Abstract
Disease due to nontypeable Haemophilus influenzae begins with colonization of the upper respiratory tract mucosa. We recently reported that two surface-exposed high-molecular-weight proteins (HMW1 and HMW2) expressed by a prototypic strain of nontypeable H. influenzae mediate attachment to cultured epithelial cells. In the present study, we examined the nature of the epithelial cell receptor with which HMW1 interacts. Both proteinase K pretreatment and periodate oxidation of epithelial monolayers resulted in a marked decrease in HMW1-mediated binding, suggesting interaction with a glycoprotein structure. Treatment with peptide-N-glycosidase F produced a similar decrease in attachment and thereby provided further evidence for this conclusion. Desialylation of the epithelial cell surface also reduced binding, implying the presence of sialic acid in the receptor structure. Furthermore, lectins specific for terminal alpha 2-3-linked sialic acid were capable of inhibiting HMW1-mediated attachment. In summary, our results indicate that the HMW1 adhesin interacts with a glycoprotein receptor containing N-linked oligosaccharide chains with sialic acid in an alpha 2-3 configuration.
Full text
PDF

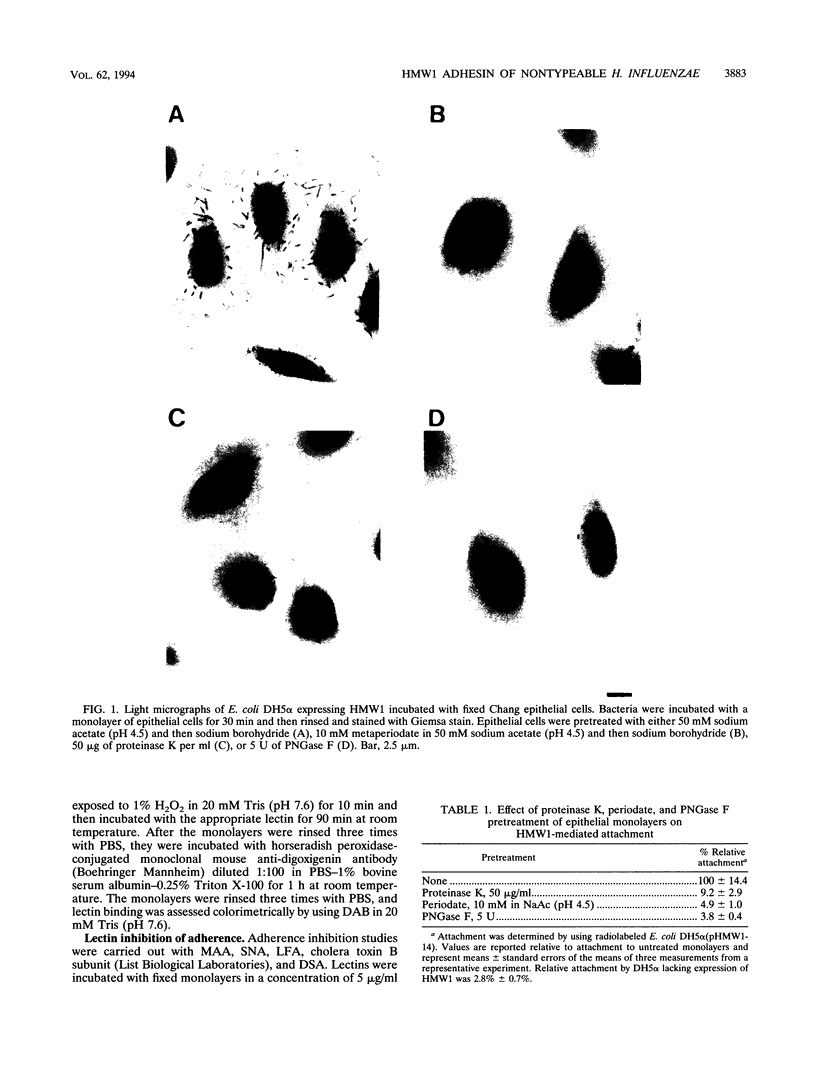

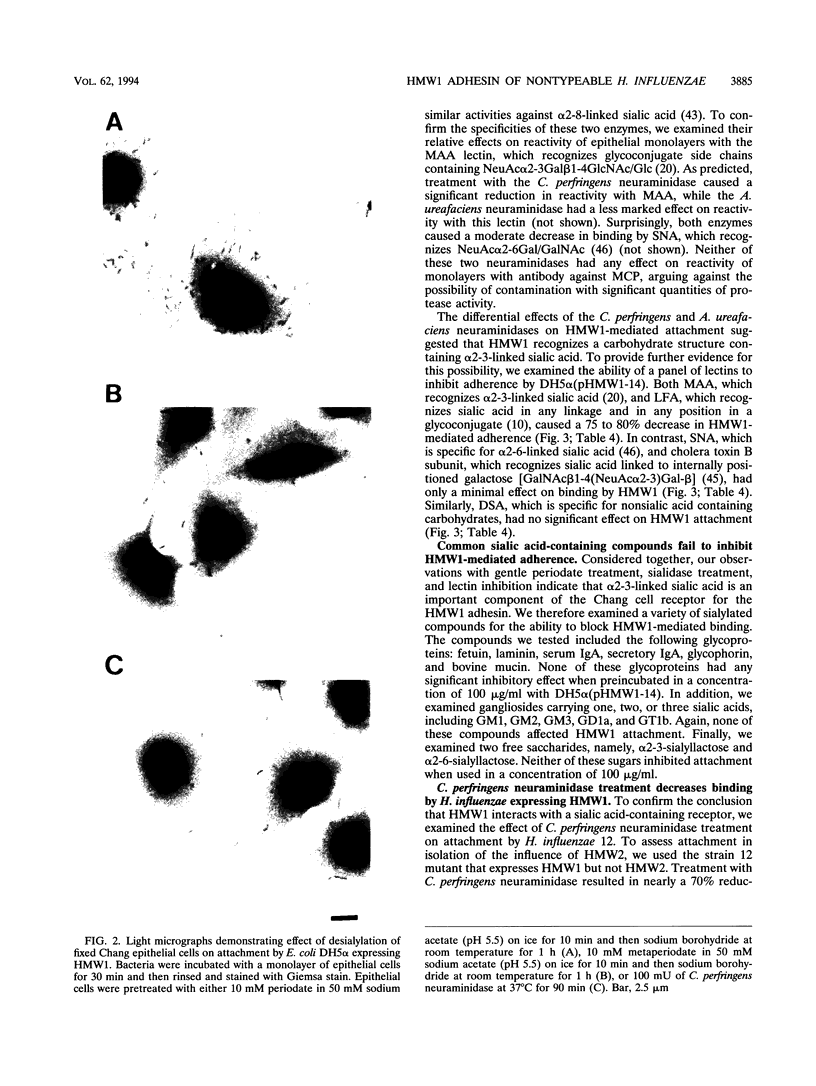

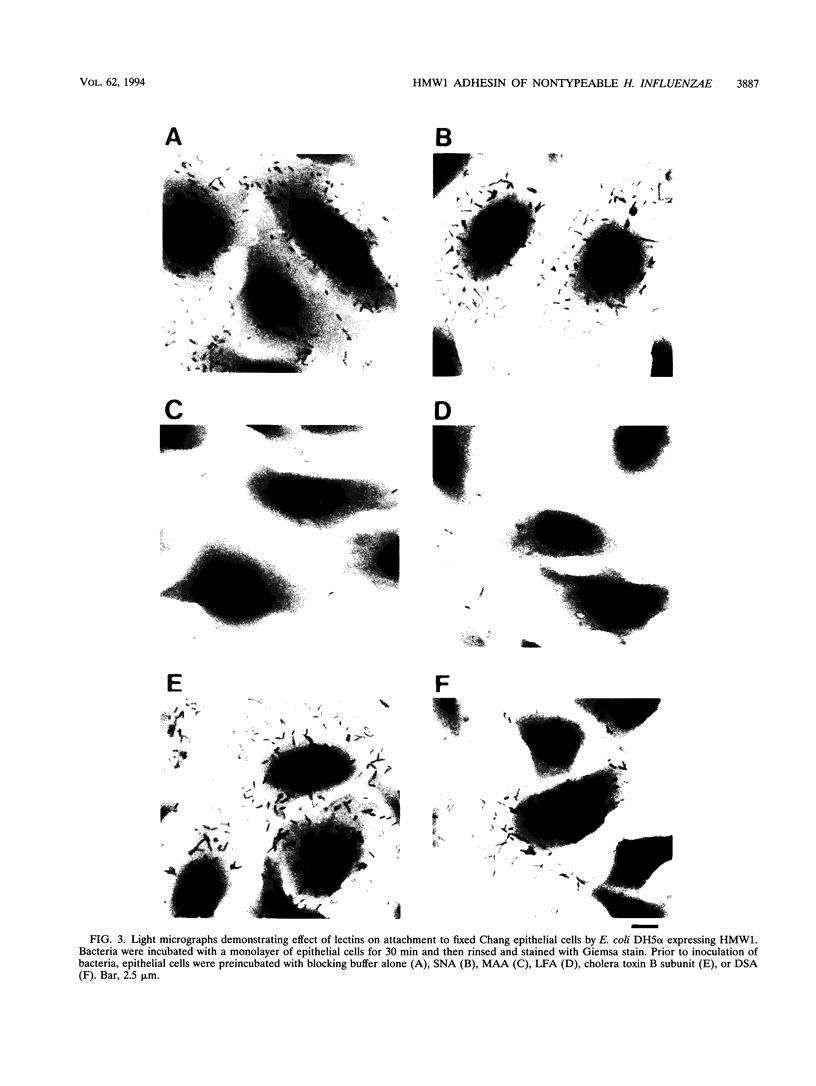


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Bodor F. F. Development of serum bactericidal activity following nontypable Haemophilus influenzae acute otitis media. Pediatr Infect Dis J. 1990 May;9(5):333–339. doi: 10.1097/00006454-199005000-00006. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992 Apr;60(4):1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Brandley B. K., Swiedler S. J., Robbins P. W. Carbohydrate ligands of the LEC cell adhesion molecules. Cell. 1990 Nov 30;63(5):861–863. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Cho S. W., Oglesby T. J., Hsi B. L., Adams E. M., Atkinson J. P. Characterization of three monoclonal antibodies to membrane co-factor protein (MCP) of the complement system and quantification of MCP by radioassay. Clin Exp Immunol. 