Abstract
Background: To explore the influence of ovarian cancer histotype on the effectiveness of adjuvant radiotherapy (RT).
Methods: A review of a population-based experience included all referred women with no reported macroscopic residuum following primary surgery who underwent adjuvant platin-based chemotherapy (CT), with or without sequential RT, and for whom it was possible to assign histotype according to the contemporary criteria.
Results: Seven hundred and three subjects were eligible, of these 351 received RT. For those with apparent stage I and II tumors, the cohort with clear cell (C), endometrioid (E), and mucinous (M) disease who additionally received RT exhibited a 40% reduction in disease-specific mortality and a 43% reduction in overall mortality.
Conclusions: The curability of those with stage I and II C-, E-, and M-type ovarian carcinomas was enhanced by RT-containing adjuvant therapy. This benefit did not extend to those with stage III or serous tumors. These findings necessitate reassessments of the role of RT and of the nonselective surgical and CT approaches that have characterized ovarian cancer care.
Keywords: adjuvant radiotherapy, histology, minimal residual disease, outcomes, ovarian cancer, predictive factors
introduction
While the search for better anticancer treatment is pursued, it remains important to use available modalities to maximum selective benefit. For women with ovarian cancers, this issue is of considerable current importance, now that the collective of ‘ovarian epithelial tumors’ is recognized to comprise a number of distinct diseases, each histotype with unique biology, anatomy, risks, and chemotherapy (CT) responsiveness [1–3]. Just as subtypes of breast carcinoma are now treated differently, it is likely that different ovarian carcinoma subtypes will best be treated with subtype-specific treatment protocols. The imperative is to consider these subtypes anew, to identify exploitable histotype-specific predictive factors, especially given the frustratingly low and unimproved cure rate for ovarian cancers [4].
Six decades ago, Paterson [5] wrote of the role of radiotherapy (RT) in the management of ovarian tumors, confessing ‘ignorance of any dependable relationship between [radiation] sensitivity and histological type’. Little has since been reported that addresses Paterson’s concern; a recent systematic overview of radiation therapy effects in ovarian cancer [6] makes no reference to this issue. The subsequent report of Nagai et al. [7] is particularly provocative; they showed in clear cell cancer of the ovary that whole abdominopelvic radiotherapy (wapRT), as an adjunct to surgery, produced a dramatic improvement in 5-year disease-free survival over that seen with platin-containing CT: 81% versus 25%, P = 0.006. This observation prompted us to conduct a reassessment of outcomes in a carefully characterized population, looking for any predictive associations between ovarian cancer histotype and RT effectiveness that might inform a more selective treatment approach.
methods
subjects
The BC Cancer Agency (BCCA) is a multicentered institution charged with guiding cancer care for the now ∼4 million residents of Canada’s westernmost province. All newly referred cases are reviewed by a disease-site multidisciplinary Tumour Group panel. The result has been an unusual consistency of care, population-wide, which provides an opportunity to link patient and disease characteristics, treatment policies, and outcomes.
A 1985 assessment of prognostic factors in ovarian cancer [8] led to a risk classification [9] that since has directed postsurgical therapy in British Columbia [10]. Risk class has been assigned postoperatively based on apparent International Federation of Gynecology and Obstetrics (FIGO) stage, tumor grade, and the reported presence or absence of macroscopic residuum—histotype has not been a determinant. Those with stage I, grade 1 lesions (without rupture, dense adherence or positive cytology—‘low-risk’ disease) were not to be offered postoperative therapy. Women with either macroscopic residuum or stage IV (‘extreme risk’) disease were offered platin-based palliative CT. The remainder, with no reported macroscopic residuum (‘moderate-risk’ and ‘high-risk’ disease), were offered curative-intent therapy options. Except for a period when RT was not used in those with ‘high-risk’ lesions [11], these comprised standard-dose platin-containing CT in sequence with wapRT or three additional cycles of CT in place of RT. The risk classification and treatment regimens are detailed in Table 1.
Table 1.
