Abstract
Notch has multiple roles in the development of the Drosophila melanogaster wing imaginal disc. It helps specify the dorsal–ventral compartment border, and it is needed for the wing margin, veins, and sensory organs. Here we present evidence for a new role: stimulating growth in response to Hedgehog. We show that Notch signaling is activated in the cells of the anterior–posterior organizer that produce the region between wing veins 3 and 4, and we describe strong genetic interactions between the gene that encodes the Hedgehog pathway activator Smoothened and the Notch pathway genes Notch, presenilin, and Suppressor of Hairless and the Enhancer of split complex. This work thus reveals a novel collaboration by the Hedgehog and Notch pathways that regulates proliferation in the 3–4 intervein region independently of Decapentaplegic.
THE cell-signaling pathways that control cell fate, proliferation, and patterning during development are unexpectedly few in number. They are used extensively among most organs and tissues and are highly conserved among metazoans. The key pathways are Hedgehog (Hh), Janus kinase/signal transducers and activators of transcription (Jak/STAT), several receptor tyrosine kinases [e.g., fibroblast growth factor (FGF) and epidermal growth factor (EGF)], transforming growth factor-β/Decapentaplegic (TGF-β/Dpp), Wnt/Wingless (Wg), and Notch (N). Evidence that cross-regulatory interactions between these signaling pathways are essential to their roles has emerged with our improved understanding of how these pathways are constituted, how they are activated, and the responses that they elicit (Hurlbut et al. 2007). In this article, we report that activation of the N pathway downstream of Hh contributes to growth and patterning in the anterior–posterior (AP) organizer region of the Drosophila wing.
Hh helps to direct development in most metazoan tissues, and its role in setting up and maintaining the wing-disc AP organizer (the region that produces the area that includes wing veins 3 and 4) has been particularly well characterized. Hh protein is produced by and exported from wing-disc posterior compartment cells and can traverse many cells to be taken up by anterior cells across the compartment border (Tabata and Kornberg 1994; Chen and Struhl 1996). Paracrine Hh signaling in these target cells engages the Patched (Ptc) receptor and activates the Smoothened (Smo) protein, which initiates a series of post-translational modifications of components of the Hh signaling transduction pathway (reviewed by Wilson and Chuang 2010). The output of this cascade changes the form and intracellular distribution of the Cubitus interruptus (Ci) protein (Aza-Blanc et al. 1997), which in the absence of Hh signaling is either a captive, inactive component of a cytoplasmic multi-protein complex or a proteolytically cleaved fragment that functions as a nuclear transcriptional repressor (CiRep). Hh signal transduction inhibits repressor formation and transforms Ci in the cytoplasmic complex to an active transcription factor (CiAct). CiAct upregulates or induces expression of a number of target genes, including ptc, dpp, and vein (vn) (Basler and Struhl 1994; Tabata and Kornberg 1994; Schnepp et al. 1996; Biehs et al. 1998; Amin et al. 1999); Vn is an EGF ligand (Wessells et al. 1999). Dpp expressed in the band of Hh-receiving cells adjacent to the AP compartment border disseminates to target cells in both compartments (Lecuit et al. 1996; Nellen et al. 1996), and by regulating their proliferation and identity, embodies much of the functionality of the AP organizer. Dpp does not, however, control all proliferation in the wing pouch: cells in the AP organizer region show a direct dependence on Hh (Mullor et al. 1997; Strigini and Cohen 1997). How Hh carries out this role is not well understood.
The role of Hh signaling at the compartment border has been defined by both loss-of-function and gain-of-function conditions. Expression of dpp decreases if Hh signaling is reduced (Strigini and Cohen 1997), and the consequence is less growth. For example, if CiRep levels are increased significantly, disc growth is reduced and wings are small (Aza-Blanc et al. 1997; Ng 2007). Less severe reductions in Hh signaling lead to more subtle phenotypes. The disc cells adjacent to the compartment border produce the region between wing veins 3 and 4, and these are the cells that are most active in Hh signaling. Proliferation in this region is strongly reduced if Hh signaling in the disc is compromised, although the wings may be otherwise normal in size and pattern. Mutants defective for fused (fu) and collier/knot (col) have wings that are representative of this effect. Fused is a serine/threonine protein kinase that, together with Ci, is a component of the cytoplasmic Hh signaling complex, and it is required for Hh signal transduction (Robbins et al. 1997). Col is a transcription factor and a transcriptional target of hh signaling (Nestoras et al. 1997; Vervoort et al. 1999).
If, on the other hand, Hh function in discs is increased, discs grow excessively. Broadly expressed ectopic CiAct leads to exceptionally large discs and large, abnormally patterned wings (Ng 2007). Ectopic expression of Hh in clones also causes extra growth, but with such localized expression that patterned outgrowths and even wing duplications can result (Tabata et al. 1995; Zecca et al. 1995). These wing duplications are a consequence of the influence of an ectopic developmental organizer that is induced at the site of ectopic paracrine Hh signaling where Hh-expressing cells abut cells that do not express Hh (Tabata et al. 1995).