1991 Feb;83(2):257–261. doi: 10.1111/j.1365-2249.1991.tb05624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S. Cloning of genes determining the production of mannose-resistant fimbriae in a uropathogenic strain of Escherichia coli belonging to serogroup O6. Infect Immun. 1982 Nov;38(2):739–744. doi: 10.1128/iai.38.2.739-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk P., Hoskins L. C., Larson G. Bacteria of the human intestinal microbiota produce glycosidases specific for lacto-series glycosphingolipids. J Biochem. 1990 Sep;108(3):466–474. doi: 10.1093/oxfordjournals.jbchem.a123223. [DOI] [PubMed] [Google Scholar]
- Falk P., Roth K. A., Borén T., Westblom T. U., Gordon J. I., Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Lauffenburger M., Sasaki H., Rogers M. E., Dell A. Structures of novel sialylated O-linked oligosaccharides isolated from human erythrocyte glycophorins. J Biol Chem. 1987 Sep 5;262(25):11952–11957. [PubMed] [Google Scholar]
- Hansen A. P., Sheikh S. P. Y2 receptor proteins for peptide YY and neuropeptide Y. Characterization as N-linked complex glycoproteins. FEBS Lett. 1992 Jul 20;306(2-3):147–150. doi: 10.1016/0014-5793(92)80987-r. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Abraham S., Caparon M., Falk P., St Geme J. W., 3rd, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993 Jun 4;73(5):887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbs R. N., Goldstein I. J., Ratcliffe R. M., Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J Biol Chem. 1991 Jan 5;266(1):83–88. [PubMed] [Google Scholar]
- Knibbs R. N., Perini F., Goldstein I. J. Structure of the major concanavalin A reactive oligosaccharides of the extracellular matrix component laminin. Biochemistry. 1989 Jul 25;28(15):6379–6392. doi: 10.1021/bi00441a034. [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Atkinson J. P. Membrane cofactor protein. Curr Top Microbiol Immunol. 1992;178:45–60. doi: 10.1007/978-3-642-77014-2_4. [DOI] [PubMed] [Google Scholar]
