Abstract
BACKGROUND
High-density lipoprotein (HDL) may provide cardiovascular protection by promoting reverse cholesterol transport from macrophages. We hypothesized that the capacity of HDL to accept cholesterol from macrophages would serve as a predictor of atherosclerotic burden.
METHODS
We measured cholesterol efflux capacity in 203 healthy volunteers who underwent assessment of carotid artery intima–media thickness, 442 patients with angiographically confirmed coronary artery disease, and 351 patients without such angiographically confirmed disease. We quantified efflux capacity by using a validated ex vivo system that involved incubation of macrophages with apolipoprotein B–depleted serum from the study participants.
RESULTS
The levels of HDL cholesterol and apolipoprotein A-I were significant determinants of cholesterol efflux capacity but accounted for less than 40% of the observed variation. An inverse relationship was noted between efflux capacity and carotid intima–media thickness both before and after adjustment for the HDL cholesterol level. Furthermore, efflux capacity was a strong inverse predictor of coronary disease status (adjusted odds ratio for coronary disease per 1-SD increase in efflux capacity, 0.70; 95% confidence interval [CI], 0.59 to 0.83; P<0.001). This relationship was attenuated, but remained significant, after additional adjustment for the HDL cholesterol level (odds ratio per 1-SD increase, 0.75; 95% CI, 0.63 to 0.90; P = 0.002) or apolipoprotein A-I level (odds ratio per 1-SD increase, 0.74; 95% CI, 0.61 to 0.89; P = 0.002). Additional studies showed enhanced efflux capacity in patients with the metabolic syndrome and low HDL cholesterol levels who were treated with pioglitazone, but not in patients with hypercholesterolemia who were treated with statins.
CONCLUSIONS
Cholesterol efflux capacity from macrophages, a metric of HDL function, has a strong inverse association with both carotid intima–media thickness and the likelihood of angiographic coronary artery disease, independently of the HDL cholesterol level. (Funded by the National Heart, Lung, and Blood Institute and others.)
A robust inverse association between the level of high-density lipoprotein (HDL) cholesterol and the risk of cardiovascular disease has fostered intensive research seeking to target HDL metabolism for therapeutic gain.1,2 However, some findings have called into question the hypothesis that pharmacologic increases in HDL cholesterol levels are necessarily beneficial. Several therapies, including nicotinic acid and fibric acid derivatives, increase HDL cholesterol levels, but linking these increases to clinical risk reduction has proved challenging.3,4 Most strikingly, an inhibitor of cholesteryl ester transfer protein (CETP) was associated with an increase in the number of cardiovascular events, despite a 72% increase in HDL cholesterol levels.5 Finally, neither rare nor common genetic variants associated with HDL cholesterol levels have been strongly linked to coronary disease.6–9
The static measurement of HDL cholesterol levels has inherent limitations as a metric of the functional effects of HDL in vivo. Reports of marked heterogeneity in the particle composition and biologic properties of HDL have reinforced a need for validated assays of HDL function.10 HDL-mediated atheroprotection is most likely pleiotropic in nature, but the ability of HDL to promote reverse cholesterol transport by accepting cholesterol from lipid-laden macrophages (termed “cholesterol efflux capacity”) is thought to play a key role.11,12
We hypothesized that cholesterol efflux capacity is a determinant of atherosclerotic burden that is independent of the HDL cholesterol level. This study was designed to explore the pairwise relationships of efflux capacity with the level of HDL cholesterol and two measures of atherosclerosis — carotid artery intima–media thickness and angiographically confirmed coronary artery disease.