BC Cancer Agency (BCCA) treatment protocols
| BCCA treatment protocols for no macroscopic residuum ovarian cancers | ||
| Date initiated | Risk groups | |
| Moderate | High | |
| Stage I, grade 2; stage IIa, grade 1 or 2 | Stage I and IIa, grade 3; stage III, any grade | |
| 1984 | Cisplatin × 3, followed by radiotherapy | Cisplatin/cyclophosphamide × 3, then radiotherapy, followed by cisplatin/cyclophosphamide × 3 |
| 1989 | Cisplatin × 6 | |
| 1994 | Cisplatin/etoposide × 3, followed by radiotherapy or default to carboplatin × 6 | |
| 1999 | Carboplatin/paclitaxel × 3, followed by radiotherapy or default to carboplatin/paclitaxel × 6 or default to carboplatin × 6 | |
If sharp dissection was used to remove the tumor, BCCA assigned a minimal stage of IIb [13].
This review focuses on those women without macroscopic residuum who received CT, with or without RT. Approval for this review was obtained from the University of British Columbia/BCCA Research Ethics Board. Those funding this endeavor had no role in its design, execution, or interpretation.
case identification and confirmation
Eligible subjects were identified by a stepwise process, excluding nonovarian primaries, tumors of nonepithelial histology, noninvasive (borderline) lesions, and macroscopic residuum epithelial malignancies.
The records of potentially eligible patients were reviewed (JLS), and data abstracted. Each apparently eligible case was then independently reviewed (KDS), and any discrepant data rationalized. The assignment of stage and risk group was retrospective to ensure a consistent classification over time. The institutional convention has been to assign lesions that required sharp dissection for removal as at least stage IIb [13]. Nodal dissection had not been routine.
Surgical comorbidity was estimated by assessing five factors: the extent of prior abdominopelvic surgeries, pre-existing adhesions, the presence of endometriosis, concurrent retroperitoneal surgical dissection, and concurrent surgery of the intestinal or urinary tracts.
Diagnostic specimens had been assessed by a BCCA onco-pathologist at the time of referral. All cases for which slides were obtainable were reviewed (CBG and MK) for histotype and grade, as described previously [12]. This allowed application of current classification criteria [1, 2] and assignment into the following histotypes: serous (S), clear cell (C), endometrioid (E), mucinous (M), and other (O), the latter a heterogeneous group including transitional cell, squamous cell, and unclassifiable carcinomas. Serous carcinomas were divided into low and high grade [12].
In order to maximize the sample size of those with ‘verified’ histological diagnoses, the set analyzed for survival includes not only all reviewed cases but also those cases not reviewed if the original diagnosis had been C or M, as these assignments rarely changed upon reassessment [12], whereas assignments to S and E proved less reliable (>98% of tumors originally diagnosed as C remained as C after review, while the corresponding figures for S and E were 81% and 67%, respectively). Thus, S, E, and O histotypes are underrepresented in the sample. An effort was made to identify the initial site of disease progression.
treatment delivery and toxicity
CT guidelines were followed uniformly. The recommendation for RT was dependent on the individual radiation oncologist’s choice, as a consensus among them was not achieved, and the evidence supporting RT use insufficient for a mandate. Whether adjuvant RT was advised, accepted or declined was recorded. The standard prescription was 2250 cGy to the whole pelvis in 10 fractions over 2 weeks, followed by 2250 cGy to the whole abdomen and pelvis in 22 fractions over 4.5 weeks. There was no shielding of the liver, and the kidneys were typically shielded with posterior blocks limiting the renal dose to 1700 cGy. Whether or not the prescription was completed (and if not, why not), and chronic toxic effects of any grade attributable to RT were recorded using Radiation Therapy Oncology Group (RTOG)/European Organisation for Research and Treatment of Cancer (EORTC) criteria [14] (see Table 2).
Table 2.
