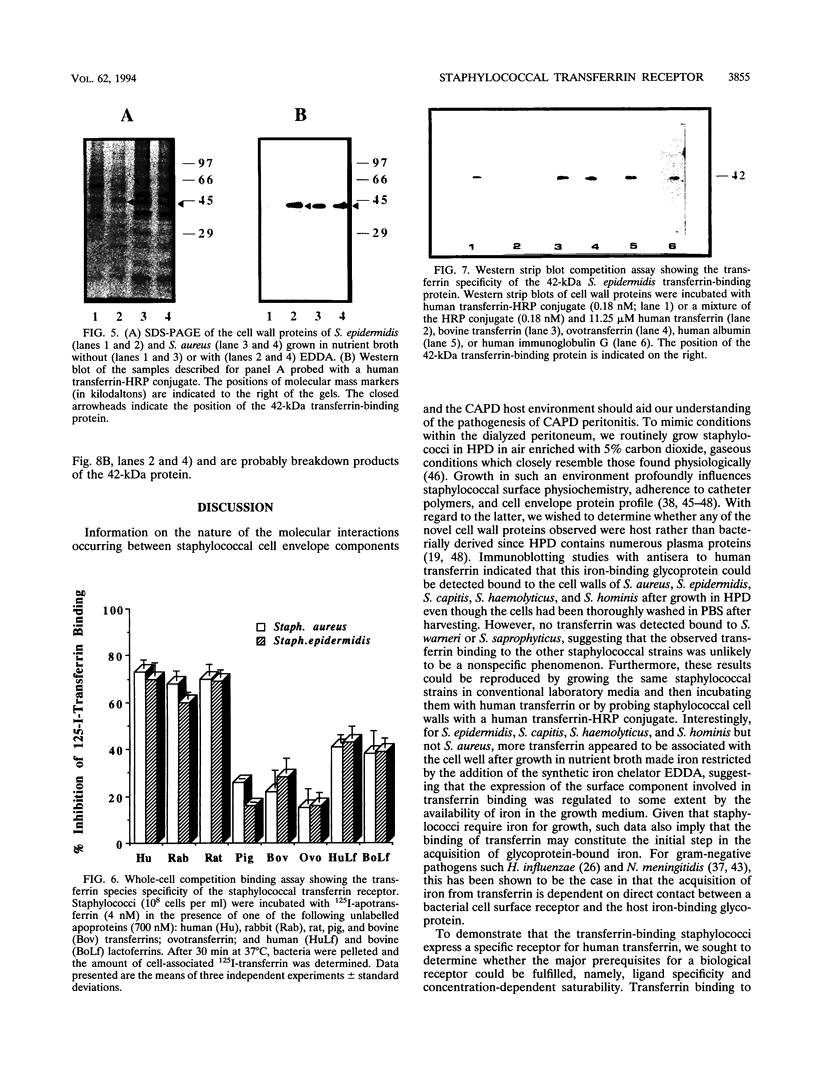
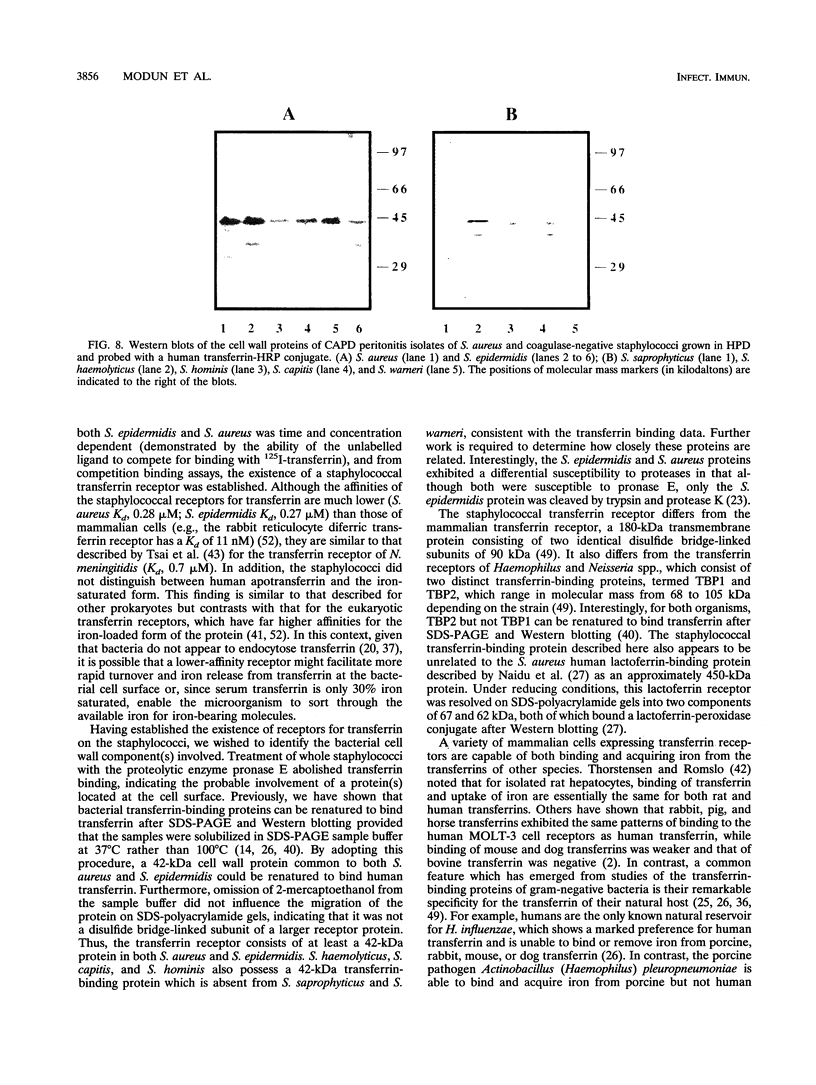
Staphylococcus aureus and the coagulase-negative staphylococci are commonly responsible for peritonitis in renal patients undergoing continuous ambulatory peritoneal dialysis. To simulate growth conditions in vivo, staphylococci isolated from peritoneal infections were cultured in used human peritoneal dialysate (HPD). Immunoblotting experiments using cell wall preparations from these staphylococci revealed the presence of the host iron-binding glycoprotein transferrin bound to S. aureus, S. epidermidis, S. capitis, S. haemolyticus, and S. hominis but not to S. warneri or S. saprophyticus. Similar results were obtained by incubating broth-grown staphylococci with human transferrin, although, in contrast to S. aureus, the coagulase-negative staphylococci bound more transferrin after growth in iron-restricted broth. To determine whether the staphylococci express a saturable specific receptor for human transferrin, the interaction of human 125I-transferrin with the staphylococci was examined. Both S. aureus and S. epidermidis bound the radiolabelled iron-saturated ligand in a time- and concentration-dependent manner. From competition binding assays, the affinity (Kd) and number of receptors were estimated for S. epidermidis (Kd, 0.27 microM; 4,200 receptors per cell) and S. aureus (Kd, 0.28 microM; 4,200 receptors per cell). S. epidermidis but not S. aureus receptor activity was partially iron regulated. Human apotransferrin and iron-saturated transferrin and rabbit and rat transferrins competed equally well for the staphylococcal receptor. Bovine and porcine transferrins and ovotransferrin as well as human and bovine lactoferrins were much less effective at competing with human transferrin. Treatment of whole staphylococci with protease abolished transferrin binding, indicating the involvement of cell surface protein. Western blots (immunoblots) of cell wall preparations probed with human transferrin revealed the presence of a 42-kDa transferrin-binding protein common to both S. aureus and S. epidermidis. On Western strip blots, the binding of human transferrin to this protein was blocked by labelled human transferrin but not by albumin, immunoglobulin G, or bovine transferrin or ovotransferrin. To assess the conservation of the 42-kDa transferrin-binding protein, cell wall proteins of S. epidermidis, S. haemolyticus, S. capitis, S. hominis, S. warneri, and S. saprophyticus were Western blotted and probed with human transferrin. Only S. warneri and S. saprophyticus lacked the 42-kDa wall protein, consistent with their inability to bind transferrin. These data show that the staphylococci express a specific receptor for human transferrin based at least in part on a common 42-kDa cell wall protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock J. H., Williams P. H., Licéaga J., Wooldridge K. G. Relative availability of transferrin-bound iron and cell-derived iron to aerobactin-producing and enterochelin-producing strains of Escherichia coli and to other microorganisms. Infect Immun. 