Abstract
Background: To study the impact of the dietary antioxidant quercetin on risk of gastric adenocarcinoma.
Patients and methods: Using data from a large Swedish population-based case–control study of gastric cancer (505 cases and 1116 controls), we studied the association between quercetin and risk of anatomic (cardia/noncardia) and histological (intestinal and diffuse) subtypes of gastric cancer.
Results: We found strong inverse associations between quercetin and the risk of noncardia gastric adenocarcinoma, with an adjusted odds ratio (OR) of 0.57 (95% confidence interval 0.40–0.83) for the highest quintile (≥11.9 mg) of daily quercetin intake relative to the lowest quintile of intake (<4 mg quercetin/day), supported by a significant decreasing linear trend (P value < 0.001). Similar findings were observed for the intestinal and diffuse subtype. For cardia cancer, we found a less evident and nonsignificant inverse relationship. The protection of quercetin appeared to be stronger among female smokers, with the OR leveled of at values <0.2 in quintiles 3–5 (>6 mg quercetin/day).
Conclusions: High dietary quercetin intake is inversely related to the risk of noncardia gastric adenocarcinoma, and the protection appears to be particularly strong for women exposed to oxidative stress, such as tobacco smoking.
Keywords: antioxidants, case–control study, gastric cancer, quercetin, Sweden
introduction
Stomach cancer is the fourth most common cancer and the second most common cause of cancer death worldwide [1]. The huge geographic and gender discrepancies (gastric cancer being eight times more common among Asian men than among North American women) indicate the importance of lifestyle and environmental factors, as well as the potential for prevention through change in exposures. Prevention opportunities are particularly important, given the dismal overall prognosis and the nature of the disease making early diagnosis rare [2, 3]. Since Helicobacter pylori infection was proposed as a necessary cause of noncardia gastric cancer [4–6] through inflammation and oxidative stress [7–9], the role of bioactive redox substances has emerged as highly relevant. While contrasting results from intervention trials have cast doubt on the positive influence of antioxidants on human health [10] and gastric cancer prevention [11, 12], only a few antioxidants (mainly β-carotene, vitamins A, C and E), out of the hundreds present in plant foods, have actually been investigated thoroughly. In a recent study, we measured a marker of antioxidant function, the total radical-trapping antioxidant potential (TRAP) of plant foods, and we showed that a diet with high total antioxidant capacity (TAC) was associated with >30% reduction in gastric cancer risk [13]. The risk reduction was even stronger among individuals whose gastric mucosa was likely to be under oxidative stress, i.e. long-term smokers and those infected with H. pylori [13]. Still, only some of the reduced cancer risk could be attributed to vitamins C and E and β-carotene, while a significant part remained unexplained [14]. Since flavonoids are major determinants of the in vitro TAC of plant products [15, 16], some of the preventive effect of plant foods on gastric tumors could be attributable to quercetin, a main dietary source of flavonoids [17], despite contradictory evidence from other cancer studies [18–21]. Quercetin not only possess strong antioxidant properties through free radical scavenging [15] but also reduces inflammation and inhibits cell proliferation and angiogenesis [22].
Thus, we investigated the association between quercetin dietary intake and gastric cancer risk using data from a large population-based case–control study conducted in Sweden, with detailed information both on known important covariates and on gastric cancer subtypes.
patients and methods
This study was conducted in two Swedish regions (five counties with a total population of 1.3 million) with differing gastric cancer incidence. The study base consisted of all individuals aged 40–79 years, born in Sweden and living in the study counties from February 1989 through January 1995. The methods have been described in detail previously [14]. Briefly, we identified all patients with newly diagnosed gastric adenocarcinoma through a comprehensive case ascertainment scheme involving contact persons at all hospitals in the study area, hospital pathology departments and regional cancer registries. Our completeness slightly exceeded that of the Swedish Cancer Register [23]. Of 908 patients who met the eligibility criteria, 567 agreed to participate in an interview. Reasons for nonparticipation were early death/very advanced disease (270), mental or physical illness other than gastric cancer (40), and patient refusal (28), while 3 could not be located.
