Abstract
In the tumor microenvironment, monocytes respond to paracrine stimuli from breast cancer cells by secreting molecules that participate in breast cancer growth, invasion, intravasation and metastasis. Here we examined the effects of media conditioned by MDA-MB-231 human breast carcinoma cells (231-CM) on expression and secretion of proteases and secretion of cytokines by U937 human monocytes. We found that 231-CM increased U937: 1) proliferation; 2) expression, activity and secretion of the cysteine protease cathepsin B (CTSB); 3) secretion of matrix metalloproteinases (MMP)-2 and -9; and 4) secretion of interleukin-6 (IL-6) and insulin-like growth factor binding protein-1 (IGFBP-1). We further demonstrated by western blotting and enzymatic activity assays that the increases in CTSB secretion and activity induced by 231-CM could be reduced by neutralizing antibodies against IL-6. Our data suggest a role for IL-6 in increased monocyte expression and secretion of CTSB in response to soluble factors secreted by breast cancer cells.
Key Words: Monocytes, Breast cancer, IL-6, Proteases, Tumor microenvironment
Introduction
Inflammatory cells enhance tumor growth, invasion and metastasis [1]. Infiltration of monocytes correlates with poor prognosis of breast cancers [2, 3]. The monocytes are recruited by cytokines and chemokines such as macrophage colony stimulating factor (M-CSF/CSF-1) [4, 5] and monocyte chemoattractant protein (MCP-1) [6] that are highly expressed by the breast tumor cells. In the tumor microenvironment, the monocytes undergo activation and differentiation and are designated tumor-educated or tumor-associated macrophages (TAMs) (for review, see [7] and [8]).
The immunological functions of macrophages include phagocytosis, antigen presentation, production of cytokines and proteases and recruitment of T-cells to sites of inflammation [9, 10]. In the tumor microenvironment, TAMs play dual roles as: 1) 'classically activated macrophages' that secrete pro-inflammatory mediators and recruit T-cells as in an early inflammatory response [5], and 2) 'regulatory macrophages' that express antiinflammatory cytokines and increase tumor growth, invasion and metastasis [11]. Advances in intravital imaging have provided new insights into the relationship between tumor cell migration and invasion by TAMs in murine mammary tumor models, establishing that macrophages are present in high numbers at the invasive margins of tumors and perivascularly in association with intravasating tumor cells [12]. Indeed, TAMs have been suggested to be “obligate partners for tumor cell migration, invasion, and metastasis” [13].
Invasion of breast cancer cells is augmented by proteases secreted from TAMs [3, 14, 15]. For example, cathepsin B (CTSB), a lysosomal cysteine protease that has been characterized as a “multi functional” enzyme in cancer [16], is highly expressed in TAMs isolated from MMTV-PyMT murine mammary carcinomas [5]. When MMTV-PyMT mice are crossed with mice deficient in CTSB, the absence of CTSB in the TAMs correlates with a reduction in lung metastasis [17]. TAM CTSB may increase invasion directly or indirectly by activating latent or inactive matrix metalloproteinases (MMPs) [18], secretion of which (e.g., proMMP-2 and proMMP-9) has been associated with increased invasion of breast cancer cells [14].
Using a live cell imaging assay for proteolysis developed in our laboratory [19, 20, 21, 22], we have shown that co-culturing macrophages with breast carcinoma cells increases degradation of the basement membrane protein type IV collagen and that this degradation is reduced by inhibitors of MMPs and CTSB [21]. Therefore, our aim in the present study was to determine how breast cancer cells modulate expression and activity of the proteases CTSB, MMP-2 and MMP-9 in human monocytes. Our results revealed that incubation of human monocytes with conditioned media of breast cancer cells increased monocyte: 1) proliferation; 2) expression, secretion and activity of CTSB; 3) secretion of MMP-2 and -9; and 4) secretion of IL-6 and insulin-like growth factor binding protein-1 (IGFBP-1). Neutralizing antibodies against IL-6 reduced the increases in CTSB expression, secretion and activity.
Materials and Methods
Reagents
Fetal bovine serum (FBS) was from Invitrogen (Carlsbad, CA); pepsin was from Roche (Indianapolis, IN); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Invitrogen (Carlsbad, CA). Polyclonal rabbit anti-human CTSB antibody was prepared in our laboratory [23]. Horseradish peroxidase-labeled goat anti-rabbit IgG and micro-bicinchoninic acid protein assay kits were from Pierce Biotechnology (Rockford, IL). Human recombinant MMP-2 and MMP-9 enzymes were a kind gift from Dr. Rafael Fridman (Wayne State University, Detroit, MI). Western blotting detection kits were from Amersham Pharmacia Biotechnologies (Piscataway, NJ). IL-6 neutralizing antibody, Quantikine MMP-2 Immunoassay and MMP-9 Fluorokine E immunoassay kits were from R&D Systems (Minneapolis, USA); and ChemiArray™ Human Cytokine Antibody Array V kit was from Chemicon (Temecula, CA, USA). Unless otherwise stated all other reagents were from Sigma (St. Louis, MO).
