Abstract
Protein kinase C (PKC) is activated by lipid second messengers or redox action, raising the question whether these activation modes involve the same or alternate mechanisms. Here we show that both lipid activators and oxidation target the zinc-finger domains of PKC, suggesting a unifying activation mechanism. We found that lipid agonist-binding or redox action leads to zinc release and disassembly of zinc fingers, thus triggering large-scale unfolding that underlies conversion to the active enzyme. These results suggest that PKC zinc fingers, originally considered purely structural devices, are in fact redox-sensitive flexible hinges, whose conformation is controlled both by redox conditions and lipid agonists. Antioxid. Redox Signal. 14, 757–766.
Introduction
Historically, the term “zinc-finger” refers to zinc-coordinated protein modules associated with mammalian transcription factors. The defining features are sets of two cysteines and two histidines, each recruited from different parts of a single polypeptide chain and arranged at their core into a tetrahedron. The extraordinarily high binding affinity of zinc chelated by these four ligands endows such protein folds with a high degree of rigidity, allowing protruding peptide loops of variable composition to intercalate finger-like fashion into DNA—hence the name (4, 27).
Far from restricted to transcription factors, zinc fingers occur in diverse classes of other pro- and eukaryotic proteins, including chaperones, kinases, ligases, or DNA processing enzymes (5, 9, 19, 32). While the basic principle of a zinc coordinated, rigid peptide core is maintained, diverse tetrahedral configurations have evolved that employ varied combinations of cysteine and histidine residues (26). These include the intricate structures found in serine/threonine kinases and other signaling molecules that contain complex arrangements of dual zinc-coordination centers, resembling butterflies (37). Further, tandem arrangements of two, three, or up to nine zinc fingers are not infrequent, but the utility of such concatenations is not fully understood.
The zinc fingers of serine/threonine kinases, such as protein kinase C (PKC), have no relevance for DNA binding but are thought to arrange proteins into specific tertiary conformations, not unlike disulfide bonds in extracellular proteins. However, zinc fingers depend on reducing conditions prevalent in the cytosol. They theoretically share with disulfide bonds the capacity for redox-mediated conformation change, although the mechanism of their disassembly is different from that of disulfide bridges (36). While the latter depend on reduction, the ionic nature of zinc coordination requires oxidation of critical cysteine residues. Because loss of key thiolate anions compromises the high-affinity chelate bond, conditions conducive to conformation change theoretically exist. Indeed one of us (U.J.) demonstrated this paradigm in the bacterial chaperone, heat-shock protein-33 (Hsp33) (19). This protein contains a zinc finger, composed of four highly conserved cysteines chelated by a single zinc ion, which stabilizes the inactive chaperone and acts as a redox sensor. Under conditions of oxidative stress, two intramolecular disulfide bonds form, causing zinc release and dramatic conformational rearrangements, leading to the full chaperone activity (11, 16).
Eleven PKC isoforms and three Raf kinases share with Hsp33 both the zinc fingers in their regulatory domains and the ability to respond to oxidative stress (22, 31). The nonclassical activation mechanism of these enzymes by redox chemistry, requiring neither lipid second messengers nor activated ras, is obscure, both with respect to the nature of oxidizing agents and the covalent modification effected. However, escape from autoinhibition and conversion to the active form requires large-scale unfolding (15), reminiscent of the redox-mediated activation of Hsp33. Loss of zinc coordination in response to redox action or phorbol ester stimulation was inferred from findings that activated PKCs shed significant amounts of zinc ions (21, 24), providing a first indication that the two activation mechanisms potentially share some common features. Progress was, however, hampered by the large size of PKC and inadequacies of contemporary physicochemical methods to study their internal dynamics.
In the present model study we furnish support for the hypothesis that mammalian zinc fingers are operational orthologs of bacterial ones. Completely different from Hsp33 in peptide configuration and butterfly-like architecture, PKC zinc centers, nonetheless, function as redox-sensitive, flexible hinges. Under oxidative stress conditions, PKC zinc fingers shed zinc and disassemble. We demonstrate that PKC-activating lipid second messengers, including phosphatidyl-serine (PS), diacyl glycerol (DAG), and its pharmacomimetics, phorbol esters, act likewise by dissociating PKC zinc centers, and that in full-length PKC zinc release correlates with acquisition of enzymatic competence. By substituting the PKC zinc finger for its bacterial ortholog, we created chimeric Hsp33 reporter proteins that retain full responsiveness to oxidation and acquire responsiveness to cognate lipid second messengers. On the basis of collective findings, we propose that PKC zinc fingers, contrary to earlier beliefs, are not static, redox-inert, and rigid folds but constitute flexible elements that on cue undergo biologically relevant conformational rearrangements, necessary for the activation of PKC.