- Manzi A. E., Dell A., Azadi P., Varki A. Studies of naturally occurring modifications of sialic acids by fast-atom bombardment-mass spectrometry. Analysis of positional isomers by periodate cleavage. J Biol Chem. 1990 May 15;265(14):8094–8107. [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991 Feb;62(1):59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- Menozzi F. D., Mutombo R., Renauld G., Gantiez C., Hannah J. H., Leininger E., Brennan M. J., Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994 Mar;62(3):769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Mizuochi T., Kobata A. Structures of the carbohydrate moieties of secretory component purified from human milk. J Biol Chem. 1982 Aug 25;257(16):9612–9621. [PubMed] [Google Scholar]
- Moolenaar C. E., Muller E. J., Schol D. J., Figdor C. G., Bock E., Bitter-Suermann D., Michalides R. J. Expression of neural cell adhesion molecule-related sialoglycoprotein in small cell lung cancer and neuroblastoma cell lines H69 and CHP-212. Cancer Res. 1990 Feb 15;50(4):1102–1106. [PubMed] [Google Scholar]
- Murphy T. F., Bernstein J. M., Dryja D. M., Campagnari A. A., Apicella M. A. Outer membrane protein and lipooligosaccharide analysis of paired nasopharyngeal and middle ear isolates in otitis media due to nontypable Haemophilus influenzae: pathogenetic and epidemiological observations. J Infect Dis. 1987 Nov;156(5):723–731. doi: 10.1093/infdis/156.5.723. [DOI] [PubMed] [Google Scholar]
- Noel G. J., Barenkamp S. J., St Geme J. W., 3rd, Haining W. N., Mosser D. M. High-molecular-weight surface-exposed proteins of Haemophilus influenzae mediate binding to macrophages. J Infect Dis. 1994 Feb;169(2):425–429. doi: 10.1093/infdis/169.2.425. [DOI] [PubMed] [Google Scholar]
- Ofek I., Sharon N. Adhesins as lectins: specificity and role in infection. Curr Top Microbiol Immunol. 1990;151:91–113. doi: 10.1007/978-3-642-74703-8_5. [DOI] [PubMed] [Google Scholar]
- Pierce-Cretel A., Pamblanco M., Strecker G., Montreuil J., Spik G., Dorland L., Van Halbeek H., Vliegenthart J. F. Primary structure of the N-glycosidically linked sialoglycans of secretory immunoglobulins A from human milk. Eur J Biochem. 1982 Jul;125(2):383–388. doi: 10.1111/j.1432-1033.1982.tb06694.x. [DOI] [PubMed] [Google Scholar]
