Abstract
Background: The American College of Surgeons Oncology Group sought to confirm the efficacy of a novel interferon-based chemoradiation regimen in a multicenter phase II trial.
Patients and methods: Patients with resected (R0/R1) adenocarcinoma of the pancreatic head were treated with adjuvant interferon-alfa-2b (3 million units s.c. on days 1, 3, and 5 of each week for 5.5 weeks), cisplatin (30 mg/m2 i.v. weekly for 6 weeks), and continuous infusion 5-fluorouracil (5-FU; 175 mg·m2/day for 38 days) concurrently with external-beam radiation (50.4 Gy). Chemoradiation was followed by two 6-week courses of continuous infusion 5-FU (200 mg·m2/day). The primary study end point was 18-month overall survival from protocol enrollment (OS18); an OS18 ≥65% was considered a positive study outcome.
Results: Eighty-nine patients were enrolled. Eighty-four patients were assessable for toxicity. The all-cause grade ≥3 toxicity rate was 95% (80 patients) during therapy. No long-term toxicity or toxicity-related deaths were noted. At 36-month median follow-up, the OS18 was 69% [95% confidence interval (CI) 60% to 80%]; the median disease-free survival and overall survival were 14.1 months (95% CI 11.0–20.1 months) and 25.4 months (95% CI 23.4–34.1 months), respectively.
Conclusions: Notwithstanding promising multi-institutional efficacy results, further development of this regimen will require additional modifications to mitigate toxic effects.
Keywords: adenocarcinoma, chemoradiation, cisplatin, interferon, pancreas, surgery
introduction
Each year, ∼5000 patients with pancreatic cancer undergo pancreaticoduodenectomy (PD) in the United States. Despite significant reductions in perioperative morbidity and mortality associated with PD over the past two decades [1], survival following PD remains poor, with most studies reporting a median survival of 11–18 months and a 5-year survival rate of ≥10% [2–4]. The use of adjuvant chemotherapy [gemcitabine or 5-fluorouracil (5-FU)/leucovorin] after surgery has been shown to significantly increase survival compared with surgery alone—in two randomized trials [4, 5], the median overall survival (OS) duration with adjuvant chemotherapy was 23 months—but major progress has been elusive.
In 1995, Virginia Mason Medical Center (VMMC) embarked on a phase II trial of adjuvant chemoradiation after pancreatic cancer resection. Cisplatin, interferon-alfa-2b, and continuous infusion 5-FU were given concurrently with radiation therapy, followed by additional continuous infusion 5-FU. These agents were chosen in part because of their efficacy as radiosensitizers [6–8] and the synergy between interferon-alfa-2b and the other agents in experimental animal systems [9–11]. The results of the VMMC trial were encouraging [12, 13]. A median OS of >32 months and a 5-year OS rate of 55% were achieved among the 43 patients who underwent surgery at VMMC—many of whom had overall unfavorable pathologic features at PD [e.g. 85% had positive lymph nodes; 26% had positive (R1) surgical margins]. However, the regimen’s toxicity was significant. Although no treatment-related deaths occurred, grade 3 or 4 toxicity was observed in 67% of the patients and 43% were hospitalized for toxic effects.
The favorable results at VMMC provided considerable hope and prompted interest in a multi-institutional trial to determine whether the VMMC regimen could be safely administered outside of a single-institution setting and whether comparable survival outcomes could be achieved. In 2003, the American College of Surgeons Oncology Group (ACOSOG) embarked on a multicenter phase II trial (Z05031) of the VMMC adjuvant therapy regimen. The primary objective of the trial was to estimate OS at 18 months (OS18) following postoperative protocol-based adjuvant chemoradiation therapy. Secondary objectives included (i) determination of the frequency and severity of acute and late toxic effects, (ii) estimation of disease-free survival rates, and (iii) analysis of post-treatment disease recurrence patterns.
patients and methods
patient eligibility
Major eligibility criteria for this trial included (i) age ≥18 years; (ii) documented Eastern Cooperative Oncology Group performance status of zero or one; (iii) pathologic stage T1–3, N0–1, and M0 adenocarcinoma of the pancreatic head in patients who had undergone a potentially curative grossly complete (R0 or R1) PD; and (iv) recovery sufficient to enable adjuvant therapy initiation within 56 days of PD. Additional eligibility requirements included adequate bone marrow function (white blood cell count ≥3000/μl, absolute neutrophil count >1500/μl, hemoglobin >9.5 mg/dl, platelet count ≥100 000/μl); adequate hepatic function [total bilirubin ≤3 mg/dl, aspartate aminotransferase ≤2.0 times the institutional upper limit of normal (ULN), alanine aminotransferase ≤2.0 times ULN, alkaline phosphatase ≤2.0 times ULN], and adequate renal function (serum creatinine ≤1.5 times ULN).
