Abstract
Norepinephrine and serotonin involvement in nociceptive functions is supported by observations of analgesic effects of norepinephrine transporter (NET) and serotonin transporter (SERT) inhibitors such as amitriptyline. However, the relative contribution of NET and SERT to baseline nociception, as well as amitriptyline analgesia, is unclear. Amitriptyline and morphine analgesia in wild-type (WT) mice and littermates with gene knockout (KO) of SERT, NET or both transporters was conducted using the hotplate and tail-flick tests. Hypoalgesia was observed in NET KO mice, and to a lesser extent in SERT KO mice. The magnitude of this hypoalgesia in NET KO mice was so profound that it limited the assessment of drug-induced analgesia. Nonetheless, the necessary exclusion of these subjects because of profound baseline hypoalgesia strongly supports the role of norepinephrine and NET in basal nociceptive behavior while indicating a much smaller role for serotonin and SERT. To further clarify the role of NET and SERT in basal nociceptive sensitivity further experiments were conducted in SERT KO and NET KO mice across a range of temperatures. NET KO mice were again found to have pronounced thermal hypoalgesia compared to WT mice in both the hotplate and tail-flick tests, and only limited effects were observed in SERT KO mice. Furthermore, in the acetic acid writhing test of visceral nociception pronounced hypoalgesia was again found in NET KO mice, but no effect in SERT KO mice. As some of these effects may have resulted from developmental consequences of NET KO, the effects of the selective NET blocker nisoxetine and the selective SERT blocker fluoxetine were also examined in WT mice: only nisoxetine produced analgesia in these mice. Collectively these data suggest that NET has a far greater role in determining baseline analgesia, and perhaps other analgesic effects, than SERT.
Keywords: Knockout Mice, Serotonin Transporter, Norepinephrine Transporter, Analgesia, Nociception, Amitriptyline
Introduction
Descending monoaminergic projections have long been known to comprise part of descending endogenous analgesia circuitry and to play a role in opiate mediated analgesia (for review see (Sawynok and Reid 1989). These descending circuits are downstream mediators of descending opiate-mediated supraspinal analgesia, which can be impaired by norepinephrine (NE) or serotonin (5-HT) lesions (Zhong et al. 1985; Sawynok and Reid 1987; 1989) and NE or 5-HT receptor antagonists (Yaksh 1979; Jensen and Yaksh 1986; Wigdor and Wilcox 1987; Arts et al. 1991). Electrophysiological experiments support this circuitry model, showing that centrally injected morphine causes serotonin release in the spinal cord (Yaksh and Tyce 1979), and direct stimulation of pontine NE cell groups or the 5-HT containing nucleus raphé magnus produce analgesic responses (Aimone et al. 1987; Yeomans et al. 1992), which are reversed by NE and 5-HT antagonists (Aimone et al. 1987).
Drugs that potentiate the action of 5-HT or NE in the synapse, such as reuptake blockers, potentiate morphine analgesia (Malseed and Goldstein 1979; Liu and Wang 1981; Botney and Fields 1983; Rigal et al. 1983; Taiwo et al. 1985; Sacerdote et al. 1987; Ventafridda et al. 1990; Sawynok and Reid 1992; Eisenach and Gebhart 1995; Gatch et al. 1998; Luccarini et al. 2004). Drugs affecting monoaminergic function also have analgesic properties when administered alone, in particular the tricyclic antidepressants (TCAs; (Pasternak et al. 1983; Hwang and Wilcox 1987; Sacerdote et al. 1987; Hersh and Kaplan 1990; Tura and Tura 1990; Ventafridda et al. 1990; Ardid et al. 1991; Acton et al. 1992; Ardid et al. 1992; Ardid et al. 1995; Sawynok et al. 1999; Korzeniewska-Rybicka and Plaznik 2000; Sawynok and Reid 2001), including amitriptyline (Spiegel et al. 1983; Sacerdote et al. 1987; Tura and Tura 1990; Ventafridda et al. 1990; Eisenach and Gebhart 1995; Abdi et al. 1998; Esser and Sawynok 1999; Sawynok et al. 1999; Esser and Sawynok 2000; Korzeniewska-Rybicka and Plaznik 2000; Esser et al. 2001; Sawynok and Reid 2001; Heughan and Sawynok 2002; Khan et al. 2002; Oatway et al. 2003; Luccarini et al. 2004; Sudoh et al. 2004), which have been shown to be clinically effective analgesics (Watson 1984; Young and Clarke 1985; Levine et al. 1986; Max et al. 1987; Frank et al. 1988; Onghena and Van Houdenhove 1992; Watson et al. 1992; Gordon et al. 1994; Nicolodi and Sicuteri 1996; Richeimer et al. 1997; Gerner et al. 2003) also see reviews by (Egbunike and Chaffee 1990; Carter and Sullivan 2002). Since TCAs affect both the NE and 5-HT systems, there is debate as to which system primarily mediates its antinociceptive properties (Carter and Sullivan 2002), or indeed whether some other action of these drugs accounts for their analgesic properties (Galeotti et al. 2001; Sawynok et al. 2001).
Transgenic KO mice have been developed for what are considered to be the main molecular targets of amitriptyline, NET (Xu et al. 2000) and SERT (Bengel et al. 1998). Therefore an examination of thermal nociception in the hotplate and tail-flick tests was conducted in NET-SERT double KO mice to assess amitriptyline- and morphine-induced analgesia. In accordance with the suggestions of some authors (Wigdor and Wilcox 1987) our expectation would be that there would be a greater effect of NET KO than SERT KO on these analgesic effects, and upon baseline analgesia. Baseline nociception has already been demonstrated to be mildly affected in NET KO mice (Bohn et al. 2000), although this initial analysis did not compare the effects of NET KO and SERT KO, and examined nociception under limited conditions. As will be seen, our own studies were found to be limited because of a more profound hypoalgesia in NET KO mice than reported in Bohn et al. (2000). Therefore, additional studies were conducted to examine baseline nociception across a broader range of conditions in NET KO and SERT KO mice in these tests, as well as the acetic acid test of visceral nociception. Finally, because these effects may have been a consequence of the gene KO during development, rather than the elimination of the gene in adult mice, the effects of selective NET blockade with nisoxetine and selective SERT blockade with fluoxetine were examined in WT mice.
Methods
Subjects
SERT (Bengel et al. 1998) and NET (Xu et al. 2000) KO mice have been described previously, as was the combined NET-SERT (Hall et al. 2002) and DAT-SERT (Sora et al. 2001) used in these experiments. Mice used in the first experiment were all from the combined NET-SERT line. Briefly, combined SERT-NET KO mice were generated initially by crosses of the single KO lines producing double heterozygote KO mice in the F1generation, with combined double homozygote mice apparent in the F2 generation. These mice were thus of the same mixed C57BL/6J-129Sv background as the original single KO strains. In experiments with double KO mice breeding was largely from NET +/− SERT +/− crosses. Some subtle abnormalities of motor function, not previously reported, were observed in NET KO and combined KO mice (see below). In the basal nociception experiments NET KO mice and WT littermates were from the NET-SERT line, while SERT KO mice and WT littermates were from the DAT-SERT KO line (Sora et al. 2001). SERT +/+ and SERT −/− mice were bred from SERT +/− crosses (NET +/+), while NET +/+ and NET −/− mice were bred from NET +/− crosses (SERT +/+). In each case WT littermate controls were used for all comparisons. During initial analgesia testing NET −/− mice were observed to exhibit mild Straub tail and hind-limb clasping. Because of the possibility of general motor impairments NET KO and SERT KO mice, and littermate controls, were assessed in two standard motor coordination paradigms: the screen hang test and the accelerating rotorod test.
Mice were genotyped by PCR, using two internal primers, one targeted at the NEO gene and one targeted at the WT gene, and one external primer, which generated two products identifying the WT and KO genes for each gene targeted (NET and SERT) as previously described (Perona et al. 2008). Mice were housed under standard laboratory conditions under a 12:12 LD cycle (lights on at 0800h), temperature maintained at 18–22 °C, 30–40% humidity, with food and water available ad libitum. After weaning mice were housed 3–4 per cage until testing at 12–16 weeks of age. Both male and female mice were used. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and all applicable NIH Guidelines, under protocols approved by the NIDA-IRP Institutional Animal Care and Use Committee.
Experiment 1: Analgesic effects of Morphine and Amitriptyline
Subjects
NET-SERT double KO mice from all 9 potential genotype combinations were tested for morphine (N=11–21 per genotype) and amitriptyline (N=10–15 per genotype) analgesia.
Procedure
Mice were tested for thermal nociceptive responses using the hotplate and the tail-flick tests. The hotplate temperature was maintained at 55 °C throughout testing, and the tail-flick temperature was maintained at 53 °C throughout testing. In the hot plate procedure mice were placed on a hotplate and latency to lick the hind paw or jump was recorded with a maximal latency of 30 sec after which the subjects were removed from the apparatus if there was no response. After each assessment in the hotplate, thermal nociception was assessed in the tail- flick test. For tail-flick testing, mice were loosely wrapped in an absorbent towel and the tail immersed in 2 centimeters of water in a water bath. This procedure was repeated every 20 minutes, always immediately after testing in the hotplate. The tail-flick latency was recorded with a maximal latency of 15 sec after which the subject’s tail was removed from the water if there was no response. After establishment of baseline nociception (3–4 assessments), an ascending, cumulative dose-effect regimen was used as previously described (Elmer et al. 1995; Sora et al. 2001) in separate groups of mice to assess morphine and amitriptyline analgesia. Morphine analgesia was assessed in naïve mice injected at 20 min intervals with an ascending cumulative dose regimen that produced total drug doses of 0.3, 1.0, 3.0, 10.0 and 20.0 mg/kg morphine s.c. Hotplate and tail-flick responses were tested 20 min after each injection, and the next dose administered. Amitriptyline analgesia was assessed in naïve mice injected i.p. at 20 min intervals using an ascending cumulative dose regimen that produced total drug doses of 5.0, 10.0, 20.0 and 40.0 mg/kg amitriptyline. Hotplate and tail-flick responses were tested 20 min after each injection, and the next dose administered.
Experiment 2: Thermal Nociceptive threshold in NET KO and SERT KO mice
Subjects
Sensitivity to thermal nociception was examined in NET +/+, +/− and −/− mice (N=8–10 mice per genotype), and in SERT +/+, +/− and −/− mice (N=9–10 per genotype).
