Abstract
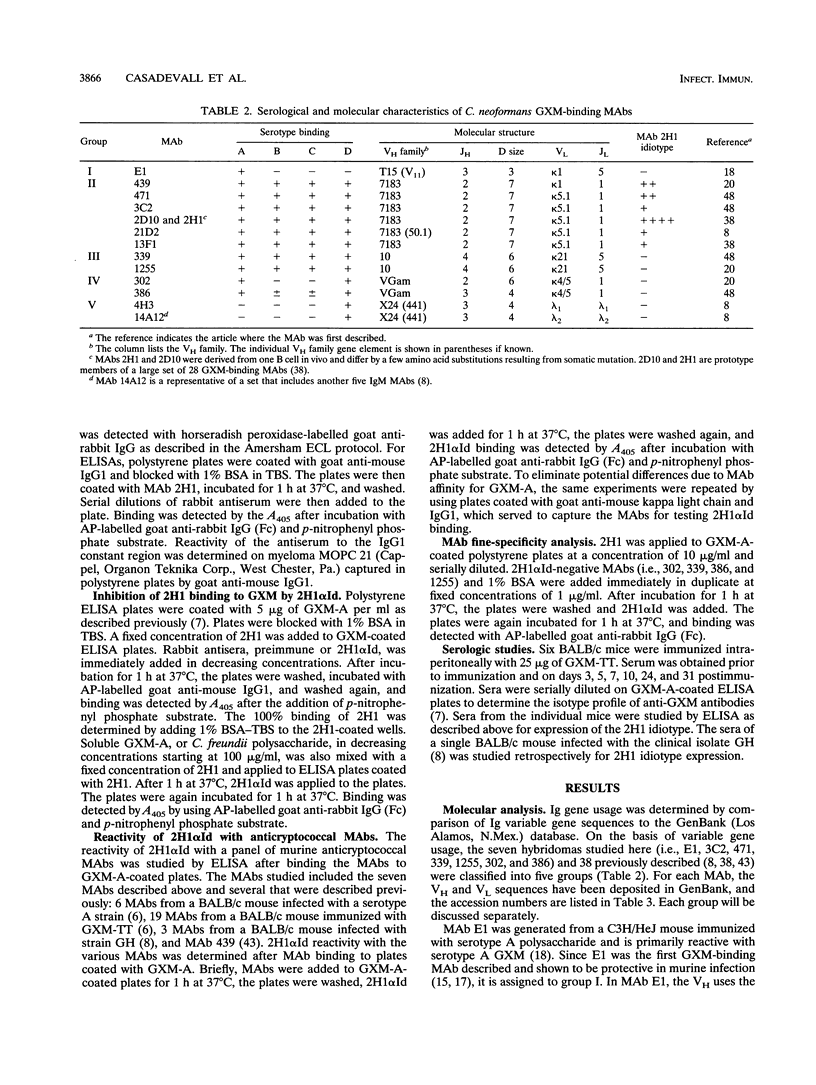
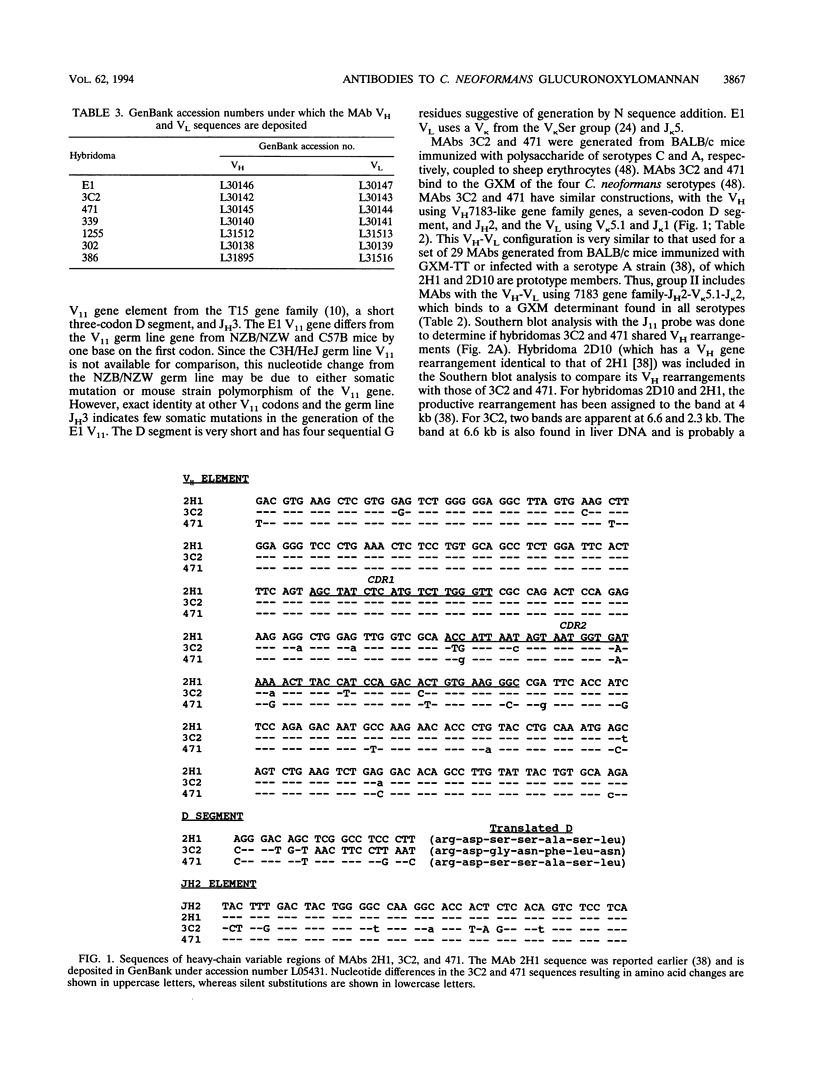
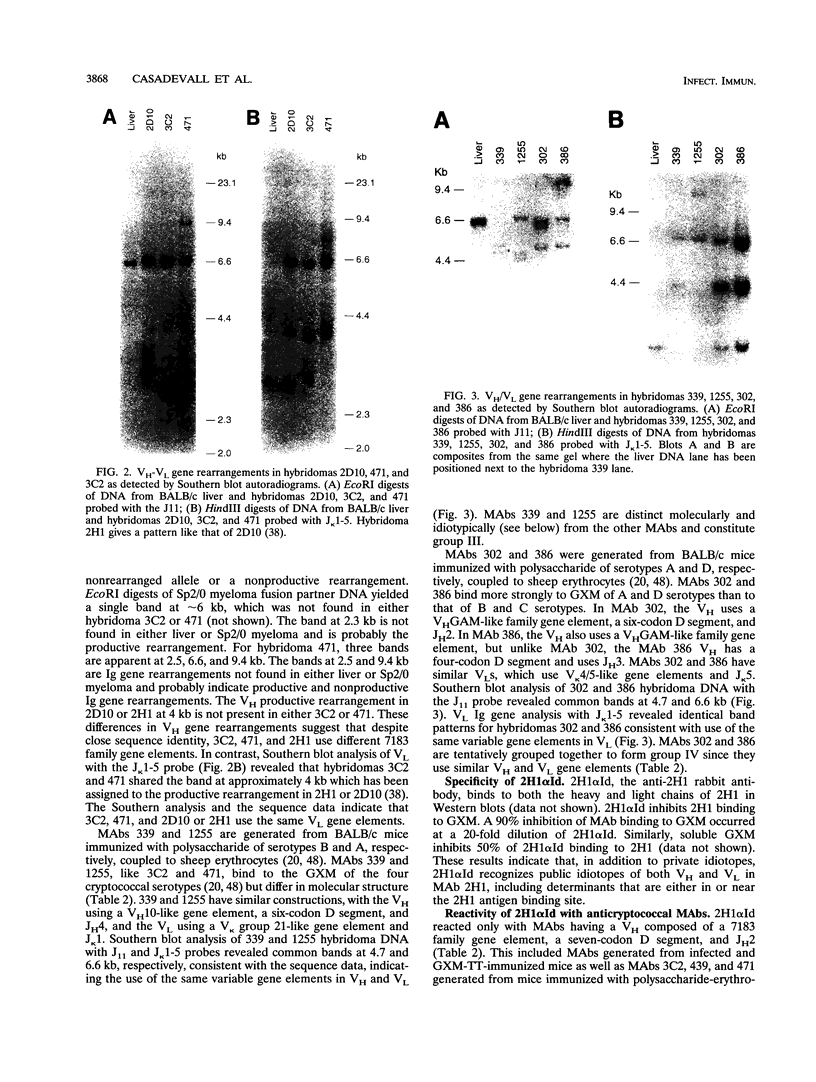
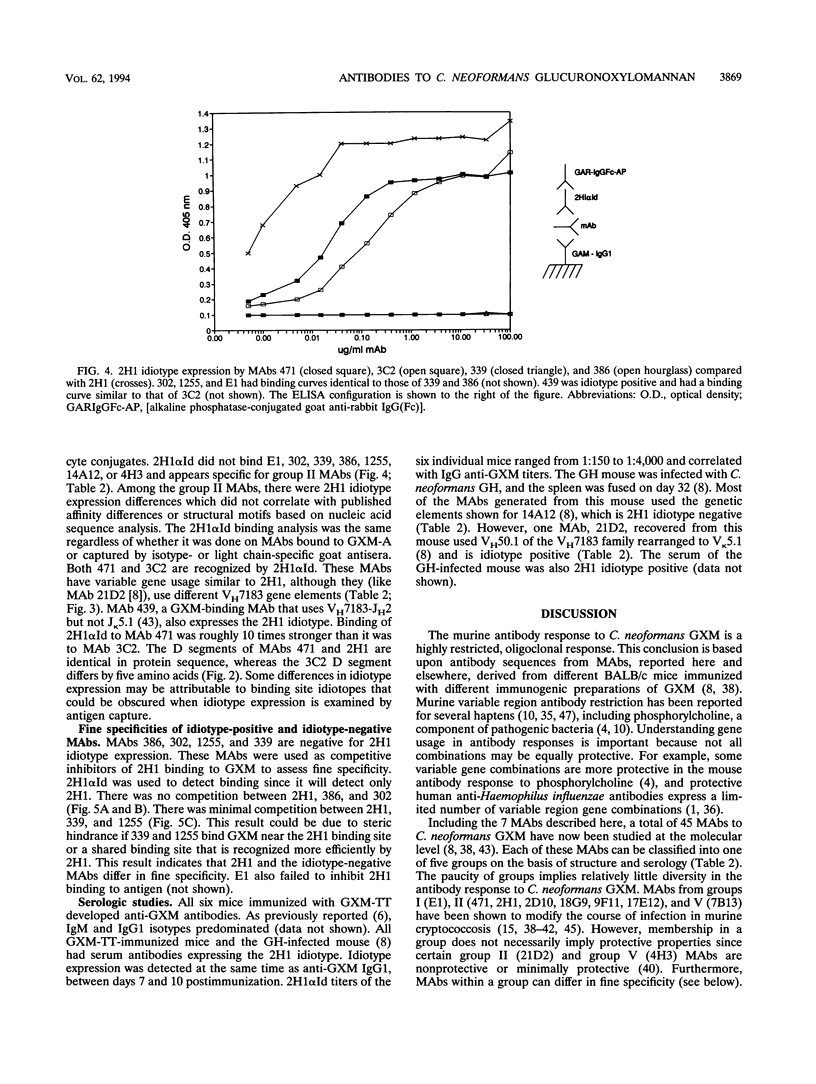
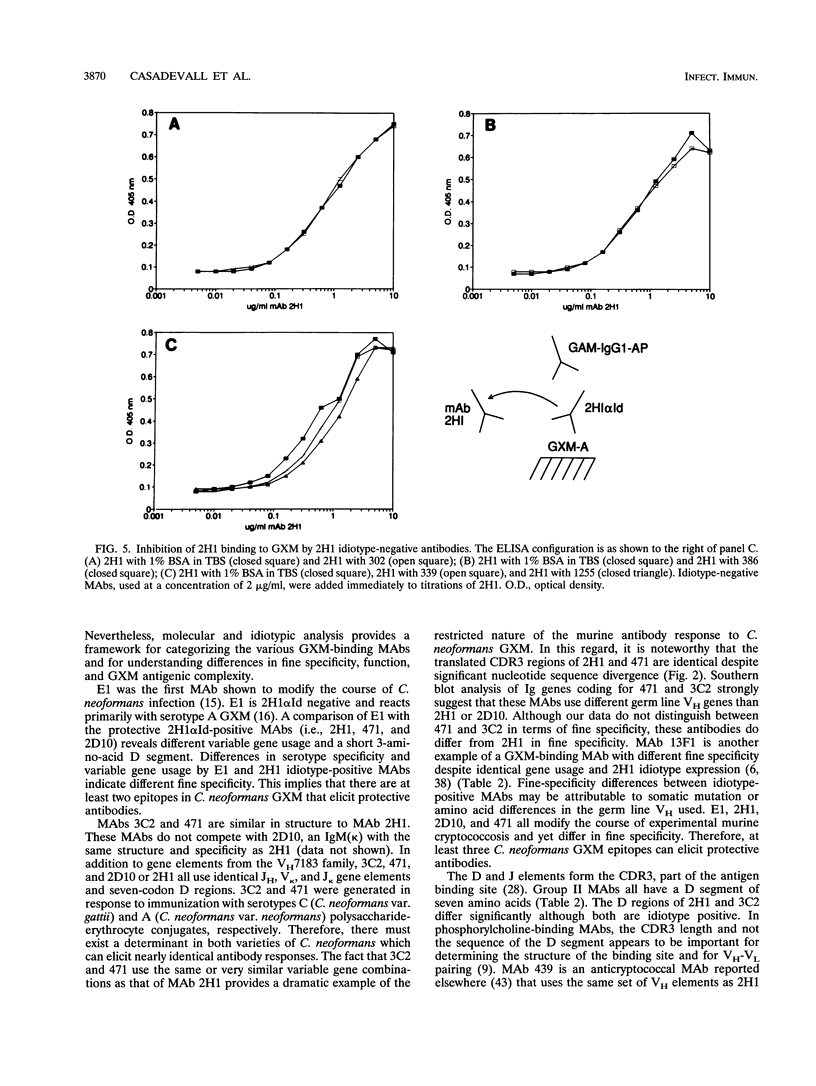
Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan (GXM) form the basis of two potential therapeutic intervention strategies, i.e., conjugate vaccines and passive antibody therapy. To better understand the molecular basis of the antibody response, the heavy- and light-chain immunoglobulin variable region (VH and VL, respectively) sequences of seven monoclonal antibodies (MAbs) to GXM were determined. Rabbit anti-idiotypic serum was made to the previously characterized murine MAb 2H1 and used to study MAb 2H1 idiotype expression in other GXM-binding MAbs and immune sera. MAb E1 originated from a C3H/HeJ mouse immunized with C. neoformans serotype A polysaccharide. MAbs 471, 1255, 339, 3C2, 386, and 302 originated from BALB/c mice immunized with polysaccharide of serotypes A, A, B, C, D, and D, respectively, conjugated to sheep erythrocytes. In the E1, VH uses V11 from the T15 gene family and JH3 and has a D segment of three amino acids, and the VL uses a VKSer-like gene family element and JK5. In MAbs 471 and 3C2, the VH uses VH7183-like gene family elements and JH2 and has D segments of seven amino acids, and the VL uses VK5.1 and JK1. In MAbs 1255 and 339, the VH uses VH10-like gene elements and JH4 and has six codon D segments, and the VL uses a VK21-like gene element and JK5. In MAbs 302 and 386, respectively, the VH uses VHGAM-like gene elements and JH2 and JH3 and has six and four codon D segments, and VL uses VK4/5-like gene elements and JK1.VH usage, MAb 2H1 idiotype expression, and fine specificity mapping define a