Abstract
Amyloid-β oligomers may cause cognitive deficits in Alzheimer's disease by impairing neuronal NMDA-type glutamate receptors, whose function is regulated by the receptor tyrosine kinase EphB2. Here we show that amyloid-β oligomers bind to the fibronectin repeats domain of EphB2 and trigger EphB2 degradation in the proteasome. To determine the pathogenic importance of EphB2 depletions in Alzheimer's disease and related models, we used lentiviral constructs to reduce or increase neuronal expression of EphB2 in memory centres of the mouse brain. In nontransgenic mice, knockdown of EphB2 mediated by short hairpin RNA reduced NMDA receptor currents and impaired long-term potentiation in the dentate gyrus, which are important for memory formation. Increasing EphB2 expression in the dentate gyrus of human amyloid precursor protein transgenic mice reversed deficits in NMDA receptor-dependent long-term potentiation and memory impairments. Thus, depletion of EphB2 is critical in amyloid-β-induced neuronal dysfunction. Increasing EphB2 levels or function could be beneficial in Alzheimer's disease.
Soluble amyloid-β oligomers may contribute to learning and memory deficits in Alzheimer's disease by inhibiting NMDA-receptor-dependent long-term potentiation (LTP)1–3, thought to underlie memory formation4. In Alzheimer's disease, hippocampal NMDA-receptor-subunit levels are reduced5, and protein levels and the phosphorylation status of NMDA-receptor subunits NR1, NR2A and NR2B correlate with cognitive performance6. Human amyloid precursor protein (hAPP) transgenic mice with high brain levels of amyloid-β oligomers have reduced hippocampal levels of tyrosine-phosphorylated NMDA receptors and key components of NMDA-receptor-dependent signalling pathways7,8. Alzheimer's disease patients and hAPP mice have hippocampal depletions of the receptor tyrosine kinase EphB29, which regulates NMDA-receptor trafficking and function by interacting with NMDA receptors and Src-mediated tyrosine phosphorylation10–13. EphB2 regulates NMDA-receptor-dependent Ca2+ influx and downstream transcription factors involved in LTP formation12, such as Fos, which is depleted in the dentate gyrus of hAPP mice. Mice lacking EphB210,14 or Fos15 have impaired NMDA-receptor-dependent LTP and memory deficits. We hypothesized that EphB2 depletion in Alzheimer's disease-related models is caused by amyloid-β oligomers and that reductions in EphB2 contribute to amyloid-β-induced deficits in synaptic plasticity and cognitive functions (Supplementary Fig. 1). Here we confirm these hypotheses and show that reversing EphB2 depletion in the dentate gyrus of hAPP mice reverses LTP and memory impairments.
Amyloid-β oligomers bind to EphB2
To determine if amyloid-β oligomers interact directly with EphB2, we measured binding of biotinylated synthetic amyloid-β1–42 oligomers to a purified recombinant EphB2–Fc chimaera. Biotinylated amyloid-β oligomers and EphB2–Fc were pulled down together by avidin agarose beads (Supplementary Fig. 2a, b) and co-immunoprecipitated under cell-free conditions (Supplementary Fig. 2c, d). EphB2 and amyloid-β oligomers also co-immunoprecipitated from homogenates of primary neurons (Supplementary Fig. 2e–g). Thus, amyloid-β oligomers may interact directly with the extracellular region of EphB2.
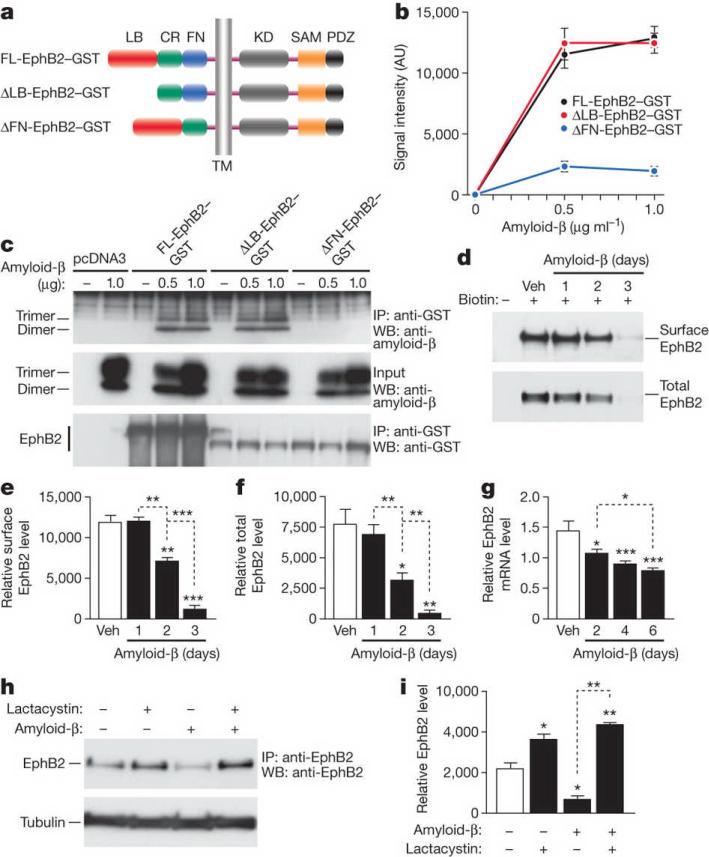
This region comprises a ligand-binding (LB) domain, a cysteine-rich (CR) domain, and a fibronectin type III repeats (FN) domain (Fig. 1a). To determine which domain mediates the interaction with amyloid-β oligomers, we generated EphB2–GST deletion mutants lacking the LB domain (DLB-EphB2) or the FN domain (DFN-EphB2) (Fig. 1a). Amyloid-β oligomers bound to FL-EphB2 and DLB-EphB2 but not DFN-EphB2 (Fig. 1b, c), indicating that the FN domain is critical for their interaction with EphB2.