Radiation Therapy Oncology Group (RTOG)/European Organisation for Research and Treatment of Cancer (EORTC) late radiation morbidity scoring scheme [14]
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Bladder | Slight atrophy; microscopic hematuria | Moderate frequency/telangiectasia; intermittent hematuria | Severe frequency/telangiectasia/dysuria; frequent hematuria | Necrosis; contracted (capacity <100 ml); severe hemorrhagic cystitis |
| Bone | Asymptomatic; reduced density | Moderate pain; irregular sclerosis | Severe pain; dense sclerosis | Necrosis/spontaneous fracture |
| Lung | Asymptomatic or mild symptoms; slight radiographic appearances | Moderate symptomatic fibrosis (cough); patchy radiographic appearances | Severe symptomatic fibrosis; dense radiographic changes | Respiratory insufficiency; ventilation |
| Mucous membrane/skin | Slight atrophy | Mild atrophy and telangiectasia | Marked atrophy, severe telangiectasia | Ulceration |
| Small/large intestine | Mild diarrhea/cramping/ discharge or bleeding | Moderate diarrhea and colic; excessive mucus | Obstruction or bleeding requiring surgery | Necrosis/perforation/fistula |
statistical analysis
A survival regression tree analysis based on recursive partitioning [15] was carried out. Fisher’s exact test was used to assess statistical associations [16].
A survival analysis was conducted, using Cox’s proportional hazards regression, to examine the effects of RT, adjusting for other factors [17], including age, stage, histotype and grade at review, peritoneal cytology, tumor rupture, ascites volume, and preoperative CA 125. Survivals were determined from the date of pathological diagnosis. Time to death due to ovarian cancer was considered for disease-specific survival (DSS) and deaths due to other causes were censored in this case; that due to any cause was considered for the overall survival (OS) analysis. The effect of RT was examined by first selecting the best models for other factors and then adding the treatment factor to the model. The Akaike Information Criterion was used to select the best model [18]. Survival curves were obtained using the Kaplan–Meier method [19].
results
case identification
The review encompasses 1 January 1984 through 31 December 2003. During this interval, 6359 women with ovarian neoplasms were reported to the BC Cancer Registry (reporting of all cancer diagnoses is mandatory in BC), of whom 4198 were referred to the BCCA, a rate of 66%. Of these, 1022 were confirmed to have invasive epithelial ovarian carcinomas with no reported macroscopic residuum. Of this group, 860 received CT. Of these, a total of 662 underwent histological review: 281 S, 172 C, 150 E, 35 M, and 24 O. One hundred and ninety-eight were not reviewed and had been initially reported as 91 S, 25 C, 49 E, 16 M, and 17 O. Thus, the ‘verified’ dataset (all reviewed plus unreviewed C and M) includes 281 S, 197 C, 150 E, 51 M, and 24 O—a total of 703 for this analysis, 82% of the target cohort. Their demographics and disease characteristics are summarized in Table 3.
Table 3.
Study population (N = 703)
| Clinicopathological variable | Category | n (%) | CT | CRT |
| Age (years), P < 0.0001 | Younger than 65 | 503 (72) | 211 | 292 |
| 65 or older | 200 (28) | 140 | 60 | |
| Preop CA 125 (mmol/l), P = 0.23 | ≤70 kU/l | 144 (20) | 67 | 77 |
| >70 kU/l | 233 (33) | 127 | 106 | |
| No data | 326 (46) | 157 | 169 | |
| FIGO stage, P < 0.0001 | I | 264 (38) | 103 | 161 |
| II | 310 (44) | 156 | 154 | |
| III | 129 (18) | 92 | 37 | |
| Rupture, P = 0.06 | None | 347 (49) | 188 | 159 |
| Preoperative | 83 (12) | 32 | 51 | |
| Intraoperative | 266 (38) | 127 | 139 | |
| Unknown | 7 (<1) | 4 | 3 | |
| Histotype, P = 0.002 | S | 281 (40) | 158 | 123 |
| C | 197 (28) | 97 | 100 | |
| E | 150 (21) | 58 | 92 | |
| M | 51 (7) | 22 | 29 | |
| O | 24 (3) | 17 | 7 | |
| Cytology, P = 0.93 | Negative | 389 (55) | 192 | 197 |
| Positive | 161 (23) | 81 | 80 | |
| Unknown | 153 (22) | 79 | 74 | |
| Grade at review, P = 0.14 | 1 | 124 (18) | 51 | 73 |
| 2 | 145 (21) | 73 | 72 | |
| 3 | 391 (56) | 207 | 184 | |
| Unknown | 43 (6) | 20 | 23 |
FIGO, International Federation of Gynecology and Obstetrics.