Baseline postoperative computed tomography (CT) of the chest and CT or magnetic resonance imaging of the abdomen/pelvis were required before registration to exclude the possibility of residual local disease or early development of metastatic disease. No specific presurgical or postsurgical serum carbohydrate antigen 19.9 value was required. Patients with prior malignancies, excluding successfully treated cervical carcinoma, lobular carcinoma of the breast, or nonmelanoma skin cancer, must have undergone potentially curative therapy with no evidence of malignancy for at least 5 years.
The trial excluded patients with adenosquamous carcinoma, ampullary carcinoma, carcinoid tumor, cystadenocarcinoma, cystadenoma, distal common bile duct carcinoma, duodenal carcinoma, or neuroendocrine tumors. Pregnant or lactating patients were excluded. Patients could not have had any treatment of pancreatic cancer other than recent surgery. Patients who had received prior radiation treatment to the chest, abdomen, or pelvis or any biological/immunologic therapies (for any condition) were also excluded.
All patients provided written informed consent before trial enrollment. The Human Investigations Committee at each participating institution approved the trial.
adjuvant treatment
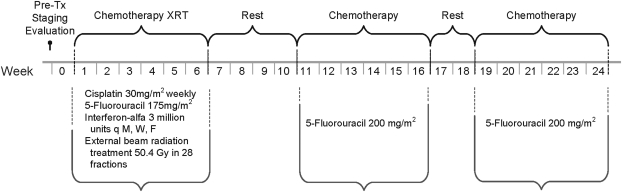
The treatment schema is outlined in Figure 1. 5-FU was infused continuously via an ambulatory infusion pump into a central venous catheter at a dosage of 175 mg·m2/day for 38 consecutive days. Cisplatin (30 mg/m2 i.v.) was given weekly on the first day of each week of the 5.5-week cycle (six total administrations). Interferon-alfa-2b (3 million units) was given s.c. on days 1, 3, and 5 of each week for 5.5 weeks. The chemotherapy was administered concurrently with external-beam radiation (50.4 Gy total; 28 fractions at 1.8 Gy/fraction), which was given on Monday to Friday for 5.5 weeks. In the event of grade 3 or 4 hematologic toxicity, all therapy (including radiation treatment) was held until resolution.
Figure 1.
Treatment schema for American College of Surgeons Oncology Group trial Z05031, a phase II trial of adjuvant interferon-based chemoradiation for patients with resected pancreatic adenocarcinoma. XRT, radiation therapy; i.v.; s.c.; q M, W, F, every Monday, Wednesday, and Friday.
Radiotherapy was planned by three-dimensional conformal techniques. The clinical target volume encompassed the areas believed to be at risk for the highest concentration of microscopic residual tumor and included the regions of the pancreaticojejunostomy, hepaticojejunostomy, proximal celiac and superior mesentery arteries, and retroperitoneal lymph nodes. In an effort to reduce toxicity over that observed in the Virginia Mason pilot experience, the radiation treatment volume was designed to be slightly smaller than that used in Radiation Therapy Oncology Group (RTOG) trial 9704 [14] and the daily dose was decreased from 2 Gy/fraction (used in the Virginia Mason pilot experience) to 1.8 Gy per fraction for this protocol
Two additional cycles of 5-FU were administered beginning 4–6 weeks after completion of chemoradiation. Patients received 5-FU (200 mg·m2/day continuously for 6 weeks via central venous catheter) with 2 weeks of rest between cycles.