Cell Lines and Culture
The MDA-MB-231 human breast carcinoma cell line [24] was purchased from American Type Culture Collection (Rockville, MD) as was the U937 human monocytic cell line, which was isolated in 1974 by Sundstrom and Nilsson [25] from a pleural effusion of a histiocytic lymphoma patient. Both cell lines were maintained in RPMI-1640 complete medium (i.e., supplemented with 10% FBS, 2 mML-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES and 1.0 mM Na-pyruvate). U937 cells possess monocytic characteristics and differentiate into mature macrophages in response to different stimulus such as cytokines, phorbol myristate acetate (PMA) and vitamin D3 [26].
Preparation of 231-CM
MDA-MB-231 cells were seeded at 2.5 × 105 cells/ml in RPMI-1640 complete medium into T-75 tissue culture flasks and grown to approximately 80% confluency. Cells in each T75 flask were washed twice with phosphate-buffered saline (PBS) and incubated in 10 ml of serum-free RPMI-1640 medium overnight (18 h). Overnight media were collected and centrifuged at 700 g at room temperature for 5 min to pellet cells. The supernatant was collected and centrifuged at 2000 g at 4 °C for 10 min to remove cell debris. Ten ml of the supernatant was concentrated 10 fold (10X) to a final volume of 1 ml using Amicon Ultracell 10K filters (Millipore, Billerica, MA), and designated as 231-CM. We prepared different concentrations from the 10X concentrated 231-CM by diluting the media 1 in 3, 1 in 4 and 1 in 6 with RPMI-1640 complete medium containing 5% FBS.
Cell Proliferation Assay
Proliferation of control and 231-CM-treated U937 cells was quantified using a colorimetric MTT assay as previously described [27] and cell growth curve [28]. For MTT assay, 5.0 × 103 cells in 100 μl of RPMI-1640 media were seeded per well in 96 well plates. At 3, 5 and 7 da, 10 μl of 5 mg/ml MTT was added to each well and incubated for 4 h at 37°C. Then 100 μl of 20% sodium dodecyl sulfate (SDS) was added to each well and absorbance was measured at 570 nm using a Tecan Spectrafluor Plus plate reader (Tecan, Durham, NC). For cell growth curves, U937 cells were seeded in triplicate at a density of 50,000 cells/well in 96-well plates in the absence (control) and presence (treated) of different dilutions of 231-CM. After 0, 3, 5 and 7 da, samples were collected the adherent cells were trypsinized and combined with media containing suspended cells. Collected cells were centrifuged for 5 min at 1000g and counted with a hemacytometer using Trypan blue to distinguish dead from viable cells and growth curves were drawn.
Treatment of U937 Human Monocytes with 231-CM
U937 cells were seeded at 2.5 × 105 cells/ml in RPMI-1640 complete medium containing 5% FBS in the absence (control) or presence of various dilutions of 231-CM (see above). At 3, 5 and 7 da, both non-adherent and adherent cells were collected, washed twice with PBS and reseeded in serum-free media overnight. Overnight conditioned media were then collected and centrifuged at 4°C at 700 g for 5 min to obtain non-adherent cells. The supernatant was re-centrifuged at 2000 g for 10 min and then concentrated using Amicon Ultracell 10K filters (Millipore, Billerica, MA). The non-adherent U937 cells were washed twice in cold PBS and solubilized in 150 μl lysis buffer [250 mM sucrose, 25 mM 2-(N-morpholino) ethane sulfonic acid, pH 7.5, 1 mM ethylenediaminetetraacetic acid, 0.1% Triton X-100]. The adherent U937 cells were harvested on ice into 200 μl lysis buffer by scraping with a rubber policeman and added to cell lysates of corresponding non-adherent U937 cells. Lysates were sonicated on ice five times at 5 sec intervals using a 50 W Ultrasonicator. Protein concentrations were determined using a micro-bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions, and DNA concentrations quantified as previously described [29].
CTSB Activity Assay
Activity of CTSB (active and latent) in U937 cell lysates and conditioned media was assessed as previously described [30] using a fluorometric CTSB-selective substrate Z-Arg-Arg-NHMec (Bachem, Torrence, CA). Latent CTSB in the U937 conditioned media was activated with pepsin as previously described [31]. CTSB activity was expressed as picomoles of NHMec formed per min per μg DNA.
SDS-Polyacrylamide Gel Electrophoresis (PAGE) and Immunoblotting
Samples were equally loaded (20 μg protein/well), separated by 12% SDS-PAGE under reducing conditions and transferred onto nitrocellulose membranes. Membranes were probed with a polyclonal anti-human CTSB antibody (1:4,000) and a secondary antibody conjugated with horseradish peroxidase (1:10,000) in Tris-buffered saline wash buffer (20 mM Tris, pH 7.5, 0.5 M NaCl) containing 0.5% Tween 20 and 5% (w/v) non-fat dry milk. After washing, bound antibodies were detected by enhanced chemiluminescence according to the manufacturer's guidelines.
