Abstract
Familial hypercholesterolemia (FH) is an autosomal codominant disease characterized by high concentrations of proatherogenic lipoproteins and premature atherosclerosis secondary to low-density lipoprotein (LDL) receptor deficiency. We examined a novel cell therapy strategy for the treatment of FH in the Watanabe heritable hyperlipidemic (WHHL) rabbit, an animal model for homozygous FH. We delivered human adipose tissue-derived multilineage progenitor cells (hADMPCs) via portal vein and followed by immunosuppressive regimen to avoid xenogenic rejection. Transplantation of hADMPCs resulted in significant reductions in total cholesterol, and the reductions were observed within 4 weeks and maintained for 12 weeks. 125I-LDL turnover study showed that the rate of LDL clearance was significantly higher in the WHHL rabbits with transplanted hADMPCs than those without transplanted. After transplantation hADMPCs were localized in the portal triad, subsequently integrated into the hepatic parenchyma. The integrated cells expressed human albumin, human alpha-1-antitrypsin, human Factor IX, human LDL receptors, and human bile salt export pump, indicating that the transplanted hADMPCs resided, survived, and showed hepatocytic differentiation in vivo and lowered serum cholesterol in the WHHL rabbits. These results suggested that hADMPC transplantation could correct the metabolic defects and be a novel therapy for inherited liver diseases.
Introduction
Familial hypercholesterolemia (FH) is characterized by premature and accelerated development of atherosclerotic lesions caused by elevated levels of cholesterol-rich lipoproteins in plasma. The disease is caused by mutations in the low-density lipoprotein (LDL) receptor gene that result in a significant decrease in receptor-mediated uptake of lipoproteins from the circulation.1–3 Patients homozygous for defects in LDL receptors have serum cholesterol levels 5–10 times those of normal and suffer as early as the first two decades of life from complications such as coronary artery disease.4,5 In homozygous FH patients, conventional drug therapy cannot treat the condition, and therapeutic recourses are limited to chronic plasmapheresis or orthotopic liver transplantation.1 Although liver transplants lower LDL levels, the procedure is life threatening; in addition, donor livers are in short supply. Cellular transplantation has been proposed to provide functional LDL receptors for the treatment of hypercholesterolemia. Transplantation of allogenic and xenogenic hepatocytes has been shown to be effective in lowering serum cholesterol in the Watanabe heritable hyperlipidemic (WHHL) rabbit,6–9 which is an animal model for homozygous FH. Further, a number of gene therapy approaches have shown some promises in animal models and human,10–13 and the therapies will cure a number of patients with FH in near future. As an alternative to whole-organ transplantation and/or gene therapy, we have investigated the ability of human adipose tissue-derived multilineage progenitor cells (hADMPCs) to differentiate into hepatocytes in vitro and to replace critical liver functions14 as well as previous reports,15,16 because the in vitro differentiation of hADMPCs into various kinds of cell types in now well reported and hADMPCs can be easily and safely obtained in large quantities without serious ethics issues.17,18 In this study, we are investigating whether hADMPCs could differentiate into hepatocytes in vivo and replace critical liver functions as considerable therapeutic potential for cellular replacement.
Materials and Methods
Cells
hADMPCs were prepared as described previously19 with some modifications.14,17,18 Adipose tissues from human subjects were resected during plastic surgery in five subjects (four males and one female, age, 20–60 years) as excess discards. Ten to 50 g of subcutaneous adipose tissue was collected from each subject. All subjects provided informed consent. The protocol was approved by the Review Board for Human Research of Kobe University Graduate School of Medicine, Osaka University Graduate School of Medicine, and Foundation for Biomedical Research and Innovation. After five to six passages, the hADMPCs were used for transplantation. Human cryopreserved hepatocytes were purchased from Invitrogen (Lot number: HuP81) and cultured as indicated by the manufacturer's protocol. Human adipose tissue-derived fibroblastic cells were obtained according to previous report.20
Flow cytometric analysis
hADMPCs isolated from adipose tissue were characterized by flow cytometry. Cells were detached from culture dishes by 0.25% trypsin/ethylenediaminetetraacetic acid (EDTA) and suspended in Dulbecco's phosphate-buffered saline (DPBS; Nacalai Tesque) containing 0.1% fetal bovine serum. Aliquots (5 × 105 cells) were incubated for 30 min at 4°C with fluorescein isothiocyanate-conjugated mouse monoclonal antibodies to human CD31 (BD PharMingen), CD105 (Ancell Corporation), CD133 (R&D Systems), phycoerythrin-conjugated mouse monoclonal antibodies to human CD29, CD34, CD45, CD73 (BD PharMingen), CD44, or CD166 (Ancell). Isotype-identical antibodies served as controls. Further, the cells were incubated with mouse monoclonal antibodies against human stage-specific embryonic antigen-4 (from Chemicon International, Inc.), ABCG-2, or CD117 (BD PharMingen) with nonspecific mouse antibody used as a negative control. After washing with DPBS, cells were incubated with phycoerythrin-labeled goat anti-mouse Ig antibody (BD PharMingen) for 30 min at 4°C. After three washes, cells were resuspended in DPBS and analyzed by flow cytometry using a FACSCalibur flow cytometer and CellQuest Pro software (BD Biosciences).