Results and Discussion
Zinc-finger domains shed zinc in response to oxidation or phorbol binding
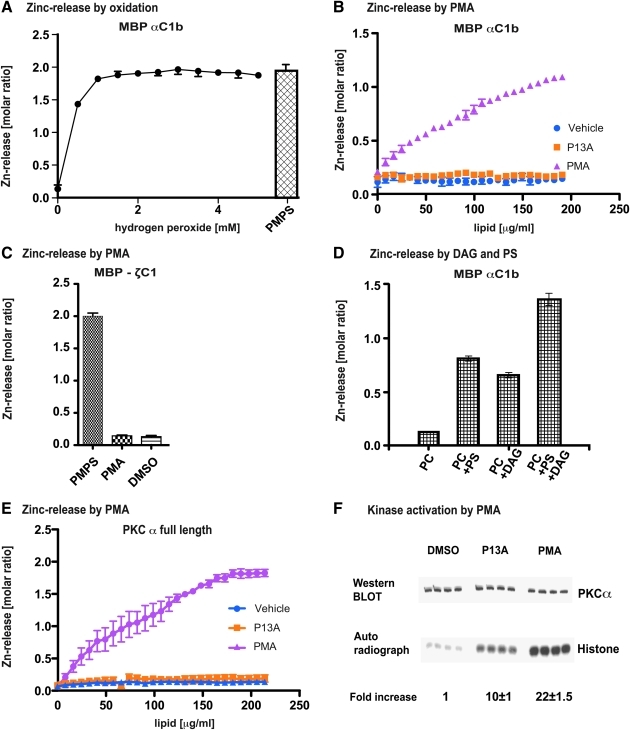
The defining feature of zinc fingers is the tetrahedral architecture of Zn2+ ions centered within four equidistant negatively charged cysteine/histidine combinations. The ionic field effect of extraordinary strength stabilizes the tertiary conformation of cytosolic proteins. Because the zinc-finger fold depends on the coordination of zinc by cysteine-thiolate anions, oxidizing conditions are expected to release Zn2+ ions from high-affinity chelation. We verified this prediction using the colorimetric zinc-release assay, based on the 4-(2-pyridylazo)-resorcinol (PAR) indicator dye (10). The PKCα C1b zinc-finger domain was expressed as a fusion protein (designated maltose-binding protein [MBP]-PKCαC1b). The purified recombinant protein, containing two zinc equivalents, was subjected to peroxide-mediated oxidation. Incubation with <1 mM hydrogen peroxide produced the release of 100% of Zn2+ within 5 min at 37°C, relative to the total sulfhydryl-bound Zn2+ displaced by p-mercurimethyl-phenyl-sulfonate (PMPS), a potent sulfhydryl poison (14) (Fig. 1A).
FIG. 1.
Zinc release by oxidation and lipid second messengers. (A) The zinc finger of maltose-binding protein (MBP)–protein kinase C α (PKCα) C1b is redox sensitive. Purified MBP-PKCα C1b fusion protein was incubated with increasing doses of H2O2 for 5 min at 37°C. Zinc release was monitored colorimetrically. Oxidation caused the release of two molar equivalents of Zn2+. PMPS is a reagent that forms thiol-mercaptide bonds with cysteines, thereby releasing all bound zinc from cysteine-containing zinc centers. Incubation of the MBP-PKCα C1b fusion protein with PMPS resulted in the release of two molar equivalents of Zn2+, indicating that both zinc-coordination centers were intact in the starting material. (B) The zinc finger of MBP-PKCα C1b responds to phorbol ester binding by releasing zinc. Purified MBP-PKCα C1b fusion protein was incubated with increasing doses of phorbol-12-myristoyl-13-acetate (PMA) or phorbol-13-acetate (P13A) for 5 min at 37°C. Zinc release was monitored colorimetrically. PMA caused the release of one zinc equivalent from MBP-PKCα C1b at maximum dose (200 μg/ml), whereas the weak phorbol agonist, P13A, was ineffective at similar concentrations. (C) The zinc finger of MBP-PKCζ C1 does not respond to phorbol ester binding. Purified MBP-PKCζ C1 fusion protein, containing a PMA nonbinding zinc finger, was incubated with increasing doses of PMA for 5 min at 37°C. Zinc release was monitored colorimetrically (titration not shown). The fusion protein failed to yield free Zn2+ upon stimulation with 200 μg/ml PMA. PMPS released two zinc equivalents. (D) The zinc finger of MBP-PKCα C1b responds to di-acyl-glycerol (DAG) and phosphatidyl-serine (PS) binding by releasing zinc. Both DAG and PS, applied with phosphocholine-based lipid vesicles, caused substantial zinc release from MBP-PKCα C1b fusion protein. The combination of DAG and PS released one Zn2+ equivalent, comparable to the molar ratio freed by PMA (as shown in B). (E) The zinc finger of full-length PKCα responds to phorbol ester binding by releasing zinc. Purified full-length PKCα, expressed in insect cells, was incubated with increasing doses of PMA or P13 A for 5 min at 37°C. Zinc release was monitored colorimetrically. PKCα responded to stimulation with PMA, but not P13A, and released at 200 μg/ml PMA two of its four zinc equivalents. Vehicle control was negative. (F) Full-length PKCα was activated by phorbol ester. Purified full-length PKCα, expressed in insect cells, was incubated with 200 μg/ml PMA or P13 A for 5 min at 37°C. Kinase activity was determined by measuring phosphotransferase capacity with histone as substrate. Upon stimulation with PMA full-length PKCα gained 22-fold higher phosphotransferase activity, whereas P13A produced less than half of this activity (p > 0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The second messenger mimetic, phorbol-12-myristoyl-13-acetate (PMA), was reported to cause PKCα to shed half its zinc content in vivo (24). These findings raised the question whether lipid-binding resulted in loss of zinc coordination. The high-affinity binding site for lipid second messengers was mapped to the second δC1b zinc center (37). We hypothesized that long-chain fatty acids of agonists disturb the tetrahedral symmetry on which zinc-finger integrity depends, collapsing the ionic field effect. We tested this possibility with the zinc-release assay, using the MBP-PKCαC1b fusion protein. Incubation with graded doses of PMA at 25°C resulted in the specific release of half the bound Zn2+ in vitro. Phorbol-13-acetate (P13A), a PKC-agonist with much lower potency than PMA but equally high binding affinity to PKC (37), did not cause detectable Zn2+ release at similar conditions (Fig. 1B), suggesting that the myristoyl chain was instrumental for zinc release. We demonstrated selectivity of PMA by testing MBP-PKCζ fusion protein, not known to bind PMA (30), and did not observe any zinc release (Fig. 1C). Oxidation of MBP-PKCζ with peroxide, however, yielded maximal zinc release (data not shown).