Gelatin Zymography
MMP-2 and MMP-9 enzymatic activities in U937 media samples were determined by SDS-PAGE gelatin zymography [32]. Briefly, samples were denatured without reducing or heating and electrophoresed in 10% SDS-PAGE containing 1% gelatin (w/v) at 4°C for 1 h. Gels were subsequently incubated twice for 15 min in renaturation buffer containing 2.5% Triton X-100 at room temperature, washed twice with water and incubated overnight at 37°C in developing buffer [5 mM CaCl2, 0.05% Brij 35, and 50 mM Tris (pH 7.8)]. Thereafter, gels were stained with 0.5% Coomassie brilliant blue R-250 in 50% methanol and 10% acetic acid for 1 h and then de-stained in a 50% methanol and 10% acetic acid solution. Clear bands represent areas of proteolytic activity. Human recombinant MMP-2 and MMP-9 were loaded separately as positive controls.
Enzyme Linked Immunosorbent Assay
Total MMP-2 (i.e., latent and active) in U937 media was quantified using a Quantikine MMP-2 Immunoassay kit according to the manufacturer's instructions. Active MMP-9 in U937 media was quantified using a Fluorokine E immunoassay kit according to the manufacturer's instructions.
Cytokine Antibody Arrays
Cytokines secreted by U937 cells were detected in U937 media samples, respectively, using a ChemiArrayTM Human Cytokine Antibody Array V kit according to the manufacturer's instructions.
IL-6 Neutralizing Antibody (IL-6-NAb) Treatment
U937 cells were grown in RPMI complete medium containing 5% FBS in the absence (control) and the presence of 231-CM diluted 1 in 3 with RPMI complete medium containing 5% FBS. After 3 da, cells were washed twice with PBS, resuspended in serum-free media with and without 2 μg/ml IL-6-NAb. Following an overnight incubation, cells and media were collected and prepared for SDS-PAGE and CTSB activity assays as described above.
Statistical Analysis
Data are expressed as mean ± standard deviation (S.D.). Statistical significance was determined using Student's t test with p = 0.05 considered significant.
Results
231-CM increased proliferation of U937 cells
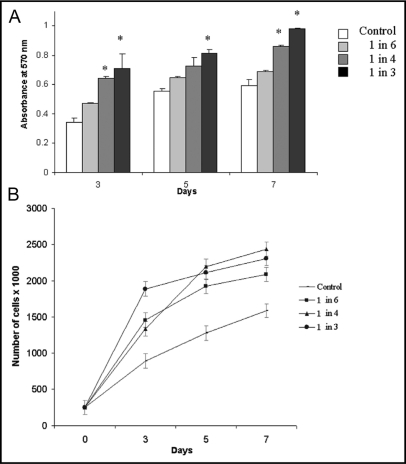
To investigate whether soluble factors secreted by breast carcinoma cells affect proliferation of human monocytes, we grew U937 cells in the presence or absence of media conditioned by MDA-MB-231 cells (231-CM; diluted 1:6, 1:4 and 1:3 in complete medium) for 3, 5 or 7 da. We observed an increase in the proliferation of U937 cells in the presence of different dilutions of 231-CM at 3, 5 and 7 da using MTT assay (Fig. 1A) and growth curves (Fig. 1B).
Fig. 1.
Proliferation of U937 cells was increased by growth in 231-CM. U937 cells were grown in the absence (control) or presence of different concentrations of 231-CM for 3, 5 and 7 da. Proliferation was assessed both by MTT (A) and growth curve (B) assays. Bars represent absorbance (at 570 nm), which is proportional to cell proliferation (A) and growth curve represents number of living cells (B). Data represent mean ± S.D. of three independent experiments; *, p = 0.05 (as compared to the control at each time interval).
231-CM increased CTSB expression, activity and secretion by U937 cells
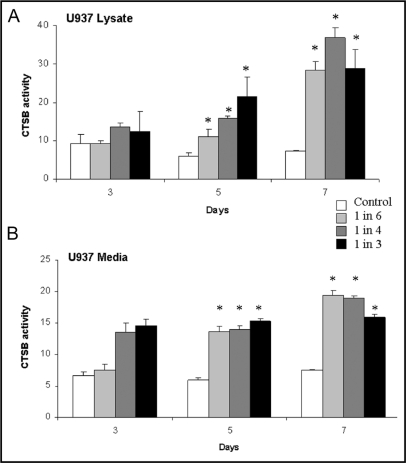
To determine whether soluble factors secreted by breast carcinoma cells affect CTSB in monocytes, we grew U937 cells in the presence or absence of 231-CM (diluted 1:6, 1:4 and 1:3 in complete medium) for 3, 5 or 7 da followed by 24 h in serum-free RPMI medium and determined the levels of expression, activity and secretion of CTSB. At 5 da, incubation with 231-CM resulted in increased levels of mature single chain CTSB in U937 cell lysates and of proCTSB in U937 media (data not shown). Using a fluorometric activity assay, we demonstrated significant dose-dependent increases in CTSB activity in U937 cell lysates at 5 da with further increases at 7 da (Fig. 2A). We also evaluated changes in secretion of proCTSB by assaying pepsin-activatable CTSB activity, i.e., latent proCTSB, in the U937 media.
Fig. 2.