minimum of three GXM epitopes which elicit protective antibodies. The results confirm that the antibody response is highly restricted, suggest a close relationship between molecular structure and serological properties, and provide insight into protein structural motifs important for GXM binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adderson E. E., Shackelford P. G., Insel R. A., Quinn A., Wilson P. M., Carroll W. L. Immunoglobulin light chain variable region gene sequences for human antibodies to Haemophilus influenzae type b capsular polysaccharide are dominated by a limited number of V kappa and V lambda segments and VJ combinations. J Clin Invest. 1992 Mar;89(3):729–738. doi: 10.1172/JCI115649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell C. M., Irwin D. C., Goodnight J., Stein K. E. Strain-dependent restricted VH and VL usage by anti-bacterial levan monoclonal antibodies. J Immunol. 1992 Jun 15;148(12):3864–3872. [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Mukherjee J., Devi S. J., Schneerson R., Robbins J. B., Scharff M. D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992 Jun;165(6):1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Mukherjee J., Scharff M. D. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992 Sep 18;154(1):27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Scharff M. D. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991 Jul 1;174(1):151–160. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. H., Claflin J. L., Potter M., Rudikoff S. Polymorphism in anti-phosphocholine antibodies reflecting evolution of immunoglobulin families. J Exp Med. 1983 Jan 1;157(1):98–113. doi: 10.1084/jem.157.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S. J., Schneerson R., Egan W., Ulrich T. J., Bryla D., Robbins J. B., Bennett J. E. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991 Oct;59(10):3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Charreire J., Contrepois A., Carbon C., Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987 Mar;55(3):749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Charreire J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J Infect Dis. 1991 May;163(5):1114–1120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- Dromer F., Gueho E., Ronin O., Dupont B. Serotyping of Cryptococcus neoformans by using a monoclonal antibody specific for capsular polysaccharide. J Clin Microbiol. 1993 Feb;31(2):359–363. doi: 10.1128/jcm.31.2.359-363.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Perronne C., Barge J., Vilde J. L., Yeni P. Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989 Dec;78(3):412–417. [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Salamero J., Contrepois A., Carbon C., Yeni P. Production, characterization, and antibody specificity of a mouse monoclonal antibody reactive with Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Mar;55(3):742–748. doi: 10.1128/iai.55.3.742-748.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Yeni P., Charreire J. Genetic control of the humoral response to cryptococcal capsular polysaccharide in mice. Immunogenetics. 1988;28(6):417–424. doi: 10.1007/BF00355373. [DOI] [PubMed] [Google Scholar]
- Eckert T. F., Kozel T. R. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Aug;55(8):1895–1899. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADEBUSCH H. H. Passive immunization against Cryptococcus neoformans. Proc Soc Exp Biol Med. 1958 Jul;98(3):611–614. doi: 10.3181/00379727-98-24123. [DOI] [PubMed] [Google Scholar]