METHODS
STUDY DESIGN
We tested our hypotheses in two independent populations, both enrolled at the University of Pennsylvania Medical Center. The first cohort consisted of 203 healthy white subjects recruited to explore the relationship between HDL-related biomarkers and subclinical atherosclerosis, as assessed by carotid intima–media thickness. Race was self-determined. Exclusion criteria were a history of coronary artery disease, current smoking, and diabetes. Details of the measurements of carotid intima–media thickness are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
The second population consisted of persons selected from a previously described cohort of patients who were undergoing cardiac catheterization.13,14 A case–control sample was selected that involved 793 white patients (442 case patients and 351 control patients; race was self-determined). Patients were excluded if catheterization was performed during an acute coronary syndrome. Patients with luminal stenosis of more than 50% in a major coronary vessel were classified as having coronary artery disease; control patients had no evidence of coronary disease at the time of angiography and no history of myocardial infarction.
The assay was also performed on available samples from patients enrolled in two previously reported pharmacologic-intervention trials. The first analysis included 39 patients from a trial involving 60 persons with the metabolic syndrome who had been randomly assigned to 12 weeks of treatment with either pioglitazone or placebo.15 The second analysis included 99 patients from a trial involving 120 persons with hyperlipidemia who had been randomly assigned to 16 weeks of therapy with 40 mg of pravastatin, 10 mg of atorvastatin, 80 mg of atorvastatin, or placebo.16
ASSESSMENT OF CHOLESTEROL EFFLUX CAPACITY
Cholesterol efflux capacity was quantified in blood samples from the cohort of healthy volunteers as described previously.17 This assay quantifies total efflux mediated by pathways of known relevance in cholesterol efflux from macrophages (i.e., ATP-binding cassette transporter A1 [ABCA1] and G1 [ABCG1], scavenger receptor B1, and aqueous diffusion).17 Each sample was run in triplicate, with a mean coefficient of variation of 4.3%. Values were normalized by dividing the efflux capacity of individual patients by the efflux capacity of a serum pool run with each assay.
Cholesterol efflux capacity in the coronary disease and pharmacologic-study cohorts was quantified with the use of a slightly modified method designed to increase throughput. J774 cells, derived from a murine macrophage cell line, were plated and radiolabeled with 2 μCi of 3H-cholesterol per milliliter. ABCA1 was up-regulated by means of a 6-hour incubation with 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP. Subsequently, efflux mediums containing 2.8% apolipoprotein B–depleted serum were added for 4 hours. All steps were performed in the presence of the acyl–coenzyme A:cholesterol acyltransferase inhibitor CP113,818 (2 μg per milliliter). In a pilot study involving serum samples from 20 healthy volunteers, results from the original assay procedure17 and the modified method were strongly correlated (r = 0.85).
Liquid scintillation counting was used to quantify the efflux of radioactive cholesterol from the cells. The quantity of radioactive cholesterol incorporated into cellular lipids was calculated by means of isopropanol extraction of control wells not exposed to patient serum. Percent efflux was calculated by the following formula: [(microcuries of 3H-cholesterol in mediums containing 2.8% apolipoprotein B–depleted serum – microcuries of 3H-cholesterol in serum-free mediums) ÷ microcuries of 3H-cholesterol in cells extracted before the efflux step] × 100. All assays were performed in duplicate. To correct for interassay variation across plates, a pooled serum control from five healthy volunteers was included on each plate, and values for serum samples from patients were normalized to this pooled value in subsequent analyses. Additional studies that were performed to validate the measurement of cholesterol efflux capacity are described in the Supplementary Appendix.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distributions. The association of efflux capacity with clinical and HDL-related variables was assessed with the use of Pearson’s correlation coefficients, with partial correlation coefficients after adjustment for HDL cholesterol level also reported.
Linear regression was used to characterize the relationship between efflux capacity and carotid intima–media thickness. Age, sex, systolic blood pressure, and levels of glycated hemoglobin, low-density lipoprotein (LDL) cholesterol, HDL cholesterol, and apolipoprotein A-I were included as covariates. Beta coefficients are reported for a 1-SD increase for continuous variables.