Figure 1. Amyloid-β oligomers bind to the fibronectin repeats domain of EphB2 and cause degradation of EphB2 in the proteasome.
a, Domain structure of full-length (FL) EphB2 and deletion constructs. Ligand-binding (LB) domain, cysteine-rich (CR) region, fibronectin type III repeats (FN) domain, transmembrane (TM) region, tyrosine kinase (KD) domain, sterile alpha motif (SAM) domain, and PSD95, DLG and ZO1 (PDZ) domain. b, Binding of amyloid-β dimers and trimers to different EphB2 constructs. See Supplementary Table 2 for experimental details pertaining to data shown in figures. AU, arbitrary units. c, Representative western blot (WB). IP, immunoprecipitation. d–f, Amyloid-β-induced depletion of EphB2. Primary rat neurons were treated with amyloid-β or vehicle (Veh), and surface and total levels of EphB2 were determined by western blots. Representative western blots are shown in d. e, f, Quantification of surface (e) and total (f) levels of EphB2. g, EphB2 mRNA levels in primary neurons treated with amyloid-β or vehicle. h, i, Lactacystin blocks amyloid-β-induced depletion of EphB2 in primary neurons. Representative western blot (h) and quantification of signals (i). For all experiments, n = 3–6 wells per condition from three independent experiments. *P < 0.05, **P < 0.001, ***P < 0.0001 versus empty bars or as indicated by brackets (Tukey test). Values are means ± s.e.m.
Deleting FN domain did not affect trafficking of EphB2 to the cell surface (Supplementary Fig. 3a). FL-EphB2 and DFN-EphB2 both phosphorylated the NMDA-receptor subunit NR1 after stimulation with the EphB2 ligand, Fc-ephrin-B2 (Supplementary Fig. 3b–d). Thus, deleting the FN domain did not eliminate the kinase function of EphB2. Deleting the LB domain prevented Fc-ephrin-B2-induced phosphorylation of NR1 (Supplementary Fig. 3b–d).
Mechanisms of amyloid-β-induced EphB2 depletion
At 3–4 but not 2 months of age, EphB2 messenger RNA (mRNA) and protein levels in the hippocampus were lower in hAPP mice than in nontransgenic controls, and were lower in humans with Alzheimer's disease than in nondemented controls (data not shown), consistent with previous findings9.
As reported by others16, we observed a doublet of putative EphB2 carboxy-terminal fragments (CTFs) of 45–50 kDa in hippocampi of hAPP mice and nontransgenic controls on western blots (not shown). Relative to nontransgenic controls, hAPP mice showed a comparable decrease in CTFs and FL-EphB2 (not shown) and no difference in the ratio of CTF1+CTF2:FL−EphB2 (hAPP, 2.7 ± 0.36; nontransgenic, 2.3 ± 0.59; P = 0.55 by t test). Thus, pathologically raised levels of amyloid-β do not affect EphB2 cleavage into CTFs.
Treating primary neuronal cultures from wild-type rats with naturally secreted amyloid-β oligomers caused severe EphB2 depletions by 3 days (Fig. 1d–f). Amyloid-β oligomers reduced EphB2 mRNA levels (Fig. 1g), but the reduction was subtle and unlikely to account for the severe EphB2 protein depletion.
Amyloid-β-induced depletion of EphB2 was blocked by the proteasome inhibitor lactacystin (Fig. 1h, i). Bafilomycin, an inhibitor of endosomal acidification, had no effect (Supplementary Fig. 4b, c). Compared with amyloid-β treatment alone, treatment of cells with lactacystin alone or together with amyloid-β increased ubiquitinated EphB2 (Supplementary Fig. 4a). These results indicate that amyloid-β depletes neuronal EphB2 mainly by enhancing its proteasomal degradation.