CTSB activity in U937 cells was increased by growth in 231-CM. U937 cells were grown in the absence (control) or presence of different concentrations of 231-CM for 3, 5 and 7 da (treated). CTSB activity in cell lysates (A) and in media (B) was measured against Z-Arg-Arg-NHMec and is expressed as pmol/min/μg DNA. Activity in the media was measured after pepsin activation of proCTSB as described in Materials and Methods. Data represent mean ± S.D. of at least three independent experiments; *, p = 0.05 (as compared to the control at each time interval).
Significant increases in secretion of proCTSB were induced by incubation with 231-CM at all times analyzed, yet were independent of the concentration of 231-CM (Fig. 2B). Our results indicate that soluble factors secreted from MDA-MB-231 carcinoma cells can modulate levels of CTSB in U937 cells.
231-CM increased secretion of MMP-2 and -9 by U937 cells
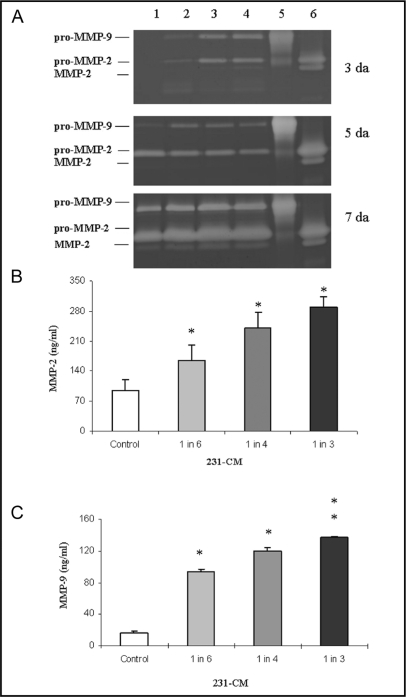
To determine whether soluble factors secreted by breast carcinoma cells affect the gelatinases MMP-2 and MMP-9 in monocytes, we used gelatin zymography and immunoassays. We detected a time-dependent increase in secretion of proMMP-2 and proMMP-9 from U937 cells grown in the presence of 231-CM (Fig. 3A). Only latent MMP-9 could be detected by zymography, whereas both latent and active forms of MMP-2 were detected.
Fig. 3.
Gelatinase activity and expression by U937 cells was increased in a time- and dose-dependent manner by growth in 231-CM. (A) Representative zymogram from three independent experiments depicting gelatinolytic activity of proMMP-2 (72 kDa), MMP-2 (62 kDa) and proMMP-9 (92 kDa) in U937 cells. Samples were loaded equally according to protein content and separated on 10% SDS-PAGE containing 1% gelatin (w/v). White bands indicate regions in which gelatin has been hydrolyzed with lanes 5 and 6 being control lanes for gelatinolytic activity of recombinant proMMP-9 and -2, respectively. Lane 1 represents overnight serum-free conditioned media of untreated U937 cells (control); lanes 2, 3 and 4 represent serum-free media of U937 cells grown with different concentrations of 231-CM (1 in 6, 1 in 4 and 1 in 3, respectively) for 3, 5 and 7 da. (B and C) Dose-dependent increase in secretion of total (active and inactive) MMP-2 (ng/ml) and active MMP-9 (ng/ml), respectively, from U937 cells after 7 da of growth with 231-CM as compared to the control U937 grown in complete RPMI media. Data represent results of at least 3 independent experiments and are presented as mean ± S.D.; *, p = 0.05 and **, p = 0.001.
In addition, we analyzed secretions from U937 cells with immunosorbent assays for total (active and inactive) MMP-2 or active MMP-9. We detected a significant dose-dependent increase in the secretion of total MMP-2 (Fig. 3B) and active MMP-9 (Fig. 3C) from U937 cells that had been incubated with 231-CM. Our results indicate that soluble factors secreted from MDA-MB-231 carcinoma cells can modulate secretion of MMP-2 and -9 from U937 cells. However, using a gelatin zymography assay, we did not detect MMP-2 and -9 in 231-CM, which indicates that MDA-MB-231 does not secrete MMP2 and 9 (data not shown).
231-CM altered the cytokine profile of U937 cells
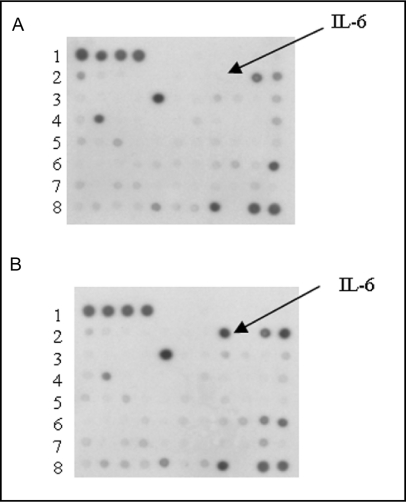
To determine whether 231-CM induces secretion of cytokines from U937 cells, we analyzed the cytokine profile of control U937 cells (Fig. 4A) and U937 cells grown with 231-CM for a 7 da period (Fig. 4B) and then incubated overnight in serum-free medium. Cytokine antibody arrays revealed that U93 7 cells grown with 231-CM exhibited increased secretion of IL-6 and IGFBP-1, which was not detected in control U937 cells. While secretion of other cytokines such as RANTES and I-309 was down-regulated, on the other hand osteoprotegrin and NAP-2 were up-regulated in U937 cells grown with 231-CM.
