Abstract
Objective
Anti-inflammatory actions of peroxisome proliferator-activated receptor (PPAR)-γ agonists such as pioglitazone (PIO) may underlie their reported but incompletely understood repression of atherosclerosis. This molecular imaging study investigated the effects of pioglitazone on plaque matrix metalloproteinase (MMP) and macrophage responses in vivo.
Methods and Results
In vitro, pioglitazone suppressed MMP-9 mRNA expression in murine peritoneal macrophages (P<0.05). To assess pioglitazone's effects on plaque inflammation, nondiabetic apoE−/− mice on high-cholesterol diet (HCD) received a MMP-activatable fluorescence imaging agent and a spectrally-distinct macrophage-avid fluorescent nanoparticle. After 24 hours, mice underwent survival dual-target intravital fluorescence microscopy (IVFM) of carotid arterial plaques. These mice were then randomized to HCD or HCD+PIO 0.012% for 8 weeks, followed by a second IVFM study of the same carotid plaque. In the HCD group, in vivo MMP and macrophage target-to-background ratios (TBRs) increased similarly (P<0.01 vs. baseline). In contrast, pioglitazone reduced MMP and macrophage TBRs (P<0.01 vs. HCD). Changes in MMP and macrophage signals correlated strongly (r-values≥0.75). Microscopy demonstrated MMP and macrophage reductions in pioglitazone-treated mice, as well as a PIO-modulated increase in plaque collagen.
Conclusions
Serial optical molecular imaging demonstrates that plaque MMP and macrophage activity in vivo intensify with hypercholesterolemia and are reduced by pioglitazone therapy.
Keywords: atherosclerosis, pioglitazone, inflammation, molecular imaging, fluorescence
INTRODUCTION
Inflammation is involved in all stages of atherosclerosis including foam cell formation, plaque progression, and ultimately plaque disruption and thrombus formation.1 Systemic inflammation correlates with an increased rate of fatal cardiovascular disease (CVD) events,2,3 even in statin-treated patients achieving target serum cholesterol levels.4 Accordingly, pharmacological strategies to reduce key inflammatory targets such as plaque macrophages and plaque proteases appear promising for reducing CVD events.5
Although in use as an anti-diabetic agent, the thiazolidinedione (TZD) pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist, is also an intriguing inflammatory-modulating agent that may reduce cardiovascular death, myocardial infarction, and stroke.6,7 PPAR-γ is a ligand-activated, nuclear receptor/transcription factor that regulates adipogenesis, insulin sensitivity, and lipid metabolism. In addition PPAR-γ agonists exert diverse anti-inflammatory actions important in atherosclerosis, including 1) reducing destabilizing matrix metalloproteinases (MMP) in macrophages, vascular smooth muscle cells, and endothelial cells; and 2) reducing the recruitment of T cells and monocytes/macrophages, key effector cells in atherosclerosis.8–10
Despite ex vivo evidence linking pioglitazone to reduced atheroma inflammation, the anti-inflammatory effects of pioglitazone on plaque MMP activity and macrophages have not been spatially mapped, tracked, and quantified in vivo. In addition, questions exist regarding potential clinical differences among the TZDs in terms of cardiovascular effects, suggesting that in vivo approaches may be needed to resolve such issues. Furthermore, while PPAR-γ agonists such as pioglitazone decrease destabilizing MMP expression in atheromata, it is unknown whether this reflects primarily 1) reduced MMP expression from a stable number of plaque macrophages; or 2) reduced numbers of macrophages, the cell responsible for the majority of MMP expression in atheromata.11
To address these open questions, here we harness serial dual-target fluorescence molecular imaging to 1) perform longitudinal, spatial assessment of pioglitazone- and hypercholesterolemic-modulated alterations of plaque inflammation and to 2) investigate whether changes in plaque MMP activity track with changes in plaque macrophage activity, as assessed by in vivo imaging and ex vivo molecular analyses. Using this novel strategy, our findings provide unequivocal in vivo evidence that pioglitazone reduces plaque inflammation, and that decreases in plaque MMP activity correlate strongly with reductions in plaque macrophage phagocytic activity.
METHODS
In vitro studies (mRNA expression, Western blotting), fluorescence microscopy, image analysis, histopathology, plasma measurements and the MMP and macrophage molecular imaging agents are detailed in the Supplement.