EphB2 depletion impairs synaptic plasticity
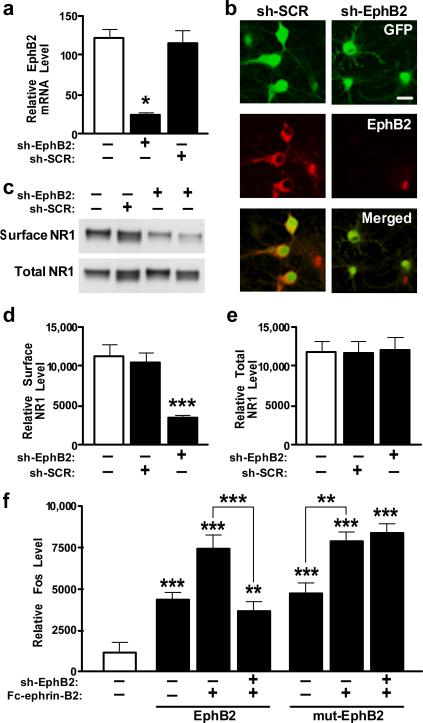
To determine if EphB2 depletion interferes with NMDA-receptor-dependent functions, we generated lentiviral vectors expressing green fluorescent protein (GFP) and anti-EphB2 shRNA (Lenti-sh-EphB2–GFP) or scrambled control shRNA (Lenti-sh-SCR–GFP). In neuronal cultures, Lenti-sh-EphB2–GFP reduced EphB2 mRNA and protein levels (Fig. 2a, b) and surface levels of NR1 (Fig. 2c–e). In cultures co-infected with a mutant EphB2 construct whose mRNA is resistant to sh-EphB2 (Lenti-mut-EphB2–Flag) and Lenti-sh-EphB2–GFP, EphB2 and surface NR1 were not reduced (Supplementary Fig. 5), excluding an off-target effect. Next we examined the effects of sh-EphB2 on expression of the immediate-early gene c-fos, which depends on NMDA receptors and is regulated by EphB212. Anti-EphB2 shRNA prevented Fc-ephrin-B2-induced increases in Fos expression in neurons expressing wild-type EphB2, but not in neurons expressing mutant EphB2 (Fig. 2f). Thus, depleting EphB2 reduces surface NR1 expression and impairs NMDA-receptor-dependent gene expression.
Figure 2. Knockdown of EphB2 reduces surface NR1 levels and Fc-ephrin-B2-dependent Fos expression.
a, b, EphB2 expression is reduced in primary neurons infected with Lenti-sh-EphB2–GFP as determined by RT–qPCR (a) or EphB2 immunostaining (b). Scale bar, 20 mm. c–e, Reduction of EphB2 levels by Lenti-sh-EphB2–GFP and effect on surface NR1 levels. f, shRNA against wild-type but not mutated EphB2 reduces Fc-ephrin-B2-dependent Fos expression. Primary rat neurons were co-infected or not with Lenti-sh-EphB2–GFP (sh-EphB2) in combination with either Lenti-EphB2 encoding wild-type EphB2 or Lenti-mut-EphB2 (mut-EphB2) encoding a mutated EphB2 mRNA that is not recognized by sh-EphB2. Four days later, cells were stimulated with clustered multimeric recombinant Fc-ephrin-B2 ligand to activate EphB2. n = 3–6 wells per condition from three independent experiments. *P < 0.05, **P < 0.001, ***P < 0.0001 versus empty bar or as indicated by brackets (Tukey's test). Values are means ± s.e.m.
To explore whether EphB2 depletion accounts for LTP deficits in hAPP mice8, we reduced EphB2 in the dentate gyrus of nontransgenic mice. Although granule cells are not very susceptible to degeneration in Alzheimer's disease, perforant path to granule cell synapses are affected early and severely17,18.
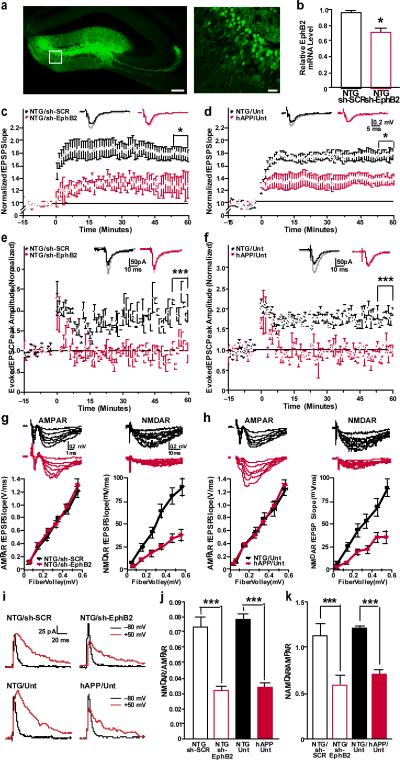
Two anti-EphB2 shRNAs reduced EphB2 mRNA and protein levels in neuronal culture (Supplementary Fig. 6). Mice injected with lentiviral vectors expressing sh-EphB2-308–GFP (Fig. 3a, b) or sh-EphB2-306–GFP (Supplementary Fig. 7a, b) had lower EphB2 mRNA levels in the dentate gyrus than controls. Transduction efficiencies (Supplementary Fig. 8) were 50–74% (mean ± s.e.m., 62.4 ± 6.2; n = 7 mice), consistent with other reports19,20.