Procedure
Thermal nociception was assessed as in the hotplate and tail-flick paradigms as described above for Experiment 1 except that mice were tested across a range of temperatures. In the hotplate the initial temperature was 47 °C. The procedure was repeated every 20 minutes, increasing the temperature by 1 °C between each test. The final temperature was 54 °C. After each assessment in the hotplate, thermal nociception was assessed in the tail-flick test. The initial temperature of the water bath was 45 °C. This procedure was repeated every 20 minutes, always immediately after testing in the hotplate, increasing the temperature by 1 °C between each test. The final temperature was 52 °C.
Experiment 3: Visceral Nociception
Subjects
Sensitivity to visceral nociception was examined in NET +/+, +/− and −/− mice (N=6–10 mice per genotype), and in SERT +/+, +/− and −/− mice (N=9–10 per genotype).
Procedure
Subjects were in injected with 0.7 % acetic acid intraperitoneally in a volume of 10 mL per kg and placed in Plexiglas chambers (18 cm × 18 cm × 18 cm) for observation. The number of abdominal contractions was counted for 20 minutes post-injection. Acetic acid was prepared fresh daily from glacial acetic acid and dissolved in isotonic saline.
Experiment 4: Nociceptive effects of Nisoxetine and Fluoxetine
Subjects
WT mice from the DAT-SERT double KO line were tested for the analgesic effects of nisoxetine and fluoxetine (N=9–10 mice/drug/test).
Procedure
The analgesic effects of nisoxetine (5–40 mg/kg IP) and fluoxetine (5–40 mg/kg IP) were examined in the hotplate and tail-flick tests as described above for morphine and amitriptyline using an ascending dose regimen. Baseline analgesia was initially established by testing the mice repeatedly, every 20 minutes, until a stable baseline was established (3–4 baseline assessments). 20 minutes before each baseline test subjects were injected with saline. After the last baseline test subjects received the first drug injection and injections were given after each subsequent test producing cumulative drug doses of 5, 10, 20 and 40 mg/kg for both nisoxetine and fluoxetine.
Experiment 5: Tests of Motor Function
Subjects
NET +/+, +/− and −/− mice were examined in the accelerating rotorod and screen hang tests (N=12–16 per genotype). SERT +/+, +/− and −/− mice were examined in the accelerating rotorod (N=10 per genotype) and screen hang tests (N=11–13 per genotype).
Accelerating Rotorod
Motor coordination was initially tested on an accelerating rotorod device (Rota-Rod; Ugo Basile, Comerio, Italy). Mice were tested once per day for three days. After placement on the device the rotations accelerated from 1 to 30 rotations per minute over 5 minutes. The latency to fall from the wheel was measured (maximum latency 300 sec).
Screen Hang Test
The day after the last rotorod test mice were tested for muscle strength in the screen hang test by placement on a wire cage top (1 cm between bars, 3 mm thickness). The cage was then inverted and positioned on top of a Plexiglas open field filled with 2 inches of bedding material. The latency to fall was measured (maximum latency 120 sec).
Drugs
Morphine sulphate (NIDA IRP Drug Supply Program) was dissolved in saline and administered in a volume of 1.0 ml/kg s.c. Amitriptyline (RBI, Natick, MA), fluoxetine (Sigma, St. Louis, MO) and nisoxetine (Sigma, St. Louis, MO) were dissolved in saline and administered in a volume of 1.0 ml/kg i.p.
Statistical analyses
For Experiment 1 nociceptive responses were assessed using latency as the dependent measure: hind paw lick or jump for the hotplate test and tail withdrawal for the tail-flick test. Baseline analgesia was taken as the average of the last two baseline measurements. Data from the hotplate and tail-flick tests were analyzed separately. Baseline analgesia data were analyzed by ANOVA with the between subjects factors of NET GENOTYPE and SERT GENOTYPE. Antinociceptive effects of morphine and amitriptyline were evaluated using percent maximal antinociception: 100 x{[(the behavioral response latency after drug) - (the baseline behavioral response latency)]/[(maximal cut-off time) - (the baseline behavioral response latency)]}. The maximal cut-off time was 30 seconds for the hotplate test and 15 seconds for the tail-flick test. Data were subjected to ANOVA with the between subjects factors of NET GENOTYPE and SERT GENOTYPE and the within subjects factor of DOSE. A number of mice, primarily in the NET KO groups, had to be excluded from the analysis of morphine or amitriptyline analgesia because they did not exhibit baseline nociceptive responses at all, under the conditions used in this study. When the baseline nociceptive latencies where greater than 2/3 of the maximal cut-off time, the subject was excluded from further analgesic testing. The exclusion of subjects from analgesia testing is summarized in Table 1. Data from Experiment 2 were analyzed separately for NET KO and SERT KO mice by ANOVA with the between subjects factors of NET GENOTYPE and SERT GENOTYPE respectively, and the additional within subjects factor of TEMPERATURE. Data from the hotplate and tail-flick tests were analyzed separately. Data from Experiment 3 were analyzed separately for NET KO and SERT KO mice by ANOVA with the between subjects factors of NET GENOTYPE and SERT GENOTYPE respectively. Data from Experiment 4 were analyzed by 1-way ANOVA with the between subjects factor of DOSE. Fluoxetine and Nisoxetine effects in the hotplate and tail-flick paradigms were analyzed separately. Data from Experiment 5 were analyzed separately in NET KO and SERT KO mice by ANOVA with the between subjects factors of NET GENOTYPE and SERT GENOTYPE respectively. Rotorod data included the additional within-subjects factor of DAY. Post hoc comparisons were made using Scheffe’s comparisons.
Table 1.
Percent of subjects excluded from analgesia testing because of high baseline analgesia (> 2/3 of maximum) for all genotypes.
| MORPHINE | AMITRIPTYLINE | |||
|---|---|---|---|---|
| GENOTYPE | HOT PLATE | TAIL FLICK | HOT PLATE | TAIL FLICK |
| NET +/+ SERT +/+ | 0 | 0 | 7 | 29 |
| NET +/+ SERT +/− | 31 | 8 | 0 | 0 |
| NET +/+ SERT −/− | 22 | 11 | 0 | 7 |
| NET +/− SERT +/+ | 33 | 6 | 7 | 20 |
| NET +/− SERT +/− | 10 | 14 | 8 | 15 |
| NET +/− SERT −/− | 5 | 5 | 15 | 15 |
| NET −/− SERT +/+ | 64 | 45 | 30 | 40 |
| NET −/− SERT +/− | 56 | 50 | 27 | 73 |
| NET −/− SERT −/− | 57 | 52 | 50 | 67 |
Results
Experiment 1: Analgesic effects of Morphine and Amitriptyline
Baseline nociception in Morphine-treated NET-SERT KO mice
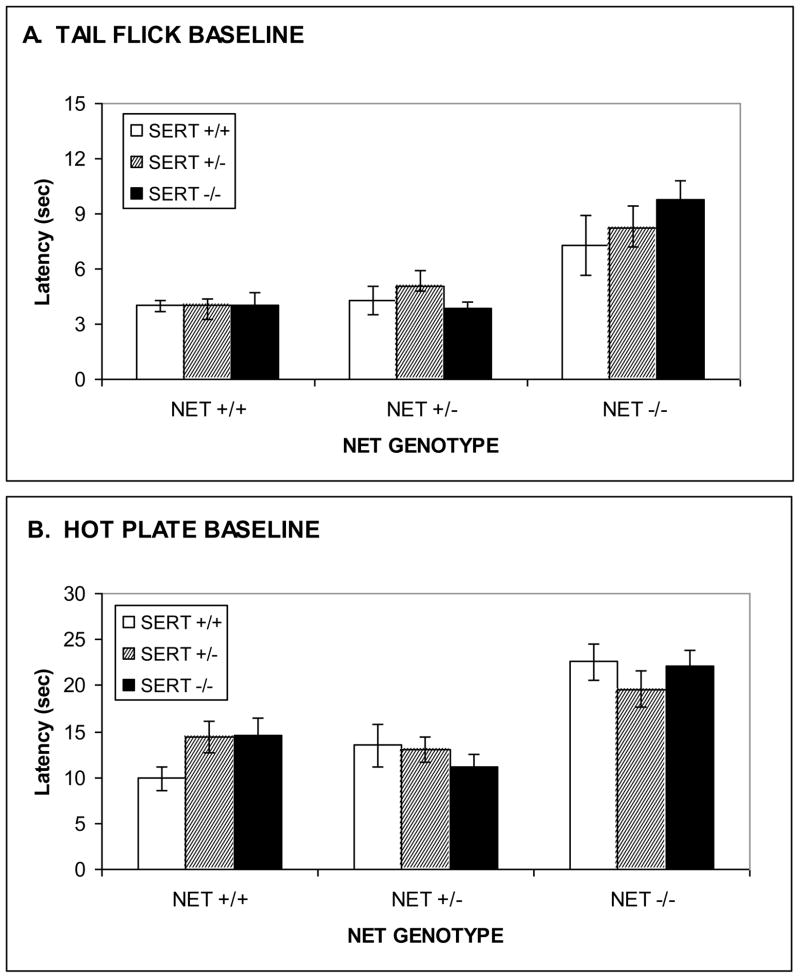
Profound baseline hypoalgesia was observed to result from NET KO in both the 55 °C hotplate and 53 °C tail-flick tests. Hypoalgesia was observed in NET KO mice for both hotplate (Fig. 1A; F[2,142]=22.4, p<0.0001), and tail-flick (Fig. 1B; F[2,142]=23.0, p<0.0001) tests. By contrast no significant changes in baseline analgesia were observed to result from SERT KO for the hotplate (Fig. 1A; F[2,142]=0.1, ns) or tail-flick (Fig. 1B; F[2,142]=0.6, ns) tests. There were no statistically significant interactions, for either test, between NET GENOTYPE and SERT GENOTYPE, although ceiling effects in NET/SERT double KO mice relative to NET KO alone cannot be ruled out given the high baseline analgesia resulting from NET KO alone, resulting in reduced power detect such an interaction. One logical suggestion might be that hypoalgesia in NET KO mice might result from enhanced μ opioid receptor function, but this hypoalgesia was not naloxone reversible (data not presented). The hypoalgesia in NET KO mice was so pronounced that a much larger percentage of subjects had to be excluded from subsequent analgesia testing for the NET KO and NET-SERT KO mice (50–60%) than for the other genotypes (0–20%).