Patients were followed every 2 months for their first 2 years after surgery, every 3 months in year 3, and then every 6 months in years 4 and 5. All visits included a history and physical examination, laboratory studies, and chest X-ray. CT was repeated 2 and 4 months following completion of study treatment and every 6 months thereafter. The appearance of new low-density liver or peritoneal lesions with characteristics of metastatic lesions was considered evidence of recurrent disease.
adverse events
Adverse events were graded using the Common Terminology Criteria for Adverse Events Version 3.0 [15]. Adverse events were recorded from enrollment to 30 days following the end of all study treatments (regardless of attribution). When the same toxicity occurred multiple times in the same patient, only the highest grade was used for toxicity data collection
statistical analysis
The trial’s primary end point was OS18 rate: the proportion of eligible and assessable patients who were alive 18 months from their dates of trial registration. The historical value for OS18 rate n this patient population was assumed to be 50%. The study was designed to accrue 93 assessable patients, which would yield 90% power to detect an absolute increase in OS18 rate of ≥15% (i.e. to detect an OS18 rate of ≥65%) with a 0.05 one-sided significance level. The corresponding decision rule was that if ≥55 patients of the 93 were alive 18 months after registration, there would be sufficient evidence of treatment efficacy to warrant further investigation.
The trial was reviewed by a Data Monitoring Committee every 6 months. Owing to toxicity concerns, the committee recommended the trial be halted before attaining its target accrual. At the time of study closure, 89 of 93 planned patients had been accrued. Nevertheless, because >55 patients lived at least 18 months, the trial remained statistically valid.
Cumulative time-to-event experiences for OS and disease-free survival were estimated using the Kaplan–Meier method. Specifically, the Kaplan–Meier estimator was used to generate point estimates for OS18, median OS, disease-free survival, and corresponding 95% confidence intervals (CIs). Patients who died without disease recurrence were considered to have experienced recurrence at the time of death for the disease-free survival analysis, and patients who were alive at their last follow-up were censored at that time for the OS analysis. Survival was measured from both date of surgery and date of trial registration. The log-rank test was used to examine potential predictors of OS. All analyses were carried out using SAS version 9 (SAS Institute Inc., Cary, NC).
results
patient characteristics
Eighty-nine patients at 15 different centers (range 2–14 patients per site) entered the trial from November 2003 to November 2005. The patients’ clinicopathologic factors are outlined in Table 1. The patients’ median age was 59 years (range 41–82 years). Ninety-three percent of patients had American Joint Committee on Cancer (6th edition) [16] stage II disease (73% had stage IIB), 73% had node-positive disease, and 25% had microscopically positive surgical margins (R1).
Table 1.
Distribution of clinicopathologic factors in the study population
| Variable | Subjects evaluable for response (n = 80) | All subjects (n = 89) |
| Age (years) | ||
| Median | 59 | 59 |
| Range | 41–82 | 41–82 |
| Sex | ||
| Female | 42 (53) | 44 (49) |
| Male | 38 (48) | 45 (51) |
| Racea | ||
| White | 76 (95) | 84 (94) |
| Black | 2 (3) | 2 (2) |
| Pacific Islander | 0 (0) | 0 (0) |
| Asian | 3 (4) | 4 (4) |
| Native American/Alaskan | 0 (0) | 0 (0) |
| T stage | ||
| T1 | 5 (6) | 5 (6) |
| T2 | 9 (11) | 10 (11) |
| T3 | 66 (83) | 74 (83) |
| N stage | ||
| N0 | 22 (28) | 24 (27) |
| N1 | 58 (73) | 65 (73) |
| Overall stage | ||
| IA | 4 (5) | 4 (4) |
| IB | 2 (3) | 3 (3) |
| IIA | 16 (20) | 17 (19) |
| IIB | 58 (73) | 65 (73) |
| ECOG performance status | ||
| 0 | 44 (55) | 48 (54) |
| 1 | 36 (45) | 41 (46) |
| Margin statusb | ||
| R0 | 60 (75) | – |
| R1 | 20 (25) | – |
| ≥3 cm primary lesionb | 41 (51.3) | – |
| Gradeb | ||
| 1 | 6 (8) | – |
| 2 | 37 (46) | – |
| 3 | 34 (43) | – |
| 4 | 3 (4) | – |
Values are no. of patients (%) unless otherwise indicated.
One patient chose two races.
For margin status, tumor size, and grade, data were abstracted only for the evaluable subjects.
ECOG, Eastern Cooperative Oncology Group
toxicity
Of the 89 patients enrolled, 84 patients were assessable for toxicity. Five patients were not treated (four patients because of ineligible histology and one for unknown reasons). The majority of grade 3 or 4 toxic effects occurred during chemoradiation. Grade 3 and 4 toxicity results are summarized in Table 2. Ninety-five percent of patients experienced a grade 3 or 4 acute toxicity (66% had grade 3 and 29% had grade 4). All toxic effects were reversible, and no treatment-related deaths occurred. No long-term toxic effects were identified during the follow-up.