Logistic regression was used to estimate the association between cholesterol efflux capacity and coronary artery disease status after adjustment for age, sex, smoking status, presence or absence of diabetes, presence or absence of hypertension, and LDL cholesterol level. HDL cholesterol and apolipoprotein A-I levels were added in subsequent models. Adjusted odds ratios are reported both for a 1-SD increase in efflux capacity and across quartiles. Model performance was assessed according to discrimination, by means of the area under the receiver-operating-characteristic curve (AUC); calibration, as indicated by the Hosmer–Lemeshow goodness-of-fit statistic; and predictive accuracy, as described by integrated-discrimination-improvement indexes.18
Paired t-tests were used to assess the effect of pharmacologic interventions on efflux capacity. These changes were compared with placebo with the use of analysis of covariance, which included the patient’s baseline value and the treatment group as covariates.
All reported P values are two-tailed, with a P value of 0.05 indicating statistical significance. Analyses were performed with the use of Stata software, version 9.0 (Stata), JMP software, version 8.0 (SAS Institute), or R software, version 2.10 (R Development Core Team).
RESULTS
ASSOCIATION OF CHOLESTEROL EFFLUX CAPACITY WITH SUBCLINICAL ATHEROSCLEROSIS
The characteristics of the 203 healthy volunteers are shown in Table 1; 33% of the cohort members reported that they had previously smoked. Mean values for systolic blood pressure and glycated hemoglobin were 124 mm Hg and 5.5%, respectively. The mean values for cholesterol efflux capacity were 11% (range, 5 to 23) before normalization and 0.77 (range, 0.36 to 1.68) after normalization. Scatterplot analyses showed significant correlations between cholesterol efflux capacity and the levels of both HDL cholesterol (r = 0.58) and apolipoprotein A-I (r = 0.63) (see Fig. 4 in the Supplementary Appendix).
Table 1.
Baseline Characteristics of the Healthy-Volunteer and Case–Control Cohorts.*
| Variable | Healthy-Volunteer Cohort (N = 203) | Case–Control Cohort | |
|---|---|---|---|
| Controls (N = 351) | Patients with Angiographically Confirmed Coronary Artery Disease (N = 442) | ||
| Age — yr | 51±8 | 62±9 | 57±9 |
| Male sex — no. (%) | 110 (54.2) | 170 (48.4) | 303 (68.6) |
| Diabetes — no. (%) | NA | 45 (12.8) | 102 (23.1) |
| Hypertension — no. (%) | NA | 166 (47.3) | 267 (60.0) |
| Current smoking — no. (%) | NA | 143 (40.7) | 216 (48.9) |
| Previous myocardial infarction — no. (%) | NA | 0 | 71 (16.1) |
| Lipid levels — mg/dl | |||
| Total cholesterol | 203±36 | 179±38 | 175±39 |
| Low-density lipoprotein cholesterol | 123±30 | 108±32 | 105±32 |
| High-density lipoprotein cholesterol | 56±18 | 50±15 | 44±12 |
| Triglycerides | |||
| Median | 90 | 91 | 112 |
| Interquartile range | 63–135 | 64–135 | 79–162 |
| Phospholipids | 238±39 | 196±36 | 191±38 |
| Apolipoproteins — mg/dl | |||
| Apolipoprotein A-I | 137±26 | 128±30 | 117±27 |
| Apolipoprotein A-II | 34±6 | 30±6 | 30±6 |
| Apolipoprotein E | |||
| Median | 3.9 | 4.3 | 4.0 |
| Interquartile range | 3.4–4.5 | 3.3–5.3 | 3.2–5.1 |
Plus–minus values are means ±SD. NA denotes not applicable. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
The mean carotid intima–media thickness was 0.66±0.13 mm. Age, systolic blood pressure, and glycated hemoglobin level were each associated with increased carotid intima–media thickness in bivariate analyses (P<0.05 for each comparison). No significant relationship between HDL cholesterol level and carotid intima–media thickness was noted in either unadjusted or adjusted models (P=0.37 and P=0.73, respectively). In contrast, a significant inverse relationship between cholesterol efflux capacity and carotid intima–media thickness was observed that was robust with additional adjustment for the HDL cholesterol or apolipoprotein A-I level (Table 2).