All tumors were uniformly classified according to tumor subsite (cardia versus noncardia) where cardia cancers were defined as centered within 1 cm proximal and 2 cm distal of the origin of the longitudinal gastric folds [24]. Tumors were also classified according to their predominant histological pattern and divided into intestinal (n = 337), diffuse (n = 184) or as being of mixed type (n = 37) when different patterns were exhibited equally. In nine patients, the histological type could not be determined with certainty [14, 25]. Two controls per case were randomly selected from age- and gender-strata in the continuously updated computerized population register and frequency matched to cases. Of 1534 control subjects invited to participate, 1165 were interviewed. Reasons for nonparticipation were refusal (245) and mental or physical illness (other than gastric cancer, 90), while 34 could not be located for interview.
collection of exposure data
Professional interviewers from the Statistics Sweden conducted face-to-face interviews to assess dietary habits 20 years before interview using a food-frequency questionnaire validated for consistency and reliability [26], including 45 foods and beverages [27]. Due to the severity of the disease, it was not always possible to blind case–control status, but the interviewers were unaware of the study hypotheses and trained to treat all subjects uniformly. We did not use substitute responders.
The nine predetermined response categories of consumption ranged from ‘never’ to ‘twice daily or more’, for each dietary item. With the exception for bread and beverages, we did not ask about portion size but instead computed average daily intake of calories and nutrients by multiplying frequency data with standard portion sizes and then linking each item to a food composition database developed at the Swedish National Food Administration [27].
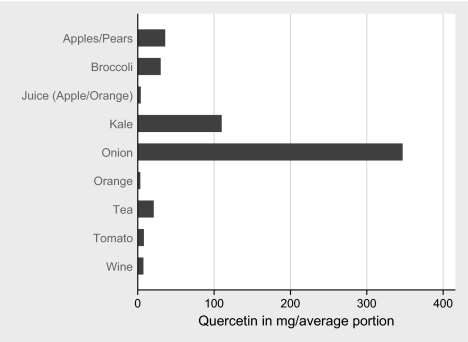
The average content of quercetin in different types of fruits, vegetables, wine, tea, coffee and fruit juices as determined by Hertog et al. [28, 29] is displayed in Figure 1. During the exposure period of interest (around 1970), green tea was basically nonexistent on the Swedish market, thus all tea was assumed to be black. Similarly, since practically all wine consumed in Sweden at the time was sold through government monopoly retail, where sales constituted 2/3 red wine and 1/3 white [30], 2/3 of the quercetin content of red wine was multiplied with the frequency of wine intake to get wine attributable quercetin intake. Other potential sources of quercetin, such as berries and olives, were judged to be of minor or no importance.
Figure 1.
Average content in mg of quercetin in different types of fruits, vegetables, wine, tea, coffee and fruit juices.
Since we lacked information on the exact number of cigarettes per day, cigarette smoking was divided into three categories (never, <30 and ≥30 years) instead of pack-years and tobacco use was classified as never versus ever smokers when testing for interaction. Occupational titles were used to create a socioeconomic status score (1–4) for unskilled or skilled manual work, low or high nonmanual work [31] and a weighed lifetime average of the socioeconomic scores was then computed according to Hansson et al. [27]. Other covariates included number of siblings, body mass index [BMI; weight (kilograms)/height (square meter)], use of table salt and number of daily meals 20 years before interview.
During the last years of fieldwork, we collected blood samples from cases (n = 298) and controls (n = 244, participation rate = 70.5% of those asked to provide sera). Serum immunoglobulin G (IgG) antibodies to H. pylori were determined using HM Cap® ELISA (sensitivity ≥98% and specificity 96%–100%) [32, 33] but since IgG antibodies to H. pylori can disappear spontaneously from atrophic stomachs, we also used immunoblot (IB) technique [34] to detect CagA antibodies that remain longer, as signs of previous H. pylori infection, and classified subjects using this combined information as never [enzyme-linked immunosorbent assay (ELISA) −/IB−] versus ever (ELISA +/IB−, ELISA −/IB+ and ELISA +/IB+) infected by H. pylori. Antigens were derived from H. pylori strain NCTC 11637 [35, 36].
statistical analysis
Of 1732 interviewed cases and controls, 62 who reported a history of partial distal gastric resection (which strongly modified the association with our main exposures), another 35 subjects with missing answers to ≥12% of the dietary questions and 14 who reported an energy intake <700 kcal/day were excluded. This left 505 cases (cardia n = 81, distal n = 420 and undeterminable subsite n = 4) and 1116 controls to be included in the general multivariate analyses. The stratified analyses of antibodies against H. pylori and CagA included 267 cases and 237 controls.