Table 2.
Grade 3 and 4 adverse events in patients who received therapy (n = 84)
| Body system/category | Adverse event | No. patients (%) |
|
| Grade 3 | Grade 4 | ||
| Hematologic | Anemia | 2 (2) | 0 (0) |
| Leukopenia | 41 (49) | 8 (10) | |
| Lymphopenia | 11 (13) | 5 (6) | |
| Neutropenia | 24 (29) | 5 (6) | |
| Thrombocytopenia | 9 (11) | 0 (0) | |
| Hemorrhage | Hemoptysis | 1 (1) | 0 (0) |
| Hepatic | Elevated ALT | 2 (2) | 1 (1) |
| Elevated AST | 3 (4) | 1 (1) | |
| Elevated bilirubin | 1 (1) | 0 (0) | |
| Hypoalbuminemia | 4 (5) | 0 (0) | |
| Infection | Febrile neutropenia | 3 (4) | 1 (1) |
| Infection | 4 (5) | 0 (0) | |
| Metabolic/laboratory | Hyperglycemia | 8 (10) | 0 (0) |
| Hyperkalemia | 1 (1) | 0 (0) | |
| Hypocalcemia | 7 (8) | 3 (4) | |
| Hypokalemia | 15 (18) | 4 (5) | |
| Hypomagnesemia | 8 (10) | 2 (2) | |
| Hyponatremia | 2 (2) | 0 (0) | |
| Hypophosphatemia | 2 (2) | 0 (0) | |
| Elevated ALK phosphatase | 1 (1) | 0 (0) | |
| Neurologic | Depression | 1 (1) | 0 (0) |
| Neurosensory | 1 (1) | 0 (0) | |
| Syncope | 2 (2) | 0 (0) | |
| Pain | Pain, abdominal | 8 (10) | 1 (1) |
| Pain, back | 1 (1) | 0 (0) | |
| Pain, gingivitis | 1 (1) | 0 (0) | |
| Renal/genitourinary | Polyuria | 1 (1) | 0 (0) |
| Cardiovascular | QTc interval prolongation | 1 (1) | 0 (0) |
| Hypertension | 1 (1) | 0 (0) | |
| Hypotension | 3 (4) | 0 (0) | |
| Sinus bradycardia | 1 (1) | 0 (0) | |
| Thrombosis | 2 (2) | 1 (1) | |
| Coagulation | Elevated PTT | 1 (1) | 0 (0) |
| Elevated prothrombin time | 2 (2) | 0 (0) | |
| Constitutional symptoms | Fatigue | 14 (17) | 0 (0) |
| Weight loss | 6 (7) | 0 (0) | |
| Dermatologic/skin | Alopecia | 1 (1) | 0 (0) |
| Rash/desquamation | 3 (4) | 0 (0) | |
| RXN, hand/foot syndrome | 9 (11) | 0 (0) | |
| Pruritus | 1 (1) | 0 (0) | |
| Skin irritation | 1 (1) | 0 (0) | |
| Gastrointestinal | Anorexia | 22 (26) | 1 (1) |
| Dehydration | 18 (21) | 2 (2) | |
| Diarrhea | 16 (19) | 4 (5) | |
| Dysphagia | 2 (2) | 0 (0) | |
| Hemorrhoids | 1 (1) | 0 (0) | |
| Nausea | 25 (30) | 0 (0) | |
| Mucositis | 19 (23) | 1 (1) | |
| Vomiting | 13 (15) | 2 (2) | |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALK, alkaline; QTc, Q-T corrected; PTT, partial thromboplastin time; RXN, reaction.
Grades 3 and 4 hematologic toxic effects included neutropenia in 35% of patients and thrombocytopenia in 11%. However, the incidence of febrile neutropenia was low (5%), and only one significant bleeding event was reported. The most common grade 3 or 4 nonhematologic toxic effects were gastrointestinal toxic effects and electrolyte disturbances: nausea (30%), anorexia (27%), dehydration (24%), diarrhea (24%), mucositis (24%), vomiting (18%), hypokalemia (23%), hypocalcemia (12%), and hypomagnesemia (12%).