Table 2.
Beta Coefficients for the Association between Cholesterol Efflux Capacity and Carotid Intima–Media Thickness.
| Linear-Regression Covariates* | Beta Coefficient per 1-SD Increase in Efflux Capacity (95% CI) | P Value |
|---|---|---|
| Age and sex | −0.02 (−0.04 to −0.003) | 0.02 |
| Age, sex, and cardiovascular risk factors | −0.02 (−0.04 to −0.004) | 0.02 |
| Age, sex, cardiovascular risk factors, and high-density lipoprotein cholesterol | −0.03 (−0.06 to −0.01) | 0.003 |
| Age, sex, cardiovascular risk factors, and apolipoprotein A-I | −0.04 (−0.06 to −0.01) | 0.005 |
Cardiovascular risk factors were systolic blood pressure, glycated hemoglobin, and low-density lipoprotein cholesterol.
ASSOCIATION OF CHOLESTEROL EFFLUX CAPACITY WITH CORONARY ARTERY DISEASE
The characteristics of the case–control cohort are provided in Table 1. Distributions of cholesterol efflux capacity among the case patients and control patients are shown in Figure 5 in the Supplementary Appendix.
As compared with control patients, patients with coronary artery disease had significantly lower levels of not only HDL cholesterol and apolipoprotein A-I (P<0.001 for each comparison) but also normalized cholesterol efflux capacity (mean value for case patients, 0.82; mean value for control patients, 0.90; P<0.001). The HDL cholesterol level was the strongest predictor of efflux capacity (P<0.001) but accounted for only 26% of the observed variation. Male sex and current smoking were associated with decreased efflux capacity (P<0.001 and P = 0.003, respectively). Subsequent adjustment for HDL cholesterol largely attenuated the relationship between sex and efflux capacity (P = 0.14), although smoking remained a significant inverse predictor of efflux capacity (P = 0.004).
The proportion of patients with coronary disease decreased consistently with increases in cholesterol efflux capacity. In a logistic-regression analysis adjusted for age, sex, and traditional cardiovascular risk factors, an increased efflux capacity was associated with a decreased risk of coronary artery disease (odds ratio per 1-SD increase in efflux capacity, 0.70; 95% confidence interval [CI], 0.59 to 0.83; P<0.001). This relationship remained robust after the addition of HDL cholesterol level as a covariate (odds ratio per 1-SD increase, 0.75; 95% CI, 0.63 to 0.90; P=0.002). The results were similar when the apolipoprotein A-I level was substituted for the HDL cholesterol level (odds ratio per 1-SD increase, 0.74; 95% CI, 0.61 to 0.89; P = 0.002).
Division of the cohort into quartiles according to efflux capacity provided additional evidence of an inverse association between efflux capacity and coronary artery disease (Table 3). In an analysis adjusted for age and sex, the quartile of highest efflux capacity was associated with a decreased risk of coronary disease, as compared with the quartile of lowest efflux capacity (odds ratio, 0.38; 95% CI, 0.25 to 0.58; P<0.001). This result remained significant after adjustment for traditional cardiovascular risk factors, including the level of HDL cholesterol or apolipoprotein A-I (Table 3).
Table 3.
Coronary Artery Disease Status According to Quartile of Efflux Capacity.
| Variable | No. of Patients | Odds Ratio for Coronary Artery Disease (95% CI)* | ||
|---|---|---|---|---|
| Adjusted for Cardiovascular Risk Factors | Adjusted for Cardiovascular Risk Factors and HDL Cholesterol | Adjusted for Cardiovascular Risk Factors and Apolipoprotein A-I | ||
| Quartile 1 | 198 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 198 | 0.75 (0.48–1.16) | 0.79 (0.51–1.24) | 0.77 (0.49–1.21) |
| Quartile 3 | 198 | 0.58 (0.37–0.89) | 0.64 (0.41–1.00) | 0.63 (0.40–0.99) |
| Quartile 4 | 199 | 0.40 (0.25–0.63) | 0.48 (0.30–0.78) | 0.46 (0.28–0.75) |
| P value for trend | <0.001 | 0.002 | 0.002 | |
Cardiovascular risk factors included in the logistic-regression model were age, sex, smoking status, presence or absence of diabetes, presence or absence of hypertension, and low-density lipoprotein cholesterol. HDL denotes high-density lipoprotein.