Figure 1. Baseline analgesic responses in combined NET/SERT KO mice tested for morphine analgesia.
Increased baseline analgesic responses (latency) was observed in NET KO, but not SERT KO, mice for supraspinal analgesia in the hot-plate test (A) and spinal analgesia in the tail-flick test (B).
Morphine Analgesia in NET-SERT KO mice
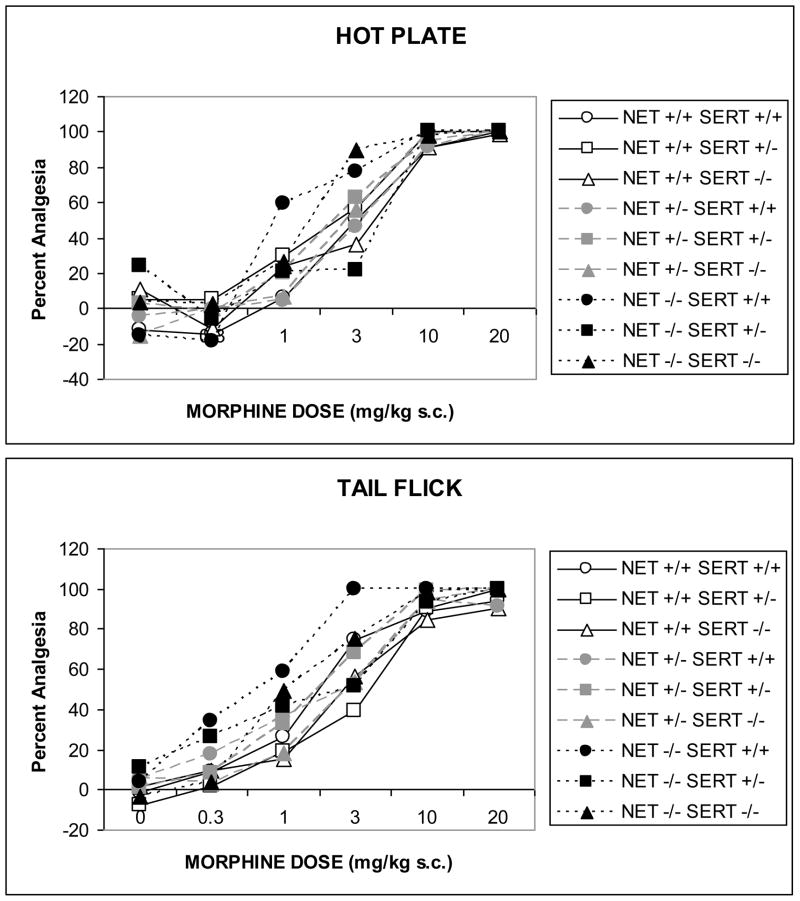
Morphine produced dose-related antinociception in the 55 °C hotplate (Fig. 2A; F[5,485]=163.2, p<0001) and 53 °C tail-flick (Fig. 2B; F[5,545]=292.7, p<0001) tests in all genotypes. The analgesic effects of morphine appeared to be somewhat enhanced in a dose-dependent manner in NET KO mice and NET x SERT KO mice, in the hotplate test but the effect of NET GENOTYPE did not reach statistical significance (NET x DOSE: F[10,485]=1.1, ns). Obviously the power to resolve such an effect was impaired by the large numbers of NET KO subjects that had to be eliminated based on high baseline analgesia. A similar effect of NET KO was observed in the tail-flick test, for which the effect of NET GENOTYPE was significant (Fig. 2B; F[2,109]=4.9, p<0.01), as well as the NET GENOTYPE x SERT GENOTYPE x DOSE interaction (F[20,545]=1.6, p<0.05). This interaction term indicates that NET KO significantly shifted the morphine dose-response curve to the left, but this effect was reversed by combination with SERT KO, even though SERT GENOTYPE alone was without effect on this measure.
Figure 2. Morphine-induced analgesia in combined NET/SERT KO mice.
The data represent analgesic responses to morphine (0–20 mg/kg SC) in NET/SERT KO mice for supraspinal analgesia in the hot-plate test (A) and spinal analgesia in the tail-flick test (B). In both tests NET KO, but not SERT KO, appeared to increase sensitivity to morphine, although this effect did not reach significance in the hot-plate test and was reversed by SERT KO in the tail-flick test.
Baseline nociception in Amitriptyline-treated NET-SERT KO mice
Hypoalgesia was observed in NET KO mice in both the hotplate (Fig 3A; F[2,103]=9.0, p<0.001), and tail-flick (Fig. 3B; F[2,103]=20.1, p<0.0001) tests. SERT KO also produced hypoalgesia in the hotplate test (Fig. 3A; F[2,103]=3.5, p<0.04), although SERT KO was without effect on baseline analgesia in the tail-flick paradigm (Fig.3B; F[2,103]=0.4, ns). The effects of NET GENOTYPE and SERT GENOTYPE were purely additive in the tail-flick paradigm; the interaction term in the ANOVA was not statistically significant (F[4,103]=0.3, ns). As before, a substantial proportion of NET KO mice had to be excluded from subsequent amitriptyline analgesia testing because of high baseline analgesia.
Figure 3. Baseline analgesic responses in combined NET/SERT KO mice tested for amitriptyline analgesia.
Baseline analgesic responses (latency) in NET/SERT KO mice for supraspinal analgesia in the hot-plate test (A) and spinal analgesia in the tail-flick test (B). NET KO produced large increases in latencies in both tests, while SERT KO produced a small increase in latencies only in the hot-plate test.
Amitriptyline Analgesia in NET-SERT KO mice
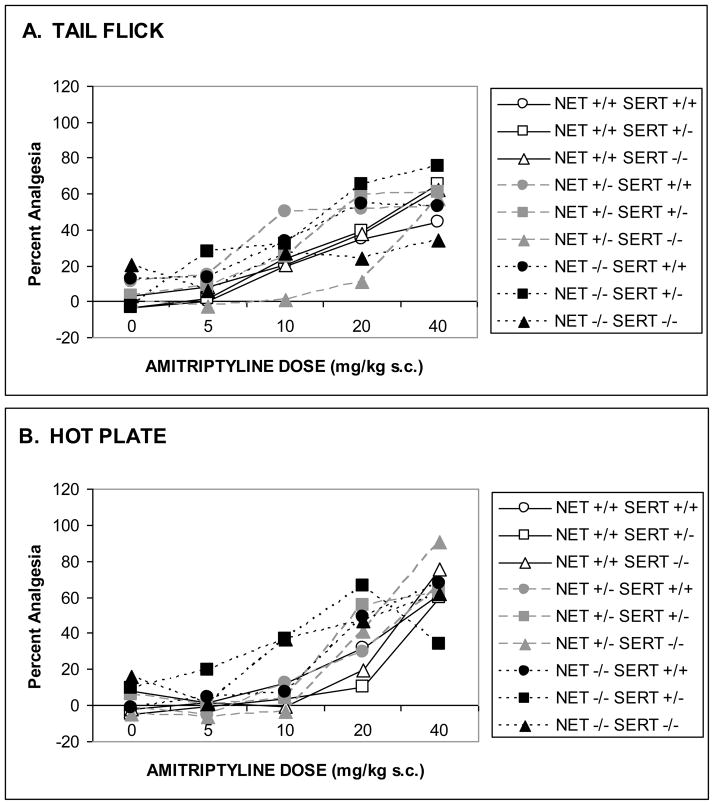
Amitriptyline produced dose-related antinociception in 55 °C hotplate (Fig. 4A; F[4,340]=74.6, p<0.0001) and 53 °C tail-flick (Fig. 4B; F[4,284]=28.8, p<0.0001) tests. Post hoc analysis by one-way ANOVA for each genotype found that all genotypes except NET −/− SERT +/− and NET −/− SERT −/− had significant amitriptyline analgesia in the hotplate test, although once again the low numbers of subjects that completed the experiment in these groups weakens such a conclusion. Nonetheless, the analgesic effects of amitriptyline were dose-dependently enhanced in NET KO mice in the hotplate test (F[8,340]=3.4, p<0.001). This is particularly obvious at the low doses (5.0 and 10.0 mg/kg). SERT KO did not affect thermal nociception significantly in the hotplate test (F[8,340]=1.5, ns). Thermal analgesia produced by amitriptyline in the tail flick-test was not affected by NET GENOTYPE (F[8,284]=0.1, ns) or SERT GENOTYPE (F[8,284]=1.5, ns). As before, post hoc analysis by one-way ANOVA for each genotype found that all genotypes except NET−/− SERT −/− had significant amitriptyline analgesia in the hot-plate test, but once again the low numbers of subjects that completed the experiment in this group weakened the power to resolve these effects.
Figure 4. Amitriptyline-induced analgesia in combined NET/SERT KO mice.
The data represent analgesic responses to amitriptyline (0–40 mg/kg IP) in NET/SERT KO mice for supraspinal analgesia in the hot-plate test (A) and spinal analgesia in the tail-flick test (B). In the hot-plate test all genotypes except NET −/− SERT +/− and NET −/− SERT −/− had significant amitriptyline analgesia. NET KO dose-dependently enhanced hot-plate analgesia. In the tail-flick test all genotypes except NET−/− SERT −/− had significant amitriptyline analgesia. As discussed in the text because of the exclusion of NET subjects because of high baseline analgesia these results must be considered tentatively.