Figure 3. Knockdown of EphB2 reduces LTP in dentate gyrus granule cells of nontransgenic mice.
a, Anti-GFP immunostaining of dentate gyrus showing infected neurons in Lenti-sh-EphB2-GFP injected mice. Right panel shows higher magnification image of boxed region on left. Scale bars: 100 mm (left), 25 mm (right). b, EphB2 mRNA levels in the entire dentate gyrus (reflecting levels in infected and uninfected cells) (n = 5–7 mice per condition). *P < 0.001 versus sh-SCR (t test). NTG, nontransgenic mice. c–f, LTP at the medial perforant path to granule cell synapse measured by field recordings (c, d) or by whole-cell patch clamp from individual GFP-positive cells (e, f) in the dentate gyrus. LTP was impaired in nontransgenic mice treated with Lenti-sh-EphB2–GFP (sh-EphB2) compared to nontransgenic mice treated with Lenti-sh-SCR–GFP (sh-SCR) (c, e). Similar LTP impairments were observed in untreated (Unt) hAPP mice (d, f) (NTG: sh-EphB2 versus hAPP: Unt). *P < 0.05, ***P < 0.001 (repeated-measures ANOVA and Bonferroni post-hoc test on the last 10 min of data). n = 8–9 slices from 3–4 mice per treatment (c) or genotype (d). g, h, Comparison of AMPA-receptor (AMPAR)-mediated (left) and NMDA-receptor (NMDAR)-mediated (right) input-output (I/O) relationships in the medial perforant path to granule cell synapses of nontransgenic mice treated with sh-EphB2 versus sh-SCR (g) and of untreated nontransgenic (NTG: Unt) versus hAPP (hAPP: Unt) mice (h). i, Example traces of evoked glutamate receptor currents from individual granule cells voltage clamped at −80 or 50 mV to measure AMPA-receptor- and NMDA-receptor-mediated currents, respectively. j, k, Summary plot of the ratios of NMDA-receptor I/O relationships to AMPA-receptor I/O relationships measured by field recordings (i) or by individual granule cells (k). ***P < 0.001 (two-way ANOVA and Bonferroni post-hoc test). n = 8–9 slices from 3–4 mice per group. Values are means ± s.e.m.
Field (Fig. 3c) and whole-cell patch-clamp recordings (Fig. 3e) from dentate gyrus granule cells in acute hippocampal slices from Lenti-sh-EphB2–GFP-injected nontransgenic mice revealed prominent LTP deficits similar to those in untreated hAPP J20 (Fig. 3d, f) and other lines of hAPP mice21,22. Lenti-sh-SCR–GFP-injected nontransgenic mice had robust LTP in the dentate gyrus (Fig. 3c, e). Whole-cell recordings from individual GFP-negative granule cells in Lenti-sh-ephB2–GFP-injected mice revealed no LTP deficits, compared with GFP-negative granule cells in untreated nontransgenic mice and GFP-positive granule cells in Lenti-sh-SCR–GFP-injected mice (P > 0.1 by repeated-measures ANOVA, n = 6 neurons from 3 mice per group; data not shown).
EphB2 depletion reduces synaptic strength
LTP at the medial perforant path to granule cell synapse depends on NMDA-receptor activity23. We determined whether impaired synaptic plasticity in sh-EphB2-treated nontransgenic and untreated hAPP mice is related to a selective impairment of these glutamate receptors. NMDA-receptor-mediated, but not a-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor–mediated synaptic transmission strength at this synapse was affected in sh-EphB2-treated nontransgenic mice (Fig. 3g) and untreated hAPP mice (Fig. 3h), as determined by field recordings and analysis of input-output (I/O) curves. These alterations markedly reduced ratios of NMDA-receptor- to AMPA-receptor-mediated synaptic strength in both groups (Fig. 3j). Similar results were obtained by whole-cell recordings from individual granule cells (Fig. 3i, k). To exclude a contribution of alterations in AMPA-receptor currents to the altered ratios, we recorded pharmacologically isolated, AMPA-receptor-mediated miniature excitatory synaptic currents (mEPSCs). The four groups of mice had comparable mEPSC peak amplitudes (Supplementary Fig. 9). Thus, like amyloid-β, EphB2 depletion probably reduces LTP by impairing NMDA-receptor function.