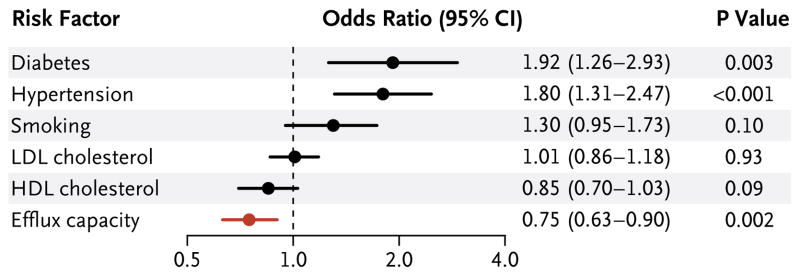
Several known cardiovascular risk factors were associated with coronary disease in logistic- regression models adjusted for age and sex. Diabetes and hypertension were each linked to an increased risk of coronary artery disease, whereas the HDL cholesterol level was inversely associated with the risk of coronary disease (odds ratio per 1-SD increase in the HDL cholesterol level, 0.71; 95% CI, 0.60 to 0.84; P<0.001). Each of the known risk factors, in addition to efflux capacity, was combined in a single logistic-regression analysis, as shown in Figure 1. In this model, which included the HDL cholesterol level, efflux capacity remained a highly significant predictor of disease status (P = 0.002).
Figure 1. Odds Ratios for Coronary Artery Disease According to Efflux Capacity and Selected Risk Factors.
The logistic-regression model was also adjusted for age and sex. Odds ratios for continuous variables are per 1-SD increase.
The inclusion of cholesterol efflux capacity resulted in moderate improvement of the overall performance of the logistic-regression model. The AUC increased from 0.705 to 0.722 (P=0.04) when efflux capacity was added to known cardiovascular risk factors. When the HDL cholesterol level was included in the baseline model, the increase in the AUC (from 0.714 to 0.723) was no longer significant (P=0.19). The addition of efflux capacity did not reduce model discrimination as assessed by goodness-of-fit statistics. Integrated-discrimination-improvement indexes were 0.022 when efflux capacity was added to a model that contained traditional risk factors and 0.011 when efflux capacity was added to a baseline model that also included HDL cholesterol levels; these changes were not significant.
HDL-RELATED PREDICTORS OF CHOLESTEROL EFFLUX CAPACITY
Additional phenotyping revealed that several HDL-associated variables, including levels of total phospholipids, apolipoprotein A-I, apolipoprotein A-II, and apolipoprotein E, were each significantly associated with cholesterol efflux capacity. These relationships were attenuated after adjustment for the HDL cholesterol level but remained significant in both cohorts (Table 1 in the Supplementary Appendix).
EFFECT OF PHARMACOLOGIC INTERVENTIONS ON CHOLESTEROL EFFLUX CAPACITY
Treatment with pioglitazone for 12 weeks resulted in a significant increase in efflux capacity, as compared with the baseline value and with the change observed in the placebo group (Table 4). In contrast, no significant change was seen in patients who were treated with statins for 16 weeks. Although both treatments were associated with small increases in the level of HDL cholesterol, no significant association was noted between a change in the HDL cholesterol level and a change in the cholesterol efflux capacity (r = 0.22 and P = 0.18 for the pioglitazone study, and r = 0.16 and P = 0.11 for the statin study).
Table 4.