Adipogenic, osteogenic, and chondrogenic differentiation procedure
For adipogenic differentiation, cells were cultured in the differentiation medium (Zen-Bio, Inc.). After 3 days, half of the medium was changed with adipocyte medium (Zen-Bio) every 2 days. Five days after differentiation, adipocytes were characterized by microscopic observation of intracellular lipid droplets by Oil Red O staining. Osteogenic differentiation was induced by culturing the cells in Dulbecco's modified Eagle's medium containing 10 nM dexamethasone, 50 mg/dL ascorbic acid 2-phosphate, 10 mM β-glycerophosphate (Sigma), and 10% fetal bovine serum. Differentiation was examined by Alizarin red staining. For Alizarin red staining, the cells were washed three times and fixed with dehydrated ethanol. After fixation, the cells were stained with 1% Alizarin red S in 0.1% NH4OH (pH 6.5) for 5 min and then washed with H2O. For chondrogenic differentiation, hADMPCs were first trypsinized and 2 × 105 cells were centrifuged at 400 g for 10 min. The resulting pellets were cultured in the chondrogenic medium (alpha-minimum essential medium (alpha-MEM) supplemented with 10 ng/mL transforming growth factor-β, 10 nM dexamethasone, 100 μM ascorbate, and 10 μL/mL 100 × ITS Solution) for 14 days. For Alcian Blue staining, nuclear counter-staining with Weigert's hematoxylin was followed by 0.5% Alcian Blue 8GX for proteoglycan-rich cartilage matrix.
hADMPC transplantation and immunosuppression regimen
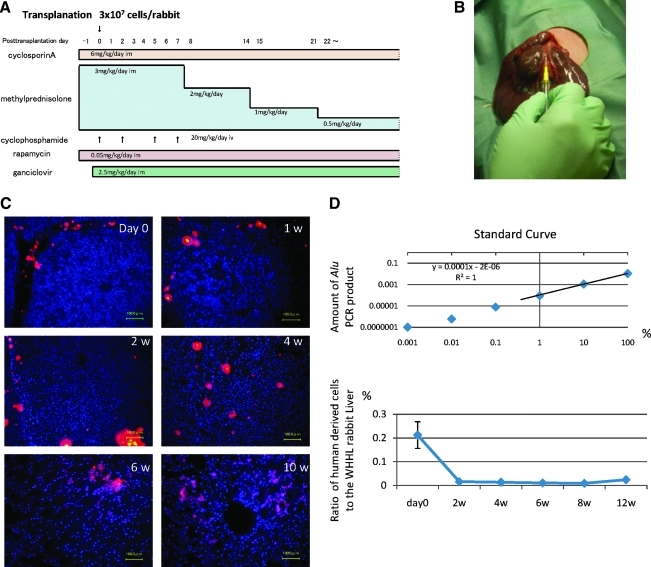
WHHL rabbits (8 weeks old; purchased from Kitayamalabes, Inc.) were anesthetized with pentobarbital (50 mg/kg). An incision distal and parallel to the lower end of the ribcage was made. The peritoneum was incised, and hADMPCs (n = 5) or human adipose tissue-derived fibroblastic cells (n = 3) (3 × 107 cells) suspended in 3 mL of Hanks' balanced salt solutions (HBSS) (20°C) or 3 mL of control saline (n = 6) were infused in 5 min into the portal vein via a 18-gauge Angiocath™ (BD). The immunosuppression regimen (Fig. 1A) consisted of the following: (1) intramuscular injection of cyclosporin A (6 mg/kg/day) daily from the day before surgery to sacrifice; (2) intramuscular injection of rapamycin (0.05 mg/kg/day) daily from the day before surgery to sacrifice; (3) methylprednisolone at 3 mg/kg/day (days 1–7), followed by tapering to 2 mg/kg/day (days 8–14), 1 mg/kg/day (days 15–21) and 0.5 mg/kg/day (day 22 to the time at sacrifice); (4) intravenous injection of cyclophosphamide (20 mg/kg/day) at days 0, 2, 5, and 7; (5) ganciclovir (2.5 mg/kg/day intramuscular injection (i.m.)) was also administrated to avoid viral infection in the immunocompromised host.
FIG. 1.
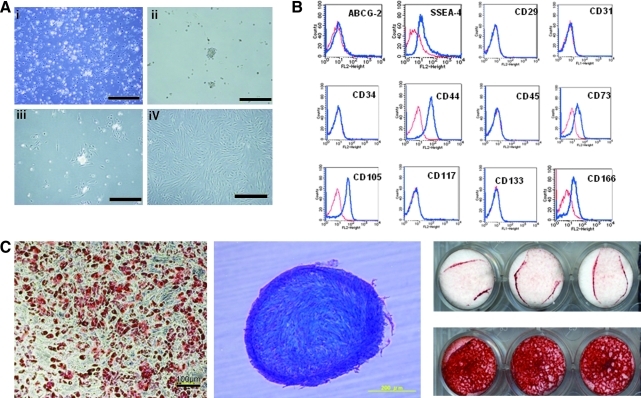
(A) Morphological characters of human adipose tissue-derived multilineage progenitor cells (hADMPCs). The cells obtained from adipose tissue were seeded and incubated for 24 h (i). After incubation, the adherent cells were treated with ethylenediaminetetraacetic acid solution, and the resulting suspended cells were replated at a density of 10,000 cells/cm2 on human fibronectin-coated dishes (BD BioCoat) (ii, iii). Within two to three passages after the initial plating of the primary culture, hADMPCs appeared as a monolayer of large flat cells (25–30 μm in diameter). As the cells approached confluence, they assumed a more spindle-shaped, fibroblastic morphology (iv). i) Bar = 499 μm, ii) bar = 201 μm, iii) bar = 502 μm and iv) bar = 202 μm. (B) Cell surface markers expressed on hADMPCs. The cells were negative for markers of the hematopoietic lineage (CD45) and of hematopoietic stem cells, ABCG-2, CD34, and CD133. They were also negative for CD31, an endothelial cell-associated marker, and the surface antigen c-Kit (CD117). However, they stained positively for a number of surface markers characteristic of mesenchymal and/or neural stem cells, but not embryonic stem (ES) cells, including CD29, CD44 (hyaluronan receptor), CD73, CD105 (endoglin), and CD166. hADMPCs also were positive for stage-specific embryonic antigen (SSEA)-4. (C) Adipocytic, chondrocytic, and osteocytic differentiation potentials of hADMPCs. Adipocytic differentiation potential of hADMPCs was confirmed by Oil Red O staining (the left panel) (bar = 100 μm). Chondrocytic differentiation potential of hADMPCs was estimated by extracellular matrices with Alcian Blue staining (the middle panel). Osteogenic differentiation potential of hADMPCs was confirmed by Alizarin red S staining for mineralized nodules (the right panel).