PMA functions as pharmacomimetic, raising the question whether the natural second messengers, DAG and PS, also destabilized PKC zinc fingers (35). Being insoluble in water, both agonists were delivered with phosphatidylcholine (PC)-based lipid vesicles (34), and both caused significant zinc release from MBP-αC1b fusion protein, although to a lesser degree than PMA (Fig. 1D). Applied in combination, their effect was additive and commensurate with that elicited by PMA. Neither PC itself nor saturated fatty acids incorporated into PC produced zinc release (data not shown).
In their natural settings, zinc fingers might be stabilized by adjacent peptide domains. It was therefore important to test the responsiveness of intact PKC to lipid second messengers. Oxidation of PKC by hydrogen peroxide in vitro inactivates the enzyme (10) presumably because of nonpermissible modifications of cysteines in the catalytic domain and elsewhere. Therefore, zinc-release tests would not be informative. However, when purified, full-length αPKC (7) was treated with PMA at 25°C, it converted to an active enzyme, as demonstrated by phosphotransfer to histone substrate, and simultaneously released two of its four zinc equivalents (Fig. 1E, F). Densitometric analysis of the histone autoradiograph indicated that PMA produced a 20-fold increase in phosphotransferase activity. P13A caused less than half of the PKCα activation achieved with PMA, and again, like in the MBP-fusion protein, no measurable zinc release (Fig. 1E, F).
Together, these results indicate that potent agonists of PKC possess the ability to displace Zn2+ from the PKCα zinc-finger domain in vitro, whether expressed as fusion protein or contained in its natural state within the full-length molecule. The results support a mechanism whereby disassembly of the zinc center is associated with the conversion of PKC from the inactive to the active enzyme, in accord with the notion that PKC activation depends on large-scale protein unfolding (15). The effective dose of P13A is lower by an order of magnitude than that of PMA, and consistent with this the activation of PKCα was attenuated. It is unclear why no Zn2+ was released or why the PAR assay failed to detect this.
The PKC zinc finger is a functional ortholog of the bacterial Hsp33 zinc finger
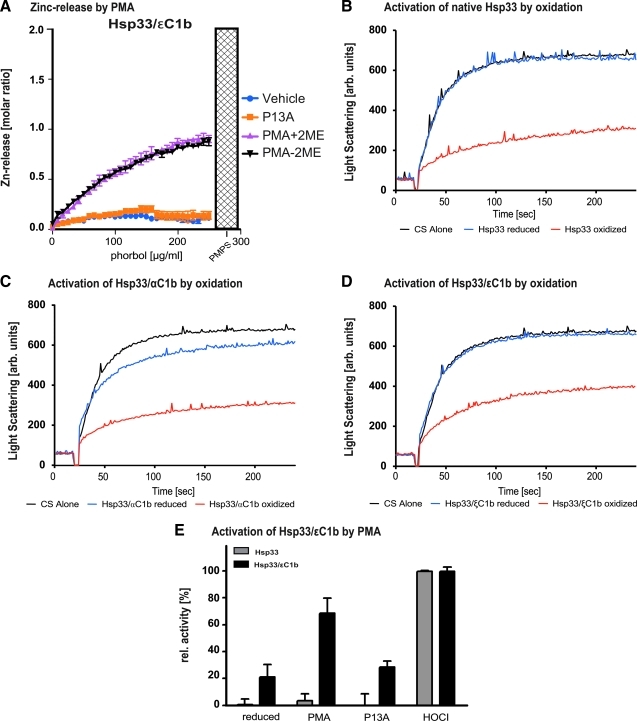
During in vivo activation, PKC undergoes a number of requisite phosphorylation events (20, 30, 33), including autophosphorylation, and translocates to diverse membrane sites (25). This complex process makes it difficult to assess to what extent the unfolding of the zinc-finger domain per se contributes to the activation process. To circumvent these potentially confounding factors, we constructed a reporter protein that allowed evaluation of the zinc-finger function unencumbered by other PKC domains. The bacterial Hsp33 chaperone is structurally completely unrelated, yet shares the prototypical, redox-sensitive zinc-finger structure with PKC (19). If the sole function of the Hsp33 zinc finger were to open on cue, enabling the protein to undergo the required rearrangements and dimerization, we reasoned that it should be possible to substitute the PKC zinc finger for the endogenous one without loss of redox sensitivity. Chimeric Hsp33/PKC C1b proteins were constructed, expressed in bacteria, and purified to homogeneity. A content of 2 molar Zn2+ equivalents indicated proper folding of zinc-coordination centers (Fig. 2A). To evaluate whether the functionality of the PKC zinc-finger domain was compromised by insertion into Hsp33, we applied the zinc release assays (14). We found that oxidation of the chimeric protein with H2O2 released 100% of its high-affinity bound Zn2+ (data not shown). Stimulation by PMA caused specific, half-maximal zinc release from the chimeric Hsp33/PKCα and Hsp33/PKCɛ proteins (Fig. 2A), comparable to the results of zinc release of recombinant MBP-PKCα C1b protein and full-length PKCα. The weak agonist, P13A, failed again to generate appreciable free Zn2+ (Fig. 2A). To guard against the possibility that reactive oxygen species (ROS) were generated during PMA stimulation, assays were performed in the presence of 5 mM β-mercapto-ethanol. Although it neutralized H2O2-mediated zinc release (data not shown), this reducing agent did not interfere with PMA freeing Zn2+ (Fig. 2A). These results demonstrate that the PKC zinc fingers retain their integrity despite their incorporation into the Hsp33 protein, but they do not tell us whether they retain their functionality as activation centers.