EphB2 rescues synaptic functions in hAPP mice
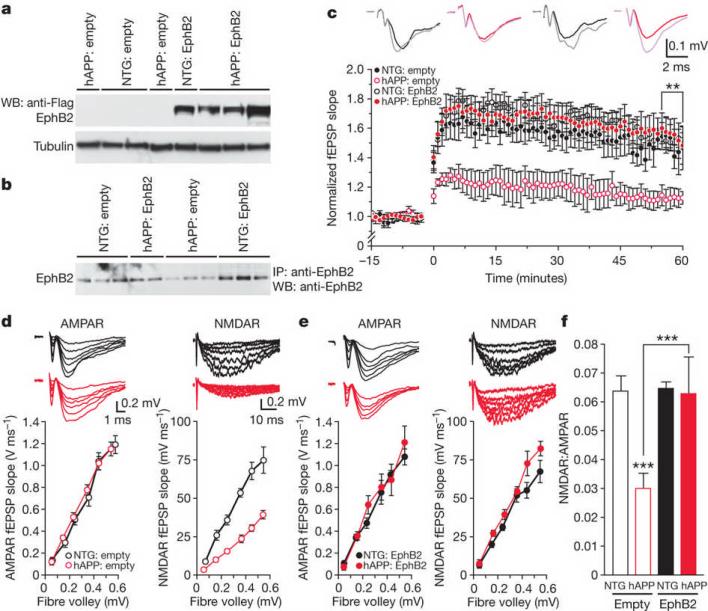
To determine if increasing EphB2 expression in the dentate gyrus of hAPP mice reverses LTP deficits, we used a lentivirus expressing EphB2–Flag (Lenti-EphB2–Flag). Lenti-EphB2–Flag-treated hAPP and nontransgenic mice had comparable EphB2–Flag expression levels in the dentate gyrus (Fig. 4a and Supplementary Fig. 10). Lenti-empty-treated nontransgenic mice and Lenti-EphB2–Flag-treated hAPP mice had comparable dentate gyrus levels of total (endogenous and exogenous) EphB2 (Fig. 4b), indicating that EphB2 levels in hAPP mice were normalized. EphB2 levels were lower in Lenti-empty-injected hAPP mice and higher in Lenti-EphB2–Flag-injected nontransgenic mice (Fig. 4b). Increasing dentate gyrus EphB2 levels reversed LTP deficits in two independent cohorts of hAPP mice but did not alter LTP in nontransgenic mice (Fig. 4c).
Figure 4. Increasing EphB2 expression rescues synaptic plasticity in hAPP mice.
a, b, Levels of EphB2–Flag (a) and total EphB2 (b) in the dentate gyrus of nontransgenic and hAPP mice injected with Lenti-empty or Lenti-EphB2–Flag (n = 9–12 mice per genotype and treatment). c, Normalization of LTP (measured as in Fig. 3c, d) in hAPP mice treated with EphB2–Flag. **P < 0.01 (repeated-measures ANOVA and Bonferroni post-hoc test on the last 10 min of data). The following ratios represent the numbers of slices per number of mice from which the recordings were obtained: nontransgenic:empty, 8/4; hAPP:empty, 6/3; nontransgenic:EphB2, 13/6; hAPP:EphB2, 20/8. d, e, Comparison of AMPA-receptor-mediated (left) and NMDA-receptor-mediated (right) I/O relationships in the medial perforant path to granule cell synapses of nontransgenic and hAPP mice treated with Lenti-empty (d) or Lenti-EphB2-Flag (e). Recording conditions were as in Fig. 3. e, f, Summary plot of the ratios of NMDA-receptor I/O relationships to AMPA-receptor I/O relationships. ***P < 0.001 (two-way ANOVA and Bonferroni post-hoc test). Number of slices per number of mice were: nontransgenic:empty, 8/4; hAPP:empty, 6/3; nontransgenic:EphB2, 6/3; hAPP:EphB2, 8/4. Values are means ± s.e.m.
Lenti-EphB2–Flag-treated mice showed a trend toward lower amyloid-β levels in the dentate gyrus (Supplementary Fig. 11), but this trend did not reach statistical significance. At analysis, hAPP mice were 4–5-months old and had not yet formed plaques, excluding EphB2 effects on plaque formation. To determine if LTP rescue was due to improved NMDA-receptor function, we again measured AMPA-receptor- and NMDA-receptor-mediated synaptic strength. Increasing EphB2 levels in the dentate gyrus of hAPP mice reversed deficits in NMDA-receptor-mediated synaptic strength without changing AMPA-receptor-mediated synaptic strength (Fig. 4d, e), normalizing the balance between them (Fig. 4f). Overexpressing EphB2 did not alter NMDA-receptor- or AMPA-receptor-mediated synaptic strength in nontransgenic mice (Fig. 4d–f).
Increasing EphB2 expression in granule cells did not reverse impairments in paired pulse modification at perforant path to granule cell synapses (Supplementary Fig. 12a) or in synaptic strength at Schaffer collateral to CA1 pyramidal cell synapses (Supplementary Fig. 12b, c).
EphB2 ameliorates cognitive deficits in hAPP mice
To determine if increasing EphB2 levels in the dentate gyrus also reverses learning and memory deficits in hAPP mice24–27, we injected Lenti-EphB2–Flag or Lenti-empty bilaterally into the dentate gyrus of hAPP and nontransgenic mice and analysed them behaviourally 2 months later.
Spatial learning and memory in the Morris water maze is strongly affected by dentate gyrus impairments28. In the spatial, hidden-platform component, Lenti-EphB2–Flag-treated but not Lenti-empty-treated hAPP mice performed at control levels (Fig. 5a, b). Overexpressing EphB2 did not alter learning in nontransgenic mice (Fig. 5a, b). All groups of mice learned similarly well in the cued-platform component (data not shown).