DNA extraction and quantification of human-derived cells
Total DNA of WHHL rabbit liver, which was obtained at the time just after hADMPC transplantation, and 2, 4, 8, and 12 weeks after transplantation, were isolated using a NucleoSpin Tissue kit (Macherey-Nagel) according to the manufacturer's instructions. hADMPCs and rabbit hepatocytes were mixed at the ratios of 100:0 (100%), 10:90 (10%), 1:99 (1%), 0.1:99.9 (0.1%), 0.01:99.99 (0.01%), and 0.001:99.999 (0.001%), and DNA was isolated. Seven hundred nanograms of each samples of extracted DNA was quantified by real-time polymerase chain reaction (PCR) using the ABI Prism 7900 Sequence Detection System (Applied Biosystems), primers for the 82 bp Alu amplicon (forward, 5′-GTCAGGAGATCGAGACCATCCC; reverse, 5′-CCACTACGCCCGGCTAATTT), and SYBR Green (TOYOBO) dye using a previously published protocol.21,22 Reactions were performed in quadruplicate and the Alu levels were calculated by the standard curve.
Assay for lipid profiling
Serum samples were obtained from nonfasting rabbits before and after transplantation. Serum total cholesterol was measured in each sample using assay kits from Wako Pure Chemical Industries. Serum lipoproteins were analyzed by an on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides by high-performance liquid chromatography at Skylight Biotech, according to the procedure as described.23
Immunohistochemical staining of WHHL rabbit liver sections
The WHHL livers were harvested and fixed immediately with 10% formalin. They were placed into optimal cutting temperature compound (Sakura Finetechnical Co.), frozen immediately, and then sectioned at 7 μm thickness. The sections were then incubated with blocking solution (Blocking one; Nacalai Tesque) for 1 h. The samples were incubated with rabbit anti-human-specific albumin antibody (MBL), rabbit anti-human-specific alpha 1 anti-trypsin antibody, and rabbit anti-LDL receptor antibody, followed by Alexa Fluor 488-labeled goat anti-rabbit IgG (Molecular Probes). To show the colocalization of human CD90 and albumin, the samples were incubated with the rabbit anti-human CD90 monoclonal antibody (Epitomics, Inc.) and then with Alexa Fluor 488-labeled goat anti-rabbit IgG (Molecular Probes), and washed extensively. Then, the specimens were incubated with rabbit anti-human-specific albumin antibody (MBL), followed by Alexa Fluor 546-labeled goat anti-rabbit IgG (Molecular Probes). The treated sample was examined with a BioZero laser scanning microscope (Keyence).
PCR analysis of WHHL rabbit liver for human liver-specific genes
Total RNAs of WHHL rabbit liver, hADMPCs, and human hepatocytes were isolated using an RNAeasy kit (Qiagen). After treatment with DNase, the cDNA was synthesized using Superscript III RNase H-minus Reverse Transcriptase (Invitrogen). Real-time PCR was performed using the ABI Prism 7900 Sequence Detection System (Applied Biosystems). About 20 × Assays-on-Demand™ Gene Expression Assay Mix for human alpha-1-antitrypsin (Hs01097800_m1), human albumin (Hs00609411_m1), human factor 9, human GATA-binding protein 4 (GATA4) (Hs00171403_m1), human hepatocyte nuclear factor 3 beta (Hs00232764_m1), human LDL receptor (Hs00181192_m1), and human glyceraldehyde-3-phosphate dehydrogenase (Hs99999905_m1) were obtained from Applied Biosystems. It was confirmed that human detectors and rabbit detectors do not cross-react with the other species. TaqMan® Universal PCR Master Mix, No AmpErase® UNG (2 × ), was also purchased from Applied Biosystems. Reactions were performed in quadruplicate and the mRNA levels were normalized relative to human glyceraldehyde-3-phosphate dehydrogenase expression. To confirm that hADMPCs differentiated into hepatocytes in vivo, the cells before transplantation and human primary hepatocytes (Invitrogen, Lot number; HuP81) were applied for quantitative PCR as control.
Clearance of 125I-LDL from rabbit serum
WHHL rabbits (8 weeks old) were anesthetized with pentobarbital (50 mg/kg). The peritoneum was incised and hADMPCs (high-dose; 3 × 107 cells/rabbit, n = 2, low-dose; 5 × 106 cells/rabbit, n = 2) suspended in 3 mL of HBSS (20°C) (n = 5) or 3 mL of control saline (n = 2) were infused into the portal vein via a 18-gauge Angiocath (BD). The rabbits were immunosuppressed using the protocol illustrated in Figure 1A. Eight weeks later, the animals were tested by the LDL turnover assay. 125I human LDL (BT-913R, Lot No. 9130709; Biomedical Technologies Inc.) was delivered via the marginal ear vein of the WHHL rabbits and normal control rabbits in physiological saline containing 2 mg/mL bovine serum albumin. Blood was collected from the opposite ear after injection at 5 min, 1 h, 2 h, 4 h, 6 h, and 28 h. 125I-labeled apolipoprotein B-containing LDL was precipitated with 20% of trichloroacetic acid (Wako Pure Chemical Industries) (serum; 320 μL, 100% w/v trichloroacetic acid (TCA) 80 μL), and then the precipitants were applied for counting.