The serous cases included 18 low-grade carcinomas. These demonstrated a tendency to extraovarian spread (10 stage II and 8 stage III), as is seen for high-grade serous carcinomas, so the two were combined.
treatment delivery and toxicity
Three hundred and eighty-eight women (55% of the total) were offered RT, 351 (90%) accepted the recommendation; 340 (97%) were prescribed wapRT, and of these 305 (90%) completed their planned course of therapy. The reasons for treatment abandonment (n = 35) were acute toxicity in 26 (particularly intestinal symptoms and thrombocytopenia of grade 2 or higher; 9 and 6 events, respectively), patient decision in 4, undocumented reasons in 3, and disease progression in 2 patients. Recorded chronic toxic effects of any grade for those completing wapRT are summarized in Table 4. The frequency of moderate (grade 3 RTOG), severe (grade 4), and fatal (grade 5) toxic effects overall were 3%, 1%, and <1%, respectively.
Table 4.
Number of chronic toxic events reported in those who started RT (N = 351)
| RTOG toxicity grade |
|||||
| 1 | 2 | 3 | 4 | 5 | |
| Bladder | 4 | 2 | 2 | 1a | |
| Bone | 1 | 2 | |||
| Lung | 2 | 2 | |||
| Skin/mucosa | 10 | 1 | 1 | ||
| Intestine | 123 | 30 | 9 | 2 | |
| Other | 1 | 2a | |||
Grade 5 toxic effects included one fatal transfusion-related hepatitis and two events of secondary malignancies potentially related to RT (one bladder cancer and in-field soft tissue sarcoma).
RTOG, Radiation Therapy Oncology Group.
Patients who had undergone more extensive prior abdominal surgeries had a higher rate of any degree of RT-related chronic intestinal toxicity than those with minor or no prior procedures (62% versus 43%, P = 0.02); no other surgical factors were predictive.
survival
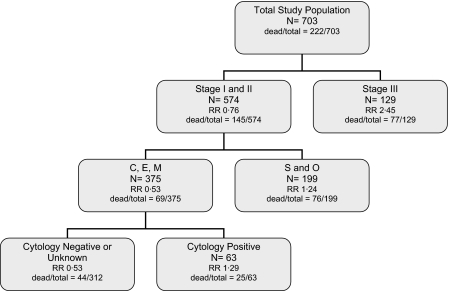
A survival regression tree for DSS is displayed in Figure 1. The results indicate that the stage is the most important prognostic factor, with stage III patients having a poor outcome compared with stage I or II. For those with stage I or II disease, histotype is next most important, clustering C, E, and M into one group against S and O, with the former having a much better outcome. Within the C, E, and M cluster, positive peritoneal cytology indicates a poorer prognosis.
Figure 1.
Disease-specific survival regression analysis.
The results of the DSS analysis, with the risk relative to CT only, adjusting for all other factors, show:
overall no chemoradiotherapy (CRT) effect [relative risk (RR) = 0.95, 95% confidence interval (CI) 0.70–1.27];
within stage III, a nonsignificant adverse effect of CRT, a 56% increase in disease-specific mortality (DSM) (RR = 1.56, 95% CI 0.93–2.65);
within stages I and II, a nonsignificant benefit of CRT, a 25% reduction in DSM (RR = 0.75, 95% CI 0.53–1.06);
within stages I and II, and the S and O histotype cluster, no effect of CRT (RR = 0.98, 95% CI 0.60–1.60);
within stages I and II, and the C, E, and M histotype cluster, a significant 40% reduction in DSM with CRT (RR = 0.60, 95% CI 0.37–0.98).
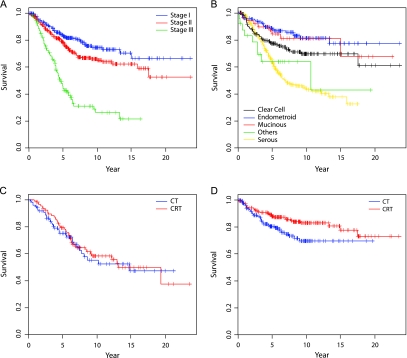
Curves depicting stage-specific survival, histotype-specific survival, and DSS in the stage I and II histotype clusters are illustrated in Figure 2A–D.