FIG. 2.
Chaperone activity of heat-shock protein-33 (Hsp33)-PKC chimeras is regulated by oxidation and by second messengers. (A) The Hsp33/ɛC1b reporter protein responds to PMA by half-maximal zinc release. Purified Hsp33/ɛC1b protein was titrated with PMA or P13A under the conditions described in (B). This resulted at maximum PMA concentration (200 μg/ml) in the release of two molar equivalents. PMPS yielded the expected four zinc equivalents. The PMA effect was not mediated by oxidation since the presence of β-mercaptoethanol did not diminish PMA effectiveness. P13 A did not cause zinc release. Chaperone activity of native Escherichia coli Hsp33 (B), the chimeric Hsp33/αC1b protein (C), or the chimeric Hsp33ɛC1b mutant protein (D). The influence of these proteins on the aggregation of chemically denatured citrate synthase (CS) at 30°C was tested. Light scattering of CS in the absence of any chaperones (black traces), in the presence of reduced, inactive chaperones (blue traces), or in the presence of oxidized chaperones, which were treated with 2 mM H2O2 at 43°C for 3 h (red traces), was monitored. (E) Effect of second messengers on the chaperone activity of Hsp33 and the chimeric Hsp33ɛC1b mutant protein. The reduced proteins were incubated in PMA, P13A, or HOCl for 3 h at 30°C. Then, the proteins were diluted 1:166-fold into the assay buffer and their effects on the aggregation of chemically denatured CS was determined as described. The residual concentrations of PMA, P13A or HOCl present in the assay buffer, did not have any significant effect on the light scattering signal of CS alone. Presence of PKCɛ C1b zinc finger conferred responsiveness to PMA at 30°C, but not to P13A. Native Hsp33 was not responsive to PMA. Both native and chimeric Hsp33 proteins were activated by hypochlorite (HOCl). Results presented are representative of at least three repeat experiments.
The purpose of the Hsp33 chaperone is to protect proteins against nonspecific aggregation specifically under oxidative stress conditions (16). Activation of Hsp33 requires oxidative zinc release from the zinc center, whose unfolding destabilizes an adjacent linker region, which, in turn, unfolds and exposes a hydrophobic surface, suggested to represent the high-affinity binding site for misfolded protein substrates (16). We evaluated the chaperone activity of the Hsp33 chimera by a light scattering assay based on the ability of activated Hsp33 to prevent the aggregation of chemically unfolded citrate synthase (CS) (6). Under reducing conditions, wild-type Hsp33 is unable to prevent the aggregation of denatured CS (Fig. 2B, compare black and blue trace). However, H2O2 (or HOCl)-activated Hsp33 prevents the aggregation of CS (Fig. 2B, red trace). Likewise, we found that the chimeric PKC/Hsp33 proteins (Fig. 2C, D) were inactive under reducing conditions, but responded to redox activation like the wild-type form (see also Fig. 2E). These results demonstrate that PKC's butterfly zinc finger serves as a redox-sensitive, autonomous folding unit, which can readily substitute for Hsp33's zinc finger.
If zinc-finger disassembly were the key initiator of linker region destabilization and large-scale Hsp33 remodeling (12), we would also expect PMA to accomplish chaperone activation. Indeed PMA efficiently activated the chimeric chaperones but not native Hsp33, yielding a threefold increase over background (Fig. 2E). The weak agonist P13A produced only marginal chaperone activation. In summary, our results show that the bacterial and mammalian zinc-finger modules are interchangeable. Considering that zinc is released during PMA activation without oxidation of the cysteines (Fig. 2A), our results strongly imply that the disassembly of the zinc-coordination centers is an early step in a cascade of events leading to and possibly preceding full activation of the chaperone. Neither the origin of the zinc-finger module nor the mode of triggering, whether by oxidation or by physical means, appears to change this outcome.