Uptake of DiO-labeled LDL by transplants ex vivo
Human LDL (1.019–1.063 g/mL) was isolated by sequential ultracentrifugation from normolipidemic donors as previously described,24 dialyzed against saline-EDTA, and then sterilized by filtration through a 0.2 μm filter. Lipoproteins were labeled with 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO; Sigma) by incubating the LDL in 0.5% bovine serum albumin/PBS with 100 mL DiO in dimethyl sulfoxide (3 mg/mL) for 8 h at 37°C. The lipoproteins were obtained by sequential ultra centrifugation (1.019–1.063 g/mL) as described,14 and then dialyzed against PBS and filtered before use. To evaluate the uptake of DiO-LDL by transplants ex vivo, thin-sliced WHHL rabbit liver tissue were incubated with serum-free Dulbecco's modified Eagle's medium containing 10 μg/mL DiO-LDL for 24 h at 37°C. Finally, the incubated slices were rinsed, fixed with 10% formalin, sectioned into 5 μm thickness, and mounted with PermaFlour (Japan Tanner Corporation). The slides were examined using a BioZero laser scanning microscope (Kyence).
Statistical analysis
Values were expressed as mean ± standard error of the mean. Differences between mean values of treated and untreated groups were evaluated using the Student's t-test. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS Statistics 17.0 package (SPSS Inc.).
Results
Characteristics of hADMPCs
The cells obtained from adipose tissue were seeded and incubated for 24 h (Fig. 1Ai). After incubation, the adherent cells were treated with EDTA solution, and the resulting suspended cells were replated at a density of 10,000 cells/cm2 on human fibronectin-coated dishes (BD BioCoat) (Fig. 1Aii and 1Aiii). Within two to three passages after the initial plating of the primary culture, hADMPCs appeared as a monolayer of large flat cells (25–30 μm in diameter). As the cells approached confluence, they assumed a more spindle-shaped, fibroblastic morphology (Fig. 1Aiv). After passaging five to six times, the hADMPCs were applied for transplantation. We used flow cytometry to assess markers expressed by hADMPCs (Fig. 1B). The cells were negative for markers of the hematopoietic lineage (CD45) and of hematopoietic stem cells, ABCG-2, CD34, and CD133. They were also negative for CD31, an endothelial cell-associated marker and the surface antigen c-Kit (CD117). However, they stained positively for a number of surface markers characteristic of mesenchymal and/or neural stem cells, but not embryonic stem cells, including CD29, CD44 (hyaluronan receptor), CD73, CD105 (endoglin), and CD166. hADMPCs also were positive for stage-specific embryonic antigen-4. Next, adipogenic, osteogenic, and chondrogenic differentiation potential of hADMPCs were examined (Fig. 1C). Adipogenic differentiation was induced by culture with differentiation medium containing 1-methyl-3-isobutylxanthine (a peroxisome proliferator-activated receptor γ agonist), dexamethasone, and insulin. Induction was confirmed by the accumulation of intracellular lipid droplets that were stained with Oil Red O. After 7-day induction for osteogenesis, hADMPCs were stained with Alizarin red S for mineralized nodules. hADMPCs showed intense Alcian Blue staining, indicating chondrogenic induction capability of hADMPCs.
Serum cholesterol in WHHL rabbit with transplants
hADMPCs were separated from human subcutaneous adipose tissues, cultured for five to seven passages, and applied for transplantation into WHHL rabbits. WHHL rabbits received immunosuppressants and an antiviral agent as illustrated in Figure 2A, and then were transplanted 3 × 107 hADMPCs by portal vein infusion (Fig. 2B). At the day of and 1, 2, 4, 6, and 10 weeks after transplantation of hADMPCs via the portal vein, we examined whether the cells reside or not in the liver after transplantation. Typical distribution patterns of transplanted hADMPCs were followed in Figure 2C. DiI-fluorescent labeled-hADMPCs resided and distributed in the portal area at the day of transplantation. Six and 10 weeks after transplantation, DiI-positive transplanted cells migrated into centrilobular direction. Next, to demonstrate certain percentage of repopulation of the transplanted cells in the liver, the ratios of human-derived cell repopulation were examined by analyzing a repetitive DNA sequence at the day of and 2, 4, 6, and 12 weeks after transplantation (Fig. 2D). To indicate standard curve, we mixed the indicated percentage of hADMPCs with rabbit hepatocytes and plotted the obtained the amount of Alu PCR products, and estimated the amount of repopulation of the transplanted cells in the liver. At the day of transplantation, the ratio of hADMPCs to whole WHHL rabbit liver cells was 0.21% ± 0.056% (mean ± standard error of the mean) and the ratio decreased to 0.016% ± 0.002%, 0.011% ± 0.001%, and 0.009% ± 0.0001% after 2, 4, and 8 weeks of transplantation, respectively. After 12 weeks of transplantation, the ratio was increased to 0.024% ± 0.00005% as indicated (Fig. 2D).
FIG. 2.