Experiment 2: Thermal Nociceptive threshold in NET KO and SERT KO mice
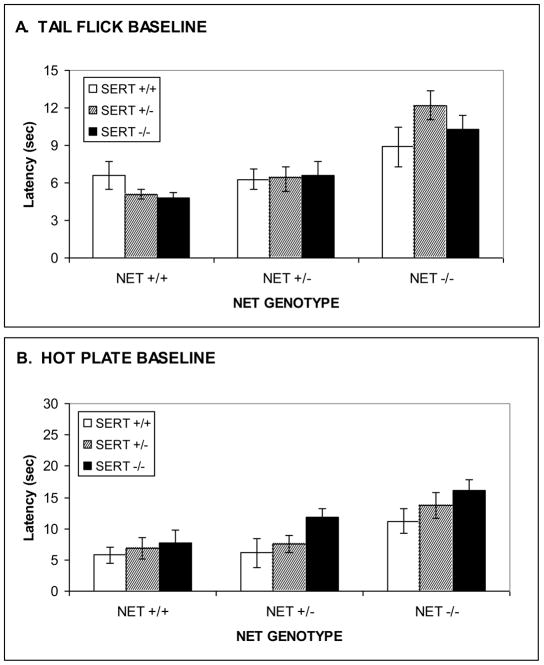
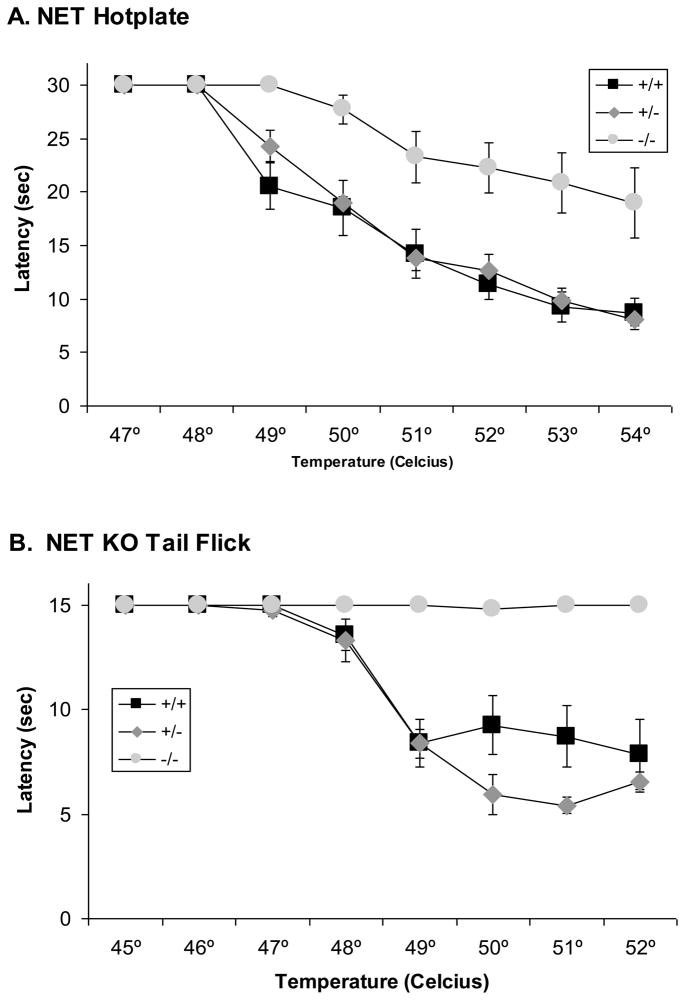
Because of the profound baseline hypoalgesia observed in the previous experiment, an additional experiment was performed to examine in more detail the sensitivity to thermal nociceptive stimuli in NET KO and SERT KO mice. In the hotplate test, initial nociceptive responses were found in NET +/+ mice at 49 °C and latencies decreased with increasing temperature to the lowest latency at 54 °C (Fig. 5A). A virtually identical pattern was observed in NET +/− mice. However, NET −/− did not exhibit any nociceptive responses at 49 °C. Nociceptive responses were found beginning at 50 °C. Latencies decreased with increasing temperature, but were substantially greater than responses in either NET +/+ or NET +/− mice at all temperatures from 49 °C to 54 °C. Thus, there were significant effects of TEMPERATURE (F[6,150]=87.9, p<0.001) and NET GENOTYPE (F[2,25]=17.7, p<0.0001) in the ANOVA, as well as a significant NET GENOTYPE x TEMPERATURE interaction (F[12,150]=3.5, p<0.0003).
Figure 5. Thermal nociceptive sensitivity in NET KO mice.
Reduced baseline nociceptive sensitivity observed in NET KO mice (+/+, +/− and −/−) for supraspinal analgesia in the hot-plate test (A), 47 ºC to 54 ºC, and spinal analgesia in the tail-flick test (B), 45 ºC to 52 ºC. Data represent response latencies (in seconds).
A similar pattern was observed in the tail-flick test. Indeed, NET −/− mice were almost totally unresponsive at all temperatures tested. Nociceptive responses were found in NET +/+ mice at 48 °C and latencies decreased with increasing temperature to the lowest latency at 51 °C (Fig. 5B). A virtually identical pattern was observed in NET +/− mice. Thus, there were significant effects of TEMPERATURE (F[6,150]=51.9, p<0.001) and NET GENOTYPE (F[2,25]=23.6, p<0.0001) in the ANOVA, as well as a significant NET GENOTYPE x TEMPERATURE interaction (F[12,150]=13.4, p<0.0001).
In the hotplate test initial nociceptive responses were found in SERT +/+ mice at 49 °C and latencies decreased with increasing temperature to the lowest latency at 54 °C (Fig. 6A). A similar pattern was observed in SERT +/− mice, indeed the latencies of SERT +/− mice were lower than SERT +/+ mice at 49 °C, but only marginally lower at higher temperatures. SERT −/− mice did not exhibit any nociceptive responses at 49 °C. Nociceptive responses were found beginning at 50 °C. Latencies decreased with increasing temperature, but were substantially greater than responses in either SERT +/+ or SERT +/− mice at 52 °C and 53 °C. Thus, there were significant effects of TEMPERATURE (F[6,156]=87.9, p<0.001) and SERT GENOTYPE (F[2,26]=9.4, p<0.001) in the ANOVA, as well as a significant SERT GENOTYPE x TEMPERATURE interaction (F[12,156]=5.4, p<0.0001).
Figure 6. Thermal nociceptive sensitivity in SERT KO mice.
No differences in baseline nociceptive sensitivity were observed in SERT KO (+/+, +/− and −/−) mice for supraspinal analgesia in the hot-plate test (A), 47 ºC to 54 ºC, or spinal analgesia in the tail-flick test (B), 45 ºC to 52 ºC. Data represent response latencies (in seconds).
A different pattern of effects was observed in the tail flick test in SERT −/− mice, which actually showed lower tail flick latencies at the highest temperatures tested. Nociceptive responses were found in SERT +/+ mice at 48 °C and latencies leveled off at approximately half maximal levels at 49 °C, with similar response between 49 °C and 52 °C (Fig. 6B). Both SERT +/− and SERT −/− mice had lower tail flick latencies at the highest temperatures tested, with the lowest latencies observed in SERT −/− mice, but the nociceptive threshold did not appear to be substantially altered. Thus, there was a significant effect of TEMPERATURE (F[6,156]=136.3, p<0.001), and SERT GENOTYPE (F[2,26]=5.9, p<0.008) in the ANOVA, as well as a significant SERT GENOTYPE x TEMPERATURE interaction (F[12,156]=3.8, p<0.0001), reflective of the lower latencies at the highest temperatures.
Experiment 3: Visceral Nociception in NET KO and SERT KO mice
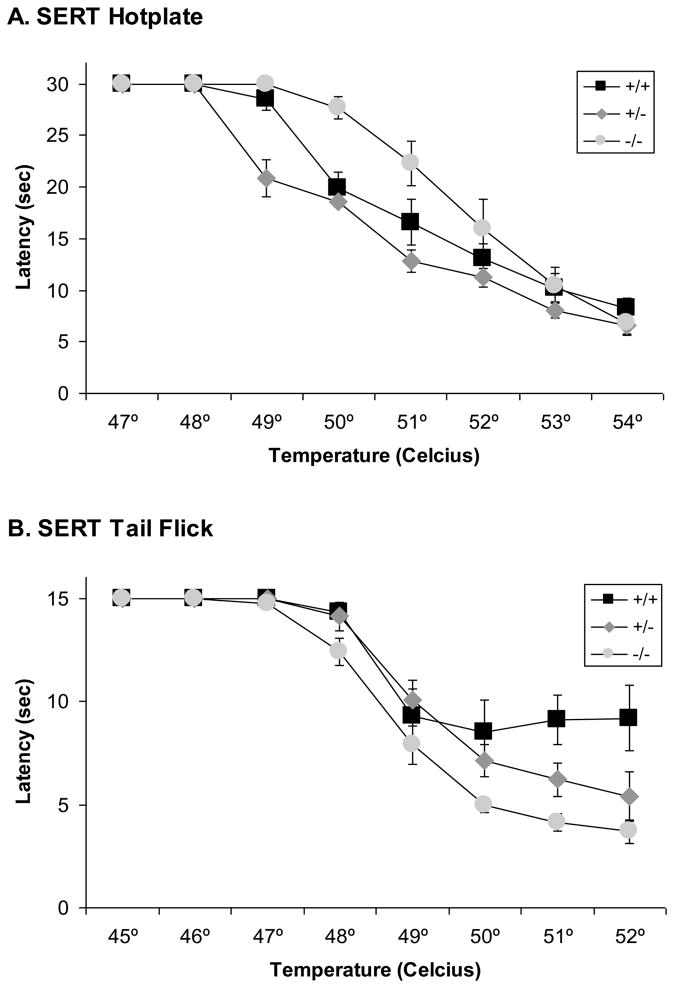
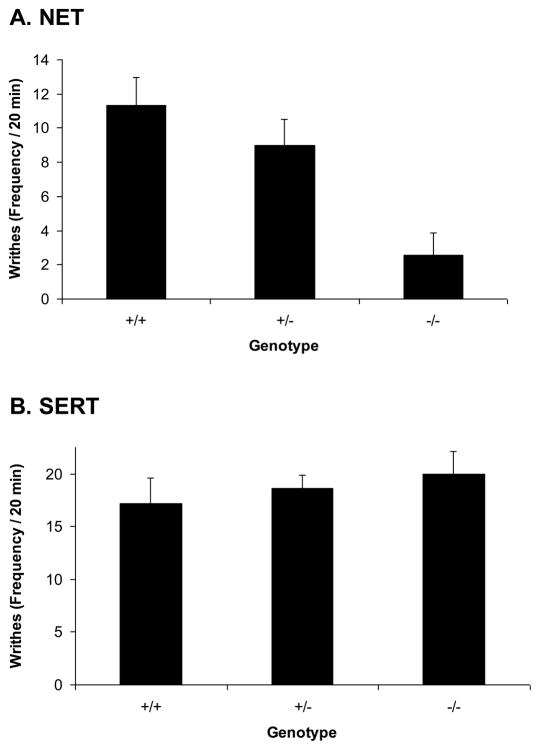
Administration of 0.7% acetic acid intraperitoneally induced distinctive writhing movements characteristic of visceral pain within about 5 minutes of injection in NET +/+ mice (Fig. 7A). The frequency of writhes was substantially reduced in NET −/− mice resulting in a significant effect of NET GENOTYPE in the ANOVA (F[2,20]=9.2, p<0.002). The frequency of writhes was slightly reduced in NET +/− mice, but not significantly compared to NET +/+ mice. Administration of 0.7% acetic acid intraperitoneally also induced distinctive writhing movements characteristic of visceral pain within about 5 minutes of injection in SERT +/+ mice (Fig. 7B). The frequency of writhes was unaffected by SERT GENOTYPE in the ANOVA (F[2,26]=0.5, ns).