Toxic effects impaired the delivery of planned treatment in most patients. Only 14 (17%) of 84 patients completed chemoradiation without interruption. Fifty-four (68%) patients received the planned radiation dose of 50.4 Gy. Of 80 patients assessable for survival, 16 (20%) received <45 Gy of radiation and/or missed at least one dose of chemotherapy during chemoradiation. Thirty-five (44%) of 80 patients did not complete all phases of protocol treatment.
survival
Eighty patients were assessable for survival. The other four treated patients were deemed ineligible for survival analysis because of protocol violations [gross residual tumor following surgery (one patient) or metastatic disease found at postsurgical restaging (three patients)]. Seventy-seven patients had been followed for at least 18 months after surgery or until death; the median follow-up of surviving patients was 36 months (range 1–55 months). At the time of this analysis, 56 patients (70%) were dead, 53 were confirmed to have died of recurrent pancreatic cancer, and 3 died without evidence of recurrence.
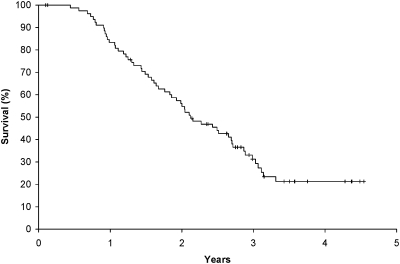
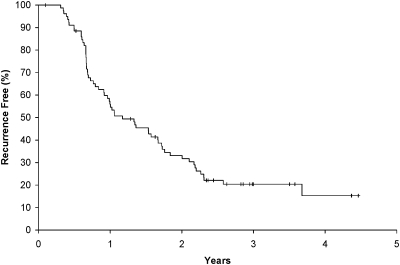
Figures 2 and 3 outline OS and disease-free survival plots. The OS18 rate (as measured from registration) for the 80 assessable patients was 69.1% (95% CI 59.6% to 80.2%). The median OSs from the date of trial registration was 25.4 months (95% CI 22.0–32.4 months). The median disease-free survival was 14.1 months (95% CI 11.0–20.1 months).
Figure 2.
Kaplan–Meier plot of overall survival (OS) for 80 eligible and assessable patients. The median OS was 25.4 months (95% confidence interval 22.0–32.4 months) from protocol registration (27.1 months from surgery).
Figure 3.
Kaplan–Meier plot of disease-free survival for 80 eligible and assessable patients. The median disease-free survival was 14.1 months (95% confidence interval 11.0–20.1 months) from surgery.
patterns of first treatment failure
Fifty-eight patients developed tumor recurrence during follow-up: 31 patients had local first recurrences, 21 patients had distant first recurrences, and 6 patients had synchronous local and distant recurrences. The local first recurrence rate was 46% (37 patients), and the systemic first recurrence rate was 34% (27 patients).
prognostic variables
None of the potential prognostic variables examined was predictive of OS, including microscopic surgical margin status (R0 versus R1, median OS 31.9 versus 18.9 months, P = 0.103), tumor grade (grade 1/2 versus grade 3/4, median OS 30.2 versus 22.7 months, P = 0.115), nodal status (N0 versus N1, median OS 30.2 versus 25.5 months, P = 0.854), and primary tumor size (<3 versus ≥3 cm, median OS 30.2 versus 23.8 months, P = 0.392).
discussion
In this multi-institutional trial, the observed OS18 rate of 69.1% met the protocol-defined standard for a regimen considered of further interest: an OS18 estimate at least 15% greater than the historical standard of 50%. Furthermore, although we acknowledge the limitations of comparisons between trials, the median OS of 25.4 months is promising in relation to the median OSs of 17–24 months reported in the chemotherapy or chemoradiation arms of phase III trials of adjuvant therapy for pancreatic cancer (Table 3) [2–5, 13, 14, 17–19]. Indeed, the 2-year OS rate of 59% observed in this trial is substantially greater than the 2-year OS rates of 37%–50% in the earlier trials—which did not employ interferon-based chemoradiation [3, 4, 14].
Table 3.
Major studies of adjuvant therapy for resected pancreatic cancer
| Study | Overall survival |
||
| Median, months | 2 Years, % | 5 Years, % | |
| GITSG (chemoradiation) [17] | 21 | 43 | 19 |
| EORTC (chemoradiation) [2] | 17.1 | 37 | 20 |
| ESPAC-1 (chemotherapy) [3] | 20.1 | 39 | 21 |
| RTOG 9704 (gemcitabine) [14] | 20.6 | 31 (3 years) | – |
| RTOG 9704 (5-FU) [14] | 16.7 | 21 (3 years) | – |
| CONKO-001 (gemcitabine) [4] | 22.8 | 48 | 21 |
| ESPAC-3 [5] | 23.3 | 48 | – |
| EORTC/FFCD/GERCOR [18] | 24.0 | 50 | – |
| VMMCa [13] | 43.7 | 56 | 44 |
| Washington, St Louis [19] | 25 | 56 | – |
| ACOSOG Z05031 | 25.4 | 59 | – |
From updated patient results (V. J. Picozzi et al., unpublished data).