The PKC zinc finger changes its conformation upon lipid agonist binding
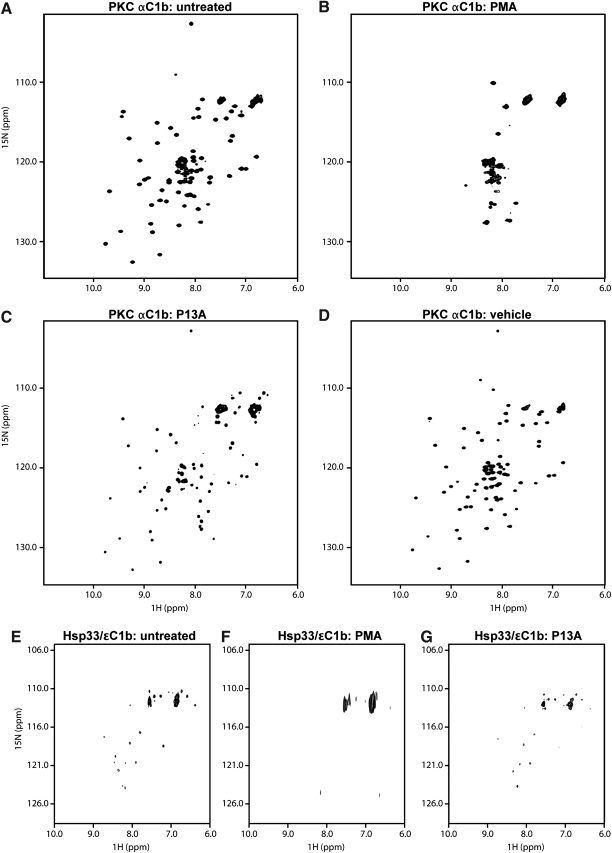
As zinc release and conversion to active chaperone implied extensive structural reorganization, we searched for direct evidence for disassembly of the zinc-finger fold. To observe conformation changes by nuclear magnetic resonance (NMR), we expressed 15N-enriched recombinant protein and recorded HSQC spectra before and after exposure to PMA. The 15N-1H HSQC spectrum of the uniformly 15N-labeled PKCα C1b revealed a well-structured domain in solution, identical with that of the published NMR structure (Fig. 3A) (12). Exposure to PMA produced a conformer that lacked dispersion, indicating loss of tertiary structure (Fig. 3B). Changes in some peak positions upon P13A exposure indicated that this lipid bound PKCα C1b, without causing loss of structure (Fig. 3C). Exposure to vehicle alone (dimethyl sulfoxide [DMSO]) did not change the HSQC spectrum (Fig. 3D).
FIG. 3.
Zinc release and chaperone activation are associated with structural changes in the zinc-finger fold. (A-D) The zinc-finger folds of PKCα C1b loose their structure upon exposure to phorbol ester. (A) The HSQC spectrum of [U-15N] PKCαC1b (250 μM) shows a well-structured fold comparable to that of published spectra [14]. (B) After stimulation with PMA (protein:PMA ratio = 1:2.6), some precipitation occurred. However, after removal of aggregates the spectrum of the supernatant showed a disordered structure. (C) Treatment with P13A (1:2.6 molar ratio) caused only minor shifts. (D) Treatment with vehicle (dimethyl sulfoxide) caused no discernible shifts. (E) The chimeric Hsp33/ɛC1b protein was uniformly labeled with [15N]L-cysteine. The HSQC spectrum of the untreated protein (100 μM) revealed signals from six cysteine residues, and additional lower intensity signals arising from scrambling of the 15N label. (F) PMA treatment (protein:PMA, 1:1.3) resulted in some precipitation, as in (B). The HSQC spectrum of the supernatant (∼80 μM) showed broadening of signals suggesting loss of structure. (G) The weak phorbol agonist, P13A, while eliciting shifts of some cysteine residues (shown at 1:2.6 molar ratio), possibly due to secondary rearrangement in Hsp33, retained the six core cysteines in the fixed positions, indicating a structurally intact zinc finger.
To evaluate the effect of PMA on the chimeric Hsp33/PKCɛ C1b protein, we used 15N-cysteine labeling that allowed selective monitoring of zinc coordination while greatly reducing spectral complexity. Because activated Hsp33 naturally is a homodimer, we eliminated the critical dimerization site E150 via mutation to arginine (11), thus minimizing interpretation errors arising from self-association of proteins. Like our observations with the PKCα C1b domain, exposure to PMA caused unraveling of the zinc-coordination site in the chimeric Hsp33 PKCɛ C1b protein (compare Fig. 3E with 3F), whereas exposure to P13A (Fig. 3G), or vehicle alone (not shown), did not cause significant changes in the HSQC spectra. Thus, our results indicate that interaction with PMA or with the natural activator of PKC, DAG, leads to the loss of tertiary structure in the zinc-finger motif of the PKC C1b domain.
Redox activation of PKCδ in mitochondria involves targeted oxidation of its zinc finger by cytochrome c
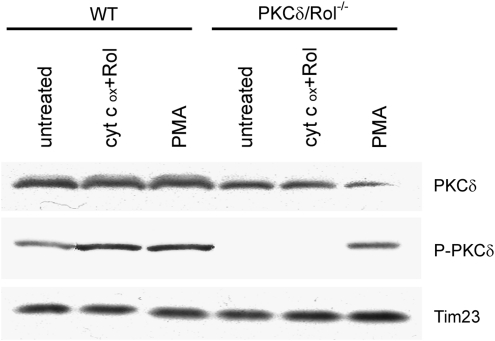
The observations that lipid second messengers and oxidation impinged on the zinc-finger domain and caused their disassembly underscored the importance of the hinge-like function for PKC activation. It is structurally and functionally well established that zinc fingers represent the activation domains where DAG and phorbol-ester bind (31). However, are these same zinc fingers the key targets for redox activation as well? If so, what is the source of oxidizing agents, and what is the molecular mechanism? It is often assumed that ROS are responsible for oxidation, but ROS lack the ability for site-specific reactions, any cysteine residue in PKC potentially offering a target. However, for the same reasons that kinases and phosphatases perform site-specific, “hand-shake”-like transactions (28) we postulated oxidation by an oxido-reductase, instead of free ROS. We recently described the first example of such site-specific redox activation of PKCδ in mitochondria (1). Cytochrome c was the oxidizing agent, but was only effective when vitamin A was present. This essential coactivator was long known to bind tightly to PKC zinc fingers, although the purpose of this association was unclear (13, 18). To test the hypothesis that redox activation involves electron transfer from the zinc finger to cytochrome c, a process in which vitamin A functions as electron carrier, we genetically ablated the retinol-binding pockets on PKCδ (2). The mutated PKCδ was no longer activated by oxidized cytochrome c (Fig. 4). Besides illuminating a new role of vitamin A in signal transduction, in the context of the present study the results strongly implicate the zinc-finger domains as the mediators of redox activation. In view of definitive structural changes observed in NMR experiments and others deduced from zinc-release studies, we propose that the dynamics of zinc fingers are important for the regulation of PKC activity.