Serial dual-target fluorescence molecular imaging of plaque inflammation
Pioglitazone's (PIO) effects on carotid plaques were investigated in apoE−/− mice (n=16 female, Jackson Laboratories). Mice consumed a high-cholesterol diet (HCD; TD88137; 42% milk fat, 0.2% cholesterol, Harlan Teklad, Madison, WI) from 10 to 26 weeks of age. Twenty-four hours before imaging, MMP and macrophage agents were co-injected (tail vein). Next apoE−/− mice underwent intravital fluorescence microscopy (IVFM #1) after surgical exposure of the right carotid artery.12 Briefly, the distal right common carotid artery was carefully separated from the periadventitial tissues by blunt dissection. For co-registration, a phantom was placed underneath the carotid artery bifurcation. After the initial IVFM study, the incisions of the 16 apoE−/− mice were surgically closed. Mice recovered from surgery without incident and were allowed water and their specified diet ad libitum. An additional group of wild-type C57BL/6J mice fed with normal chow diet (n=6) were injected with MMP and macrophage imaging agents, and served as controls.
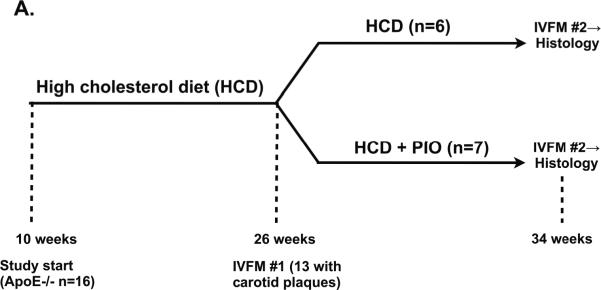
ApoE−/− mice with carotid atheromata detected on the first IVFM study (IVFM #1) were then randomized either to continued HCD or to HCD supplemented with pioglitazone (0.012% w/w) ad libitum (8 weeks, Figure 2A). After 8 weeks (34 weeks of age), apoE−/− mice were re-injected with the same dosages of the MMP and macrophage imaging agents. Twenty-four hours later, the same carotid atheroma visualized in IVFM #1 underwent a second IVFM (IVFM #2), prior to sacrifice and histopathological analyses. The Subcommittee on Research Animal Care at Massachusetts General Hospital approved all procedures.
Figure 2.
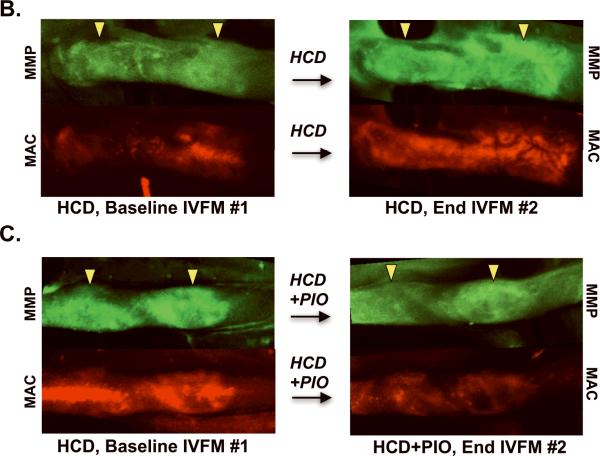
Longitudinal in vivo tracking and quantification of multi-component plaque inflammation in apoE−/− mice. The same right carotid plaque from each animal underwent serial IVFM. (A) Study schema. After 16 weeks of high-cholesterol diet (HCD), IVFM was performed. Mice with carotid plaques (n=13) were next randomized to continued HCD+/−admixed 0.012% pioglitazone (HCD+PIO), corresponding to a PIO dose of 13 mg/kg/day. After 8 weeks, a second IVFM study of the same plaque was performed. (B) In HCD-treated mice, plaque MMP and macrophage phagocytic activities (colored green and red, respectively) increased significantly over eight weeks. (C) In contrast, pioglitazone treatment reduced plaque MMP and macrophage activities. Representative images processed and windowed identically.
Intravital fluorescence microscopy (IVFM)
IVFM studies employed a multichannel laser scanning fluorescence microscope (See Supplement for details). The utilized 4χ objective (NA 0.15) provided an in-plane resolution of 13×13 μm. A plastic tube phantom (PE-10 tubing, Becton Dickinson, Franklin Lakes, NJ) was placed underneath carotid artery bifurcation and served to co-register the imaging fields of the 2 IVFM datasets. The plaque target-to-background ratio (TBR) was calculated as the ratio of plaque signal intensity to the adjacent vessel background signal intensity (see Supplement for details).