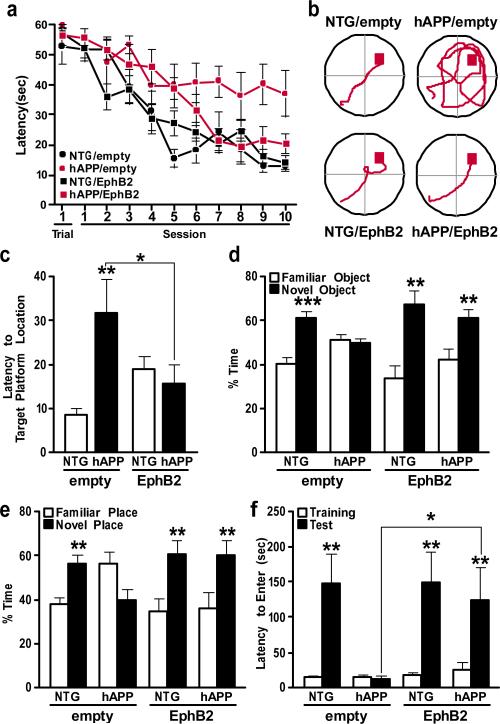
Figure 5. Increasing EphB2 expression in the dentate gyrus ameliorates learning and memory deficits in hAPP mice.
a, Learning curves during spatial training in the Morris water maze. The latency for each mouse to reach the hidden platform was recorded. Trial 1 represents performance on the first trial, and subsequent sessions represent the average of two training trials. Lenti-empty treated hAPP mice had longer latencies and travelled farther (not shown) to find the hidden platform than all other groups (P < 0.0001, repeated-measures ANOVA). b, Representative paths from the last session of hidden-platform training. c, Time it took mice to reach the target platform location during a probe trial (platform removed) 24 h after the last hidden-platform training. *P < 0.05, **P < 0.01 versus first bar or as indicated by bracket (one-way ANOVA followed by Bonferroni post-hoc test). d, Object recognition memory as reflected by the percentage of time mice spent exploring a familiar versus a novel object during a 10-min test session. **P < 0.01, ***P < 0.001 versus familiar object (paired t test). e, Spatial location memory as reflected by the percentage of time mice spent exploring familiar objects whose locations were or were not altered. **P < 0.01 versus familiar place (t test). f, Passive avoidance memory. *P < 0.05, **P < 0.01, versus training or as indicated by bracket (one-way nonparametric Kruskal-Wallis test followed by Dunn's post test). n = 9–12 mice per genotype and treatment. Values are means ± s.e.m.
In a probe trial, Lenti-empty-treated but not Lenti-EphB2–Flag-treated hAPP mice took longer to reach the original platform location than Lenti-empty-treated nontransgenic controls (Fig. 5c). Lenti-EphB2–Flag-treated nontransgenic mice performed slightly worse than Lenti-empty-treated nontransgenic mice (Fig. 5c) (P = 1.0 by one-way ANOVA and Bonferroni post-hoc test).
In the novel object recognition test, Lenti-EphB2-treated but not Lenti-empty-treated hAPP mice spent more time exploring the novel object (Fig. 5d). In the novel place recognition test, Lenti-EphB2-treated but not Lenti-empty-treated hAPP mice spent more time exploring the object whose location had changed (Fig. 5e). Thus, increasing EphB2 expression in the dentate gyrus of hAPP mice ameliorates deficits in both spatial and nonspatial learning and memory.
Finally, we assessed passive avoidance learning, which depends at least partly on hippocampal functions29,30. During training, escape latencies were similar across groups (Fig. 5f). However, 24 h later, Lenti-empty-treated hAPP mice were severely impaired, whereas all other groups performed well (Fig. 5f). Increasing dentate gyrus EphB2 levels in hAPP mice did not reverse behavioural deficits that were probably caused by impairments of other brain regions, including hyperactivity in the open field and disinhibition in the elevated plus maze (Supplementary Fig. 13).
Discussion
Our study shows that EphB2 depletion contributes to amyloid-β-induced neuronal deficits and cognitive dysfunction. Reducing neuronal EphB2 levels caused functional deficits similar to those caused by amyloid-β, including deficits in NMDA-receptor-dependent synaptic strength and gene expression and impaired LTP and memory. Increasing neuronal EphB2 levels in hAPP mice reversed these deficits, indicating that EphB2 impairment is necessary and sufficient to elicit them and that increasing EphB2 activity counteracts amyloid-β-induced neuronal dysfunction. Consistent with a previous report9, EphB2 depletion in memory-related brain regions was detected not only in hAPP mice, but also in humans with Alzheimer's disease, underlining the potential clinical relevance of our findings. Our data further indicate that the depletion of EphB2 by amyloid-β oligomers involves direct binding of amyloid-β oligomers to the FN repeats domain of EphB2 and EphB2 degradation in the proteasome. Reduction of EphB2 mRNA may have an additional role.
Our results and those of others indicate that neuronal EphB2 depletion causes deficits in learning and memory by impairing NMDA-receptor functions (Supplementary Fig. 1). EphB2 modulates NMDA receptors by tyrosine phosphorylation and recruits active NMDA receptors to excitatory synapses10–12. EphB2-deficient mice have LTP deficits10,14 and fewer NR1 subunits in the postsynaptic density10. Our results are consistent with these findings, although LTP deficits after shRNA knockdown of EphB2 in adult nontransgenic mice were more severe than those in EphB2-deficient mice. Other members of the large Eph family might partially compensate for EphB2 ablation during early development. The more severe deficits after acute EphB2 knockdown in adults probably reflect the lack of such compensation. Modulation of other LTP-related proteins also results in different outcomes, depending on when it is initiated31.