GITSG, Gastrointestinal Study Group; EORTC, European Organization for Research and Treatment of Cancer; ESPAC, European Study Group for Pancreas Cancer; RTOG, Radiation Therapy Oncology Group; FFCD, Federation Francophone de la Cancerologie Digestive; GERCOR, Groupe Cooperateur Multidisciplinaire en Oncologie; VMMC, Virginia Mason Medical Center; ACOSOG, American College of Surgeons Oncology Group.
The survival results from the current multi-institutional trial were, however, similar to the promising results of two single-institution trials of adjuvant interferon-based chemoradiation [13, 19]. An updated unpublished analysis of the VMMC patient population with longer follow-up (7–13 years) demonstrated a median OS from the date of surgery of 43.7 months and 2- and 5-year OS rates of 56% and 44%, respectively (V. J. Picozzi, Y. Hashimoto, L. W. Traverso et al., unpublished data). Linehan et al. [19] at Washington University employed a similar chemoradiation regimen (with a slightly lower dose of cisplatin) in 53 patients with pancreatic cancer (8 of whom had pancreatic body or tail primaries). Seventy percent (37) of their patients completed all planned therapy, the incidence of grade 3/4 toxic effects was 68% during both chemoradiation and chemotherapy (most toxic effects during chemotherapy were hematologic), and no deaths due to acute toxicity were reported. The median OS from the date of surgery was 25 months (95% CI 21.5–48.5 months); 2- and 3-year OS rates were 56% and 41%, respectively. In aggregate, the data from the present trial and the VMMC and Washington University trials provide a relatively robust body of phase II trial evidence confirming the efficacy of interferon-based chemoradiation as adjuvant therapy for pancreatic cancer.
The mechanism by which this regimen may improve outcomes is not clear. Conceivably, the improved OS results could arise from more potent radiosensitization than offered by 5-FU alone; the activity of a platinol/fluoropyrimidine drug combination [as suggested by the Charite Onkologie (CONKO)-003 trial [20]]; the antineoplastic, antiangiogenic, and/or immunostimulatory [21] properties of interferon-alfa-2b; or some other, unrecognized mechanism.
The considerable toxic effects of interferon-based chemoradiation are a consistent theme of the cumulative experience to date. Indeed, the high rates of grades 3 and 4 toxic effects observed in this trial led the ACOSOG Data Monitoring Committee to close the trial slightly short of its 93 patient target. Almost all patients experienced some high-grade toxicity, and nearly half of patients failed to complete all phases of the planned treatment. Thus, it seems clear that further development of this regimen will require additional changes to mitigate toxic effects. Administering the regimen preoperatively might also improve tolerability.
Several aspects of the toxicity profile of adjuvant interferon-based chemoradiation warrant consideration. First, no irreversible toxic effects and no treatment-related deaths occurred in this trial. In the context of the relatively favorable outcome results observed and the typically high relapse rates and associated mortality rates of patients with resected pancreatic adenocarcinoma, manageable toxic effects should not lead to abject rejection of this regimen. Second, it is important to note that many of the phase III trials of adjuvant therapy for localized pancreatic cancer were also characterized by high toxicity rates and difficulty with completion of all components of treatment [2–4]. For example, only 62% of patients received all six cycles of gemcitabine therapy in the CONKO-001 trial [4]. The hematologic toxic effects observed in the current trial were similar to those seen in the gemcitabine arm of the RTOG 9704 trial [14] (e.g. 35% versus 43% grade ≥3 neutropenia, respectively) but were of little clinical consequence beyond their impact on therapy administration. Finally, the toxicity issues associated with this regimen do not appear insurmountable. In fact, indications were that the experience required for safe delivery of this regimen can be ‘exported’ to other institutions for broader usage. For example, the incidence of gastrointestinal toxicity in ACOSOG Z05031 was similar to that seen in the VMMC trial [13] (e.g. 30% versus 33% versus 30% grade ≥3 nausea, respectively) despite the inexperience of the study investigators with this regimen.