Figure 2.
Disease-specific survival (DSS) curves. (A) DSS by stage. (B) DSS by histotype. (C) DSS for stage I and II, serous and other histotypes. (D) DSS for stage I and II clear cell, endometrioid, and mucinous histotypes.
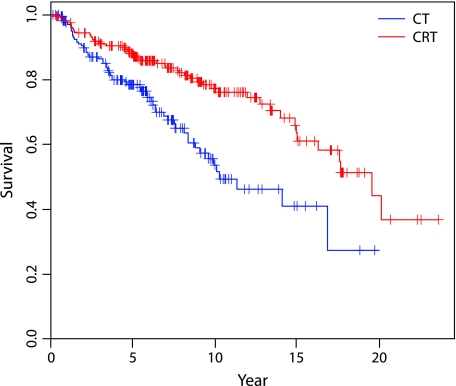
The analysis of OS provides parallel results: a significant reduction of 43% (RR = 0.57, 95% CI 0.38–0.86) in overall mortality was observed for C, E, and M patients with stage I or II disease given RT (Figure 3).
Figure 3.
Overall survival (OS) curves. OS for stage I and II clear cell, endometrioid, and mucinous histotypes.
A comparison of the reported site of initial relapse for the CT and CRT cohorts showed a significant reduction in the rate of initial pelvic relapse with RT (42% for CT versus 35% for CRT, P = 0.007), as well as for C, E, and M subjects with stage I and II diseases (25% versus 19%, P = 0.02).
discussion
Whether a treatment is advised is based on perceptions of its efficacy, safety, convenience, and economy. If treatment is of curative intent, efficacy is more important than convenience and economy, and arguably more important than safety. Persistent concerns regarding each of these factors have led to adjuvant RT for ovarian cancers being abandoned by many [20], at least in ‘advanced disease’. Our provincial experience had suggested better tolerability and therapeutic benefit [11, 21, 22], and we have continued to advocate its use but not in those with macroscopic residuum after primary surgery [23].
The realization that ‘ovarian epithelial cancer’ comprises several distinct diseases, currently best defined by histotype, necessitated a careful reassessment of the potential roles of RT in each. The failure to fully appreciate the importance of tumor histotype, both as a prognostic and predictive factor, may have been the result of the inconsistent application of unreliable definitions—problems now resolvable in practice [2, 12]. Our study examined this issue, applying modern diagnostic criteria to a large population-derived cohort of women. Each had no reported macroscopic residuum following primary surgery but had a perceived risk for relapse, by virtue of having either grade 2 or 3 lesions, tumor rupture, positive cytology, a need for sharp dissection to remove the disease, or evidence of extraovarian spread. Each received platin-based CT, and one-half also received RT.
efficacy
Our data suggest substantial benefits from RT in those with low-stage, no macroscopic residuum, nonserous disease, with a reduction of 40% in DSM. Our dataset is too small to discriminate effects among the clear cell, endometrioid, and mucinous histotypes. The improvements in survival are durable and portend cure.
Nonetheless, this conclusion is subject to the potential selection bias that applies to all retrospective observational studies. Overall, as can be seen in Table 3, older women were offered RT less frequently, a patient characteristic that covaries with stage III disease of high-grade serous histotype—prognostic factors adjusted for in our analysis. The relative enthusiasm of Tumor Group radiation oncologists to advise RT tended to be personally consistent; our ignorance during the period of study of the potential relationship between histotype and the utility of RT should minimize bias that would impact our major finding.
Additional features that strengthen our conclusions include the large sample size and that the CT and CRT cohorts were treated contemporaneously.