The reported mean caloric intake among control subjects was 1648 kcal, thus our food-frequency questions likely did not capture the entire consumption of fat and carbohydrates. However, the consumption of fruit and vegetables, i.e. the basis of our quercetin calculations, was judged to be complete. Using the logarithms of energy and quercetin intake, energy-adjusted quercetin values were calculated according to the residual method [37], and to obtain a measure of the isocaloric quercetin intake, the residuals were added to the predicted quercetin intake for an energy intake corresponding to the mean intake among control subjects. Quintiles of quercetin intake were established according to the distribution among controls and the lowest intake category was always used as reference.
Unconditional logistic regression was used to estimate odds ratios (ORs), as measures of relative risk, and corresponding 95% confidence intervals (CIs). The frequency-matching factors (age and sex) were always kept in the models; moreover, only covariates of primary biological and statistical importance were kept in the final multivariate models: socioeconomic status (high versus low), number of siblings (categorical, ≤2, 3–4, 5–6 and 7+), BMI (semicontinuous, <22.0, 22.0–23.4, 23.5–24.9, 25.0–26.9 and 27+), smoking (categorical, as described above), and energy and salt intake (both included as continuous). Two-sided P values were used in all analyses. The log-likelihood ratio test (LRT) was used to assess if the effect quercetin on the risk of gastric cancer was modified by gender, H. pylori infection and tobacco smoking. Model fitting, model dispersion and residual analysis were carried out using the deviance statistics based on the LRT and Pearson chi-squared statistics [38].
results
Table 1 shows demographics, clinical and behavioral characteristics of cases and controls according to tumor subsite. Owning to the frequency matching, age and sex distributions were similar among cases and controls, but patients with tumors in the gastric cardia were more likely to be younger and of male and had, on average, a lower socioeconomic status than those with noncardia gastric cancer. The percentage of ever smokers was lower among control subjects, as well as the percentage of ever infected with H. pylori. Controls had a lower salt intake than cases. The crude intake of major sources of quercetin intake also differed between cases and controls, cases reporting on average a lower intake of wine (grams per month) and fruit–vegetables (times per month).
Table 1.
Characteristics of study subjects
| Characteristics | Totala | Cases |
Controls | |
| Cardia | Noncardia | |||
| Number | 1621 | 81 | 420 | 1116 |
| Age (mean), years | 67.0 | 64.8 | 68.1 | 66.8 |
| Gender (males, %) | 66.7 | 80.2 | 63.1 | 66.9 |
| Number of siblingsb (mean) | 5.0 | 5.0 | 5.8 | 4.7 |
| Low socioeconomic standard (%) | 58.7 | 63.0 | 49.5 | 61.8 |
| Ever smokers (%) | 49.8 | 54.1 | 52.6 | 48.3 |
| Infected with Helicobacter pylori (%)c | 77.2 | 73.3 | 97.3 | 59.1 |
| Body mass index (median, kg/m2) | 24.5 | 25.1 | 24.7 | 24.4 |
| High salt intake (%) | 25.1 | 25.9 | 28.9 | 23.6 |
| Meals per day (mean) | 3.5 | 3.6 | 3.6 | 3.4 |
| Wine (mean, g/month) | 196.8 | 113.0 | 176.4 | 211.2 |
| Total fruit intake (mean, times/month) | 26.2 | 24.0 | 22.6 | 27.7 |
| Total vegetable intake (mean, times/month) | 37.1 | 33.5 | 34.4 | 38.4 |
| Energy intake (median, kcal/day) | 1655 | 1763 | 1650 | 1648 |
Includes all controls (1116) and all cases (505 including 4 cases with cancer subsite unknown).