RESULTS
Pioglitazone represses cytokine-induced MMP-9 expression in vitro
To determine if PPAR-γ activation by pioglitazone represses MMP-9 protein expression in vitro, isolated mouse peritoneal macrophages (MPMs) were plated and cultured in the presence of LPS and/or pioglitazone 10 μM. Pioglitazone pretreatment attenuated the LPS-augmented protein expression of MMP-9 as seen on immunoblotting (control, 34.1±2.0 arbitrary units (AU); LPS, 46.8±2.2 AU; LPS+pioglitazone, 19.1±2.9 AU, P<0.05, Supplemental Figure S1). Ponceau S staining confirmed equal supernatant protein gel loading (not shown).
Effect of pioglitazone therapy on body weight and metabolic parameters
Pioglitazone-treated apoE−/− mice (13 mg/kg/day) showed reduced plasma cholesterol levels and similar body weight, plasma glucose, insulin, and triglyceride levels as HCD-treated mice (Supplemental Table), consistent with prior studies.13
Dual-target fluorescence molecular imaging of carotid atherosclerosis reveals abundant in vivo MMP activity and macrophage signals compared to normal vessels
Survival IVFM in cholesterol-fed, 26-week-old apoE−/− mice (n=16) was performed to assess in vivo carotid plaque inflammation at baseline. Twenty-four hours prior to imaging, dual-targeted, spectrally resolved MMP activity (MMPSense680) and macrophage phagocytic activity (CLIO-Cy7) NIRF imaging agents were co-injected. At the start of the imaging session, a third spectrally distinct intravascular agent (FITC-dextran) was administered and multichannel high-resolution IVFM was performed.
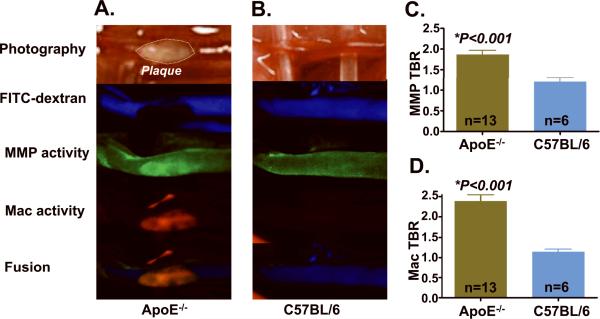
Plaques were located in the distal common carotid artery and its bifurcation. Discrete MMP (colored green) and macrophage (colored red) plaque NIRF signals were confirmed to be intravascular by FITC-dextran (colored blue) based carotid arterial angiograms. The angiogram provided precise identification of the plaque boundaries to enable accurate ROI analyses of the molecular imaging targets of MMP and macrophage activity (Figure 1). Carotid arteries of control C57BL/6J mice injected with the same agents showed mild NIR fluorescence diffusely throughout the vessel wall; no plaques were evident, as expected. Z-stacks demonstrated significantly greater MMP and macrophage signals in apoE−/− mice compared with C57BL/6J mice (MMP TBR 1.9±0.1 vs. 1.2±0.1, P<0.001; macrophage TBR 2.4±0.1 vs. 1.1±0.1, P<0.001).
Figure 1.
High-resolution in vivo fluorescence molecular imaging of multiple inflammatory targets in murine carotid atheromata. IVFM of carotid arteries revealed (A) discrete plaque-specific MMP and macrophage activity in apoE−/− mice in contrast to (B) normal carotid vessels of age- and sex-matched C57BL/6J mice. (C) The in vivo plaque MMP target-to-background ratio (TBR) in apoE−/− mice was 55% higher than in C57BL/6J mice (1.9±0.1 vs. 1.2±0.1; P<0.001). (D) Similarly, the in vivo plaque macrophage TBR was 110% greater in apoE−/− mice (2.4±0.1 vs. 1.1±0.1, P<0.001). Images within each channel were windowed and processed identically. Mac=macrophage.
Pioglitazone suppresses plaque MMP activity and macrophages in vivo
To assess the in vivo inflammation-modulating effects of hypercholesterolemia and pioglitazone therapy, a second follow-up IVFM study was performed to measure changes in carotid plaque MMP and macrophage activity over time. At 26 weeks of age, the 13 of 16 apoE−/− mice (81%) with carotid arterial plaques on baseline IVFM imaging were randomized to either continued HCD (Group 1, HCD, n=6) or to HCD admixed with pioglitazone (Group 2, HCD+PIO, n=7). The baseline MMP and macrophage plaque activities were similar between these 2 groups (baseline MMP TBR: Group 1 1.8±0.1 vs. Group 2 2.0±0.1, P=0.35; baseline macrophage TBR: Group 1 2.1±0.2 vs. Group 2 2.5±0.2, P=0.19).