This result is biologically plausible, given that nonserous lesions exhibit less occult tumor spread (the ‘upstaging’ resulting from extensive surgical exploration is a feature of serous disease [24–27]). The more ‘restricted anatomy’ of clear cell, endometrioid, and mucinous diseases would be prerequisite for a ‘regional’ treatment such as RT to have curative potential. Conversely, serous disease is characteristically widespread early in its natural history. As well, the relative ineffectiveness of CT in clear cell and mucinous cancers [3, 28] offers a greater opportunity to observe any benefit from RT. Often overlooked is the fact that no CT agent has demonstrated ‘non-cross-resistant’ activity even approaching that of RT in platin-refractory disease [28], suggesting a potential advantage from combined modality treatment. The results are strengthened further by the observed pattern of initial relapse; the low dose administered to the whole abdomen could explain the lack of impact on extrapelvic relapse and therefore why more disseminated serous tumors derive no benefit overall from RT.
safety
Quantifying the toxic effects seen with this RT prescription is important, particularly as they are generally perceived to be substantial and persistent. Retrospective analyses underestimate toxic effects, particularly chronic effects. However, certain serious adverse effects, for example, intestinal obstruction requiring surgical intervention, become evident typically within months and are not likely to have been overlooked.
The wapRT prescribed was completed in a high proportion of those with low-stage disease (93% in those with stage I and II nonserous tumors), with a modest rate of moderate, severe, or fatal toxic effects. Perhaps the infrequency of extensive primary surgical exploration in this population was a mitigating factor, as indicated by our data that show more chronic RT effects in those who had undergone more extensive prior laparotomies (and suggesting, perhaps, a need to avoid uninformative explorations in those who may benefit from RT [27, 29]). A small number of radiation oncologists were responsible for treating this series of women, a concentration of experience that may also have contributed to favorable outcomes. The observation that RT-related survival benefits are durable alleviates at least some of the anxiety about deleterious long-term effects. No quality of life or economic data were available for evaluation.
looking ahead
These positive results necessitate a prospective evaluation of RT in the diseases clustered under the collective rubric of ‘ovarian epithelial cancers’. The survival benefits apparently attributable to RT are substantial, suggesting that future studies focusing on low-stage clear cell, endometrioid, and mucinous tumors need not be large. For example, with a 5-year accrual period and 5 years of additional follow-up, ∼200 subjects per arm could detect a reduction of 40% in overall mortality (where the median survival in the control arm is ∼10 years) with 80% power and a 5% significance level—numbers well within reach of an intergroup study.
Questions regarding the RT prescription abound: Does less frequent occult dissemination of apparent low-stage clear cell, endometrioid, and mucinous tumors allow more restricted fields to be treated? Would benefits accrue to those with modest residuum confined to pelvic or nodal regions? Does each of these nonserous histotypes benefit equally from RT? Will RT be of greater benefit if delivered sooner after surgery, given the reduction in cure rates seen when its use is delayed in other diseases? [30, 31] Will concurrent CRT regimens be superior? Could RT alone be used in those with disease poorly responsive to currently used CT regimens? Are there those with a yet-to-be-defined subset of serous tumors that benefit from RT?
Until more robust data are accumulated, a pragmatic interim approach could consider:
that clear cell, endometrioid, and mucinous histotypes, for which apparent low stage is reliable, may be cured by limited surgery [31]; the cure of microscopic residuum may be enhanced by RT-containing adjuvant treatment,
that serous disease is usually disseminated at the time of diagnosis, and surgery and CT can be of considerable, although usually palliative, benefit; adjuvant RT has no evident role at this time.
The Gynecology Tumour Group of our institution has elected to continue to advise the use of CRT in women with stage Ic and II endometrioid and mucinous lesions, and all stage I and II clear cell disease, on the basis of these data and a recently published analysis of histotype-specific outcomes [32]. However, RT will be targeted to the pelvis, with consideration of extending the field to include paraaortic nodes if stage IIc (given the higher rate of occult nodal involvement reported for this stage [26]). RT will be dropped from our prescription for those with serous tumors.