Including him/herself.
Based on 267 cases and 237 controls.
Table 2 provides the adjusted OR to study the relationship between quercetin intake and risk of cardia and noncardia gastric cancer.
Table 2.
Multivariatea odds ratios (OR) and 95% confidence intervals (CI) of gastric adenocarcinoma in relation to calorie-adjusted intake of quercetin
| Quercetin intake in quintiles (mg/day) | Controls | Cardia cancer |
Noncardia cancer |
Noncardia cancer |
|||||
| Intestinal |
Diffuse |
||||||||
| Cases | OR (95% CI) | Cases | OR (95% CI) | Cases | OR (95% CI) | Cases | OR (95% CI) | ||
| 0.16–3.88 | 223 | 28 | 1.0 | 124 | 1.0 | 73 | 1.0 | 47 | 1.0 |
| 3.89–6.02 | 223 | 11 | 0.39 (0.18–0.83) | 99 | 0.76 (0.54–1.06) | 58 | 0.75 (0.50–1.12) | 29 | 0.59 (0.35–0.99) |
| 6.03–8.17 | 223 | 12 | 0.44 (0.21–0.91) | 69 | 0.52 (0.36–0.74) | 34 | 0.45 (0.28–0.71) | 27 | 0.48 (0.28–0.82) |
| 8.18–11.88 | 224 | 11 | 0.45 (0.22–0.95) | 61 | 0.50 (0.34–0.72) | 33 | 0.50 (0.31–0.80) | 23 | 0.43 (0.24–0.75) |
| ≥11.89 | 223 | 19 | 0.76 (0.40–1.44) | 67 | 0.57 (0.40–0.83) | 32 | 0.51 (0.32–0.82) | 27 | 0.54 (0.31–0.92) |
| χ2 for trend | (P value) | 0.87 (0.35) | 14.48 (<0.001) | 12.10 (<0.001) | 6.98 (0.008) | ||||
Adjusted for age, gender, socioeconomic status, number of siblings, body mass index, smoking and energy and salt intake.
We found strong inverse associations between quercetin and the risk of noncardia gastric adenocarcinoma, with an OR of 0.57 (95% CI 0.40–0.83) for the highest quantile (≥11.9 mg) of daily quercetin intake relative to the lowest quintile of intake (<4 mg quercetin/day), supported by a significant decreasing linear trend (P value < 0.001).
The pattern was essentially identical for both histological types of noncardia gastric cancer. For cardia cancer, we found a less evident inverse relationship, with an OR of 0.76 (95% CI 0.40–1.44), to contrast the highest versus the lowest intake of quercetin (P value for linear tend = 0.35). Adjustments for other dietary antioxidants (vitamin C, α-tocopherol and β-carotene) did not meaningfully change the risk (data not shown).
In Table 3, we report the results to study if the protective effect of quercetin intake was consistent in subgroups defined by gender, smoking and H. pylori status. The interaction term for gender and quercetin did not reach statistical significance (P value = 0.17), indicating that the dose–response pattern was overall similar, even though in males, the protective effect of quercetin was somewhat lower a lower intake of quercetin.
Table 3.