(A) Immunosuppression regimen. Cyclosporin A (6 mg/kg/day) and rapamycin (0.05 mg/kg/day) were administered intramuscularly daily from the day before surgery to sacrifice. Methylprednisolone was administered at 3 mg/kg/day (days 1–7), 2 mg/kg/day (days 8–14), 1 mg/kg/day (days 15–21), and 0.5 mg/kg/day (day 22 to sacrifice). Cyclophosphamide (20 mg/kg/day) was injected intravenously at days 0, 2, 5, and 7. Ganciclovir (2.5 mg/kg/day) was also injected intramuscularly to avoid viral infection in the immunocompromised host. (B) Surgical procedure. Watanabe heritable hyperlipidemic (WHHL) rabbits were anesthetized with pentobarbital. An incision was made distal and parallel to the lower end of the ribcage. The peritoneum was incised and hADMPCs, and human adipose tissue-derived fibroblastic cells (hADFCs) (3 × 107 cells/rabbit) or controls were infused into the portal vein using an 18-gauge Angiocath. (C) Localization of transplanted hADMPCs in the WHHL liver. At the day of and 1, 2, 4, 6, and 10 weeks after transplantation of DiI-labeled hADMPCs via the portal vein, the WHHL rabbit liver was examined histologically. DiI-fluorescent labeled-hADMPCs resided and distributed in the portal area at the day of transplantation. One to 2 weeks after transplantation, the DiI-stained hADMPCs-derived cells were localized near the portal areas. Four weeks after transplantation some of the DiI-stained cells resembled innate hepatocytes morphologically. Six and 10 weeks after transplantation, DiI-positive transplanted cells were dispersed in a centrilobular direction, resembling the mature innate hepatocytes. Bars = 100 μm. (D) Quantification of repopulation of the transplanted cells in the liver. The ratios of human-derived cell repopulation were examined by analyzing an Alu repetitive DNA sequence at the day of and 2, 4, 8, and 12 weeks after transplantation. In upper panel the standard curve was indicated, and in lower panel the ratio of repopulation of human cells was shown in time course after transplantation of hADMPCs.
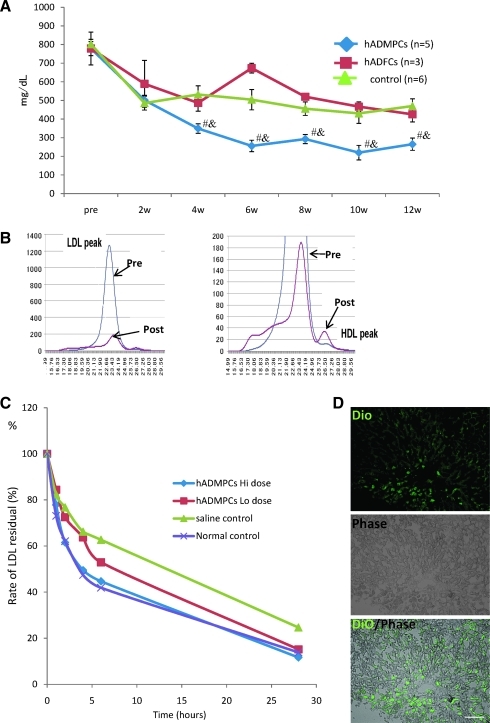
To reveal the effects of hADMPC transplantation onto the lipid profiles of the WHHL rabbit, serum cholesterol levels were monitored over 12 weeks (Fig. 3A). Significant reductions in total serum cholesterol were observed within 4 weeks of the transplantation, and the reductions were maintained for the entire period. The reduction in serum cholesterol in the animals that received hADMPC transplantation was significantly greater than that of the control group. To determine the effects of hADMPC transplantation on the fractions of high-density lipoprotein and LDL in recipient animals, fractionation by fast protein liquid chromatography was performed (Fig. 3B). Transplantation of hADMPCs resulted in marked reduction of the peak LDL-cholesterol and increment of high-density lipoprotein cholesterol fraction (right panel).
FIG. 3.
(A) Total serum cholesterol levels. hADMPC transplantation in WHHL rabbits was followed for 12 weeks. Total serum cholesterol was measured in five rabbits that each received 3 × 107 hADMPCs, three rabbits that each received 3 × 107 hADFCs, and in six rabbits that received saline (control). Bars indicated mean ± standard error of the mean (SEM) (#p < 0.05; control vs. the hADMPC-transplanted WHHL rabbit; &p < 0.05; the hADFC-transplanted WHHL rabbit vs. the hADMPC-transplanted WHHL rabbit). (B) Lipoprotein profiles in a representative WHHL rabbit with hADMPC transplantation after gel filtration. Serum samples from the WHHL rabbit before and 4 weeks after transplantation were fractionated. Note the marked reduction in low-density lipoprotein (LDL) peak and appearance of high-density lipoprotein (HDL) peak. (C) Rate of clearance of LDL from the serum of rabbits with and without transplantation of hADMPCs. Animals were injected with 125I-labeled human LDL, and the time course of clearance was monitored following trichroloacetic acid precipitation of serum at time 5 min, 1 h, 2 h, 4 h, 6 h, and 28 h. Residual 125I-LDL was expressed as percentages of that at 5 min. #p < 0.05 (control vs. the hADMPC-transplanted WHHL rabbit [low dose]) and *p < 0.05 (control vs. the hADMPC-transplanted WHHL rabbit [high dose]). (D) DiO-LDL uptake into hADMPC-derived hepatocytes in the WHHL rabbi liver. Thin-sliced recipient liver was incubated with DiO-labeled LDL in the serum-free medium for 24 h. After washing and fixation, the incubated slices were applied for fluorescent microscopy. DiO-LDL uptake cells (green) and no uptake parenchymal cells were observed in the section. Bar = 100 μm.
Next, clearance experiments were performed with human LDL to confirm that the transplanted hADMPCs contributed the fall in serum cholesterol through uptake of LDL via LDL receptors. The rate of LDL clearance was significantly higher in the WHHL rabbits with transplanted hADMPCs than WHHL rabbits without transplanted hADMPCs (Fig. 3C). Rabbits with hADMPC transplants showed ~2.4-fold (high-dose; 3 × 107 cells/rabbit) and 1.4-fold (low-dose; 5 × 106 cells/rabbit) increase in the rate of LDL cholesterol clearance.