Figure 7. Baseline visceral nociceptive responses in NET and SERT KO mice.
Reduced visceral nociceptive responses were observed in (A) NET KO mice (+/+, +/− and −/−), but not (B) SERT KO mice(+/+, +/− and −/−) after intraperitoneal injection with acetic acid. Data represent the number of writhes (abdominal contractions) observed in the first 20 minutes after injection.
Experiment 4: Nociceptive effects of Nisoxetine and Fluoxetine
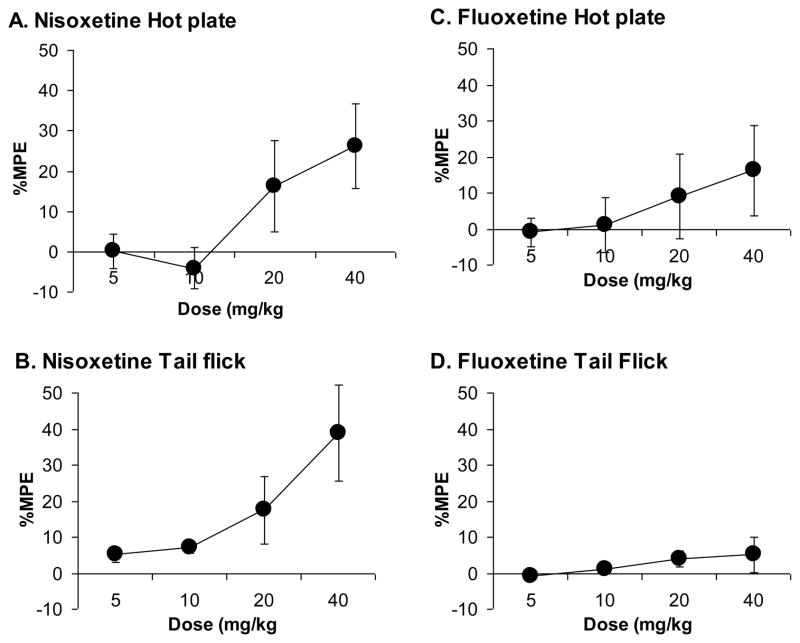
Nisoxetine produced significant analgesic effects in both the hotplate (Fig. 8A; F[3,24]=4.1, p<0.02) and tail-flick tests (Fig. 8B; F[3,27]=4.9, p<0.01). Fluoxetine failed to produce significant analgesic effects in either the hotplate (Fig. 8C; F[3,24]=1.7, ns) or tail-flick tests (Fig. 8D; F[3,27]=1.3, ns).
Figure 8. Analgesic effects of Nisoxetine but not Fluoxetine.
Nisoxetine produced significant analgesia in the hot-plate (8A) and tail-flick (8B) tests, but fluoxetine did not produce significant analgesia in either the hot-plate (8C) or tail-flick (8D) tests.
Experiment 5: Motor Tests in NET KO and SERT KO mice
No significant differences were found for either the accelerating rotorod test or the screen hang test in NET KO (Table 2) mice. SERT KO mice were found to have motor deficits in both tests (Table 3). There was a significant reduction in time on the accelerating rotorod in SERT KO mice (F[2,27]=4.3, p<0.03). There was an increase in time spent on the rotorod over the 3 days of testing resulting in a significant effect of DAY in the ANOVA (F[2,54]=3.8, p<0.03). Although the SERT GENOTYPE x DAY interaction was not significant (F[4,54]=0.6, NS), the increase in time over days was primarily observed in SERT −/− mice. Thus, there was a significant difference between SERT +/+ and SERT −/− mice on day 1 (p<0.05, Scheffe’s post hoc comparison), but not day 3. SERT +/− mice were not different from SERT +/+ mice at any point. The SERT KO mice were also impaired in the screen hang test (F[2,34]=5.2, p<0.02). Again, the SERT −/− mice, but not the SERT +/− mice were impaired compared to SERT +/+ mice (p<0.05, Scheffe’s post hoc comparison).
Table 2.
Effects of NET KO on performance in tests of motor function.
| Genotype | Accelerating Rotorod | Screen Hang | ||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| +/+ | 139.0 ± 29.8 | 172.5 ± 23.4 | 202.5 ± 24.5 | 80.7 ± 10.4 |
| +/− | 171.6 ± 22.1 | 213.3 ± 22.5 | 224.9 ± 19.4 | 100 ± 7.1 |
| −/− | 167.3 ± 29.9 | 214.8 ± 23.4 | 257 ± 22.6 | 92.7 ± 7.5 |
Table 3.
Effects of SERT KO on performance in tests of motor function.
| Genotype | Accelerating Rotorod | Screen Hang | ||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| +/+ | 244.8 ± 20.4 | 254.9 ± 21.8 | 264.3 ± 19.3 | 96.5 ± 10.0 |
| +/− | 207.2 ± 33.1 | 244.2 ± 37.4 | 264.5 ± 24.4 | 82.1 ± 13.0 |
| −/− | 137.9 ± 23.4* | 180.9 ± 26.9 | 212.2 ± 26.8 | 43.5 ± 12.0* |
p<0.05, Scheffe’s post hoc comparison.
Discussion
The main finding from these experiments is that NET gene KO produces profound baseline hypoalgesia; indeed, the hypoalgesia was so profound that it was difficult to assess morphine- and amitriptyline-induced analgesia in these mice. The magnitude of this effect was much greater than the mild hypoalgesia reported previously in NET KO mice (Bohn et al. 2000). The reason for the differences between that study and the present results are uncertain, but could involve either paradigmatic differences or genetic background differences between the two strains. However, both the Bohn et al. (2000) study and Experiment 1 utilized single temperatures that may not have provided an accurate estimation of the extent of differences in nociceptive thresholds in NET KO mice. Therefore, Experiment 2 was performed to more extensively examine differences in nociceptive thresholds in NET KO and SERT KO mice. The potential bases of the differences between these studies are discussed in detail below, as are the roles of NET and SERT in basal nociceptive thresholds. In addition to these findings with regard to the roles of these genes in baseline nociception these data also indicate roles for both NET and SERT in different aspects of morphine- and amitriptyline-induced analgesia. It is important to note that it appears unlikely that motor deficits account for these differences in NET KO mice, as no impairments were observed in the two motor tests in NET KO mice, while SERT KO mice, that showed far smaller and less ubiquitous changes in nociception, were substantially impaired in both motor tests.
There is substantial evidence for both NE and 5-HT involvement in nociception, although the relative importance of each neurotransmitter has been a matter of some debate. Descending analgesia circuits (see (Lipp 1991) for review) include both noradrenergic projections from the locus coeruleus (LC) to hindbrain and spinal levels, and serotonergic projections to spinal levels from the nucleus raphe magnus (NRM), which are both activated by periaqueductal gray (PAG) efferents. PAG stimulation leads to release of both 5-HT and NE in the spinal cord (Cui et al. 1999). Morphine injections in the PAG release 5-HT in the spinal cord (Yaksh and Tyce 1979), suggesting that 5-HT might act downstream from some opiate effects. Other descending cell groups have also been suggested to modulate nociception, including descending 5-HT projections from the rostral ventrolateral medulla (Siddall et al. 1994). Intracerebral opiates/opioids produce nociception at several of these sites as well (Jensen and Yaksh 1986; 1986), and lesions of descending bulbospinal projections eliminate some analgesic effects of TCAs (Ardid et al. 1995). There are two descending projections from the NRM, that have opposing actions on ascending pain projection neurons, and are differentially modulated by both opioidergic and noradrenergic inputs to the NRM (Bie et al. 2003). Collectively, this circuitry provides multiple potential sites of action for serotonergic and noradrenergic agents to affect nociceptive responses.
The role of SERT and NET in basal pain sensitivity
A previous study in NET KO mice (Bohn et al. 2000) found mild hypoalgesia in the tail-flick test, but not the hotplate test. In the present study, profound hypoalgesia was observed in NET KO mice in both of these tests as well as a test of visceral nociception. It appears likely that these differences can be explained in part by the different intensities of the thermal stimuli used in each of these studies. The previous study used higher intensity stimuli in both tests, with consequently lower latencies, such that even NET KO mice may have been near-maximally responsive to the nociceptive stimulus, obscuring group differences that were readily apparent in the present study when less intense nociceptive stimuli were used. However, given the magnitude of these effects, particularly in the tail-flick test, this may be only a partial explanation. It is additionally possible that differences in genetic background (e.g. particular C57B6/J and 129S alleles), in the combined double KO line used in these experiments compared to those in the original NET KO line used in the Bohn et al. (2000) study, might have enhanced the effect of NET KO on baseline pain sensitivity in the present experiments. These strains vary substantially in baseline pain sensitivity (Mogil and Wilson 1997; Mogil et al. 1999).