Multivariatea odds ratios (OR) and 95% confidence intervals (CI) of noncardia gastric adenocarcinoma associated with calorie-adjusted intake of quercetin, stratified by gender and tobacco smoking
| Quercetin intake in quintiles (mg/day) | Gender |
Smoking |
||||||
| Males |
Females |
Yes |
No |
|||||
| Co/Ca | OR (95% CI) | Co/Ca | OR (95% CI) | Co/Ca (85%) | OR (95% CI) | Co/Ca | OR (95% CI) | |
| 0.16–3.88 | 173/91 | 1.00 | 50/33 | 1.00 | 104/70 | 1.00 | 118/54 | 1.00 |
| 3.89–6.02 | 64/158 | 0.72 (0.48–1.06) | 55/35 | 0.88 (0.47–1.67) | 116/45 | 0.53 (0.33–0.86) | 107/54 | 1.04 (0.65–1.66) |
| 6.03–8.17 | 34/150 | 0.40 (0.25–0.64) | 73/35 | 0.74 (0.39–1.40) | 116/32 | 0.36 (0.21–0.60) | 106/37 | 0.75 (0.45–1.25) |
| 8.18–11.88 | 35/136 | 0.52 (0.33–0.83) | 88/26 | 0.45 (0.23–0.87) | 108/35 | 0.49 (0.29–0.81) | 115/26 | 0.49 (0.28–0.85) |
| 11.89–50.29 | 41/130 | 0.66 (0.42–1.04) | 93/26 | 0.49 (0.25–0.94) | 93/39 | 0.61 (0.40–1.01) | 130/28 | 0.53 (0.31–0.90) |
Adjusted for age, socioeconomic status, number of siblings, body mass index and energy and salt intake.
Co, controls; Ca, cases.
When focusing on the effect of quercetin and smoking, although the differences in dose–response pattern among smokers and nonsmokers looked unimpressive upon eyeball inspection, both the LRT and the Wald test indicated a significant interaction term (P = 0.05), in the fully adjusted analysis of noncardia cancer risk.
Point estimates (for both cardia and noncardia cancer) barely changed when we adjusted for H. pylori infection in the multivariate models, but since serological data were not available for all subjects, the CIs widened and became nonsignificant (data not shown).
When we stratified for H. pylori status, the estimates for noncardia cancer in positive subjects were consistent with what observed in the total study group. No estimates were produced in negative subjects due to the limited sample size (data not shown).
Formal testing of interaction across all levels of intake was statistically nonsignificant.
In Table 4, a finer stratifications on both gender and smoking indicated that in women the inverse association was almost entirely confined to ever smokers (P value for interaction = 0.01) Among the latter, the adjusted OR in the second quintile of quercetin intake, relative to the first, was 0.24 (95% CI 0.08–0.74) and remained between 0.11 and 0.19 in quintiles 3–5. No association between quercetin intake and noncardia gastric cancer risk was observed among female never smokers. A similar effect modification of quercetin could not be statistically confirmed among men (P value for interaction = 0.26).
Table 4.
Multivariatea odds ratios (OR) and 95% confidence intervals (CI) of noncardia gastric adenocarcinoma associated with calorie-adjusted intake of quercetin among female (ever) smokers versus never smokers
| Quercetin intake in quintiles (mg/day) | Female smokers |
Female nonsmokers |
||||
| Controls | Cases | OR (95% CI) | Controls | Cases | OR (95% CI) | |
| 0.16–3.88 | 10 | 19 | 1.0 | 40 | 14 | 1.00 |
| 3.89–6.02 | 22 | 10 | 0.24 (0.08–0.74) | 43 | 25 | 1.74 (0.79–3.85) |
| 6.03–8.17 | 30 | 11 | 0.16 (0.05–0.47) | 42 | 24 | 1.63 (0.72–3.69) |
| 8.18–11.88 | 28 | 8 | 0.11 (0.03–0.36) | 60 | 18 | 0.89 (0.39–2.03) |
| 11.89–50.29 | 34 | 13 | 0.19 (0.06–0.56) | 59 | 13 | 1.02 (0.44–2.35) |
Adjusted for age, socioeconomic status, number of siblings, body mass index and energy and salt intake.
discussion
Quercetin intake was strongly negatively associated with the risk of gastric cancer, particularly for noncardia gastric cancers and already at moderate levels of intake; a 50% reduction in risk was observed at the third lowest quintile of intake (6–8 mg quercetin/day), while no substantial further decline was associated with additional intake of quercetin. We also found a statistically significant interaction between quercetin intake and smoking in the fully adjusted analysis of noncardia cancer risk. We, further, found a statistically significant interaction between quercetin intake and smoking in the fully adjusted analysis of noncardia cancer risk, but differences between smokers and nonsmokers were mainly confined in the second and third quintiles of quercetin intake; in addition, when considering only women, the protective effect of quercetin on noncardia cancer risk was confined only on smokers. The last results should, of course, carefully be interpreted, in light also of the reduced sample size based on which the models were fitted.