Amyloid-β may impair LTP by inducing internalization of NMDA receptors32,33. We found that depletion of EphB2 contributes to the amyloid-β-induced decrease in NMDA receptors and that increasing EphB2 expression markedly improves LTP and memory even in the presence of high amyloid-β levels. Increased EphB2 levels probably increase surface NMDA-receptor expression. Indeed, increasing neuronal EphB2 expression reversed amyloid-β-induced deficits in NMDA-receptor-mediated synaptic strength.
Opposition of amyloid-β-induced surface depletion of NMDA receptors is the most parsimonious interpretation of the EphB2-mediated rescue effects (Supplementary Fig. 1). However, amyloid-β may impair LTP and memory through alternative processes, and increased expression of EphB2 may counteract amyloid-β effects also through downstream signalling mechanisms.
Manipulating individual functional hubs of neurons can profoundly affect a larger network34,35. Even manipulating an individual neuron can affect the global brain state36. Thus, improving the function of a subset of neurons might allow an impaired brain region to better support specific behaviours. The current study supports this hypothesis: increasing EphB2 expression in a subset of granule cells improved dentate gyrus LTP and learning and memory in hAPP mice. It remains to be determined whether EphB2 depletions contribute to amyloid-β-dependent impairments in other brain regions and whether increasing neuronal EphB2 levels in these regions is tolerated as well as it was in the dentate gyrus. If so, pharmacological treatments might be used to increase EphB2 expression or activity. Our results indicate additional entry points for interventions (Supplementary Fig. 1). For example, it may be possible to identify small molecules that block the binding of amyloid-β oligomers to EphB2's FN repeats domain, prevent proteasomal degradation of EphB2, or improve its interactions with NMDA receptors. 1555 (1997). http://dx.doi.org/10.1073/pnas.94.4.1550
METHODS SUMMARY
General
Unless indicated otherwise, all data reported in this paper were obtained in blind-coded experiments, in which the investigators who obtained the data were unaware of the specific genotype and treatment of mice, brain slices and cell cultures. For number of mice, slices and cell cultures analysed in each experiment, refer to Supplementary Table 1. For experimental details related to each figure legend, refer to Supplementary Table 2.
Experimental models
Heterozygous transgenic and nontransgenic mice were from hAPP line J207,8,37,38. Primary neuronal cultures from wild-type rats were treated with medium conditioned by CHO cells that do or do not produce human amyloid-β oligomers (Supplementary Figs 14 and 15 and refs 39, 40.).
Experimental manipulations
Lentiviral constructs directing neuronal expression of no transgene products, EphB2-Flag, or GFP in combination with anti-EphB2 shRNAs or scrambled control shRNA were injected stereotactically into the dentate gyrus of mice20,41. Neuronal cultures were infected with some of these constructs and stimulated with Fc-ephrin-B2 or Fc control12,42.
Outcome measures
The interaction between biotinylated or naturally secreted amyloid-β oligomers and EphB2 was assessed under cell-free conditions and in neuronal cultures of primary neurons or HEK cells by pull-down with avidin agarose beads43 or immunoprecipitation and western blot44. EphB2 and NR1 levels in brain tissues or neuronal cultures were determined by immunoprecipitation and western blot or western blot alone44. Corresponding transcripts were measured by quantitative polymerase chain reaction with reverse transcription (RT-qPCR). Fos expression in neuronal cultures was determined by western blot44. Field recordings8 or whole-cell patch-clamp recordings45 from acute hippocampal slices were used to determine synaptic strength (fEPSP I/O relationships; mediated by either AMPA receptors or NMDA receptors), synaptic plasticity (LTP), and NMDA-receptor:AMPA-receptor ratios of EPSCs at the medial perforant path to dentate gyrus granule cell synapses. Learning and memory were assessed in the Morris water maze, novel object recognition test, novel place recognition test, and passive avoidance test46–49. Amyloid-β levels in the dentate gyrus of hAPP-J20 mice were determined by ELISA50.
Supplementary Material
Acknowledgements
We thank I. Ethell for the plasmid encoding the Flag-tagged EphB2 receptor; D. J. Selkoe and D. Walsh for CHO-7PA2 cells; S. Finkbeiner for the plasmid encoding the NMDA receptor subunit NR1; J. Palop for comments; H. Solanoy, M. Thwin and X. Wang for technical support; G. Howard and S. Ordway for editorial review; J. Carroll for preparation of graphics; and M. Dela Cruz for administrative assistance. The study was supported by NIH grants AG011385, AG022074 and NS041787 to L.M., a fellowship from the McBean Family Foundation to M.C., and the National Center for Research Resources Grant RR18928-01 to the Gladstone Institutes.