FIG. 4.
Redox activation of PKCδ by cytochrome c in mitochondria implicates the zinc-finger domain. Embryonal fibroblasts (MEF) of wild-type mice or mice with germline deletion of the PKCδ gene were used. The latter were reconstituted with an engineered mutant form of PKCδ that does not bind vitamin A (retinol, Rol) in its zinc-finger domains (PKCδ/Rol−/− MEFs), and as the result of this defect has lost the ability to become redox activated by cytochrome c3+ in mitochondria (1,2). Mitoplasts of the WT and PKCδ/Rol-/- MEFs, prepared as described in Experimental Procedures section, were incubated with 20 mM oxidized horse heart cytochrome c plus 2 μM retinol for 10 min. Mitoplasts were analyzed by Western blot for the presence of PKCδ protein and the activated species of PKCδ using a phospho-Thr505-specific antibody as indicator (23). TIM23 was used as loading control. While PKCδ of WT genotype showed increased phosphorylation, the vitamin A-nonbinding mutant form remained unphosphorylated. Both responded to PMA by undergoing phosphorylation, indicating functional integrity, despite ablation of the vitamin A-binding site in the mutant form. These results illuminate the importance of PKC zinc-finger domains as targets of redox activation. A detailed analysis of activation of PKCδ in mitochondria was recently published (1).
Concluding Remarks and Outlook
Zinc-finger domains occur in hundreds of mammalian proteins, many of these conserved from distant evolutionary time. The question arises whether interconversion of well-ordered rigid zinc-finger domains to unstructured folds by redox mechanisms or, as now demonstrated with our reporter protein, by specific lipid binding, is more common than hitherto appreciated. Transcription factors bind DNA by deploying zinc fingers; do they not eventually need to come off their promoters? Ring fingers are thought to mediate protein–protein recognition; does this process not require regulation by alternately presenting and concealing recognition faces? Topoisomerases ratchet hand-over-hand along DNA using strings of zinc fingers; does this not imply cyclical opening and closing of zinc fingers? Future work will tell how many more zinc fingers serve as dynamic elements, and what additional means beyond redox mechanisms and lipid interactions exist to trigger conformation change of these domains.
Experimental Procedures
Chemicals and biological reagents
PAR, PMA, and P13A were purchased from Sigma (St. Louis, MO). PC and PS were from Avanti Polar Lipids (Alabaster, AL). PMPS was synthesized according to Nesmejanow (29).
Plasmids and recombinant proteins
A vector encoding the C1b domain of rat PKCα (HKFKIHTYGSPTTFCDHCGSLLYGLIHQGMKCDTCDMNVHKQCVINVPSLC) fused to MBP was kindly provided by U. Hommel (12). The PKCɛ C1b domain was fused to MBP in analogous fashion. The proteins were expressed in Escherichia coli and purified by affinity chromatography on maltose agarose according to the manufacturer's instructions (New England Biosciences, Beverley, MA). The purity, as determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, exceeded 95%. The proteins contained two equivalents of Zn2+ (see below), indicating complete folding. The NMR HSQC spectrum of the cleaved αC1b domain matched published data (12). The fusion proteins were stored with reducing agent at 4°C. Hsp33 protein was purified as described (19). The chimeric chaperones were generated by excising the endogenous zinc finger (residues 232–268, retaining the C-terminal cysteine) and inserting the murine PKC αC1b domain (residues as above, preceded by the linker amino acids, MNP) or the PKCɛ C1b domain (KFGIHNYKVPTFCDHCGSLLWGLLRQGLQCKVCKMNVHRRCETNVAPNC), preceded by the linker amino acids, NMPC. The proteins were purified from E. coli lysates by ion exchange chromatography on MonoQ agarose, yielding 95% purity and two zinc equivalents, indicating proper folding.
Zinc-release assay
Test proteins were purged of reducing agent by gel filtration and diluted to 5 μM in 50 μM Tris/HCl buffer (pH 7.5) (14). PAR was added at a final concentration of 100 μM. To determine the content of chelated Zn2+, p-mercurimethyl-phenyl-sulfonate (PMPS) was added in small increments until saturation was achieved. The PAR-Zn2+ complex was measured colorimetrically at λmax 500 nm. A Zn2+ standard curve was used to convert OD units to molarity. Data are presented as molar ratios of Zn2+ to protein.
One hundred twenty microliters of test protein (5 μM) with indicator dye was titrated at room temperature with hydrogen peroxide in water, phorbol-12-myristoyl-13-acetate (PMA), or P13A, both dissolved in DMSO. When saturation was reached, PMPS was added to release residual high-affinity bound Zn2+. All titrations were performed in triplicates. Means and standard deviations of the mean were calculated using the Student's t-test. Because DAG and PS are insoluble in water, they were supplied as lipid vesicles generated with PC as base, as described by Sando (34). Note that because of their lipid nature the amounts used are given in mg/ml. They correspond to apparent concentrations, not actual molarities.
Measurements of chaperone activity
Purified native Hsp33 and the chimeras were reduced and zinc reconstituted as described (6). About 50 μM of the reduced proteins was incubated with 2 mM H2O2, 15 μl DMSO, 240 μM PMA, or 240 μM P13A (both dissolved in DMSO) for one or 3 h at 30°C. To determine the activity of the proteins, aliquots were removed and the influence of 0.3 μM Hsp33 or the chimera on the aggregation of 0.075 μM chemically unfolded CS (Sigma) was analyzed at 30°C as described (17).