Although risk estimates were not modified after adjustment for the other antioxidants, we cannot be certain that quercetin is the critical agent among many other antioxidants and wholesome substances in quercetin-rich plant foods, the true protective substance may just covary with quercetin. In fact, we cannot even confidently rule out that residual confounding, or confounding from unmeasured factors related to a healthy lifestyle (including high consumption of quercetin-rich food), might have inflated—although perhaps not entirely explained—the observed inverse association.
However, the current results are in line with our previous findings of TRAP, ascorbic acid, β-carotene and α-tocopherol [13], indicating a stronger preventive effect of antioxidants (measured as TAC) on gastric cancer among long-term smokers and those infected with H. pylori [13]. We hypothesize that a certain minimum level of antioxidant compounds are needed to maintain our defense against oxidative stress and cancer development. The existence of a possible threshold for the presumed protective effect of dietary antioxidants could suggest the existence of regulatory hierarchies that modulate the interaction between antioxidants and oxidative stress to achieve a physiological redox balance. Consequently, those under higher oxidative pressure may require a somewhat higher intake of antioxidants to keep the redox balance and avoid oxidative stress [39]. Given the large gender discrepancy in gastric cancer incidence, we found the strong inverse association between quercetin intake and noncardia cancer among female smokers, as opposed to male smokers, very interesting. Previous studies have seen lower basic levels of antioxidants in plasma and tissues among females compared with males [40]. Recent reviews also indicate a possible difference between men and women in terms of redox enzymes (myeloperoxidase, xanthine oxidase and superoxide dismutase) as well as in the molecular targets of free radicals (NF-κB) and MAP-kinases [41]. However, the literature on this issue is incomplete and fragmentary and fails to identify a common mechanism for interactions between gender and redox status. If our results are a random finding, influenced by an endogenous redox pattern of females, or, linked to a potential effect of estrogens remains to be shown and further studies are indeed needed.
Apart from its role as an antioxidant, quercetin may also block the activation of procarcinogens by modulating the expression of cytochrome P450 enzymes [42]. Additionally, other researchers have associated quercetin with enhanced DNA repair [43] and increased expression of phase II conjugating enzymes that facilitate our elimination of carcinogenic products (e.g. polycyclic aromatic compounds from smoking and adducts from phase I metabolism), by making them more soluble and disposable by secretion [44].
Among the limitations of the present study is the investigation of a single flavonoids among at least 30 known classes. However, quercetin is widely present in many food items of plan origin, it has been more frequently studied and has a strong antioxidant capacity [45].
The potential for selection bias was strongly reduced through our population-based design and comparably high participation rates. Also, only 2.2% of the cases versus 4.7% of the controls believed in any association whatsoever between diet and disease, and the proportion who thought that diet could influence risk of cancer was limited: 0.5% of the cases and 0.3% of the controls, almost entirely undermining any fear of differential information bias (recall bias). The validity of the food-frequency questionnaire in terms of consistency of dietary recall in the distant past as well as the reliability between successive reports on nutritional levels was considered to be high [26, 27].
Important confounding is also an unlikely explanation of our results, given our careful multivariate adjustment for most known risk predictors of gastric cancer. Although the role of chance must always be taken into consideration, the inherent difficulty to assess food intake with an enormous potential for random misclassification, the consistent pattern of an inverse relationship between the antioxidant potential of plant food, here measured through quercetin, and gastric cancer should be a crude underestimation of the true protective effect.
In conclusion, although the enthusiasm for cancer prevention with dietary antioxidants was severely hampered by negative results in randomized controlled trials of galenic antioxidant supplements [42], one should not dismiss the potential for cancer prevention by dietary means. This study suggests that dietary interventions among individuals under oxidative stress and very low intake of plant foods, particularly women, might decrease their gastric cancer risk.
funding
National Cancer Institute (R01 CA 50959); the Swedish Cancer Society. Clerici foundation fellowship to RB (Italian Institute of Culture, Stockholm, Sweden, 2009).
disclosure
None of the authors declare conflicts of interest.