- GORDON M. A., LAPA E. SERUM PROTEIN ENHANCEMENT OF ANTIBIOTIC THERAPY IN CRYPTOCOCCOSIS. J Infect Dis. 1964 Oct;114:373–377. doi: 10.1093/infdis/114.4.373. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Goldrick M. M., Boyd R. T., Ponath P. D., Lou S. Y., Gottlieb P. D. Molecular genetic analysis of the V kappa Ser group associated with two mouse light chain genetic markers. Complementary DNA cloning and southern hybridization analysis. J Exp Med. 1985 Aug 1;162(2):713–728. doi: 10.1084/jem.162.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill J. R., Hague M., Drutz D. J. Passive immunization in murine cryptococcosis. Sabouraudia. 1981 Dec;19(4):237–244. doi: 10.1080/00362178185380411. [DOI] [PubMed] [Google Scholar]
- Ikeda R., Shinoda T., Fukazawa Y., Kaufman L. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J Clin Microbiol. 1982 Jul;16(1):22–29. doi: 10.1128/jcm.16.1.22-29.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Hermerath C. A. Benzoquinone activation of Cryptococcus neoformans capsular polysaccharide for construction of an immunoaffinity column. J Immunol Methods. 1988 Feb 24;107(1):53–58. doi: 10.1016/0022-1759(88)90008-7. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. Role of the capsule in phagocytosis of Cryptococcus neoformans. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S436–S439. doi: 10.1093/cid/10.supplement_2.s436. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Kozel T. R., Edman J. C., Polacheck I., Ellis D., Shinoda T., Dromer F. Recent advances in biology and immunology of Cryptococcus neoformans. J Med Vet Mycol. 