To evaluate the uptake of DiO-LDL by transplants ex vivo, thin-sliced WHHL rabbit liver was incubated with DiO-labeled LDL for 24 h and the uptake was examined as clearance experiment (Fig. 3D). DiO-LDL was uptaken by some but not all of the cells in the WHHL rabbit liver transplanted with hADMPCs. The DiO-LDL-uptaking cells were seen dispersed, contacted, and integrated among the nonuptaking parenchymal cells, suggesting that hADMPCs differentiated into hepatocytes in vivo, lowered of serum cholesterol via LDL uptake.
hADMPCs reside, survive, and differentiate into hepatocytes in vivo
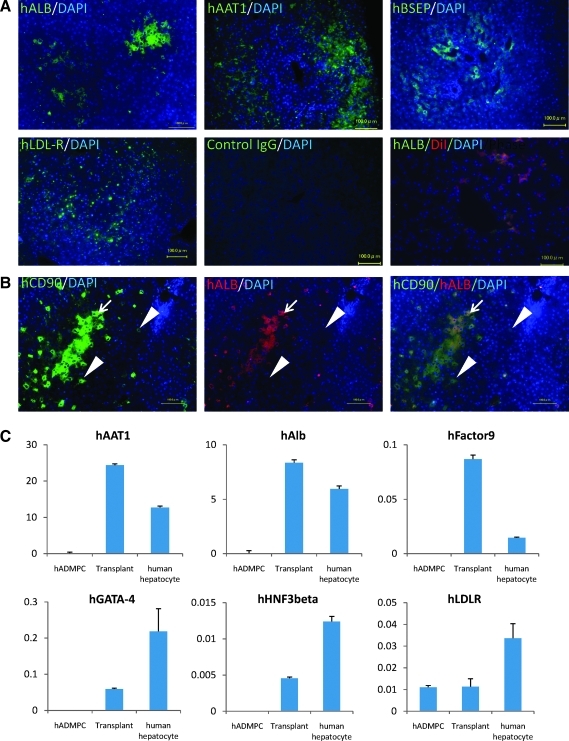
After establishment of the graft as indicated by long-term lowering of serum cholesterol, human-specific hepatocytic proteins, such as albumin, alpha-1-antitrypsin, bile salt export pump, and LDL-receptor, positive cells were identified dispersed within perivenous regions of the liver parenchyma, where they have contacted and integrated among the host cells (Fig. 4A), with cell–cell interactions conserved between hADMPC-derived hepatocytes and diseased hepatocytes pair. Ten weeks after transplantation of DiI-prestained hADMPCs, copresence of human albumin (green) and pretreated DiI-fluorescence (red) on the same cells was observed (Fig. 4A), indicating the transplanted hADMPCs might differentiate into hepatocyte-like cells. To confirm transplanted hADMPCs might differentiate into hepatocyte-like cells and to reveal the efficacy of differentiation, the colocalization of human CD90 and human albumin was examined. As shown in Figure 4B, almost but not all human CD90-positive cells expressed human albumin, indicating that about 80% or more of transplanted hADMPCs could differentiated into human albumin-positive hepatocyte-like cells 12 weeks after transplantation. Next, to confirm the differentiation of hADMPCs into hepatocytes in vivo, expression of hepatocyte markers was analyzed by quantitative RT-PCR. The WHHL rabbit liver that was transplanted with hADMPCs expressed higher levels of human-specific alpha-1-antitrypsin, albumin, and coagulation factor IX than hADMPCs (Fig. 4C). The expression levels of human GATA-4, human hepatocyte nuclear factor 3 beta, and LDL-receptor were also higher in the WHHL rabbit liver than hADMPCs (Fig. 4C). These results indicate that hADMPCs differentiate into mature hepatocytes in vivo.
FIG. 4.
(A) Immunohistochemical identification of human hepatocytic marker cells in liver sections of WHHL rabbits after hADMPC transplantation. Twelve weeks after hADMPC transplantation, human albumin-, human alpha-1-antitrypsin-, human bile salt export pump (BSEP)-, and LDL-receptor-positive cells were dispersed within the perivenous regions of the liver parenchyma, where they made contact with and integrated among the host cells with cell–cell interactions between hADMPC-derived cells and diseased hepatocytes pair. Ten weeks after transplantation of DiI-stained hADMPCs, copresence of human albumin (green) and pretreated DiI-fluorescence (red) on the same cells was observed. Bar = 100 μm. (B) Differentiation of transplanted hADMPCs into hepatocyte-like cells. Twelve weeks after transplantation, almost but not all human CD90-positive cells expressed human albumin, indicating that major population of transplanted hADMPCs could differentiate into hepatocyte-like cells (left panel: human CD90; middle panel: human albumin; right panel: merge). Arrows indicate human CD90 and human albumin double-positive cells; arrowheads indicate human CD90-positive but human albumin-negative cells. (C) Human hepatic gene expression in WHHL rabbit liver after hADMPC transplantation. RNA was prepared from the WHHL rabbit liver 12 weeks after hADMPC transplantation. We used the following hepatic markers: human alpha-1-antitrypsin, human albumin, human factor IX, human GATA-binding protein 4 (GATA-4), human hepatocyte nuclear factor 3 (HNF-3) beta, and human LDL-receptor. Their expression levels were examined by quantitative real time-polymerase chain reaction (RT-PCR) using Assays-on-Demand Gene Expression Assay Mix. The livers of WHHL rabbits that received saline (n = 3) were negative for human hepatic genes. The mRNA levels were normalized based on human glyceraldehyde-3-phosphate dehydrogenase expression as housekeeping gene and data are mean ± SEM of triplicate experiments. The livers of WHHL rabbits that received hADMPC transplantation (n = 3) were positive for human hepatic genes, and their expression levels were similar to those of human primary hepatocytes but not hADMPCs per se. Data are mean ± SEM.