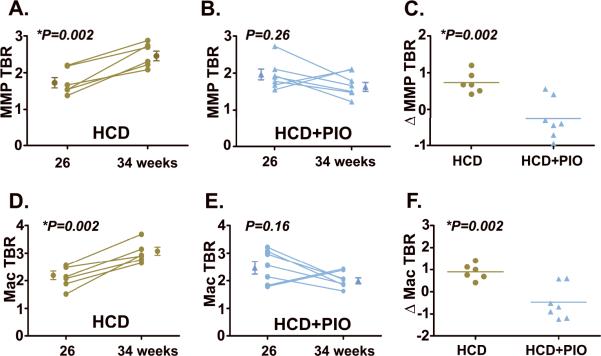
After 8 additional weeks, the same plaque that was imaged in IVFM #1 underwent repeat multichannel IVFM #2. The plaques of mice with continued HCD (Group 1) demonstrated significantly increased MMP and macrophage signals over baseline (MMP TBR, 1.8±0.1 at 26 weeks→2.5±0.1 at 34 weeks, P=0.002; macrophage TBR, 2.1±0.2 at 26 weeks→3.0±0.2 at 34 weeks, P=0.002; Figures 2 and 3). In contradistinction, plaques in the pioglitazone group (Group 2, HCD+PIO) showed trends of reduction for both plaque MMP and macrophage activity (MMP TBR 2.0±0.1 at 26 weeks→1.7±0.1 at 34 weeks, P=0.26; macrophage TBR 2.5±0.2 at 26 weeks→ 2.0±0.1 at 34 weeks, P=0.16).
Figure 3.
Quantification of longitudinal changes in plaque MMP and macrophage activity. (A,D) In HCD-treated mice, plaque MMP and macrophage activity increased over 8 weeks (MMP TBR increase 42%, P=0.002, macrophage TBR increase 43%, P=0.002). (B,E) In contrast, pioglitazone treatment (HCD+PIO) reduced plaque inflammation (MMP TBR decrease 13%, P=0.26, macrophage TBR decrease 19%,P=0.16). (C,F) Adjusting for baseline TBR values, pioglitazone significantly reduced changes in plaque inflammation (ΔTBRs), in contrast to the HCD control group (ΔMMP TBR: HCD +0.7±0.1 vs. −0.3±0.2 HCD+PIO, P=0.002; ΔMac TBR: HCD +0.9±0.1 vs. −0.5±0.3 HCD+PIO, P=0.002).
The natural history of in vivo plaque MMP and macrophage activity over the 8-week study period differed significantly between the two groups, with increased inflammation in the HCD group and reduced inflammation in the HCD+PIO group (ΔMMP TBR: HCD +0.7±0.1 vs. −0.3±0.2 HCD+PIO, P=0.002; ΔMac TBR: HCD +0.9±0.1 vs. −0.5±0.3 HCD+PIO, P=0.002; Figures 3C and 3F).
Plaque MMP activity exhibits a linear relationship with plaque macrophages
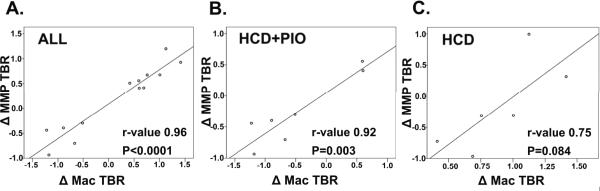
To better understand the relationship between plaque MMP activity and plaque macrophage activity in vivo, Pearson correlation coefficients between the ΔMMP TBR and ΔMac TBR were derived (Figure 4). In the entire cohort, a strong relationship existed between the ΔMMP TBR and ΔMac TBR (r=0.96, P<0.0001). Subgroup analyses revealed a stronger correlation in the PIO-modulated group (r=0.92,P=0.003) compared to the HCD group (r=0.75, P=0.084).
Figure 4.
Correlational analyses of in vivo plaque MMP activity and plaque macrophage content for (A) the entire group, (B) HCD+PIO subgroup and (C) HCD subgroup. A strong correlation between the ΔMMP TBR and ΔMac TBR (r=0.96, P<0.001) was present for the entire group. Subgroup analyses showed a stronger correlation in the PIO-modulated group (r=0.92) compared to the HCD group (r=0.75).