Acknowledgments
We are indebted to Anders Lindgren for review and pathohistological classification of the tissue specimens.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Hansson LE, Sparen P, Nyren O. Survival in stomach cancer is improving: results of a Nationwide Population-Based Swedish Study. Ann Surgy. 1999;230:162. doi: 10.1097/00000658-199908000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology. 2000;58:96–107. doi: 10.1159/000012086. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Arndt V, Stegmaier C, et al. Is Helicobacter pylori Infection a Necessary Condition for Noncardia Gastric Cancer? Am J Epidemiol. 2004;159:252–258. doi: 10.1093/aje/kwh039. [DOI] [PubMed] [Google Scholar]
- 5.Ekström AM, Held M, Hansson LE, et al. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784–791. doi: 10.1053/gast.2001.27999. [DOI] [PubMed] [Google Scholar]
- 6.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Eng J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 7.Davies GR, Simmonds NJ, Stevens TR, et al. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G626–G634. doi: 10.1152/ajpgi.2001.281.3.G626. [DOI] [PubMed] [Google Scholar]
- 9.Touati E, Michel V, Thiberge JM, et al. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology. 2003;124:1408–1419. doi: 10.1016/s0016-5085(03)00266-x. [DOI] [PubMed] [Google Scholar]
- 10.Vivekananthan DP, Penn MS, Sapp SK, et al. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomized trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 11.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 12.Czernichow S, Galan P, Hercberg S. Antioxidant supplements for prevention of gastrointestinal cancers. Lancet. 2005;365:470–471. doi: 10.1016/S0140-6736(05)17857-X. [DOI] [PubMed] [Google Scholar]
- 13.Serafini M, Bellocco R, Wolk A, Ekström AM. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology. 2002;123:985–991. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- 14.Ekström A, Serafini M, Nyrén O, et al. Dietary antioxidant intake and the risk of cardia cancer and non-cardia cancer of the intestinal and diffuse types—a population-based case-control study in Sweden. Int J Cancer. 2000;87:133–140. [PubMed] [Google Scholar]
- 15.Kim D, Lee CY. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit Rev Food Sci Nutr. 2004;44:253–273. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- 16.Vinson JA, Hontz AB. Phenol antioxidant index: comparative antioxidant effectiveness of red and white wines. J Agr Food Chem. 1995;43:401–403. [Google Scholar]
- 17.Karakaya S. Bioavailability of phenolic compounds. Crit Rev Food Sci Nutr. 2004;44:453–464. doi: 10.1080/10408690490886683. [DOI] [PubMed] [Google Scholar]
- 18.Hertog MG, Hollman PC. Potential health effects of the dietary flavonol quercetin. Eur J Clin Nutr. 1996;50:63–71. [PubMed] [Google Scholar]
- 19.Goldbohm RA, Hertog MG, Brants HA, et al. Consumption of black tea and cancer risk: a prospective cohort study. J Natl Cancer Inst. 1996;88:93–100. doi: 10.1093/jnci/88.2.93. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Closas R, Gonzalez CA, Agudo A, Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 1999;10:71–75. doi: 10.1023/a:1008867108960. [DOI] [PubMed] [Google Scholar]
- 21.Lagiou P, Samoli E, Lagiou A, et al. Flavonoids, vitamin C and adenocarcinoma of the stomach. Cancer Causes Control. 2004;15:67–72. doi: 10.1023/B:CACO.0000016619.18041.b0. [DOI] [PubMed] [Google Scholar]
- 22.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 23.Ekström AM, Signorello LB, Hansson LE, et al. Evaluating gastric cancer misclassification: a potential explanation for the rise in cardia cancer incidence. J Natl Cancer Inst. 1999;5:786–790. doi: 10.1093/jnci/91.9.786. [DOI] [PubMed] [Google Scholar]
- 24.Misumi A, Murakama A, Harada K, et al. Definition of carcinoma of the castric cardia. Langenbecks Arch Chir. 1989;374:221–226. doi: 10.1007/BF01359557. [DOI] [PubMed] [Google Scholar]