Our findings also bring into question the nonselective surgical and CT approaches that have characterized ovarian cancer care and are of potential clinical importance for those with other tumors, which have been collectively defined by presumed organ of origin rather than underlying biological features.
contributors
KDS: Concept; design; data collection, assembly, analysis, and interpretation; writing. JLS: Data collection and assembly; interpretation; writing. CBG: Data collection, assembly, analysis and interpretation; writing. MK: Data collection, assembly, analysis and interpretation; writing. PJH: Data interpretation; writing. FW: Data interpretation, writing. NDL: Data assembly, analysis and interpretation; writing.
funding
National Cancer Institute of Canada (017051); Michael Smith Foundation for Health Research Unit (INRUA006045 to CBG); unrestricted educational grant from sanofi aventis Canada Inc. MK received fellowship support from Eli Lilly Canada.
disclosure
None of the authors declare conflicts of interest.
Acknowledgments
The authors wish to acknowledge the dedicated clinicians of the BCCA Gynecology Tumour Group who supervised the care of the patients attending our facilities during the interval of this study. Initial statistical analyses undertaken by V. Moravan provided a foundation for much of this work. The corresponding author has had full access to all the data in this study and maintains final responsibility for submission to this publication.
References
- 1.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5(12) doi: 10.1371/journal.pmed.0050232. e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009;40(9):1213–1223. doi: 10.1016/j.humpath.2009.04.017. doi:10.1016/j.humpath.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Fountain J, Trimble E, Birrer MJ. Summary and discussion of session recommendations. Gynecol Oncol. 2006;103(2 Suppl 1):S23–S25. doi: 10.1016/j.ygyno.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Cronin KA, Johnson KA, et al. Improved survival time: what can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer. 2008;112(10):2289–2300. doi: 10.1002/cncr.23425. doi:10.1002/cncr.23425. [DOI] [PubMed] [Google Scholar]
- 5.Paterson R. The Treatment of Malignant Disease by Radium and X-rays. 1st edition. London: Edward Arnold (Publishers) Ltd; 1948. [Google Scholar]
- 6.Einhorn N, Trope C, Ridderheim M, et al. A systematic overview of radiation therapy effects in ovarian cancer. Acta Oncologica. 2003;42(5/6):562–566. doi: 10.1080/02841860310014426. [DOI] [PubMed] [Google Scholar]
- 7.Nagai N, Inamine M, Hirakawa M, et al. Postoperative whole abdominal radiotherapy in clear cell adenocarcinoma of the ovary. Gynecol Oncol. 2007;107:469–473. doi: 10.1016/j.ygyno.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 8.Swenerton KD, Hislop TG, Spinelli J, et al. Ovarian carcinoma: a multivariate analysis of prognostic factors. Obstet Gynecol. 1985;65:264–270. [PubMed] [Google Scholar]
- 9.Swenerton K. Prognostic indices in ovarian cancer: their significance in treatment planning. Acta Obstet Gynecol Scand. 1992;155(Suppl):67–74. doi: 10.1111/j.1600-0412.1992.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 10.BCCA Cancer Management Guidelines. http://www.bccancer.bc.ca?HPI?CancerManagementGuidelines?Gynecology/default.htm. (19 July 2010, date last accessed) [Google Scholar]
- 11.Hoskins PJ, Swenerton KD, Wong F, et al. Platinum plus cyclophosphamide plus radiotherapy is superior to platinum alone in 'high-risk' epithelial ovarian cancer (residual negative and either stage I or II, grade 3, or stage III, any grade) Int J Gynecol Cancer. 1995;5(2):134–142. doi: 10.1046/j.1525-1438.1995.05020134.x. [DOI] [PubMed] [Google Scholar]
- 12.Gilks CB, Ionescu DN, Kalloger SE, et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39(8):1239–1251. doi: 10.1016/j.humpath.2008.01.003. doi:10.1016/j.humpath.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Dembo AJ, Davy M, Stenwig AE, et al. Prognostic factors in patients with stage I epithelial ovarian cancer. Obstet Gynecol. 1990;75:263–273. [PubMed] [Google Scholar]