1992;30 (Suppl 1):133–142. [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P., Maziarz R. T. Killing of Cryptococcus neoformans by human peripheral blood mononuclear cells stimulated in culture. J Infect Dis. 1991 May;163(5):1108–1113. doi: 10.1093/infdis/163.5.1108. [DOI] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh D. Y., Bothwell A. L., White-Scharf M. E., Imanishi-Kari T., Baltimore D. Molecular basis of a mouse strain-specific anti-hapten response. Cell. 1983 May;33(1):85–93. doi: 10.1016/0092-8674(83)90337-9. [DOI] [PubMed] [Google Scholar]
- Lucas A. H. Expression of crossreactive idiotypes by human antibodies specific for the capsular polysaccharide of Hemophilus influenzae B. J Clin Invest. 1988 Feb;81(2):480–486. doi: 10.1172/JCI113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn B. A., Yancopoulos G. D., Barth J. E., Bona C. A., Alt F. W. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990 Mar 1;171(3):843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Casadevall A., Scharff M. D. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J Exp Med. 1993 Apr 1;177(4):1105–1116. doi: 10.1084/jem.177.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Pirofski L. A., Scharff M. D., Casadevall A. Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3636–3640. doi: 10.1073/pnas.90.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Scharff M. D., Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992 Nov;60(11):4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Zuckier L. S., Scharff M. D., Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994 Mar;38(3):580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Lee S., Mukherjee J., Scharff M. D., Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994 Mar;62(3):1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson E. W., Welch W. H., Kozel T. R. Protein-polysaccharide interactions. A monoclonal antibody specific for the capsular polysaccharide of Cryptococcus neoformans. J Biol Chem. 1994 Jan 21;269(3):1858–1864. [PubMed] [Google Scholar]