Pioglitazone reduces corresponding histological, microscopic and molecular measures of plaque inflammation
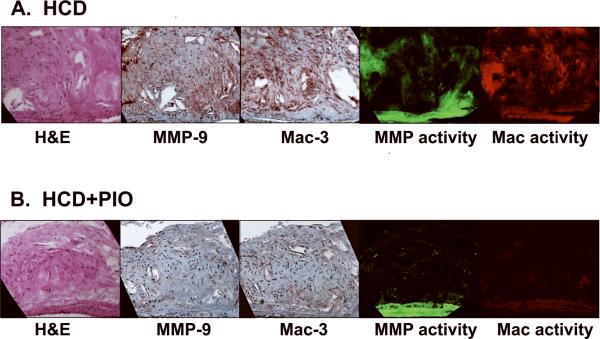
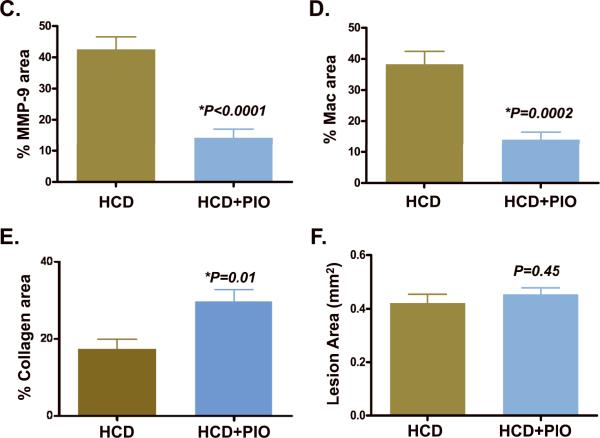
Compared to carotid atherosclerotic plaques of HCD-fed apoE−/− mice, plaques of pioglitazone-treated HCD-fed apoE−/− mice contained reduced MMP-9 and macrophage presence (%MMP-9 staining: HCD, 42.6±4.0% vs. HCD+PIO, 14.3±2.8% (66% reduction), P<0.0001; %Mac staining: HCD, 38.3±4.2% vs. HCD+PIO, 14.1±2.4% (63% reduction), P=0.0002; Figure 5). On multichannel fluorescence microscopy, carotid plaque sections revealed colocalization of MMP activity and macrophage molecular imaging signals. In contrast to HCD animals, pioglitazone-treated animals had substantially reduced plaque MMP activity and macrophage signals. Similar levels of NIR autofluorescence emanated from the medial fibers of plaques from both groups.
Figure 5.
Correlative microscopy of representative carotid plaque sections. Images (×200) from left-to-right demonstrate adjacent sections of H&E stain, immunoreactive MMP-9, immunoreactive macrophages (Mac-3), NIRF MMP activity (680 nm), and NIRF macrophage phagocytic activity (750nm). (A) In HCD-fed apoE−/− control mice, abundant MMP-9 and macrophage immunohistochemical expression (red brown color) colocalized with respective microscopic NIRF molecular imaging signals. (B) In pioglitazone-treated mice (HCD+PIO), however, carotid plaque sections showed reduced MMP-9 and macrophage immunohistochemical and NIRF microscopic signals. (C–E) Quantitative histological analyses of carotid and aortic plaque sections (N=26 and N=39 high-powered fields analyzed, respectively). Pioglitazone therapy proportionally decreased the percentage of carotid plaque (C) MMP-9 and (D) macrophage expression compared to HCD control mice (66% and 63% respectively, P<0.05 for each). (E) PIO reduced aortic plaque collagen content detected by Masson's trichrome stain (% collagen area HCD+PIO 29.7±3.2% vs. HCD 17.3±2.6%, P=0.01) (F) Intimal aortic lesion areas were similar (HCD+PIO, 0.45±0.02 mm2 vs. HCD, 0.42±0.03 mm2, P=0.45).
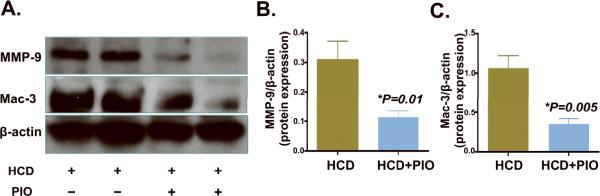
Immunoblots of MMP-9 and macrophage expression were next examined to evaluate whether pioglitazone reduced aortic vessel wall MMP-9 and macrophage-specific protein levels. Consistent with the IVFM, histological, and fluorescence microscopy studies, pioglitazone reduced aortic MMP-9 and mac-3 protein levels in apoE−/− mice, (63% and 67% reductions, P=0.01 and P=0.005 respectively, Figure 6).
Figure 6.
Immunoblots of aortic Mac-3 and MMP-9 protein in HCD-fed (n=6) or pioglitazone-treated (n=7) apoE−/− mice. Resected aortas underwent immunoblot analysis of Mac-3, MMP-9 and β-actin protein levels. (A) Representative data from the HCD group (2 mice shown) and HCD+PIO (2 mice shown). In pioglitazone-treated animals, aortic (B) MMP-9 and (C) macrophages were significantly and similarly reduced compared to the HCD control group (P=0.01 and P=0.005, respectively).