Fig. 4.
Increased secretion of IL-6 and IGFBP-1 from U937 cells grown in 231-CM. (A) Representative cytokine antibody array of media conditioned by untreated U937 cells (control). (B) Representative cytokine antibody array of media conditioned by U937 cells grown in 231-CM (1 in 4 dilution) for 7 da prior to incubating overnight in serum-free medium. Increases in IGFBP-1 (lane 6 spot 10); osteoprotegrin (lane 8 spot 2) and NAP-2 (lane 7 spot 10); and a decrease in RANTES (lane 4 spot 2) and I-309, (lane 2 spot 1) were observed.
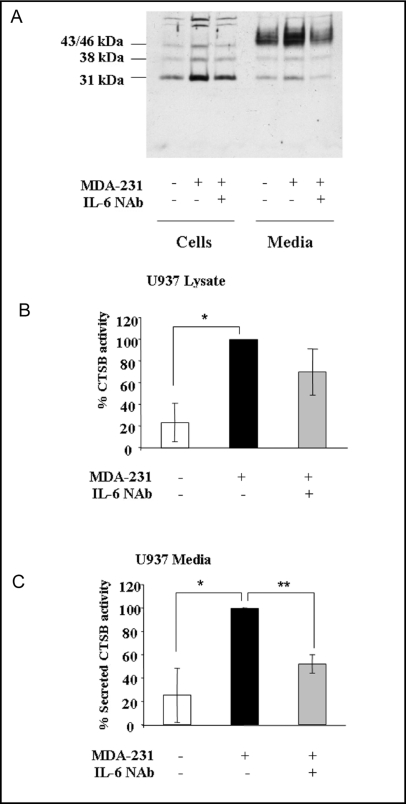
Neutralizing antibodies to IL-6 reduced 231-CM induced increases in CTSB expression, activity and secretion by U937 cells
Since IL-6 was the predominant cytokine expressed in U937 cells grown with 231-CM, we directly assessed the effects of this cytokine on CTSB expression by U937 cells. U937 cells were incubated with 231-CM in the presence and absence of an IL-6 NAb for 5 da and then incubated overnight in serum-free medium. Immunoblotting analysis revealed an increase in expression of the single chain active form of CTSB (31 kDa) in U937 cell lysates and in secretion of proCTSB (43/46 kDa) from U937 cells incubated with 231-CM (Fig. 5A). Addition of IL-6 NAb reduced the levels of CTSB expression and secretion to levels comparable to those found in control cells. The changes in protein levels of CTSB correlated with increases in CTSB activity in U937 cell lysates (Fig. 5B) and secretion of pepsin-activatable proCTSB from U937 cells (Fig. 5C). In contrast, IL-6 NAb did not affect secretion of either MMP-2 or -9, nor did a neutralizing antibody to IGFBP-1 have an effect on CTSB expression, activity and secretion or MMP-2 and -9 secretion (data not shown). Thus, our data are consistent with IL-6 being one soluble factor secreted from MDA-MB-231 breast carcinoma cells that can modulate levels of CTSB in U937 cells.
Fig. 5.
IL-6-NAb reduced 231-CM induced increases in CTSB expression, secretion and activity by U937 cells. U937 cells were grown for 5 da in the absence (control) or presence of 231-CM or 231-CM plus 4 μg/ml IL-6 NAb. Cells were washed and incubated in serum-free medium overnight prior to collecting cell lysates and media. (A) Representative immunoblot of cell lysates and overnight serum-free conditioned media for CTSB. CTSB activity in cell lysates (B) and in media (C) was measured against Z-Arg-Arg-NHMec and is expressed as pmol/min/μg DNA. Specific activity of CTSB is shown as a percentage of that measured in U937 cells treated with 231- CM. Activity in the media was measured after pepsin activation of proCTSB as described in Materials and Methods. Data represent mean ± S.D. of at least three independent experiments; *, p = 0.05; ** p = 0.01.
Discussion
Increased infiltration of TAMs into breast carcinomas correlates with poor prognosis [33]; however, the mechanisms for this effect remain unclear. Genes that function to suppress immune activation, promote extracellular matrix (ECM) remodeling and tumor angiogenesis are upregulated in TAMs [5]. In the present study, since the cysteine protease CTSB and the gelatinases MMP-2 and -9 participate in ECM remodeling and angiogenesis (for review, [16, 34, 35, 36]) we examined whether breast carcinoma cells are able to modulate their expression and secretion in monocytes. We determined that 231-CM increased expression, secretion and activity of CTSB and secretion of MMP-2 and -9 in U937 human monocytic cells. These results are consistent with crosstalk between carcinoma cells and macrophages within the tumor microenvironment being able to modulate CTSB expression through activation of monocytes. We have previously shown that phorbol ester activation of human monocytes increases CTSB expression [37] and activity [21]. The induction of CTSB is not species-specific as TAMS in the mouse mammary tumor-polyoma middle T antigen (MMTV-PyMT) transgenic mouse model for mammary carcinoma express higher levels of CTSB than do macrophages distant from the tumor [38]. Furthermore, TAM CTSB has been causally linked to metastasis: lung metastasis is reduced in MMTV-PyMT mice crossed with CTSB-deficient mice [17]. We therefore speculate that increased CTSB expression in TAMs is a component of the tumor microenvironment of metastasis recently identified as a prognostic marker for prediction of hematogenous dissemination and distant metastasis [39].