Effect of Pharmacologic Interventions on Cholesterol Efflux Capacity.*
| Pharmacologic Intervention | No. of Patients | Percent Change in Cholesterol Efflux Capacity (95% CI) | P Value | |
|---|---|---|---|---|
| vs. Baseline | vs. Placebo | |||
| Thiazolidinedione | ||||
| Pioglitazone | 16 | 11.3 (1.8 to 20.8) | 0.02 | 0.04 |
| Placebo | 23 | 0.0 (−6.2 to 6.1) | 0.99 | |
| Statin | ||||
| Pravastatin, 40 mg | 23 | −0.4 (−6.5 to 5.6) | 0.88 | 0.71 |
| Atorvastatin, 10 mg | 26 | 2.7 (−4.8 to 10.2) | 0.47 | 0.81 |
| Atorvastatin, 80 mg | 25 | −2.5 (−9.1 to 4.1) | 0.45 | 0.38 |
| Placebo | 25 | −1.1 (−6.5 to 4.2) | 0.66 | |
Patients treated with pioglitazone received 30 mg per day for 6 weeks, followed by 45 mg per day for an additional 6 weeks. Patients treated with statins received continuous therapy at a fixed dose for 16 weeks.
DISCUSSION
In this study, we found that the ability of HDL to promote cholesterol efflux from macrophages was strongly and inversely associated with both subclinical atherosclerosis and obstructive coronary artery disease. These associations persisted after adjustment for traditional cardiovascular risk factors, including the levels of HDL cholesterol and apolipoprotein A-I.
Although cholesterol efflux from macrophages represents only a small fraction of overall flux through the reverse-cholesterol-transport pathway, it is probably the component that is most relevant to atheroprotection.19 We used an assay that integrates the pathways known to mediate cholesterol efflux from macrophages (i.e., ABCA1, ABCG1, scavenger receptor B1, and aqueous diffusion). Only a small part of the observed relationship between cholesterol efflux capacity and atherosclerosis was explained by variation in HDL cholesterol levels. Indeed, efflux capacity served as the stronger predictor of both carotid intima–media thickness and coronary disease status in regression models that included both variables.
Small studies conducted with the use of a rat hepatoma cell line have shown decreased efflux capacity of whole serum or plasma in patients with coronary disease, diabetes, or diabetic ne-phropathy.20–22 Similarly, an autopsy study of nonhuman primates showed an inverse correlation between atherosclerotic burden and efflux capacity.23 In contrast, analyses that used human skin fibroblasts as the cholesterol source showed no significant differences according to the presence or absence of diabetes24 or the presence or absence of the metabolic syndrome.25 An analysis of patients undergoing coronary angiography also showed no significant variation in efflux capacity from fibroblasts according to disease status, disease severity, or risk of future events.26 In contrast with our findings, those studies have shown no relationship or only minimal relationship between whole-serum efflux capacity and the level of HDL cholesterol or apolipoprotein A-I. These discrepancies may reflect the nature of the cell line and the acceptor chosen for the ex vivo assessment of cholesterol efflux capacity. In this study, our goal was to assess the capacity of the whole HDL fraction to promote cholesterol efflux from macrophages.
A substantial body of evidence suggests that cholesterol efflux capacity, an integrated measure of HDL quantity and quality, is reflective of the role of HDL in atheroprotection. Cholesterol efflux has been shown to protect macrophages from LDL-induced apoptosis and to enhance endothelial function.27–29 In vivo studies in mice have indicated that ABCA1 and ABCG1 play a key role in facilitating cholesterol efflux and reverse cholesterol transport.30 Mice that are deficient in these proteins have marked increases in foam-cell accumulation and atherosclerosis, providing compelling evidence that the macrophage efflux pathway is antiatherogenic in vivo.31,32 Similarly, a study of patients with rare ABCA1 mutations showed an inverse relationship between cellular cholesterol efflux and carotid intima–media thickness.33
Given the substantial heterogeneity in the particle size, charge, and protein composition of HDL, it may not be surprising that HDL cholesterol levels are a poor surrogate for cholesterol efflux capacity. A recent report suggests that interindividual differences in the pre-β (lipid-poor) apolipoprotein A-I particle concentration explain some of the observed variation.17 In the present study, associations between multiple lipid-related variables and efflux capacity remained significant after adjustment for the HDL cholesterol level. These findings are consistent with previous analyses that implicate HDL-associated apolipoprotein E and phospholipids as mediators of cholesterol efflux.34,35 The attenuated efflux capacity noted in smokers is worthy of additional follow-up and may be related to apolipoprotein A-I oxidation.36 Future studies may prove fruitful in elucidating additional components of HDL that determine cholesterol efflux capacity.