- 14.RTOG/EORTC late radiation morbidity scoring schema. http://www.rtog.org/members/toxicity/late.html. (19 July 2010, date last accessed) [Google Scholar]
- 15.Therneau T, Atkinson E. Technical Report Series No. 61, An introduction to recursive partition using the RPART routines. September 3, 1997. Rochester, Mayo Foundation. [Google Scholar]
- 16.Agresti A. Categorical Data Analysis. New York: Wiley; 2002. [Google Scholar]
- 17.Cox DR, Oakes D. Analysis of Survival Data. 21st edn. Boca Raton: CRC Press;; 1984. [Google Scholar]
- 18.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical-Theoretic Approach. 2nd edn. New York: Springer-Verlag;; 2002. [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley; 2002. [Google Scholar]
- 20.Shelley WE, Starreveld AA, Carmichael JA, et al. Toxicity of abdominopelvic radiation in advanced ovarian carcinoma patients after cisplatin/cyclophosphamide therapy and second-look laparotomy. Obstet Gynecol. 1988;71(3 Pt 1):327–332. [PubMed] [Google Scholar]
- 21.Hoskins PJ, Swenerton KD, Manji M, et al. ‘Moderate-risk’ ovarian cancer (stage I, grade 2; stage II, grade 1 or 2) treated with cisplatin chemotherapy (single agent or combination) and pelvi-abdominal irradiation. Int J Gynecol Cancer. 1994;4(4):272–278. doi: 10.1046/j.1525-1438.1994.04040272.x. [DOI] [PubMed] [Google Scholar]
- 22.Swenerton KD, Gilks B, Le N. Epithelial ovarian cancer without macroscopic residual: long-term, population-based outcomes. Proc Am Soc Clin Oncol. 2004;23:460. [Google Scholar]
- 23.Hoskins PJ, O'Reilly SE, Swenerton KD, et al. Ten-year outcome of patients with advanced epithelial ovarian carcinoma treated with cisplatin-based multimodality therapy. J Clin Oncol. 1992;10(10):1561–1568. doi: 10.1200/JCO.1992.10.10.1561. [DOI] [PubMed] [Google Scholar]
- 24.Faught W, Le T, Fung Kee Fung M, et al. Early ovarian cancer: what is the staging impact of retroperitoneal node sampling? J Obstet Gynaecol Can. 2003;25(1):18–21. doi: 10.1016/s1701-2163(16)31078-7. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Ohwada M, Yamada T, et al. Lymph node metastasis in stage I epithelial ovarian cancer. Gynecol Oncol. 2000;79(2):305–308. doi: 10.1006/gyno.2000.5951. doi:10.1006/gyno.2000.5951. [DOI] [PubMed] [Google Scholar]
- 26.Fournier M, Stoeckle E, Guyon F, et al. Lymph node involvement in epithelial ovarian cancer: sites and risk factors in a series of 355 patients. Int J Gynecol Cancer. 2009;19(8):1307–1313. doi: 10.1111/IGC.0b013e3181b8a07c. [DOI] [PubMed] [Google Scholar]
- 27.Nomura H, Tsuda H, Susumu N, et al. Lymph node metastasis in grossly apparent stages I and II epithelial ovarian cancer. Int J Gynecol Cancer. 2010;20:341–345. doi: 10.1111/IGC.0b013e3181cf6271. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara K, Suzuki S, Yoden E, et al. Local radiation therapy for localized relapsed or refractory ovarian cancer patients with or without symptoms after chemotherapy. Int J Gynecol Cancer. 2002;12(3):250–256. doi: 10.1046/j.1525-1438.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 29.Takano M, Sugiyama T, Yaegashi N, et al. The impact of complete surgical staging upon survival in early-stage ovarian clear cell carcinoma: a multi-institutional retrospective study. Int J Gynecol Cancer. 2009;19(8):1353–1357. doi: 10.1111/IGC.0b013e3181a83f4f. [DOI] [PubMed] [Google Scholar]
- 30.Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1–4. doi: 10.1016/j.radonc.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol. 2009;27(1):16–23. doi: 10.1200/JCO.2008.18.1891. [DOI] [PubMed] [Google Scholar]
- 32.Kobel M, Kalloger SE, Santos JL, et al. Tumor type and substage predict survival in stage I and II ovarian carcinoma: insights and implications. Gynecol Oncol. 2010;116:50–56. doi: 10.1016/j.ygyno.2009.09.029. [DOI] [PubMed] [Google Scholar]