- Pierce-Crétel A., Decottignies J. P., Wieruszeski J. M., Strecker G., Montreuil J., Spik G. Primary structure of twenty three neutral and monosialylated oligosaccharides O-glycosidically linked to the human secretory immunoglobulin A hinge region determined by a combination of permethylation analysis and 400-MHz 1H-NMR spectroscopy. Eur J Biochem. 1989 Jun 15;182(2):457–476. doi: 10.1111/j.1432-1033.1989.tb14853.x. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Phillips M. L., Wayner E., Nudelman E., Singhal A. K., Hakomori S., Paulson J. C. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S. M., Yin Y., Rodzinski E., Tuomanen E. I., Masure H. R. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993 Jul;61(7):2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Roth J., Zuber C. Immunoelectron microscopic investigation of surface coat of Wilms tumor cells. Dense lamina is composed of highly sialylated neural cell adhesion molecule. Lab Invest. 1990 Jan;62(1):55–60. [PubMed] [Google Scholar]
- Rutishauser U., Acheson A., Hall A. K., Mann D. M., Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988 Apr 1;240(4848):53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Neural cell adhesion molecule as a regulator of cell-cell interactions. Adv Exp Med Biol. 1990;265:179–183. doi: 10.1007/978-1-4757-5876-4_17. [DOI] [PubMed] [Google Scholar]
- Savage A. V., Donoghue C. M., D'Arcy S. M., Koeleman C. A., van den Eijnden D. H. Structure determination of five sialylated trisaccharides with core types 1, 3 or 5 isolated from bovine submaxillary mucin. Eur J Biochem. 1990 Sep 11;192(2):427–432. doi: 10.1111/j.1432-1033.1990.tb19244.x. [DOI] [PubMed] [Google Scholar]