NMR spectroscopy
Uniformly 15N-labeled proteins were expressed in E. coli using M9 medium with 15NH4Cl as the sole nitrogen source. Selectively15N-cysteine labeled proteins were expressed in Luria broth medium supplemented with 0.1g/l 15N-cysteine according to the method of Englander et al. (8). For NMR experiments, PKCα C1b was expressed as a fusion protein with a N-terminal his-MBP tag (3) and cleaved from the affinity tag using thrombin. NMR experiments were performed at 0.8 mM and 0.1 mM concentrations of PKCα C1b and Hsp33/PKCɛ C1b, respectively, in 50 mM Tris (pH 7.5) buffer containing 100 mM NaCl, 10 mM β-mercaptoethanol, and 20 mM ZnSO4 at 25°C. All experiments were performed on Bruker 700 or 800 MHz spectrometers equipped with cryoprobes.
For titrations, stock solutions of both PMA and P13A were made in DMSO at a concentration of 10 mg/ml. These were added to protein samples in 1 μl increments with mixing at each step.
Mitoplast preparation for cytochrome c import
Mitoplast for cytochrome c import were prepared from embryonal fibroblasts (MEFs) cells that were cultured for 18h in serum-free transferrin, linoleic acid, bovine serum albumin medium (1). Mitochondria were isolated by homogenization of the cell pellets in buffer A (0.32 M sucrose, 1 mM EDTA, and 10 mM Tris-HCl, pH 7.4) followed by centrifugation at 1000g for 5 min. Supernatant containing mitochondria was then centrifuged at 20,000g for 10 min, and mitochondria were resuspended at 1 mg/ml in MAITE buffer (10 mM Tris-HCl, pH 7.4; 25 mM sucrose; 75 mM sorbitol; 100 mM KCl; 10 mM K2HPO4; 0.05 mM EDTA; 5 mM MgCl2; 1 mg/ml BSA). Digitonin (1 mg digitonin/5 mg mitochondrial protein) was added, and the mixture incubated on ice for 45 min. Then, the combination of 20 mM cytochrome c plus 2 μM retinol was added and the mitoplasts were incubated at 37°C for 10 min. Pyruvate+malate-driven respiration was measured to assure functionality (data not shown) using 50 μg of the mitoplast preparation, as described (1).
Immunoblot analysis
To determine the phosphorylation levels of PKCδ in mitoplast, 50 μg of protein was separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electroblotted onto polyvinylidene fluoride filters (BioRad, Hercules, CA) and immunoblotted with the appropriate antibodies.
Abbreviations Used
- CS
citrate synthase
- DAG
diacyl glycerol
- DMSO
dimethyl sulfoxide
- Hsp33
heat-shock protein-33
- MBP
maltose-binding protein
- NMR
nuclear magnetic resonance
- P13A
phorbol-13-diacetate
- PAR
4-(2-pyridylazo)-resorcinol
- PC
phosphatidyl choline
- PKC
protein kinase C
- PMA
phorbol-12-myristoyl-13-acetate
- PMPS
p-mercurimethyl-phenyl-sulfonate
- PS
phosphatidyl-serine
- ROS
reactive oxygen species
Acknowledgments
The authors thank Dr. Ulrich Hommel for the generous gift of the expression vector encoding the PKC delta C1b maltose-binding protein fusion protein. The baculovirus expression vector for full-length PKAα was kindly donated by Drs. Ushio Kikkawa and Yoshitaka Ono. This work was supported by National Institutes of Health grants CA 089362 and DK 069348 (to U.H.) and GM065318 (to U.J.).
References
- 1.Acin-Perez R. Hoyos B. Gong J. Vinogradov V. Fischman DA. Leitges M. Borhan B. Starkov A. Manfredi G. Hammerling U. Regulation of intermediary metabolism by the PKCδ signalosome in mitochondria. FASEB J. 2010;24:5033–5042. doi: 10.1096/fj.10-166934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acin-Perez R. Hoyos B. Zhao F. Vinogradov V. Fischman DA. Harris RA. Leitges M. Wongsiriroy N. Blaner WS. Manfredi G. Hammerling U. Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis. FASEB J. 2009;24:627–632. doi: 10.1096/fj.09-142281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandrov A. Dutta K. Pascal SM. MBP fusion protein with a viral protease cleavage site: one-step cleavage/purification of insoluble proteins. Biotechniques. 2001;30:1194–1198. doi: 10.2144/01306bm01. [DOI] [PubMed] [Google Scholar]
- 4.Berg JM. Zinc finger domains—hypotheses and current knowledge. Ann Rev Biophys Biophys Chem. 1990;19:405–421. doi: 10.1146/annurev.bb.19.060190.002201. [DOI] [PubMed] [Google Scholar]
- 5.Bish RA. Myers MP. Werner helicase-interacting protein 1 binds polyubiquitin via its zinc finger domain. J Biol Chem. 2007;282:23184–23193. doi: 10.1074/jbc.M701042200. [DOI] [PubMed] [Google Scholar]
- 6.Buchner J. Grallert H. Jakob U. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 1998;290:323–338. doi: 10.1016/s0076-6879(98)90029-5. [DOI] [PubMed] [Google Scholar]
- 7.Burns DJ. Bloomenthal J. Lee MH. Bell RM. Expression of the alpha, beta II, and gamma protein kinase C isozymes in the baculovirus-insect cell expression system. Purification and characterization of the individual isoforms. J Biol Chem. 1990;265:12044–12051. [PubMed] [Google Scholar]
- 8.Englander J. Cohen L. Arshava B. Estephan R. Becker JM. Naider F. Selective labeling of a membrane peptide with 15N-amino acids using cells grown in rich medium. Biopolymers. 2006;84:508–518. doi: 10.1002/bip.20546. [DOI] [PubMed] [Google Scholar]