Consistent with the finding of enhanced basal pain thresholds in NET KO mice, which have greatly elevated extracellular NE levels (Xu et al. 2000), is the hyperalgesia produced by dopamine β-hydroxylase (DBH) gene knockout (Jasmin et al. 2002) which eliminates NE from the nervous system. There is also substantial pharmacological evidence that NE systems are involved in setting baseline nociceptive tone. For instance, intrathecal NE (Eide and Hole 1992), and administration of the α2 adrenergic receptor agonist clonidine, is analgesic (Davis et al. 1991; Eide and Hole 1992; Naguib and Yaksh 1994; Eisenach and Gebhart 1995; Eisenach 1996; De Kock et al. 1997; Buerkle and Yaksh 1998; Eisenach et al. 1998; Chiari et al. 1999; Pan et al. 1999; Baker et al. 2004). Stimulation of α2 adrenergic receptors can also reverse the hyperalgesia observed in DBH KO mice (Jasmin et al. 2002). Treatment with antisense oligonucleotides targeting the α subunit of Gi1/2/3 proteins coupled to α2 adrenergic receptors also reduces the analgesic effects of amitriptyline (Ghelardini et al. 2002).
In addition to the pronounced hypoalgesia observed in NET −/− mice, differences in baseline pain sensitivity were also observed in SERT −/− mice, although to a far lesser degree. Hypoalgesia was observed in the hotplate test in SERT −/− mice, although at the highest temperature all genotypes responded equally. In the tail-flick test SERT −/− mice actually responded faster although this appeared to be more of a decrease in the minimal tail flick latency and did not seem to be substantially dependent on temperature. Although some effects were observed in the baseline habituation prior to morphine or amitriptyline treatment the higher temperatures used in those experiments generally negated all but the extremely pronounced NET −/− effects. These data are contrary to a previous study that found no differences in baseline hypoalgesia in SERT KO mice to several types of nociceptive stimuli (Vogel et al. 2003). The mice used in those experiments were on a C57Bl/6J background, a strain known to have very low nociceptive thresholds (Mogil and Wilson 1997; Mogil et al. 1999), in contrast to the mixed background used in the present experiments. Nonetheless, this serves to indicate that the role of SERT in determining baseline sensitivity is modest, and perhaps more important when interacting with other factors. Indeed, although SERT KO did not affect baseline pain sensitivity, it did greatly attenuate thermal hyperalgesia induced by sciatic nerve ligation (Vogel et al. 2003) and lead to hyperalgesia in the hot plate test when combined with deletion of a single BDNF gene allele (Ren-Patterson et al. 2005).
The role of SERT and NET in amitriptyline-induced analgesia
Several TCA compounds (Spiegel et al. 1983; Hwang and Wilcox 1987; Sacerdote et al. 1987; Hersh and Kaplan 1990; Tura and Tura 1990; Ventafridda et al. 1990; Ardid et al. 1991; Acton et al. 1992; Ardid et al. 1992; Ardid et al. 1995; Sawynok et al. 1999; Korzeniewska-Rybicka and Plaznik 2000; Sawynok and Reid 2001), including amitriptyline (Spiegel et al. 1983; Sacerdote et al. 1987; Tura and Tura 1990; Ventafridda et al. 1990; Eisenach and Gebhart 1995; Abdi et al. 1998; Esser and Sawynok 1999; Sawynok et al. 1999; Esser and Sawynok 2000; Korzeniewska-Rybicka and Plaznik 2000; Esser et al. 2001; Sawynok and Reid 2001; Heughan and Sawynok 2002; Khan et al. 2002; Oatway et al. 2003; Luccarini et al. 2004; Sudoh et al. 2004), have analgesic, antiallodynic or antihyperalgesic effects in animal models. These preclinical findings are consistent with clinical data showing that administration of several TCAs produce clinically significant analgesic, antiallodynic or antihyperalgesic effects in humans (Watson 1984; Young and Clarke 1985; Levine et al. 1986; Max et al. 1987; Frank et al. 1988; Onghena and Van Houdenhove 1992; Watson et al. 1992; Gordon et al. 1994; Nicolodi and Sicuteri 1996; Richeimer et al. 1997; Gerner et al. 2003); also see reviews by (Egbunike and Chaffee 1990; Carter and Sullivan 2002), including amitriptyline (Frank et al. 1988; Nicolodi and Sicuteri 1996; Gerner et al. 2003). Although data from both animal and human studies support the potential involvement of both NE and 5-HT in the analgesic effects of TCAs, one study that compared the effects of selective NET, SERT and DAT reuptake blockers found weak analgesic effects only for fluoxetine (Gatch et al. 1998), which may indicate that actions at multiple monoamine transporters are necessary for pronounced analgesic effects. However, we examined the analgesic effects of selective SERT blocker fluoxetine and did not observe significant analgesic effects, but significant analgesia was produced by the selective NET blocker nisoxetine. Obviously further work is necessary to determine why such contradictory results were obtained from the Gatch et al (1998) study, but given some of the other observations presented here it may be likely that genetic background is an important factor.
In part, the intention of the experimental design used in the present experiments was to determine the requirement of SERT and/or NET for the analgesic effects of amitriptyline, in the same way that we have used these knockouts to determine the role of these monoamine targets in the rewarding effects of cocaine (Sora et al. 1998; Sora et al. 2001; Hall et al. 2002). This is an important question since a number of non-monoaminergic mechanisms have been proposed for the analgesic effects of TCAs, including blockade of voltage-gated sodium channels (Pancrazio et al. 1998), NMDA glutamate receptor antagonism (Eisenach and Gebhart 1995), and interaction with adenosine mechanisms (Sawynok et al. 1999). Nonetheless, one possibility was that under baseline conditions monoamine transporter KO mice might behave as though they have been treated with amitriptyline, obscuring subsequent effects of drug treatment. Under baseline conditions extracellular levels of NE are elevated in NET KO mice (Mathews et al. 2004), and 5-HT levels are elevated in SERT KO mice (Mathews et al. 2004; Shen et al. 2004). Thus, although neither SERT nor NET KO individually had pronounced effects on amitriptyline analgesia per se, it might be considered that these effects were already present, particularly in NET KO mice because of the pronounced baseline hypoalgesia that was observed. Although post hoc analysis found that double KO mice did not have significant analgesia, this finding must be treated with a great degree of caution because baseline hypoalgesia necessitated dropping so many subjects from the study. It would then appear likely that NET blockade by amitriptyline is a likely mediator of a substantial portion of the analgesic effects of amitriptyline; however, other experimental approaches will be necessary to confirm this hypothesis.
The role of SERT and NET in morphine-induced analgesia
Several TCA compounds (Rigal et al. 1983; Taiwo et al. 1985; Max et al. 1987; Sacerdote et al. 1987; Ventafridda et al. 1990; Sawynok and Reid 1992; Gatch et al. 1998), including amitriptyline (Malseed and Goldstein 1979; Liu and Wang 1981; Botney and Fields 1983; Taiwo et al. 1985; Sacerdote et al. 1987; Ventafridda et al. 1990; Eisenach and Gebhart 1995; Galeotti et al. 1996; Luccarini et al. 2004), enhance morphine-induced analgesia in animal models. This is consistent with clinical data, which has demonstrated that several TCAs potentiate morphine-induced analgesia in humans (Levine et al. 1986; Gordon et al. 1993). Consistent with this modulatory role of NE and 5-HT systems both NE and 5-HT antagonists can reduce the antinociceptive effects of morphine (Jensen and Yaksh 1986; Wigdor and Wilcox 1987; Arts et al. 1991). Indeed, several TCAs increase β-endorphin levels in the hypothalamus (Sacerdote et al. 1987). Consistent with a previous finding in NET KO mice (Bohn et al. 2000), the present results also found that NET KO did produce a small enhancement of morphine analgesia, which appeared to be reversed in NET/SERT combined KO mice. However, as stated previously, conclusions about the effects of morphine in NET KO mice based on the present results must be interpreted cautiously due to the necessity of removing so many NET KO mice from those portions of the study.
Constitutive knockouts are likely to induce a variety of types of compensatory changes. Changes in many systems that might be most relevant to nociception and analgesia have not yet been examined in NET and SERT KO mice. However, a potentially informative comparison may be to the effects of long-term antidepressant treatment. Chronic treatment with amitriptyline increases spinal, hypothalamic and cerebral cortical levels of leu-enkephalin, and spinal and hypothalamic levels of met-enkephalin (Hamon et al. 1987), and increases spinal delta and mu receptor binding (Hamon et al. 1987). It remains to be seen whether such changes are observed in NET KO mice. In a study specifically examining the analgesic effects of selective monoamine reuptake blockers, only those acting at SERT, and not at NET or DAT, potentiated the effects of opiate agonists in a manner similar to TCAs (Gatch et al. 1998). This was dissimilar to the current study where SERT KO had no effect on morphine analgesia.
Conclusions
The present data support the importance of both NE and 5-HT in different aspects of nociceptive function. NET, SERT and combined NET/SERT gene KO had differential effects on basal, morphine- and amitriptyline-stimulated analgesia. NET gene KO had a pronounced influence on baseline thermal and visceral nociception, while SERT KO had rather marginal and inconsistent effects. Indeed, the effect of NET KO was so great that it impaired the ability to subsequently observe pharmacologically induced analgesia in these subjects. Nonetheless, consistent with previous results (Bohn et al. 2000), we were able to observe enhanced morphine induced analgesia in NET KO mice. Although one of the initial goals of this study, to examine the role of NET and SERT genes in amitriptyline-induced analgesia, was compromised by the profound hypoalgesia in NET KO mice, it did appear that deletion of both genes eliminated the analgesic effects of amitriptyline. The effects of NET deletion may have been less pronounced because of the baseline hypoalgesia observed in these subjects (the NET portion of those analgesic effects was already present, thus negating the effects of amitriptyline). Although any such conclusion must be interpreted with great caution given the considerations discussed at length above, deletion of either NET or SERT alone was not sufficient to eliminate amitriptyline analgesia which may indicate that the presence of either gene is sufficient for amitriptyline analgesia, and furthermore other potential molecular targets of amitriptyline may not be relevant. A definitive conclusion regarding this issue will necessitate an alternative approach.