1991 Sep;59(9):3185–3190. doi: 10.1128/iai.59.9.3185-3190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Bártek J., Viklický V., Stratil A. Phylogenetically more conservative epitopes among monoclonal antibody-defined antigenic sites of human transferrin are involved in receptor binding. Br J Haematol. 1985 Mar;59(3):435–441. doi: 10.1111/j.1365-2141.1985.tb07330.x. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Fischetti V. A. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988 May;56(5):1061–1065. doi: 10.1128/iai.56.5.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R. Proteins of iron storage and transport. Adv Protein Chem. 1990;40:281–363. doi: 10.1016/s0065-3233(08)60288-0. [DOI] [PubMed] [Google Scholar]
- DeBlasi A., O'Reilly K., Motulsky H. J. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989 Jun;10(6):227–229. doi: 10.1016/0165-6147(89)90266-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. C., Caamano D. L., Schryvers A. B. Identification and characterization of a porcine-specific transferrin receptor in Actinobacillus pleuropneumoniae. Mol Microbiol. 1990 Jul;4(7):1173–1179. doi: 10.1111/j.1365-2958.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Gorringe A. R., Woods G., Robinson A. Growth and siderophore production by Bordetella pertussis under iron-restricted conditions. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):101–105. doi: 10.1016/0378-1097(90)90265-r. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Iron and bacterial virulence--a brief overview. Biol Met. 1991;4(1):7–13. doi: 10.1007/BF01135551. [DOI] [PubMed] [Google Scholar]
- Holland J., Langford P. R., Towner K. J., Williams P. Evidence for in vivo expression of transferrin-binding proteins in Haemophilus influenzae type b. Infect Immun. 1992 Jul;60(7):2986–2991. doi: 10.1128/iai.60.7.2986-2991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konetschny-Rapp S., Jung G., Meiwes J., Zähner H. Staphyloferrin A: a structurally new siderophore from staphylococci. Eur J Biochem. 1990 Jul 20;191(1):65–74. doi: 10.1111/j.1432-1033.1990.tb19094.x. [DOI] [PubMed] [Google Scholar]
- Marcelis J. H., den Daas-Slagt H. J., Hoogkamp-Korstanje J. A. Iron requirement and chelator production of staphylococci, Streptococcus faecalis and enterobacteriaceae. Antonie Van Leeuwenhoek. 1978;44(3-4):257–267. doi: 10.1007/BF00394304. [DOI] [PubMed] [Google Scholar]
- McGregor S. J., Brock J. H., Briggs J. D., Junor B. J. Longitudinal study of peritoneal defence mechanisms in patients on continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int. 1989;9(2):115–119. [PubMed] [Google Scholar]
- McKenna W. R., Mickelsen P. A., Sparling P. F., Dyer D. W. Iron uptake from lactoferrin and transferrin by Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):785–791. doi: 10.1128/iai.56.4.785-791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiwes J., Fiedler H. P., Haag H., Zähner H., Konetschny-Rapp S., Jung G. Isolation and characterization of staphyloferrin A, a compound with siderophore activity from Staphylococcus hyicus DSM 20459. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):201–205. doi: 10.1111/j.1574-6968.1990.tb13863.x. [DOI] [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991 Nov;59(11):3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modun B., Williams P., Pike W. J., Cockayne A., Arbuthnott J. P., Finch R., Denyer S. P. Cell envelope proteins of Staphylococcus epidermidis grown in vivo in a peritoneal chamber implant. Infect Immun. 1992 Jun;60(6):2551–2553. doi: 10.1128/iai.60.6.2551-2553.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Siderophore-independent acquisition of transferrin-bound iron by Haemophilus influenzae type b. J Gen Microbiol. 1990 May;136(5):927–933. doi: 10.1099/00221287-136-5-927. [DOI] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Utilization of transferrin-bound iron by Haemophilus species of human and porcine origins. FEMS Microbiol Lett. 1989 Nov;53(1-2):123–127. doi: 10.1016/0378-1097(89)90378-9. [DOI] [PubMed] [Google Scholar]
- Naidu A. S., Andersson M., Forsgren A. Identification of a human lactoferrin-binding protein in Staphylococcus aureus. J Med Microbiol. 1992 Mar;36(3):177–183. doi: 10.1099/00222615-36-3-177. [DOI] [PubMed] [Google Scholar]
- Naidu A. S., Miedzobrodzki J., Andersson M., Nilsson L. E., Forsgren A., Watts J. L. Bovine lactoferrin binding to six species of coagulase-negative staphylococci isolated from bovine intramammary infections. J Clin Microbiol. 1990 Oct;28(10):2312–2319. doi: 10.1128/jcm.28.10.2312-2319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W. C. Systematics and the natural history of staphylococci. 2. Soc Appl Bacteriol Symp Ser. 1990;19:39S–48S. doi: 10.1111/j.1365-2672.1990.tb01796.x. [DOI] [PubMed] [Google Scholar]
- Redhead K., Hill T. Acquisition of iron from transferrin by Bordetella pertussis. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):303–307. doi: 10.1016/0378-1097(91)90570-z. [DOI] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- SCHADE A. L. SIGNIFICANCE OF SERUM IRON FOR THE GROWTH, BIOLOGICAL CHARACTERISTICS, AND METABOLISM OF STAPHYLOCOCCUS AUREUS. Biochem Z. 1963;338:140–148. [PubMed] [Google Scholar]
- Schade A. L., Caroline L. An Iron-binding Component in Human Blood Plasma. Science. 1946 Oct 11;104(2702):340–341. doi: 10.1126/science.104.2702.340. [DOI] [PubMed] [Google Scholar]
- Schade A. L., Caroline L. RAW HEN EGG WHITE AND THE ROLE OF IRON IN GROWTH INHIBITION OF SHIGELLA DYSENTERIAE, STAPHYLOCOCCUS AUREUS, ESCHERICHIA COLI AND SACCHAROMYCES CEREVISIAE. Science. 1944 Jul 7;100(2584):14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- Schneewind O., Model P., Fischetti V. A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992 Jul 24;70(2):267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Lee B. C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989 Mar;35(3):409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. G., Wilcox M. H., Williams P., Finch R. G., Denyer S. P. Characterization of cell envelope proteins of Staphylococcus epidermidis cultured in human peritoneal dialysate. Infect Immun. 1991 Feb;59(2):617–624. doi: 10.1128/iai.59.2.617-624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. C. Infections in continuous ambulatory peritoneal dialysis. J Med Microbiol. 1988 Sep;27(1):1–9. doi: 10.1099/00222615-27-1-1. [DOI] [PubMed] [Google Scholar]