These results could be important in the assessment of new therapies targeting HDL metabolism and reverse cholesterol transport. We have shown the feasibility of this approach in blood samples from two previously reported, small, placebo-controlled trials. The moderate relationships noted between changes in HDL cholesterol levels and changes in efflux capacity reinforce the idea that these two metrics provide complementary information. We noted increased efflux capacity after therapy with pioglitazone, a phenomenon that could be related to enhanced transcription of apolipoprotein A-I.37 In contrast, no such increase was noted after patients had been treated with statins, a finding that is consistent with the concept that statins most likely exert therapeutic benefit by means of a mechanism that is distinct from the promotion of cholesterol efflux. Our demonstration that cholesterol efflux capacity is associated with atherosclerosis in humans helps support the use of this measure in guiding the development of new HDL-targeted therapies for humans.
One limitation of this study is its cross-sectional approach. Another limitation is that although our assessment of cholesterol efflux capacity reflects the ability to mobilize free cholesterol from macrophages, it does not capture variation in the reverse-cholesterol-transport pathway in terms of cellular components (i.e., the hydrolysis of cholesteryl esters and the status of endogenous macrophage cholesterol transporters) or terminal components (i.e., uptake into the liver and biliary excretion).38
In conclusion, cholesterol efflux capacity, a key metric of HDL function, is not explained simply by circulating levels of HDL cholesterol or apolipoprotein A-I and is independently related to both the presence and the extent of atherosclerosis. These findings reinforce the concept that assessment of HDL function may prove informative in refining our understanding of HDL-mediated atheroprotection.
Supplementary Material
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (HL22633 and P50 HL70128) and the National Center for Research Resources (M01 RR00040 and UL1-RR-024134) and a Distinguished Clinical Scientist Award from the Doris Duke Charitable Foundation. Dr. Khera was supported by a medical student research fellowship from the Howard Hughes Medical Institute, and Dr. Cuchel by a K23 award (HL077146) from the NHLBI.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–63. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 3.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298:786–98. doi: 10.1001/jama.298.7.786. [Erratum, JAMA 2007;298:1516.] [DOI] [PubMed] [Google Scholar]
- 4.Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 6.Franceschini G, Sirtori CR, Capurso A, II, Weisgraber KH, Mahley RW. A-I Milano apoprotein: decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest. 1980;66:892–900. doi: 10.1172/JCI109956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabresi L, Baldassarre D, Castelnuovo S, et al. Functional lecithin:cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation. 2009;120:628–35. doi: 10.1161/CIRCULATIONAHA.108.818143. [DOI] [PubMed] [Google Scholar]
- 8.Frikke-Schmidt R, Nordestgaard BG, Stene MC, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–32. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 9.Willer CJ, Sanna S, Jackson AU, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–9. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–56. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–73. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 12.Glomset JA. The plasma lecithin: cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–67. [PubMed] [Google Scholar]
- 13.Lehrke M, Millington SC, Lefterova M, et al. CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J Am Coll Cardiol. 2007;49:442–9. doi: 10.1016/j.jacc.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Myocardial Infarction Genetics Consortium. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–41. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szapary PO, Bloedon LT, Samaha FF, et al. Effects of pioglitazone on lipoproteins, inflammatory markers, and adipo-kines in nondiabetic patients with metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:182–8. doi: 10.1161/01.ATV.0000195790.24531.4f. [DOI] [PubMed] [Google Scholar]