Acknowledgments
This work was supported by funding from the Intramural Research Program of the National Institute on Drug Abuse (USA). We gratefully acknowledge animal care support from the Charles River/Triad animal care support section, especially our breeder/geneticist Kriss Knestaut. Experiments were conducted under protocols approved by the NIDA Intramural Animal Care and Use Committee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg. 1998;87(6):1360–1366. [PubMed] [Google Scholar]
- Acton J, McKenna JE, Melzack R. Amitriptyline produces analgesia in the formalin pain test. Exp Neurol. 1992;117(1):94–96. doi: 10.1016/0014-4886(92)90116-8. [DOI] [PubMed] [Google Scholar]
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31(1):123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Ardid D, Eschalier A, Lavarenne J. Evidence for a central but not a peripheral analgesic effect of clomipramine in rats. Pain. 1991;45(1):95–100. doi: 10.1016/0304-3959(91)90169-X. [DOI] [PubMed] [Google Scholar]
- Ardid D, Jourdan D, Mestre C, Villanueva L, Le Bars D, Eschalier A. Involvement of bulbospinal pathways in the antinociceptive effect of clomipramine in the rat. Brain Res. 1995;695(2):253–256. doi: 10.1016/0006-8993(95)00826-c. [DOI] [PubMed] [Google Scholar]
- Ardid D, Marty H, Fialip J, Privat AM, Eschalier A, Lavarenne J. Comparative effects of different uptake inhibitor antidepressants in two pain tests in mice. Fundam Clin Pharmacol. 1992;6(2):75–82. doi: 10.1111/j.1472-8206.1992.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Arts KS, Holmes BB, Fujimoto JM. Differential contribution of descending serotonergic and noradrenergic systems to central Tyr-D-Ala2-Gly-NMePhe4-Gly-ol5 (DAMGO) and morphine- induced antinociception in mice. J Pharmacol Exp Ther. 1991;256(3):890–896. [PubMed] [Google Scholar]
- Baker A, Klimscha W, Eisenach JC, Li XH, Wildling E, Menth-Chiari WA, Chiari AI. Intrathecal clonidine for postoperative analgesia in elderly patients: the influence of baricity on hemodynamic and analgesic effects. Anesth Analg. 2004;99(1):128–134. doi: 10.1213/01.ANE.0000114549.17864.36. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4- methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter- deficient mice. Mol Pharmacol. 1998;53(4):649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bie B, Fields HL, Williams JT, Pan ZZ. Roles of alpha1- and alpha2-adrenoceptors in the nucleus raphe magnus in opioid analgesia and opioid abstinence-induced hyperalgesia. J Neurosci. 2003;23(21):7950–7957. doi: 10.1523/JNEUROSCI.23-21-07950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci. 2000;20(24):9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botney M, Fields HL. Amitriptyline potentiates morphine analgesia by a direct action on the central nervous system. Ann Neurol. 1983;13(2):160–164. doi: 10.1002/ana.410130209. [DOI] [PubMed] [Google Scholar]
- Buerkle H, Yaksh TL. Pharmacological evidence for different alpha 2-adrenergic receptor sites mediating analgesia and sedation in the rat. Br J Anaesth. 1998;81(2):208–215. doi: 10.1093/bja/81.2.208. [DOI] [PubMed] [Google Scholar]
- Carter GT, Sullivan MD. Antidepressants in pain management. Curr Opin Investig Drugs. 2002;3(3):454–458. [PubMed] [Google Scholar]
- Chiari A, Lorber C, Eisenach JC, Wildling E, Krenn C, Zavrsky A, Kainz C, Germann P, Klimscha W. Analgesic and hemodynamic effects of intrathecal clonidine as the sole analgesic agent during first stage of labor: a dose-response study. Anesthesiology. 1999;91(2):388–396. doi: 10.1097/00000542-199908000-00012. [DOI] [PubMed] [Google Scholar]
- Cui M, Feng Y, McAdoo DJ, Willis WD. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J Pharmacol Exp Ther. 1999;289(2):868–876. [PubMed] [Google Scholar]
- Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47(3):309–317. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- De Kock M, Wiederkher P, Laghmiche A, Scholtes JL. Epidural clonidine used as the sole analgesic agent during and after abdominal surgery. A dose-response study. Anesthesiology. 1997;86(2):285–292. doi: 10.1097/00000542-199702000-00003. [DOI] [PubMed] [Google Scholar]
- Egbunike IG, Chaffee BJ. Antidepressants in the management of chronic pain syndromes. Pharmacotherapy. 1990;10(4):262–270. [PubMed] [Google Scholar]
- Eide K, Hole K. Interactions between substance P and norepinephrine in the regulation of nociception in mouse spinal cord. Pharmacol Toxicol. 1992;70(6 Pt 1):397–401. doi: 10.1111/j.1600-0773.1992.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Eisenach JC. Three novel spinal analgesics: clonidine, neostigmine, amitriptyline. Reg Anesth. 1996;21(6 Suppl):81–83. [PubMed] [Google Scholar]
- Eisenach JC, Gebhart GF. Intrathecal amitriptyline acts as an N-methyl-D-aspartate receptor antagonist in the presence of inflammatory hyperalgesia in rats. Anesthesiology. 1995;83(5):1046–1054. doi: 10.1097/00000542-199511000-00018. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Gebhart GF. Intrathecal amitriptyline. Antinociceptive interactions with intravenous morphine and intrathecal clonidine, neostigmine, and carbamylcholine in rats. Anesthesiology. 1995;83(5):1036–1045. doi: 10.1097/00000542-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Hood DD, Curry R. Intrathecal, but not intravenous, clonidine reduces experimental thermal or capsaicin-induced pain and hyperalgesia in normal volunteers. Anesth Analg. 1998;87(3):591–596. doi: 10.1097/00000539-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Evans JL, Ladenheim B, Epstein CJ, Cadet JL. Transgenic superoxide dismutase mice differ in opioid-induced analgesia. Eur J Pharmacol. 1995;283(1–3):227–232. doi: 10.1016/0014-2999(95)00365-r. [DOI] [PubMed] [Google Scholar]
- Esser MJ, Chase T, Allen GV, Sawynok J. Chronic administration of amitriptyline and caffeine in a rat model of neuropathic pain: multiple interactions. Eur J Pharmacol. 2001;430(2–3):211–218. doi: 10.1016/s0014-2999(01)01276-6. [DOI] [PubMed] [Google Scholar]
- Esser MJ, Sawynok J. Acute amitriptyline in a rat model of neuropathic pain: differential symptom and route effects. Pain. 1999;80(3):643–653. doi: 10.1016/S0304-3959(98)00261-9. [DOI] [PubMed] [Google Scholar]
- Esser MJ, Sawynok J. Caffeine blockade of the thermal antihyperalgesic effect of acute amitriptyline in a rat model of neuropathic pain. Eur J Pharmacol. 2000;399(2–3):131–139. doi: 10.1016/s0014-2999(00)00336-8. [DOI] [PubMed] [Google Scholar]
- Frank RG, Kashani JH, Parker JC, Beck NC, Brownlee-Duffeck M, Elliott TR, Haut AE, Atwood C, Smith E, Kay DR. Antidepressant analgesia in rheumatoid arthritis. J Rheumatol. 1988;15(11):1632–1638. [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Bartolini A. Effect of pertussis toxin on morphine, diphenhydramine, baclofen, clomipramine and physostigmine antinociception. Eur J Pharmacol. 1996;308(2):125–133. doi: 10.1016/0014-2999(96)00299-3. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C, Bartolini A. Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology. 2001;40(1):75–84. doi: 10.1016/s0028-3908(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Negus SS, Mello NK. Antinociceptive effects of monoamine reuptake inhibitors administered alone or in combination with mu opioid agonists in rhesus monkeys. Psychopharmacology (Berl) 1998;135(1):99–106. doi: 10.1007/s002130050490. [DOI] [PubMed] [Google Scholar]
- Gerner P, Kao G, Srinivasa V, Narang S, Wang GK. Topical amitriptyline in healthy volunteers. Reg Anesth Pain Med. 2003;28(4):289–293. doi: 10.1016/s1098-7339(03)00209-8. [DOI] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Bartolini A. Amitriptyline and clomipramine activate Gi-protein signaling pathway in the induction of analgesia. Naunyn Schmiedebergs Arch Pharmacol. 2002;365(1):1–7. doi: 10.1007/s00210-001-0496-8. [DOI] [PubMed] [Google Scholar]
- Gordon NC, Heller PH, Gear RW, Levine JD. Temporal factors in the enhancement of morphine analgesia by desipramine. Pain. 1993;53(3):273–276. doi: 10.1016/0304-3959(93)90223-C. [DOI] [PubMed] [Google Scholar]
- Gordon NC, Heller PH, Gear RW, Levine JD. Interactions between fluoxetine and opiate analgesia for postoperative dental pain. Pain. 1994;58(1):85–88. doi: 10.1016/0304-3959(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115(1):153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Hamon M, Gozlan H, Bourgoin S, Benoliel JJ, Mauborgne A, Taquet H, Cesselin F, Mico JA. Opioid receptors and neuropeptides in the CNS in rats treated chronically with amoxapine or amitriptyline. Neuropharmacology. 1987;26(6):531–539. doi: 10.1016/0028-3908(87)90144-4. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Kaplan P. Antinociceptive action of tricyclic antidepressant drugs in the rat. Anesth Prog. 1990;37(4):186–189. [PMC free article] [PubMed] [Google Scholar]
- Heughan CE, Sawynok J. The interaction between gabapentin and amitriptyline in the rat formalin test after systemic administration. Anesth Analg. 2002;94(4):975–980. doi: 10.1097/00000539-200204000-00037. table of contents. [DOI] [PubMed] [Google Scholar]
- Hwang AS, Wilcox GL. Analgesic properties of intrathecally administered heterocyclic antidepressants. Pain. 1987;28(3):343–355. doi: 10.1016/0304-3959(87)90068-6. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Tien D, Weinshenker D, Palmiter RD, Green PG, Janni G, Ohara PT. The NK1 receptor mediates both the hyperalgesia and the resistance to morphine in mice lacking noradrenaline. Proc Natl Acad Sci U S A. 2002;99(2):1029–1034. doi: 10.1073/pnas.012598599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Res. 1986;363(1):99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of the antinociceptive action of mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rat as studied by the microinjection technique. Brain Res. 1986;372(2):301–312. doi: 10.1016/0006-8993(86)91138-8. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Examination of spinal monoamine receptors through which brainstem opiate-sensitive systems act in the rat. Brain Res. 1986;363(1):114–127. doi: 10.1016/0006-8993(86)90663-3. [DOI] [PubMed] [Google Scholar]