What steps should be taken to improve the tolerability and efficacy of this regimen, given its promise? At least three steps seem essential. First, patient selection criteria need to be improved to better identify the patients at highest risk of local recurrence and those who can tolerate chemoradiation [19, 22]. Second, finding and employing strategies to lessen toxicity, from surgery through adjuvant therapy, are critical. This step requires improved understanding by surgical, medical, and radiation oncologists of quality standards for each component of treatment and improved application of integrated supportive care strategies throughout treatment. Administering the chemoradiation regimen at a different time in the overall treatment sequence, i.e., after adjuvant chemotherapy or preoperatively, and more frequent adjustments of drug doses during treatment are also worth considering. Third, the systemic activity of the regimen must be maintained or enhanced while toxicity is diminished. The use of more contemporary drug combinations and other strategies (e.g. gemcitabine combinations, targeted therapies) in place of infusional 5-FU may be a reasonable next step in the evolution of this approach.
The patterns of initial failure observed in this trial also require careful consideration. We observed a paradoxically inverted ratio of local to systemic recurrences: a 46% local first recurrence rate and a 34% systemic first recurrence rate. This high rate of local first recurrence is particularly disappointing given that most PDs were carried out in high-volume centers by experienced surgeons (all patients were presumed to have undergone either R0 or R1 resection) and that state-of-the-art quality-controlled chemoradiation was delivered. There was, however, no quality control for surgery (which was not part of the protocol treatment), and it is likely that many patients considered to have had R0 resections had R1 or even R2 resections. Reduced radiation treatment volumes and interruption of chemoradiation owing to toxicity may also have compromised local control. We are systematically reviewing the operative reports, surgical pathology reports, and radiation quality assurance data to ascertain the extent to which suboptimal patient selection, incomplete (R2) surgery, and/or compromise in the quality of radiation treatment may have contributed to this high local recurrence rate.
From the perspective of long-term survival, many patients and physicians may believe that the significant risk of serious but reversible toxic effects is worth taking given the uniformly poor outcomes otherwise seen with this disease and the favorable survival results seen in the aggregate phase II trial experience with this adjuvant chemoradiation regimen. Others, however, may feel more comfortable with a less toxic adjuvant therapy regimen that offers a more modest survival benefit over surgery alone. Arguably, the latter approach might in fact result in the longest survival free of symptoms from the disease or its treatment. Nevertheless, further trials exploring approaches to reduce the toxic effects and enhance the efficacy of adjuvant interferon-based chemoradiation are warranted and should ideally include assessments of quality of life to better determine the overall impact of this regimen.
funding
U.S. National Institutes of Health to the American College of Surgeons Oncology Group (U10 CA 76001).
Acknowledgments
We thank the ACOSOG staff, in particular the leadership of Samuel Wells, Heidi Nelson, and David Ota and the data management of Nancy You and Ghazala Haque, for their assistance in the development of this manuscript. We also thank all of the investigators and their site research teams. These include (principal investigator in parentheses) Brigham and Women’s Hospital, Boston, MA (C.S. Fuchs); Dallas Presbyterian Hospital, Dallas, TX (LSW); Johns Hopkins Hospital, Baltimore, MD (D.A. Laheru); Massachusetts General Hospital, Boston, MA (J.W. Clark); Memorial Medical Center, Savannah, GA (S.T. Brower); Memorial Sloan-Kettering Cancer Center, New York, NY (EMO); Ohio State University Hospital, Columbus, OH (T.S. Bekaii-Saab); Roswell Park Memorial Cancer Center, Buffalo, NY (M.M. Javle); University of Florida-Shands Memorial Hospital (R.L. Marsh); University of Louisville Hospital, Louisville, KY (RCM); University of Minnesota Hospital, Minneapolis, MN (EG); University of Rochester-Strong Memorial Hospital, Rochester, NY (S.A. Ahrendt); University of Wisconsin Medical Center, Madison, WI (J.P. Thomas); Vanderbilt University Hospital, Nashville, TN (J.D. Berlin); and VMMC, Seattle, WA (VJP). Finally, we wish to thank the brave patients with pancreatic cancer and their caregivers who participated in this study. Presented in part at the 2008 GI Symposium in Orlando, Florida, and the 2008 annual meeting of the American Society of Clinical Oncology, Chicago, IL.