- 16.Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol. 2008;51:1653–62. doi: 10.1016/j.jacc.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 17.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–55. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 20.Syvänne M, Castro G, Dengremont C, et al. Cholesterol efflux from Fu5AH hepatoma cells induced by plasma of subjects with or without coronary artery disease and non-insulin-dependent diabetes: importance of LpA-I:A-II particles and phospholipid transfer protein. Atherosclerosis. 1996;127:245–53. doi: 10.1016/s0021-9150(96)05962-x. [DOI] [PubMed] [Google Scholar]
- 21.Pajunen P, Syvänne M, Castro G, Nieminen MS, Taskinen MR. Cholesterol efflux capacity in vitro predicts the severity and extent of coronary artery disease in patients with and without type 2 diabetes. Scand Cardiovasc J. 2001;35:96–100. doi: 10.1080/140174301750164736. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Tan KC, Shiu SW, Wong Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab Res Rev. 2008;24:617–23. doi: 10.1002/dmrr.895. [DOI] [PubMed] [Google Scholar]
- 23.Mikkola TS, Anthony MS, Clarkson TB, St Clair RW. Serum cholesterol efflux potential is an independent predictor of coronary artery atherosclerosis. Atherosclerosis. 2003;170:31–8. doi: 10.1016/s0021-9150(03)00247-8. [DOI] [PubMed] [Google Scholar]
- 24.de Vries R, Groen AK, Perton FG, et al. Increased cholesterol efflux from cultured fibroblasts to plasma from hyper-triglyceridemic type 2 diabetic patients: roles of pre beta-HDL, phospholipid transfer protein and cholesterol esterification. Atherosclerosis. 2008;196:733–41. doi: 10.1016/j.atherosclerosis.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Dullaart RP, Groen AK, Dallinga-Thie GM, de Vries R, Sluiter WJ, van Tol A. Fibroblast cholesterol efflux to plasma from metabolic syndrome subjects is not defective despite low high-density lipoprotein cholesterol. Eur J Endocrinol. 2008;158:53–60. doi: 10.1530/EJE-07-0451. [DOI] [PubMed] [Google Scholar]
- 26.Chirinos JA, Zambrano JP, Chakko S, et al. Ability of serum to decrease cellular acylCoA:cholesterol acyl transferase activity predicts cardiovascular outcomes. Circulation. 2005;112:2446–53. doi: 10.1161/CIRCULATIONAHA.104.521815. [DOI] [PubMed] [Google Scholar]
- 27.Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci U S A. 2007;104:15093–8. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui D, Thorp E, Li Y, et al. Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82:1040–50. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- 29.Terasaka N, Yu S, Yvan-Charvet L, et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–13. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Collins HL, Ranalletta M, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–24. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvan-Charvet L, Ranalletta M, Wang N, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–8. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Out R, Hoekstra M, Habets K, et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–64. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 33.van Dam MJ, de Groot E, Clee SM, et al. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: an observational study. Lancet. 2002;359:37–42. doi: 10.1016/S0140-6736(02)07277-X. [DOI] [PubMed] [Google Scholar]
- 34.Fournier N, Paul JL, Atger V, et al. HDL phospholipid content and composition as a major factor determining cholesterol efflux capacity from Fu5AH cells to human serum. Arterioscler Thromb Vasc Biol. 1997;17:2685–91. doi: 10.1161/01.atv.17.11.2685. [DOI] [PubMed] [Google Scholar]
- 35.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–42. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao B, Oda MN, Bergt C, et al. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281:9001–4. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang LH, Kamanna VS, Ganji SH, et al. Pioglitazone increases apolipoprotein A-I production by directly enhancing PPRE-dependent transcription in HepG2 cells. J Lipid Res. 2010;51:2211–22. doi: 10.1194/jlr.M004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khera AV, Rader DJ. Future therapeutic directions in reverse cholesterol transport. Curr Atheroscler Rep. 2010;12:73–81. doi: 10.1007/s11883-009-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.