Pioglitazone increases plaque collagen content without altering lesion size
Given reduced plaque inflammatory composition following pioglitazone therapy, we investigated whether piogitazone might induce a compensatory increase in plaque collagen content. Masson's trichrome stain for collagen in fact demonstrated increased carotid and aortic plaque collagen in the PIO group, with a 72% increase in the aortic intimal lesion collagen content (%collagen area HCD+PIO, 29.7±3.2% vs. HCD, 17.3±2.6%, P=0.01, Figure 5E and Supplemental Figure 2). Similar levels of collagen staining in the media were noted. No reduction in aortic plaque area was found (HCD+PIO, 0.45±0.02 mm2 vs. HCD, 0.42±0.03 mm2, P=0.45).
DISCUSSION
By utilizing serial, dual-targeted fluorescence molecular imaging coupled to ex vivo molecular methodologies, this study provides novel in vivo evidence that pioglitazone reduces inflammation in atherosclerosis. Plaque MMP and macrophage activity progressively increased in vivo in hypercholesterolemic apoE−/− mice, and was reduced by the TZD PPAR-γ agonist pioglitazone. Furthermore, the current study provides additional insight into the temporal relationship of MMP and macrophage activities in atherosclerosis. Specifically, we found that alterations in plaque matrix metalloproteinases were strongly linked to alterations in plaque macrophages, as assessed by in vivo activity imaging, fluorescence microscopy, immunohistochemistry, and immunoblotting.
Matrix metalloproteinases - zinc-dependent endopeptidases that digest collagen, elastin, and extracellular proteins - are implicated in plaque destabilization by promoting both expansive remodeling and fibrous cap rupture.11 In this study, in vivo functional MMP activity, rather than the sole presence of MMPs, was imaged utilizing a MMP-activatable NIRF substrate14,15 in concert with optimized intravital fluorescence microscopy (IVFM).12 Serial IVFM demonstrated that carotid plaque MMP activity increased by 42% in the HCD group (Figures 2–3). In contrast, pioglitazone treatment reduced in vivo plaque MMP activity by 13% (p<0.05 vs. HCD control group), extending a prior ex vivo pioglitazone study into the in vivo realm.16 MMP-9 was directly investigated for three reasons: 1) MMP-9 is a well-established destabilizing factor in atherogenesis, promoting both expansive remodeling and fibrous cap disruption11 2) prior work suggests TZDs may downregulate MMP-9 expression in cellular models, including macrophages,17,18 and 3) MMP-9 substantially activates the MMP imaging agent (MMPSense) in atherosclerosis,15 with recent MMP-9 gene deletion studies further supporting MMP-9 as a dominant activator of the NIRF imaging agent.19 Corroborating the in vivo molecular imaging findings, we found that pioglitazone reduced MMP-9 expression by 66% and 63% on respective immunohistochemical and immunoblotting studies of atherosclerotic tissues (Figures 5–6).
Observed reductions in plaque MMP activity in vivo could be due to a combination of 1) suppression of MMP expression from resident macrophages, as demonstrated in vitro for various PPAR-γ agonists17,18 and here specifically for the TZD pioglitazone (Supplemental Figure S1); 2) reductions in present or recruited plaque macrophages; 3) changes in MMP inhibitor levels independent of any regulation of MMP mRNA expression or protein levels; and/or 4) a change in other non-macrophage cells exerting a paracrine effect. To elucidate the relative contributions of these possibilities, we simultaneously imaged macrophage phagocytic activity using spectrally-distinct dextran-coated fluorescent nanoparticles validated for detecting plaque macrophages.20 These unmodified nanoparticles are taken up similarly by resting and activated macrophages in vitro,21 and do not target apoptotic cells.22 In the HCD group, we observed a 43% TBR increase in plaque macrophage activity (Figures 2–3), similar to the 42% TBR increase in the plaque MMP activity. In the PIO group, pioglitazone reduced macrophage activity by 19%, also similar to the observed 13% reduction in MMP activity. Concurrent fluorescence microscopy, immunohistochemical, and immunoblotting analyses confirmed that pioglitazone reduced plaque macrophage content, as noted previously ex vivo.16 Notably, pioglitazone-mediated reductions in plaque MMP activity were highly similar to reductions in plaque macrophages by all in vivo and ex vivo measures.