- Stevenson P., Williams P., Griffiths E. Common antigenic domains in transferrin-binding protein 2 of Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae type b. Infect Immun. 1992 Jun;60(6):2391–2396. doi: 10.1128/iai.60.6.2391-2396.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa U. Transferrin receptors: structure and function. Curr Top Hematol. 1985;5:127–161. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. Uptake of iron from transferrin by isolated hepatocytes. Biochim Biophys Acta. 1984 Jun 19;804(2):200–208. doi: 10.1016/0167-4889(84)90150-2. [DOI] [PubMed] [Google Scholar]
- Tsai J., Dyer D. W., Sparling P. F. Loss of transferrin receptor activity in Neisseria meningitidis correlates with inability to use transferrin as an iron source. Infect Immun. 1988 Dec;56(12):3132–3138. doi: 10.1128/iai.56.12.3132-3138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Conroy W. E., Peterson P. K. Bacterial growth and killing in chronic ambulatory peritoneal dialysis fluids. J Clin Microbiol. 1984 Aug;20(2):199–203. doi: 10.1128/jcm.20.2.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M. H., Finch R. G., Smith D. G., Williams P., Denyer S. P. Effects of carbon dioxide and sub-lethal levels of antibiotics on adherence of coagulase-negative staphylococci to polystyrene and silicone rubber. J Antimicrob Chemother. 1991 May;27(5):577–587. doi: 10.1093/jac/27.5.577. [DOI] [PubMed] [Google Scholar]
- Wilcox M. H., Smith D. G., Evans J. A., Denyer S. P., Finch R. G., Williams P. Influence of carbon dioxide on growth and antibiotic susceptibility of coagulase-negative staphylococci cultured in human peritoneal dialysate. J Clin Microbiol. 1990 Oct;28(10):2183–2186. doi: 10.1128/jcm.28.10.2183-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M. H., Williams P., Smith D. G., Modun B., Finch R. G., Denyer S. P. Variation in the expression of cell envelope proteins of coagulase-negative staphylococci cultured under iron-restricted conditions in human peritoneal dialysate. J Gen Microbiol. 1991 Nov;137(11):2561–2570. doi: 10.1099/00221287-137-11-2561. [DOI] [PubMed] [Google Scholar]
- Williams P., Griffiths E. Bacterial transferrin receptors--structure, function and contribution to virulence. Med Microbiol Immunol. 1992;181(6):301–322. doi: 10.1007/BF00191543. [DOI] [PubMed] [Google Scholar]
- Wong J. C., Holland J., Parsons T., Smith A., Williams P. Identification and characterization of an iron-regulated hemopexin receptor in Haemophilus influenzae type b. Infect Immun. 1994 Jan;62(1):48–59. doi: 10.1128/iai.62.1.48-59.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge K. G., Williams P. H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993 Nov;12(4):325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Young S. P., Bomford A., Williams R. The effect of the iron saturation of transferrin on its binding and uptake by rabbit reticulocytes. Biochem J. 1984 Apr 15;219(2):505–510. doi: 10.1042/bj2190505. [DOI] [PMC free article] [PubMed] [Google Scholar]