- Khan MA, Gerner P, Kuo Wang G. Amitriptyline for prolonged cutaneous analgesia in the rat. Anesthesiology. 2002;96(1):109–116. doi: 10.1097/00000542-200201000-00023. [DOI] [PubMed] [Google Scholar]
- Korzeniewska-Rybicka I, Plaznik A. Supraspinally mediated analgesic effect of antidepressant drugs. Pol J Pharmacol. 2000;52(2):93–99. [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Smith R, McBryde R. Desipramine enhances opiate postoperative analgesia. Pain. 1986;27(1):45–49. doi: 10.1016/0304-3959(86)90220-4. [DOI] [PubMed] [Google Scholar]
- Lipp J. Possible mechanisms of morphine analgesia. Clin Neuropharmacol. 1991;14(2):131–147. doi: 10.1097/00002826-199104000-00003. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Wang RI. Increased sensitivity of the central nervous system to morphine analgesia by amitriptyline in naive and morphine-tolerant rats. Biochem Pharmacol. 1981;30(15):2103–2109. doi: 10.1016/0006-2952(81)90229-x. [DOI] [PubMed] [Google Scholar]
- Luccarini P, Perrier L, Degoulange C, Gaydier AM, Dallel R. Synergistic antinociceptive effect of amitriptyline and morphine in the rat orofacial formalin test. Anesthesiology. 2004;100(3):690–696. doi: 10.1097/00000542-200403000-00033. [DOI] [PubMed] [Google Scholar]
- Malseed RT, Goldstein FJ. Enhancement of morphine analgesia by tricyclic antidepressants. Neuropharmacology. 1979;18(10):827–829. doi: 10.1016/0028-3908(79)90028-5. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140(1–2):169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, Dubner R. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37(4):589–596. doi: 10.1212/wnl.37.4.589. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG. Nociceptive and morphine antinociceptive sensitivity of 129 and C57BL/6 inbred mouse strains: implications for transgenic knock-out studies. Eur J Pain. 1997;1(4):293–297. doi: 10.1016/s1090-3801(97)90038-0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80(1–2):67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Naguib M, Yaksh TL. Antinociceptive effects of spinal cholinesterase inhibition and isobolographic analysis of the interaction with mu and alpha 2 receptor systems. Anesthesiology. 1994;80(6):1338–1348. doi: 10.1097/00000542-199406000-00022. [DOI] [PubMed] [Google Scholar]
- Nicolodi M, Sicuteri F. Fibromyalgia and migraine, two faces of the same mechanism. Serotonin as the common clue for pathogenesis and therapy. Adv Exp Med Biol. 1996;398:373–379. doi: 10.1007/978-1-4613-0381-7_58. [DOI] [PubMed] [Google Scholar]
- Oatway M, Reid A, Sawynok J. Peripheral antihyperalgesic and analgesic actions of ketamine and amitriptyline in a model of mild thermal injury in the rat. Anesth Analg. 2003;97(1):168–173. doi: 10.1213/01.ane.0000067406.52093.bf. table of contents. [DOI] [PubMed] [Google Scholar]
- Onghena P, Van Houdenhove B. Antidepressant-induced analgesia in chronic non-malignant pain: a meta-analysis of 39 placebo-controlled studies. Pain. 1992;49(2):205–219. doi: 10.1016/0304-3959(92)90144-Z. [DOI] [PubMed] [Google Scholar]
- Pan HL, Chen SR, Eisenach JC. Intrathecal clonidine alleviates allodynia in neuropathic rats: interaction with spinal muscarinic and nicotinic receptors. Anesthesiology. 1999;90(2):509–514. doi: 10.1097/00000542-199902000-00027. [DOI] [PubMed] [Google Scholar]
- Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C., 3rd Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 1998;284(1):208–214. [PubMed] [Google Scholar]
- Pasternak GW, Gintzler AR, Houghten RA, Ling GS, Goodman RR, Spiegel K, Nishimura S, Johnson N, Recht LD. Biochemical and pharmacological evidence for opioid receptor multiplicity in the central nervous system. Life Sci. 1983;33 (Suppl 1):167–173. doi: 10.1016/0024-3205(83)90470-8. [DOI] [PubMed] [Google Scholar]
- Perona MT, Waters S, Hall FS, Sora I, Lesch KP, Murphy DL, Caron M, Uhl GR. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav Pharmacol. 2008;19(5–6):566–574. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Sherrill S, Huang SJ, Tolliver T, Lesch KP, Lu B, Murphy DL. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J Neurosci Res. 2005;79(6):756–771. doi: 10.1002/jnr.20410. [DOI] [PubMed] [Google Scholar]
- Richeimer SH, Bajwa ZH, Kahraman SS, Ransil BJ, Warfield CA. Utilization patterns of tricyclic antidepressants in a multidisciplinary pain clinic: a survey. Clin J Pain. 1997;13(4):324–329. doi: 10.1097/00002508-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Rigal F, Eschalier A, Devoize JL, Pechadre JC. Activities of five antidepressants in a behavioral pain test in rats. Life Sci. 1983;32(26):2965–2971. doi: 10.1016/0024-3205(83)90647-1. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Brini A, Mantegazza P, Panerai AE. A role for serotonin and beta-endorphin in the analgesia induced by some tricyclic antidepressant drugs. Pharmacol Biochem Behav. 1987;26(1):153–158. doi: 10.1016/0091-3057(87)90548-x. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Esser MJ, Reid AR. Peripheral antinociceptive actions of desipramine and fluoxetine in an inflammatory and neuropathic pain test in the rat. Pain. 1999;82(2):149–158. doi: 10.1016/S0304-3959(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Esser MJ, Reid AR. Antidepressants as analgesics: an overview of central and peripheral mechanisms of action. J Psychiatry Neurosci. 2001;26(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Effect of 6-hydroxydopamine-induced lesions to ascending and descending noradrenergic pathways on morphine analgesia. Brain Res. 1987;419(1–2):156–165. doi: 10.1016/0006-8993(87)90579-8. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Lesions to ascending noradrenergic and serotonergic pathways modify antinociception produced by intracerebroventricular administration of morphine. Neuropharmacology. 1989;28(2):141–147. doi: 10.1016/0028-3908(89)90050-6. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Desipramine potentiates spinal antinociception by 5-hydroxytryptamine, morphine and adenosine. Pain. 1992;50(1):113–118. doi: 10.1016/0304-3959(92)90118-U. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Antinociception by tricyclic antidepressants in the rat formalin test: differential effects on different behaviours following systemic and spinal administration. Pain. 2001;93(1):51–59. doi: 10.1016/S0304-3959(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid AR, Esser MJ. Peripheral antinociceptive action of amitriptyline in the rat formalin test: involvement of adenosine. Pain. 1999;80(1–2):45–55. doi: 10.1016/s0304-3959(98)00195-x. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29(10):1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Polson JW, Dampney RA. Descending antinociceptive pathway from the rostral ventrolateral medulla: a correlative anatomical and physiological study. Brain Res. 1994;645(1–2):61–68. doi: 10.1016/0006-8993(94)91638-1. [DOI] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR. Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology. 2001;25(1):41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98(9):5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95(13):7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Kalb R, Pasternak GW. Analgesic activity of tricyclic antidepressants. Ann Neurol. 1983;13(4):462–465. doi: 10.1002/ana.410130418. [DOI] [PubMed] [Google Scholar]
- Sudoh Y, Desai SP, Haderer AE, Sudoh S, Gerner P, Anthony DC, De Girolami U, Wang GK. Neurologic and histopathologic evaluation after high-volume intrathecal amitriptyline. Reg Anesth Pain Med. 2004;29(5):434–440. doi: 10.1016/j.rapm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Fabian A, Pazoles CJ, Fields HL. Potentiation of morphine antinociception by monoamine reuptake inhibitors in the rat spinal cord. Pain. 1985;21(4):329–337. doi: 10.1016/0304-3959(85)90162-9. [DOI] [PubMed] [Google Scholar]
- Tura B, Tura SM. The analgesic effect of tricyclic antidepressants. Brain Res. 1990;518(1–2):19–22. doi: 10.1016/0006-8993(90)90948-b. [DOI] [PubMed] [Google Scholar]
- Ventafridda V, Bianchi M, Ripamonti C, Sacerdote P, De Conno F, Zecca E, Panerai AE. Studies on the effects of antidepressant drugs on the antinociceptive action of morphine and on plasma morphine in rat and man. Pain. 1990;43(2):155–162. doi: 10.1016/0304-3959(90)91068-T. [DOI] [PubMed] [Google Scholar]
- Vogel C, Mossner R, Gerlach M, Heinemann T, Murphy DL, Riederer P, Lesch KP, Sommer C. Absence of thermal hyperalgesia in serotonin transporter-deficient mice. J Neurosci. 2003;23(2):708–715. doi: 10.1523/JNEUROSCI.23-02-00708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CP. Therapeutic window for amitriptyline analgesia. Can Med Assoc J. 1984;130(2):105–106. [PMC free article] [PubMed] [Google Scholar]
- Watson CP, Chipman M, Reed K, Evans RJ, Birkett N. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomized, double-blind, crossover trial. Pain. 1992;48(1):29–36. doi: 10.1016/0304-3959(92)90128-X. [DOI] [PubMed] [Google Scholar]
- Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther. 1987;242(1):90–95. [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3(5):465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Direct evidence that spinal serotonin and noradrenaline terminals mediate the spinal antinociceptive effects of morphine in the periaqueductal gray. Brain Res. 1979;160(1):180–185. doi: 10.1016/0006-8993(79)90616-4. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Tyce GM. Microinjection of morphine into the periaqueductal gray evokes the release of serotonin from spinal cord. Brain Res. 1979;171(1):176–181. doi: 10.1016/0006-8993(79)90747-9. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Clark FM, Paice JA, Proudfit HK. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain. 1992;48(3):449–461. doi: 10.1016/0304-3959(92)90098-V. [DOI] [PubMed] [Google Scholar]
- Young RJ, Clarke BF. Pain relief in diabetic neuropathy: the effectiveness of imipramine and related drugs. Diabet Med. 1985;2(5):363–366. doi: 10.1111/j.1464-5491.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Zhong FX, Ji XQ, Tsou K. Intrathecal DSP4 selectively depletes spinal noradrenaline and attenuates morphine analgesia. Eur J Pharmacol. 1985;116(3):327–330. doi: 10.1016/0014-2999(85)90171-2. [DOI] [PubMed] [Google Scholar]