- Sanford J. E., Lupan D. M., Schlageter A. M., Kozel T. R. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect Immun. 1990 Jun;58(6):1919–1923. doi: 10.1128/iai.58.6.1919-1923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlageter A. M., Kozel T. R. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect Immun. 1990 Jun;58(6):1914–1918. doi: 10.1128/iai.58.6.1914-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Spiropulu C., Eppard R. A., Otteson E., Kozel T. R. Antigenic variation within serotypes of Cryptococcus neoformans detected by monoclonal antibodies specific for the capsular polysaccharide. Infect Immun. 1989 Oct;57(10):3240–3242. doi: 10.1128/iai.57.10.3240-3242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer E. D., Spitzer S. G., Freundlich L. F., Casadevall A. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet. 1993 Mar 6;341(8845):595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- Sundstrom J. B., Cherniak R. The glucuronoxylomannan of Cryptococcus neoformans serotype A is a type 2 T-cell-independent antigen. Infect Immun. 1992 Oct;60(10):4080–4087. doi: 10.1128/iai.60.10.4080-4087.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Johnson G., Kabat E. A. Length distribution of CDRH3 in antibodies. Proteins. 1993 May;16(1):1–7. doi: 10.1002/prot.340160102. [DOI] [PubMed] [Google Scholar]
- Wysocki L., Manser T., Gefter M. L. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuger A., Louie E., Holzman R. S., Simberkoff M. S., Rahal J. J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986 Feb;104(2):234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]