To further assess the relationship between in vivo plaque MMP and macrophage activity, we derived Pearson correlation coefficients (Figure 4). A significant relationship existed between the ΔMMP TBR and the ΔMac TBR (r=0.96), with a stronger correlation evident in the PIO subgroup (r=0.92) compared to the HCD subgroup (r=0.75). The ability to assess and correlate these concomitant changes in plaque inflammation stemmed from the employed serial, two-timepoint imaging methodology of the same carotid plaque for a given subject.
The integrated in vivo molecular imaging, microscopic, histological, and protein immunoblotting results suggest that pioglitazone-mediated reductions in plaque MMP activity and presence are predominantly due to reduced number of macrophages that furnish MMPs, rather then reduced MMP expression from a numerically static macrophage population. TZD-relevant mechanisms that may underlie reductions in plaque macrophages, while beyond the scope of this investigation, include 1) reduced monocyte recruitment via decreased expression of leukocyte adhesion molecules23,24 and/or decreased monocyte chemotaxis;25,26 and 2) increased TZD-mediated apoptosis of macrophages.13,27
From a translational imaging agent perspective, both macrophages and MMP activity are viable clinical atherosclerosis molecular imaging targets.28–31 Iron oxide magnetic nanoparticles are already clinically utilized in noninvasive pharmacological MRI studies of macrophage responses in the atherosclerotic plaques of carotid arteries.32 In addition, the backbone of the MMP-activatable agent has been safely tested in clinical trials, and a related cysteine protease-activatable NIRF agent is planned for clinical trials.31
From a clinical technology perspective, NIR fluorescence molecular imaging is positioned well to interrogate the human coronary arteries, via clinically translatable intravascular catheters that detect NIRF plaque inflammation through blood in coronary-sized arteries.33 From a noninvasive perspective, fluorescence molecular tomographic-based systems (three dimensional noninvasive imaging systems that reconstruct fluorescence quantitatively deep in tissue)34 can be scaled up for interrogating the human carotid arteries, with and without ultrasound integration. Advances in either of these two arenas, coupled with FDA approval of appropriate NIRF imaging agents, may enable a clinical investigation of the anti-inflammatory effects of pioglitazone in atherosclerosis.
Additional study findings merit further discussion regarding pioglitazone's effects on atherosclerosis. In contrast to a prior key atherosclerosis investigation of PPAR-γ agonists by Li et al. showing nonsignificant reductions in inflammatory plaque markers in female mice,35 this investigation found significant reductions in plaque MMP-9 and macrophage levels. Potential explanations for this difference might include the use of rosiglitazone in the prior study as opposed to pioglitazone and the prior use of LDL receptor deficient mice rather than apoE−/− mice. PIO also reduced LDL levels, as noted by others,13 and may have also contributed to the changes seen. Further mechanistic studies are needed to distinguish the relative contributions of PPAR activation, decreased inflammation and cholesterol reductions in the reductions in plaque inflammation observed here. In addition, while not the focus of this in vivo inflammation investigation, pioglitazone administered at 3× higher dose (40 mg/kg/day) has been reported to promote necrotic core formation,13 but was not found at the lower dose used here.36 Also noteworthy was our histological analysis of the carotid artery, a vessel much less investigated in murine atherosclerosis studies as opposed to the aortic root or the inominate artery. Immunohistochemical analyses of carotid plaque MMP-9 and macrophages were supplemented with more established plaque measurements, including whole aortas immunoblots, which also demonstrated reduced MMP-9 and macrophage content and plaque area. Collagen content also increased, as seen by others as well.16 Lastly, it is important to note that experimental murine pioglitazone dosages are higher than human dosages (< 1 mg/kg/day); therefore dedicated clinical studies will be necessary to assess whether pioglitazone can reduce human plaque inflammation.
Ultimately, additional clinical outcomes trials in both nondiabetic and diabetic human subjects are needed to determine the net clinical and dose-dependent effect of pioglitazone, as well as distinctions between different PPARγ agonists. The need for more specific, in vivo assessments are required given the complexity of both the atherosclerotic disease process, as well as transcriptional modifiers like PPAR agonists that have complex effects and both stabilizing and destabilizing actions. Integrated biologic and imaging studies in vivo in both mice and humans hold the potential to provide more specific and detailed information regarding anti-atherosclerotic strategies.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge Purvish Patel B.S for assistance with intravital microscopy, Gabriela Orasanu, Ph.D. for assistance with mouse peritoneal macrophage isolation, Yoshiko Iwamoto, B.S. for histological assistance, Amit Saxena Ph.D. and Brian Thompson Ph.D. for intravital imaging, and Tae-Jong Yoon, Ph.D. for assistance with image analysis.