Discussion
We have used the WHHL rabbit to study the ability of hADMPC-derived hepatocytes to lower serum cholesterol in an animal model of FH. Our results have shown that hADMPCs transplanted into the rabbit liver differentiate into hepatocytes in vivo and effectively clear LDL from the circulation.
The reductions in cholesterol brought about by the engrafted hADMPC-derived hepatocytes suggest that human LDL receptors can act as replacement for the mutant LDL receptors in the WHHL rabbit. This capacity of hADMPC-derive hepatocytes is not unexpected, as the liver is the most important site of LDL uptake, accounting for >50% of total removal from the circulation, and the liver is only organ capable of converting cholesterol to bile for excretion. The substantial decrease in serum cholesterol achieved suggests that the hADMPC-derived hepatocytes both internalize LDL and metabolize the cholesterol to bile for excretion. The correlation between cholesterol and coronary heart disease has been well documented, and decreases in serum cholesterol of the magnitude that we have demonstrated would be expected to decrease morbidity and mortality in the patients with severe FH.25
The appearance of the hADMPC-derived hepatocytes as revealed by immunohistochemistry and RT-PCR indicated that the hADMPCs differentiated into hepatocytes and integrated into the liver parenchyma. The perivenous migration of the differentiated hepatocytes derived from hADMPCs along the portal-venous axis and suggests that hADMPCs recognize conserved signals on host cells and matrix. There are some reports describing the hepatogenic differentiation potential of hADMPCs.15,16 These studies described that hepatocytes differentiated from hADMPCs ex vivo engrafted in the liver and functioned, and that the hADMPCs could be resided and changed their characters into hepatocyte-like cells only in the chemically damaged liver. These reports, revealing that hADMPCs have capabilities to differentiate into hepatocytes, hinted us that hADMPCs might differentiate into hepatocytes in liver. Hepatogenic signals from the microenvironment such as cell-to-cell connections or intermediates are probably important factors that dictate the type of functional hepatocytes in hepatic differentiation.26 We are currently investigating the mechanism for the differentiation hADMPCs into hepatocytes.
The choice of cell source is critical for realizing success in cellular therapy. Liposuction surgeries yield a massive amount of lipoaspirate adipose tissue from 100 mL to >3 L as cell sources.27 A major advantage of hADMPCs is their availability in safe and easy with few ethical issues, as compared with the shortage of human livers for orthotopic transplantation, which has been shown to be effective for the treatment of FH.25 Our serum cholesterol reduction studies and in vitro studies demonstrated that human LDL binds to the hADMPC-derived hepatocytes receptor, indicating that this therapy will be useful in humans. Previous attempts to study the efficacy of hepatocyte transplantation in the WHHL rabbit model have employed allogenic hepatocytes, xenogenic hepatocytes, or hepatocytes transduced ex vivo with a recombinant retrovirus containing the LDL receptor cDNA.6–13 The lowering effects of hepatocyte transplantation on serum cholesterol have been reported, but there was some problems. First, hepatocytes could not be expanded ex vivo with functional potentials; second, the cell viability reduced after cryopreservation; third, the many injected hepatocytes are supposed to be cleared by the reticuloendothelial system or lose viability during early phase. The rate of LDL clearance was returned to normal in LDL receptor knockout mice by introduction of an adenoviral construct containing an LDL receptor cDNA, and similar approaches have lowered serum cholesterol levels in the WHHL rabbit.10,12,13 However, sustained expression of the LDL receptor from viral vectors can be difficult to achieve.11,13 Moreover, hepatocytes derived from hADMPCs have the advantage that the LDL receptor is expressed from an endogenous gene with intact regulatory sequences. Such control of LDL receptor levels would not be expected after treatment of hypercholesterolemia with LDL receptor cDNA construct that lack the regulatory regions of the gene.28
Our experiments have shown that the hADMPCs expressed hepatocyte markers after transplantation in vivo and the integrated cells into parenchyma provide functional LDL receptors, indicating that they differentiated into hepatocytes and might lower serum cholesterol in the WHHL rabbit. These results suggested that hADMPC transplantation via portal vein could correct the metabolic defects of FH patients and that hADMPC-derived hepatocytes could function as supplier with plasma proteins derived from liver, giving us an idea that hADMPC-transplantation might be a novel cell therapy for hemophilia, alpha-1 antitrypsin deficiency, mucolipidosis, and other diseases caused by genetic defects for liver function. In near future, the therapy will be a novel therapy for kinds of inherited liver diseases.
Acknowledgments
This study was supported in part by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), RIKEN Program for Drug Discovery and Medical Technology Platforms, and Kobe Translational Research Cluster, the Knowledge Cluster Initiative, Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Disclosure Statement
All of the authors stated no conflict of interest.