- Schauer R. Biosynthesis and function of N- and O-substituted sialic acids. Glycobiology. 1991 Nov;1(5):449–452. doi: 10.1093/glycob/1.5.449. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Schengrund C. L., Ringler N. J. Binding of Vibrio cholera toxin and the heat-labile enterotoxin of Escherichia coli to GM1, derivatives of GM1, and nonlipid oligosaccharide polyvalent ligands. J Biol Chem. 1989 Aug 5;264(22):13233–13237. [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Van Damme E. J., Peumans W. J. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988 Jan 15;263(2):728–734. [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Springer T. A., Lasky L. A. Cell adhesion. Sticky sugars for selectins. Nature. 1991 Jan 17;349(6306):196–197. doi: 10.1038/349196a0. [DOI] [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Falkow S., Barenkamp S. J. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990 Dec;58(12):4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme J. W., 3rd Nontypeable Haemophilus influenzae disease: epidemiology, pathogenesis, and prospects for prevention. Infect Agents Dis. 1993 Feb;2(1):1–16. [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Strömberg N., Marklund B. I., Lund B., Ilver D., Hamers A., Gaastra W., Karlsson K. A., Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gal alpha 1-4Gal-containing isoreceptors. EMBO J. 1990 Jun;9(6):2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Teele D. W., Klein J. O., Chase C., Menyuk P., Rosner B. A. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J Infect Dis. 1990 Sep;162(3):685–694. doi: 10.1093/infdis/162.3.685. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J Biochem. 1979 Nov;86(5):1573–1585. doi: 10.1093/oxfordjournals.jbchem.a132675. [DOI] [PubMed] [Google Scholar]
- Van Strijp J. A., Russell D. G., Tuomanen E., Brown E. J., Wright S. D. Ligand specificity of purified complement receptor type three (CD11b/CD18, alpha m beta 2, Mac-1). Indirect effects of an Arg-Gly-Asp (RGD) sequence. J Immunol. 1993 Sep 15;151(6):3324–3336. [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Geelen-van den Broek L., Blaas L., van Ham M., Dankert J. Blocking of fimbria-mediated adherence of Haemophilus influenzae by sialyl gangliosides. Infect Immun. 1991 Dec;59(12):4473–4477. doi: 10.1128/iai.59.12.4473-4477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]