One potential role for CTSB is to initiate proteolytic cascades on the surface of tumor cells that result in the activation of downstream proteases such as proMMP-2 and -9 (for review, see [18, 40]). We observed increases in secretion of MMP-2 and -9 from U937 cells that had been incubated with 231-CM. Similar results have been reported for other populations of macrophages, e.g., macrophages differentiated from human peripheral blood monocytes [14] and the human THP-1 monocytic cell line [41] and linked to increased invasiveness of a variety of breast carcinoma cell lines. Thus, as with CTSB, increases in TAM expression of MMP-2 and -9 are induced by interactions between carcinoma cells and macrophages within the tumor microenvironment. Intriguingly, Hiratsuka et al. [42] report similar findings for both TAMs and endothelial cells within lung metastases. MMP-9 expression in TAMs is elevated and those TAMs, via a mechanism modulated by vascular endothelial growth factor receptor-1, increase MMP-9 expression in the endothelial cells [42]. Although the latter study did not include breast tumors, it provides further evidence that protease expression in TAMs can be induced by cross-talk among cells. A recent report by deNardo et al. [43] indicates that the cross-talk among cells in the tumor microenvironment in the MMTV-PyMT model is more extensive than previously appreciated, involving CD4+ T lymphocytes, macrophages, immature myeloid cells and carcinoma cells. Intriguingly, a subset of CD4+ T lymphocytes that express IL-17, as a result of activation by IL-6 and TGFβ, has been linked to chronic inflammation and thereby tumorigenesis (for a review on the properties and putative functions of this new cell lineage, see [44]). IL-17 [45] and other cytokines such as tumor necrosis factor-alpha [14], produced by breast carcinoma-associated macrophages, have been shown to increase expression of MMP-2 and -9 in the TAMs. Therefore, we profiled the cytokines, chemokines and growth factors secreted by U937 human monocytes that had been incubated with 231-CM.
The predominant identified cytokine secreted by U937 cells grown in 231-CM was IL-6, a pro-inflammatory cytokine that is expressed by classically activated macrophages or M1 macrophages [11] and a subset of alternatively activated macrophages or M2a macrophages [8]. Moreover, we identified IL-6 as an inducer of CTSB expression in U937 cells incubated with 231-CM. IL-6 increases proliferation and survival of breast tumor cell lines and its expression at high levels in breast tumors and patient sera is a negative prognostic marker (for review, see [46]). IL-6 has previously been found to increase expression of the proteases found to be elevated in the present study: MMP-2 and -9 in non-Hodgkin's lymphomas [47], MMP-9 in squamous cell carcinoma [48] and CTSB in myotubes [49]. The induction of protease expression by IL-6 is consistent with the recent report that IL-6 induces an epithelial-mesenchymal transition in breast carcinoma cells [50].
Secretion of others factors was also modulated by 231-CM treatments, e.g., increases in IGFBP-1, osteoprotegrin and NAP-2 and a decrease in RANTES and I-309. IL-6 has been shown to stimulate production of IGFBP-1 in the human hepatocellular liver carcinoma cell line HepG2 [51] and of IGFBP-1, -3, -4 in co-cultures of rat hepatocytes and Kupffer cells [52]. IGFBP-1 is a neutralizing protein to IGF-1, a growth factor known to stimulate cell growth, proliferation and migration ([53]). Binding of IGFBP-1 to IGF-1 reduces cellular anabolism and found to be associated with inflammatory disorders ([54]). Moreover, IGFBP-1 was shown to stimulate cellular migration by binding to a5β1 integrin [55, 56].
Osteoprotegrin and NAP-2 are cytokines associated with anti-apoptotic and cell survival mechanisms. For instance, osteoprotegrin inhibits pro-apoptotic mechanisms stimulated by RANKL and TRAIL by binding to both proteins (for review [57]). NAP-2, a chemotactic agent for neutrophils, was found to protect cells of hematopoietic progenitor cells against cytotoxicity of chemotherapeutic drugs [58]. On the other hand, RANTES and I-309 are chemokines responsible for leukocyte recruitment in the tumor microenvironment [59, 60]. Down regulation of these chemokines may reflect the immunosuppressive properties of U937 cells grown in 231-CM. The studies here were performed with only one breast cancer and one monocyte cell line and thus need to be confirmed in additional breast cancer and monocyte cell lines. Furthermore, our ongoing studies using patient samples will elucidate the role contribution of IL-6 and sIL-6R in the metastatic phenotypes breast cancer.