- 9.Gamsjaeger R. Liew CK. Loughlin FE. Crossley M. Mackay JP. Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem Sci. 2007;32:63–70. doi: 10.1016/j.tibs.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishna R. Anderson WB. Reversible oxidative activation and inactivation of protein kinase C by the mitogen/tumor promoter periodate. Arch Biochem Biophys. 1991;285:382–387. doi: 10.1016/0003-9861(91)90377-u. [DOI] [PubMed] [Google Scholar]
- 11.Graf PCF. Martinez-Yamoout M. Van Haerents S. Lilie H. Dyson HJ. Jakob U. Activation of the redox-regulated chaperone Hsp33 by domain unfolding. J Biol Chem. 2004;279:20529–20538. doi: 10.1074/jbc.M401764200. [DOI] [PubMed] [Google Scholar]
- 12.Hommel U. Zurini M. Luyten M. Solution structure of a cysteine rich domain of rat protein kinase C. Nat Struct Biol. 1994;1:383–388. doi: 10.1038/nsb0694-383. [DOI] [PubMed] [Google Scholar]
- 13.Hoyos B. Imam A. Chua R. Swenson C. Tong G-X. Levi E. Noy N. Hammerling U. The cysteine-rich regions of the regulatory domains of Raf and protein kinase C as retinoid receptors. J Exp Med. 2000;192:835–845. doi: 10.1084/jem.192.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt JB. Neece SH. Schachman HK. Ginsburg A. Mercurial promoted Zn2+ release from Escherichia coli aspartate transcarbamoylase. J Biol Chem. 1984;259:14793–14803. [PubMed] [Google Scholar]
- 15.Hurley JH. Grobler JA. Protein kinase C and phospholipase C: bilayer interactions and regulation. Curr Opin Struct Biol. 1997;7:557–565. doi: 10.1016/s0959-440x(97)80122-4. [DOI] [PubMed] [Google Scholar]
- 16.Ilbert M. Graf PCF. Jakob U. Zinc center as redox switch—new function for an old motif. Antioxid Redox Signal. 2006;8:835–846. doi: 10.1089/ars.2006.8.835. [DOI] [PubMed] [Google Scholar]
- 17.Ilbert M. Horst J. Ahrens S. Winter J. Graf PCF. Lilie H. Jakob U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat Struct Mol Biol. 2007;14:556–563. doi: 10.1038/nsmb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imam A. Hoyos B. Swenson C. Chua R. Levi E. Viriya E. Hammerling U. Retinoids as ligands and coactivators of protein kinase C alpha. FASEB J. 2001;15:28–30. doi: 10.1096/fj.00-0329fje. [DOI] [PubMed] [Google Scholar]
- 19.Jakob U. Muse W. Eser M. Bardwell JC. Chaperone activity with a redox switch. Cell. 1999;96:341–352. doi: 10.1016/s0092-8674(00)80547-4. [DOI] [PubMed] [Google Scholar]
- 20.Keranen LM. Dutil EM. Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- 21.Knapp LT. Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275:24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 22.Konishi H. Tanaka M. Takemura Y. Matsuzaki H. Ono Y. Kikkawa U. Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi H. Yamauchi E. Taniguchi H. Yamamoto T. Matsuzaki H. Takemura Y. Ohmae K. Kikkawa U. Nishizuka Y. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci U S A. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korichneva I. Hoyos B. Chua R. Levi E. Hammerling U. Zinc-release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277:44327–44331. doi: 10.1074/jbc.M205634200. [DOI] [PubMed] [Google Scholar]
- 25.Kraft AS. Anderson WB. Cooper HL. Sando JJ. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982;257:13193–13196. [PubMed] [Google Scholar]
- 26.Krishna SS. Majumdar I. Grishin NV. Structural classification of zinc fingers. Nucleic Acids Res. 2003;31:532–550. doi: 10.1093/nar/gkg161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J. Lachlan AD. Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985;4:1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesmejanow AN. Gluschnew NT. Epifansky PT. Aus dem Gebiet der organischen Quecksilber-Verbindungen. Chem Ber. 1933;67:130–134. [Google Scholar]
- 30.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 31.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 32.Ono Y. Fujii T. Igarashi K. Kuno T. Tanaka C. Kikkawa U. Nishizuka Y. Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci U S A. 1989;86:4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parekh DB. Ziegler W. Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sando JJ. Complexities in protein kinase C activity assays. Methods Mol Biol. 2003;233:45–61. doi: 10.1385/1-59259-397-6:45. [DOI] [PubMed] [Google Scholar]
- 35.Takai Y. Kishimoto A. Iwasa Y. Kawahara Y. Mori T. Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979;254:3692–3695. [PubMed] [Google Scholar]
- 36.Wedemeyer WJ. Welker E. Narayan M. Scheraga HA. Disulfide bonds and protein folding. Biochemistry. 2000;39:4207–4216. doi: 10.1021/bi992922o. [DOI] [PubMed] [Google Scholar]
- 37.Zhang G. Kazanietz MG. Blumberg PM. Hurley JH. Crystal structure of the Cys2 activator-binding domain of protein kinase C8 in complex with phorbol ester. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]