SOURCES OF FUNDING Howard Hughes Medical Institute Career Development Award (FAJ), American Heart Association Scientist Development Grant #0830352N (FAJ), Donald W. Reynolds Foundation (RW,EA,FAJ), and NIH UO1 HL080731 (RW,FAJ, JM).
Footnotes
DISCLOSURE RW, FJ – Equity Interest/Consultant, VisEn Medical; JP- Consultant, Roche, Novo Nordisc, Takeda, and Amylin Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: From pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N, Hingorani AD. C-reactive protein and prognosis in diabetes: Getting to the heart of the matter. Diabetes. 2009;58:798–799. doi: 10.2337/db08-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in c-reactive protein and ldl cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 5.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 6.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the proactive study (prospective pioglitazone clinical trial in macrovascular events): A randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 7.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 8.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 9.Lehrke M, Lazar MA. The many faces of ppar gamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Ricote M, Valledor AF, Glass CK. Decoding transcriptional programs regulated by ppars and lxrs in the macrophage: Effects on lipid homeostasis, inflammation, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:230–239. doi: 10.1161/01.ATV.0000103951.67680.B1. [DOI] [PubMed] [Google Scholar]
- 11.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69:625–635. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin k activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 13.Thorp E, Kuriakose G, Shah YM, Gonzalez FJ, Tabas I. Pioglitazone increases macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions of nondiabetic low-density lipoprotein receptor-null mice. Circulation. 2007;116:2182–2190. doi: 10.1161/CIRCULATIONAHA.107.698852. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: Visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 16.He L, Game BA, Nareika A, Garvey WT, Huang Y. Administration of pioglitazone in low-density lipoprotein receptor-deficient mice inhibits lesion progression and matrix metalloproteinase expression in advanced atherosclerotic plaques. J Cardiovasc Pharmacol. 2006;48:212–222. doi: 10.1097/01.fjc.0000248831.21973.c4. [DOI] [PubMed] [Google Scholar]
- 17.Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain ppargamma: Differentiation-dependent peroxisomal proliferator-activated receptor gamma(ppargamma) expression and reduction of mmp-9 activity through ppargamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 19.Klohs J, Baeva N, Steinbrink J, Bourayou R, Boettcher C, Royl G, Megow D, Dirnagl U, Priller J, Wunder A. In vivo near-infrared fluorescence imaging of matrix metalloproteinase activity after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1284–1292. doi: 10.1038/jcbfm.2009.51. [DOI] [PubMed] [Google Scholar]
- 20.Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- 21.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 22.Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, Dai G, Reynolds F, Grazette L, Rosenzweig A, Weissleder R, Josephson L. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54:718–724. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 23.Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 24.Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- 25.Han KH, Quehenberger O. Ligands for peroxisome proliferator-activated receptor inhibit monocyte ccr2 expression stimulated by plasma lipoproteins. Trends Cardiovasc Med. 2000;10:209–216. doi: 10.1016/s1050-1738(00)00076-1. [DOI] [PubMed] [Google Scholar]
- 26.Murao K, Imachi H, Momoi A, Sayo Y, Hosokawa H, Sato M, Ishida T, Takahara J. Thiazolidinedione inhibits the production of monocyte chemoattractant protein-1 in cytokine-treated human vascular endothelial cells. FEBS Lett. 1999;454:27–30. doi: 10.1016/s0014-5793(99)00765-6. [DOI] [PubMed] [Google Scholar]
- 27.Bodles AM, Varma V, Yao-Borengasser A, Phanavanh B, Peterson CA, McGehee RE, Jr., Rasouli N, Wabitsch M, Kern PA. Pioglitazone induces apoptosis of macrophages in human adipose tissue. J Lipid Res. 2006;47:2080–2088. doi: 10.1194/jlr.M600235-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 29.Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009;29:983–991. doi: 10.1161/ATVBAHA.108.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laufer EM, Winkens MH, Narula J, Hofstra L. Molecular imaging of macrophage cell death for the assessment of plaque vulnerability. Arterioscler Thromb Vasc Biol. 2009;29:1031–1038. doi: 10.1161/ATVBAHA.108.165522. [DOI] [PubMed] [Google Scholar]
- 31.Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: Insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1017–1024. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, UK-I JM, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH. The atheroma (atorvastatin therapy: Effects on reduction of macrophage activity) study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, Ntziachristos V, Libby P, Weissleder R. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntziachristos V, Tung CH, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med. 2002;8:757–760. doi: 10.1038/nm729. [DOI] [PubMed] [Google Scholar]
- 35.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in ldl receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorp E, Tabas I. Differential effects of pioglitazone on advanced atherosclerotic lesions. Am J Pathol. 2009;175:1348. doi: 10.2353/ajpath.2009.090483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.