References
- 1.Ghaneh P, Kawesha A, Howes N, et al. Adjuvant therapy for pancreatic cancer. World J Surg. 1999;23(9):937–945. doi: 10.1007/s002689900603. [DOI] [PubMed] [Google Scholar]
- 2.Klinkenbijl JH, Jeekel J, Sahmound T, et al. Adjuvant radio-therapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region. Ann Surg. 1999;230:776–784. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreas cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos J, Buchler M, Stocken DD, et al. ESPAC-3(v2): a multicenter, international, open-label randomized controlled phase III trial of adjuvant 5-fluorouracil/folinic acid versus gemcitabine in patients with resected pancreas cancer. J Clin Oncol. 2009;27:18s. (Suppl; Abstr. LBA4505) [Google Scholar]
- 6.Byfield JE, Calabro-Jones P, Klisak I, et al. Pharmacological requirements for obtaining sensitization of human tumor cells in vitro to combined 5-fluorouracil and x-rays. Int J Radiat Oncol Biol Phys. 1982;13:1691–1697. doi: 10.1016/0360-3016(82)90451-5. [DOI] [PubMed] [Google Scholar]
- 7.Holsti LR, Mattson K, Niiraven A, et al. Enhancement of radiation effects by alpha-interferon in the treatment of small cell lung cancer. Int J Radiat Oncol Biol Phys. 1987;13:1161–1166. doi: 10.1016/0360-3016(87)90189-1. [DOI] [PubMed] [Google Scholar]
- 8.Sischy B, Doggett RLS, Krall JM. Definitive irradiation and chemotherapy for radiosensitization in the management of anal carcinoma: interim report of Radiation Therapy Oncology Group No. 8314. J Natl Cancer Inst. 1989;81:850–856. doi: 10.1093/jnci/81.11.850. [DOI] [PubMed] [Google Scholar]
- 9.Wadler S, Wersto R, Weinberg V, et al. Interaction of fluorouracil and interferon in human colon cancer cell lines: cytotoxic and cytokinetic effects. Cancer Res. 1990;50:3473–3486. [PubMed] [Google Scholar]
- 10.Vokes EE. The promise of biochemical modulation in combined modality therapy. Semin Oncol. 1994;21(S14):29–33. [PubMed] [Google Scholar]
- 11.Ismail A, Van Groeningen CJ, Hardcastle A, et al. Modulation of fluorouracil cytotoxicity by interferon-alpha-2b and -gamma. Mol Pharmacol. 1998;53:252–261. doi: 10.1124/mol.53.2.252. [DOI] [PubMed] [Google Scholar]
- 12.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. doi: 10.1016/s0002-9610(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 13.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 14.Regine WF, Winter KA, Abrams RA. Fluorouracil vs. gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 15.Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda, MD,; 2003. [Google Scholar]
- 16.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th edition. New York: Springer-Verlag; 2002. [Google Scholar]
- 17.Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Van Laetham JL, Mornex F, Azria D, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiation after curative resection for pancreatic cancer: updated results of a randomized EORTC/FFCD/GERCOR phase II study. J Clin Oncol. 2009;27:15s. doi: 10.1200/JCO.2010.30.3446. (Suppl; Abstr 4527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linehan DC, Tan MCB, Strasberg SM, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution phase II study. Ann Surg. 2008;248:145–151. doi: 10.1097/SLA.0b013e318181e4e9. [DOI] [PubMed] [Google Scholar]
- 20.Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine-refractory pancreas cancer: final results of the CONKO 003 study. J Clin Oncol. 2008;26:14s. (Suppl; Abstr 4508) [Google Scholar]
- 21.Schmidt J, Jaeger D, Hoffman K, et al. Impact of interferon-alpha in combined chemoradioimmunotherapy for pancreatic adenocarcinoma (CapRI): first data from the immunomonitoring. J Immunother. 2007;30:108–115. doi: 10.1097/01.cji.0000211317.15278.27. [DOI] [PubMed] [Google Scholar]
- 22.Pisters PWT, Evans DB. Cisplatin, fluorouracil, interferon-α and radiation as adjuvant therapy for resected pancreatic cancer: is there a future for this regimen and/or should we change our approach to the research and treatment of patients with resected pancreatic cancer? Ann Surg. 2008;248:152–153. doi: 10.1097/SLA.0b013e3181820d35. [DOI] [PubMed] [Google Scholar]