- 25.Lauren P. Two main histological types of gastric carcinoma: diffuse and so-called intestinal type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 26.Hansson LE, Nyrén O, Bergströmm R, et al. Diet and risk of gastric cancer. A population-based case-control study in Sweden. Int J Cancer. 1993;55:181–189. doi: 10.1002/ijc.2910550203. [DOI] [PubMed] [Google Scholar]
- 27.Hansson LE, Nyren O, Bergstrom R, et al. Nutrients and gastric cancer risk. A population-based case-control study in Sweden. Int J Cancer. 1994;57:638–644. doi: 10.1002/ijc.2910570505. [DOI] [PubMed] [Google Scholar]
- 28.Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992;40:2379–2383. [Google Scholar]
- 29.Hertog MGL, Hollman PCH, van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J Agric Food Chem. 1993;41:1242–1246. [Google Scholar]
- 30.Systembolaget. 2009 Responsibility Report. http://www.systembolaget.se/NR/rdonlyres/DEAD35AA-DBA4-4790-9BDC-2FA0A6BBCEDD/0/ansvarsredovisning_2009_eng_webb.pdf (26 July 2010, date last accessed) [Google Scholar]
- 31.Statistics Sweden. Swedish socioeconomic classification. Stockholm: Reports on statistical co-ordination, 1982:4 (English summary). http://www.scb.se/Pages/PublishingCalendarViewInfo____259924.aspx?PublObjId=6607 (26 July 2010, date last accessed) [Google Scholar]
- 32.Evans DJJ, Evans DG, Graham DY, Klein PD. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology. 1989;24:1004–1008. doi: 10.1016/0016-5085(89)91616-8. [DOI] [PubMed] [Google Scholar]
- 33.Marchildon PA, Ciota LM, Zamaniyan FZ, et al. Evaluation of three commercial enzyme immunoassays compared with the 13C urea breath test for detection of Helicobacter pylori infection. J Clin Microbiol. 1996;34:1147–1152. doi: 10.1128/jcm.34.5.1147-1152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klaamas K, Held M, Wadstrom T, et al. IgG immune response to Helicobacter pylori antigens in patients with gastric cancer as defined by ELISA and immunoblotting. Int J Cancer. 1996;67:1–5. doi: 10.1002/(SICI)1097-0215(19960703)67:1<1::AID-IJC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Guruge JL, Schalen C, Nilsson I, et al. Detection of antibodies to Helicobacter pylori cell surface antigens. Scand J Infect Dis. 1990;22:457–465. doi: 10.3109/00365549009027078. [DOI] [PubMed] [Google Scholar]
- 36.Lelwala-Guruge J, Nilsson I, Ljungh A, Wadstrom T. Cell surface proteins of Helicobacter pylori as antigens in an ELISA and a comparison with three commercial ELISA. Scand J Infect Dis. 1992;24:457–465. doi: 10.3109/00365549209052632. [DOI] [PubMed] [Google Scholar]
- 37.Willett WC. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 38.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 39.Block G, Jensen CD, Morrow JD, et al. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic Biol Med. 2008;45:377–384. doi: 10.1016/j.freeradbiomed.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serafini M, Villano D, Spera G, Pellegrini N. Redox molecules and cancer prevention: the importance of understanding the role of the antioxidant network. Nutr Cancer. 2006;56:232–240. doi: 10.1207/s15327914nc5602_15. [DOI] [PubMed] [Google Scholar]
- 41.Malorni W, Campesi I, Straface E, et al. Redox features of the cell: a gender perspective. Antioxid Redox Signal. 2007;9:1779–1801. doi: 10.1089/ars.2007.1596. [DOI] [PubMed] [Google Scholar]
- 42.Scalbert A, Manach C, Morand C, Remesy C. Dietary polyphenols and prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 43.Webster RP, Gawde MD, Bhattacharya RK. Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Let. 1996;109:185–191. doi: 10.1016/s0304-3835(96)04443-6. [DOI] [PubMed] [Google Scholar]
- 44.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 45.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]