TAM-derived IL-6 and downstream pathways that are modulated by IL-6 may be targets for therapeutic intervention in breast carcinomas. Indeed, the anti-tumor agent, Yondelis or trabectedin, which is active against human breast adenocarcinomas, inhibits the production of IL-6 by both TAMs and tumor cells at sub-cytotoxic concentrations [61]. IL-6 is a growth and survival factor for tumors and has been reported to interact with the immunosuppressive cytokine IL-10 to enhance suppression of anti-tumor immune responses [8]. Here we have demonstrated that soluble factors secreted by human MDA-MB-231 breast carcinoma cells exert a paracrine effect on human U937 monocytes resulting in increased secretion of IL-6 and thereby increased expression, secretion and activity of CTSB. The high levels of expression of CTSB in TAMs [5] and the link between CTSB in TAMs and metastasis [17] suggest that a downstream consequence of targeting IL-6 may be to affect metastases as well as primary tumors.
Abbreviations
231-CM (media conditioned by MDA-MB-231 human breast carcinoma cells); CTSB (cathepsin B); ECM (extracellular matrix); FBS (fetal bovine serum); IL (interleukin); IL-6-NAb (interleukin-6 neutralizing antibody); sIL-6R (soluble form of IL-6 receptor); IGFBP-1 (insulin-like growth factor binding protein-1); MMP (metalloproteinases); MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide); PAGE (polyacrylamide gel electrophoreses); NAP-2 (neutrophil-activating peptide-2); OPTGRN (Osteoprotegerin); PBS (phosphate buffer saline); RANKL (receptor activator of NF-kappaB ligand); RANTES (regulated upon activation normal T cell expressed and secreted); SDS (sodium dodecyl sulfate); S.D. (standard deviation); TAM (tumor-associated macrophage); TRAIL (Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand).
Acknowledgements
We acknowledge with special gratitude the contribution of J. Dosescu, to these studies. The authors were supported by an Avon Foundation American Association for Cancer Research International Scholar Award in Breast Cancer Research (M.M.M.), an Avon grant # 02-2007-049 (M.M.M., B.F.S.), U.S. Public Health Service Grant CA 56586 (B.F.S.), Science and Technology Development Fund, Egypt, Grant # 343 (M.M.M.)
References
- 1.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 2.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2004;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu JL, Rak JW. Host microenvironment in breast cancer development: Inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res. 2003;5:83–88. doi: 10.1186/bcr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M. Significant correlation of monocyte chemo-attractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92:1085–1091. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 8.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.al-Sarireh B, Eremin O. Tumour-associated macrophages (tams): Disordered function, immune suppression and progressive tumour growth. J R Coll Surg Edinb. 2000;45:1–16. [PubMed] [Google Scholar]
- 10.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 11.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 13.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to tnf-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 15.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via nf-kappa b and jnk. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed MM, Sloane BF. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 17.Vasiljeva O, Korovin M, Gajda M, Brodoefel H, Bojic L, Kruger A, Schurigt U, Sevenich L, Turk B, Peters C, Reinheckel T. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin b-deficient mice. Oncogene. 2008;27:4191–4199. doi: 10.1038/onc.2008.59. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Fridman R, Mobashery S. Extracellular proteases as targets for treatment of cancer metastases. Chem Soc Rev. 2004;33:401–409. doi: 10.1039/b209224g. [DOI] [PubMed] [Google Scholar]
- 19.Sloane BF, Sameni M, Podgorski I, Cavallo-Medved D, Moin K. Functional imaging of tumor proteolysis. Annu Rev Pharmacol Toxicol. 2006;46:301–315. doi: 10.1146/annurev.pharmtox.45.120403.095853. [DOI] [PubMed] [Google Scholar]
- 20.Sameni M, Moin K, Sloane BF. Imaging proteolysis by living human breast cancer cells. Neoplasia. 2000;2:496–504. doi: 10.1038/sj.neo.7900116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sameni M, Dosescu J, Moin K, Sloane BF. Functional imaging of proteolysis: Stromal and inflammatory cells increase tumor proteolysis. Mol Imaging. 2003;2:159–175. doi: 10.1162/15353500200303136. [DOI] [PubMed] [Google Scholar]
- 22.Jedeszko C, Sameni M, Olive MB, Moin K, Sloane BF. Visualizing protease activity in living cells: From two dimensions to four dimensions. Curr Protoc Cell Biol. 2008;Chapter 4 doi: 10.1002/0471143030.cb0420s39. Unit 4 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moin K, Day NA, Sameni M, Hasnain S, Hirama T, Sloane BF. Human tumour cathepsin b. Comparison with normal liver cathepsin b. Biochem J. 1992;285:427–434. doi: 10.1042/bj2850427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cailleau R, Young R, Olive M, Reeves WJ., Jr. Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53:661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (u-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 26.Oberg F, Nilsson K. Differentiation and activation associated expression of il-6 and il-6 receptors in u-937 monocytic cells: Relationship to the expression of cd14. Growth Factors. 1992;7:85–96. doi: 10.3109/08977199209023940. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Joshi CV, Supriya P, Ajitkumar P. Growth inhibition of human promonocytic leukaemic u937 cells by interferon gamma is irreversible and not cell cycle phase-specific. Cytokine. 1999;11:673–678. doi: 10.1006/cyto.1998.0474. [DOI] [PubMed] [Google Scholar]