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
References
- 1.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nature Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 4.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Ikonomovic MD, et al. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer's disease. Exp. Neurol. 1999;160:194–204. doi: 10.1006/exnr.1999.7196. [DOI] [PubMed] [Google Scholar]
- 6.Sze C, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. N-Methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer's disease. J. Neurol. Sci. 2001;182:151–159. doi: 10.1016/s0022-510x(00)00467-6. [DOI] [PubMed] [Google Scholar]
- 7.Palop JJ, et al. Vulnerability of dentate granule cells to disruption of Arc expression in human amyloid precursor protein transgenic mice. J. Neurosci. 2005;25:9686–9693. doi: 10.1523/JNEUROSCI.2829-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon AM, et al. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer's disease. J. Alzheimers Dis. 2009;17:773–786. doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- 10.Henderson JT, et al. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32:1041–1056. doi: 10.1016/s0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- 11.Dalva MB, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 12.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Fu AK, Ip NY. Bidirectional signaling of ErbB and Eph receptors at synapses. Neuron Glia Biol. 2008;4:211–221. doi: 10.1017/S1740925X09990287. [DOI] [PubMed] [Google Scholar]
- 14.Grunwald IC, et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001;32:1027–1040. doi: 10.1016/s0896-6273(01)00550-5. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann A, et al. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J. Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litterst C, et al. Ligand binding and calcium influx induce distinct ectodomain/γ-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakabayashi K, Honer WG, Masliah E. Synapse alterations in the hippocampalentorhinal formation in Alzheimer's disease with and without Lewy body disease. Brain Res. 1994;667:24–32. doi: 10.1016/0006-8993(94)91709-4. [DOI] [PubMed] [Google Scholar]
- 18.Scheff SW, Price DA. Alzheimer's disease-related alterations in synaptic density: neocortex and hippocampus. J. Alzheimers Dis. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 19.Mueller-Steiner S, et al. Anti-amyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Sun B, et al. Imbalance between GABAergic and glutamatergic transmissions impairs adult neurogenesis in an animal model of Alzheimer's disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shemer I, et al. Non-fibrillar β-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur. J. Neurosci. 2006;23:2035–2047. doi: 10.1111/j.1460-9568.2006.04733.x. [DOI] [PubMed] [Google Scholar]
- 22.Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colino A, Malenka RC. Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro. J. Neurophysiol. 1993;69:1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
- 24.Harris JA, et al. Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer's disease are independent of caspase cleavage of the amyloid precursor protein. J. Neurosci. 2010;30:372–381. doi: 10.1523/JNEUROSCI.5341-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Mejia RO, et al. Phospholipase A2 reduction ameliorates cognitve deficits in mouse model of Alzheimer's disease. Nature Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meilandt WJ, et al. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2008;28:5007–5017. doi: 10.1523/JNEUROSCI.0590-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid b-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn. Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima R, et al. Comprehensive behavioral phenotyping of calpastatin-knockout mice. Mol. Brain. 2008;1:7. doi: 10.1186/1756-6606-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter MC, et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terashima A, et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nature Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 33.Kurup P, et al. Aβ-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J. Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonifazi P, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 35.Han JH, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 36.Li CY, Poo MM, Dan Y. Burst spiking of a single cortical neuron modifies global brain state. Science. 2009;324:643–646. doi: 10.1126/science.1169957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockenstein EM, et al. Levels and alternative splicing of amyloid β protein precursor (APP) transcripts in brains of transgenic mice and humans with Alzheimer's disease. J. Biol. Chem. 1995;270:28257–28267. doi: 10.1074/jbc.270.47.28257. [DOI] [PubMed] [Google Scholar]
- 38.Mucke L, et al. High-level neuronal expression of Ab1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koo EH, Squazzo SL. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J. Biol. Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 40.Walsh DM, et al. Naturally secreted oligomers of amyloid b protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 41.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic; 1997. [Google Scholar]
- 42.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alfa Cisse M, et al. M1 and M3 muscarinic receptors control physiological processing of cellular prion by modulating Alzheimer's disease AM17 phosphorylation and activity. J. Neurosci. 2007;27:4083–4092. doi: 10.1523/JNEUROSCI.5293-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Rush A, Rowan MJ, Anwyl R. NMDA receptor- and metabotropic glutamate receptor-dependent synaptic plasticity induced by high frequency stimulation in the rat dentate gyrus in vitro. J. Physiol. (Lond.) 2001;533:745–755. doi: 10.1111/j.1469-7793.2001.t01-1-00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raber J, et al. Hypothalamic-pituitary-adrenal function in Apoe−/− mice: possible role in behavioral and metabolic alterations. J. Neurosci. 2000;20:2064–2071. doi: 10.1523/JNEUROSCI.20-05-02064.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raber J, LeFevour A, Buttini M, Mucke L. Androgens protect against Apolipoprotein E4-induced cognitive deficits. J. Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dere E, Huston JP, De Souza Silva MA. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res. Protoc. 2005;16:10–19. doi: 10.1016/j.brainresprot.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Benice T, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 50.Johnson-Wood K, et al. Amyloid precursor protein processing and Ab42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl Acad. Sci. USA. 94:1550. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.