References
- 1.Brown M.S. Goldstein J.L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Havel R.J. Yamada N. Shames D.M. Watanabe heritable hyperlipidemic rabbit. Animal model for familial hypercholesterolemia. Arteriosclerosis. 1989;9(1 Suppl):I33. [PubMed] [Google Scholar]
- 3.Yamamoto T. Bishop R.W. Brown M.S. Goldstein J.L. Russell D.W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986;32:1230. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bujo H. Takahashi K. Saito Y. Maruyama T. Yamashita S. Matsuzawa Y. Ishibashi S. Shionoiri F. Yamada N. Kita T. Clinical features of familial hypercholesterolemia in Japan in a database from 1996–1998 by the research committee of the ministry of health, labour and welfare of Japan. J Atheroscler Thromb. 2004;11:146. doi: 10.5551/jat.11.146. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita S. Hbujo H. Arai H. Harada-Shiba M. Matsui S. Fukushima M. Saito Y. Kita T. Matsuzawa Y. Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb. 2008;15:292. doi: 10.5551/jat.e610. [DOI] [PubMed] [Google Scholar]
- 6.Gunsalus J.R. Brady D.A. Coulter S.M. Gray B.M. Edge A.S. Reduction of serum cholesterol in Watanabe rabbits by xenogeneic hepatocellular transplantation. Nat Med. 1997;3:48. doi: 10.1038/nm0197-48. [DOI] [PubMed] [Google Scholar]
- 7.Tejera M.L. Cienfuegos J.A. Maganto P. Pardo F. Santamaria L. Codesal J. De Andres S. Hernandez J.L. Castillo-Olivares J.L. Reduction of cholesterol levels following liver cell grafting in hyperlipidemic (WHHL) rabbits. Transplant Proc. 1992;24:160. [PubMed] [Google Scholar]
- 8.Wang J. Pollak R. Bartholomew A. Sustained reduction of serum cholesterol levels following allo-transplantation of parenchymal hepatocytes in Watanabe rabbits. Transplant Proc. 1991;23:894. [PubMed] [Google Scholar]
- 9.Wiederkehr J.C. Kondos G.T. Pollak R. Hepatocyte transplantation for the low-density lipoprotein receptor-deficient state. A study in the Watanabe rabbit. Transplantation. 1990;50:466. doi: 10.1097/00007890-199009000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury J.R. Grossman M. Gupta S. Chowdhury N.R. Baker J.R., Jr. Wilson J.M. Long-term improvement of hypercholesterolemia after ex vivo gene therapy in LDLR-deficient rabbits. Science. 1991;254:1802. doi: 10.1126/science.1722351. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi S. Brown M.S. Goldstein J.L. Gerard R.D. Hammer R.E. Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozarsky K.F. McKinley D.R. Austin L.L. Raper S.E. Stratford-Perricaudet L.D. Wilson J.M. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem. 1994;269:13695. [PubMed] [Google Scholar]
- 13.Wilson J.M. Chowdhury N.R. Grossman M. Wajsman R. Epstein A. Mulligan R.C. Chowdhury J.R. Temporary amelioration of hyperlipidemia in low density lipoprotein receptor-deficient rabbits transplanted with genetically modified hepatocytes. Proc Natl Acad Sci U S A. 1990;87:8437. doi: 10.1073/pnas.87.21.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okura H. Komoda H. Saga A. Kakuta-Yamamoto A. Hamada Y. Fumimoto Y. Lee C.M. Ichinose A. Sawa Y. Matsuyama A. Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2010;16:761. doi: 10.1089/ten.TEC.2009.0208. [DOI] [PubMed] [Google Scholar]
- 15.Banas A. Teratani T. Yamamoto Y. Tokuhara M. Takeshita F. Quinn G. Okochi H. Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 16.Seo M.J. Suh S.Y. Bae Y.C. Jung J.S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 17.Komoda H. Okura H. Lee C.M. Sougawa N. Iwayama T. Hashikawa T. Saga A. Yamamoto A. Ichinose A. Murakami S. Sawa Y. Matsuyama A. Reduction of N-glycolylneuraminic acid xenoantigen on human adipose tissue-derived stromal cells/mesenchymal stem cells leads to safer and more useful cell sources for various stem cell therapies. Tissue Eng Part A. 2010;16:1143. doi: 10.1089/ten.TEA.2009.0386. [DOI] [PubMed] [Google Scholar]
- 18.Okura H. Matsuyama A. Lee C.M. Saga A. Kakuta-Yamamoto A. Nagao A. Sougawa N. Sekiya N. Takekita K. Shudo Y. Miyagawa S. Komoda H. Okano T. Sawa Y. Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng Part C Methods. 2010;16:417. doi: 10.1089/ten.TEC.2009.0362. [DOI] [PubMed] [Google Scholar]
- 19.Bjorntorp P. Karlsson M. Pertoft H. Pettersson P. Sjostrom L. Smith U. Isolation and characterization of cells from rat adipose tissue developing into adipocytes. J Lipid Res. 1978;19:316. [PubMed] [Google Scholar]
- 20.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicklas J.A. Buel E. Development of an Alu-based, real-time PCR method for quantitation of human DNA in forensic samples. J Forensic Sci. 2003;48:936. [PubMed] [Google Scholar]
- 22.Opel K.L. Fleishaker E.L. Nicklas J.A. Buel E. McCord B.R. Evaluation and quantification of nuclear DNA from human telogen hairs. J Forensic Sci. 2008;53:853. doi: 10.1111/j.1556-4029.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki M. Usui S. Ishigami M. Sakai N. Nakamura T. Matsuzawa Y. Yamashita S. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol. 2005;25:578. doi: 10.1161/01.ATV.0000155017.60171.88. [DOI] [PubMed] [Google Scholar]
- 24.Bier D.M. Havel R.J. Activation of lipoprotein lipase by lipoprotein fractions of human serum. J Lipid Res. 1970;11:565. [PubMed] [Google Scholar]
- 25.Steinberg D. Witztum J.L. Current concepts. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990;264:3047. [PubMed] [Google Scholar]
- 26.Hughes R.D. Mitry R.R. Dhawan A. Hepatocyte transplantation for metabolic liver disease: UK experience. J R Soc Med. 2005;98:341. doi: 10.1258/jrsm.98.8.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilheimer D.W. Goldstein J.L. Grundy S.M. Starzl T.E. Brown M.S. Liver transplantation to provide low-density-lipoprotein receptors and lower plasma cholesterol in a child with homozygous familial hypercholesterolemia. N Engl J Med. 1984;311:1658. doi: 10.1056/NEJM198412273112603. [DOI] [PMC free article] [PubMed] [Google Scholar]