- 29.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin b, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- 30.Linebaugh BE, Sameni M, Day NA, Sloane BF, Keppler D. Exocytosis of active cathepsin b enzyme activity at ph 7.0, inhibition and molecular mass. Eur J Biochem. 1999;264:100–109. doi: 10.1046/j.1432-1327.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 31.Koblinski JE, Dosescu J, Sameni M, Moin K, Clark K, Sloane BF. Interaction of human breast fibroblasts with collagen i increases secretion of procathepsin b. J Biol Chem. 2002;277:32220–32227. doi: 10.1074/jbc.M204708200. [DOI] [PubMed] [Google Scholar]
- 32.Toth M, Gervasi DC, Fridman R. Phorbol ester-induced cell surface association of matrix metalloproteinase-9 in human mcf10a breast epithelial cells. Cancer Res. 1997;57:3159–3167. [PubMed] [Google Scholar]
- 33.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 34.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer metastasis reviews. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 35.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Handsley MM, Edwards DR. Metallo-proteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115:849–860. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 37.Berquin IM, Yan S, Katiyar K, Huang L, Sloane BF, Troen BR. Differentiating agents regulate cathepsin b gene expression in hl-60 cells. J Leukoc Biol. 1999;66:609–616. doi: 10.1002/jlb.66.4.609. [DOI] [PubMed] [Google Scholar]
- 38.Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, Peters C, Reinheckel T. Tumor cell-derived and macrophage-derived cathepsin b promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 39.Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, Jones JG. Tumor microenvironment of metastasis in human breast carcinoma: A potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15:2433–2441. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mai J, Waisman DM, Sloane BF. Cell surface complex of cathepsin b/annexin ii tetramer in malignant progression. Biochim Biophys Acta. 2000;1477:215–230. doi: 10.1016/s0167-4838(99)00274-5. [DOI] [PubMed] [Google Scholar]
- 41.Szabo KA, Singh G. Modulation of monocyte matrix metalloproteinase-2 by breast adenocarcinoma cells. Breast Cancer Res. 2005;7:R661–668. doi: 10.1186/bcr1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. Mmp9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 43.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. Cd4(+) t cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong C. Th17 cells in development: An updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. Il-17 expression by breast-cancer-associated macrophages: Il-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA, Janowska-Wieczorek A. Interleukin-6 regulation of matrix metalloproteinase (mmp-2 and mmp-9) and tissue inhibitor of metalloproteinase (timp-1) expression in malignant non-hodgkin's lymphomas. Blood. 1999;94:2080–2089. [PubMed] [Google Scholar]
- 48.Sundelin K, Roberg K, Grenman R, Hakansson L. Effects of cytokines on matrix metalloproteinase expression in oral squamous cell carcinoma in vitro. Acta Otolaryngol. 2005;125:765–773. doi: 10.1080/00016480510027484. [DOI] [PubMed] [Google Scholar]
- 49.Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins b and l, proteasome) in c2c12 myotubes. Clin Sci (Lond) 1995;89:431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samstein B, Hoimes ML, Fan J, Frost RA, Gelato MC, Lang CH. Il-6 stimulation of insulin-like growth factor binding protein (igfbp)-1 production. Biochem Biophys Res Commun. 1996;228:611–615. doi: 10.1006/bbrc.1996.1705. [DOI] [PubMed] [Google Scholar]
- 52.Lelbach A, Scharf JG, Ramadori G. Regulation of insulin-like growth factor-i and of insulin-like growth factor binding protein-1, -3 and -4 in cocultures of rat hepatocytes and kupffer cells by interleukin-6. J Hepatol. 2001;35:558–567. doi: 10.1016/s0168-8278(01)00170-2. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Yee D. Insulin-like growth factor binding protein-1 (igfbp-1) inhibits breast cancer cell motility. Cancer Res. 2002;62:4369–4375. [PubMed] [Google Scholar]
- 54.Baxter RC. Changes in the igf-igfbp axis in critical illness. Best Pract Res Clin Endocrinol Metab. 2001;15:421–434. doi: 10.1053/beem.2001.0161. [DOI] [PubMed] [Google Scholar]
- 55.Jones JI, Gockerman A, Busby WH, Jr., Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its arg-gly-asp sequence. Proc Natl Acad Sci U S A. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: Regulation by tgf-beta, igf-ii, and igfbp-1. Exp Cell Res. 1995;217:419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- 57.Zauli G, Melloni E, Capitani S, Secchiero P. Role of full-length osteoprotegerin in tumor cell biology. Cell Mol Life Sci. 2009;66:841–851. doi: 10.1007/s00018-008-8536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han ZC, Lu M, Li J, Defard M, Boval B, Schlegel N, Caen JP. Platelet factor 4 and other cxc chemokines support the survival of normal hematopoietic cells and reduce the chemosensitivity of cells to cytotoxic agents. Blood. 1997;89:2328–2335. [PubMed] [Google Scholar]
- 59.Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003;63:8360–8365. [PubMed] [Google Scholar]
- 60.Miller MD, Krangel MS. The human cytokine i-309 is a monocyte chemo-attractant. Proc Natl Acad Sci U S A. 1992;89:2950–2954. doi: 10.1073/pnas.89.7.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, de Braud F, Jimeno J, D'Incalci M. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]