N signaling also plays key roles in most developmental systems; in contrast to Hh, however, N signaling appears to involve communication between cells that either are immediately juxtaposed or are close neighbors (reviewed in Fortini 2009). N signaling often controls binary cell-fate choices by cells whose developmental potential is initially equivalent or occurs at borders between two distinct populations of cells. Signaling can be initiated by binding between two single-pass membrane proteins at the cell surface of their respective cells: N and one of its ligands, such as Delta (Dl) or Serrate (Ser). Binding causes proteolytic processing of N, leading to release of the N intracellular domain (NICD) and translocation of this N fragment to the nucleus. In the nucleus, the NICD forms complexes with transcription factors such as Suppressor of Hairless [Su(H)] to regulate target genes.
Known targets of Drosophila N include the 12 genes of the Enhancer of split [E(spl)] complex, of which 7 encode basic helix-loop-helix (bHLH) transcriptional repressors (Klambt et al. 1989; Delidakis and Artavanis-Tsakonas 1992; Knust et al. 1992; Jennings et al. 1994). Disc expression of most of these repressors is N-dependent (Singson et al. 1994; de Celis et al. 1996; Wurmbach et al. 1999). While E(spl) genes are required for some N-dependent functions, they are not required for others, indicating that the E(spl)-encoded transcription factors are not the only effectors of N signaling (de Celis et al. 1996). The E(spl) repressors appear to be partially functionally redundant, as no lethal mutations have been identified in any one (Delidakis et al. 1991; Schrons et al. 1992; Nagel et al. 2000).
Another known target is wg. In third instar wing discs, wg is expressed at the dorsal–ventral (DV) compartment border in cells with enhanced N signaling (Diaz-Benjumea and Cohen 1995; Rulifson and Blair 1995). The disc cells at the DV compartment border generate the wing margin, and loss of wing margin cells (wing notching) is one of the characteristic phenotypes of N mutants. N-induced wg expression functions to pattern growth and differentiation along the DV axis (Diaz-Benjumea and Cohen 1995), a role that is analogous to that of Dpp in the AP organizer. Later in wing development, N helps to direct formation of wing veins and wing-blade sensory organs (Shellenbarger and Mohler 1978; Parody and Muskavitch 1993). The work reported here adds another role for N in wing development.
We came upon interactions between the Hh and N pathways in the course of a genetic screen for genes that are involved in Hh signaling. This screen was conducted in a sensitized background in which the efficiency of Hh signaling in the cells of the AP organizer was reduced by expression of smo RNAi. Flies with smo function reduced in this way have reproducible abnormalities in both the state and the size of the 3–4 intervein regions. By making portions of the genome hemizygous in this sensitized background, we previously identified 26 autosomal segments that show haplo-insufficiency, and we characterized the roles of two genes that these deficiencies uncovered (Casso et al. 2008). Here, we extend this screen to the X chromosome and describe two haplo-insufficient segments on the X, as well as a new role for N signaling in the AP organizer region of the wing disc.
MATERIALS AND METHODS
Drosophila lines:
smo RNAi is described in Casso et al. (2008). Su(H)del47 [a 1881-base deletion that removes portions of Su(H) and l(2)35Bg] is a null allele. All other N pathway mutants used in this study are listed in Table 1. Two N reporters were used: Su(H)lacZ reporter [originally described as Gbe+Su(H)m8 in Furriols and Bray 2001] on the X chromosome and E(spl)m-α-GFP on the second chromosome (Castro et al. 2005). UAS-N expresses the full-length wild-type N cDNA (Lawrence et al. 2000). Two transgenes were used to express E(spl) complex HLH proteins: UAS-mΔh8 expresses mΔ and UAS-E(Spl).T3 expresses E(spl)m8.
TABLE 1.
Notch pathway genes and mutants characterized in this study
| Gene | Genetic lesion | Type | ptcGAL4 | ptcGAL4 smo RNAi | Source |
|---|---|---|---|---|---|
| N | Df(1)RR62 | Del | ND | E | BDSC |
| N | Df(1)N-8 | Del | ND | E | BDSC |
| N | Df(1)N-264-105 | Del | ND | E | BDSC |
| N | N55e11/+ | Null | ND | E | BDSC |
| N | Nfa-1 | Mut | NC | E | BDSC |
| N | Nnd-0 | Mut | NC | E | BDSC |
| N | NNIG.3936R-2 | RNAi | Fused | E | NIG |
| N | UAS-N14E | RNAi | NC | L | BDSC |
| N | UAS-NFLN | Ect exp | VT | S | A. Martinez Arias (Cambridge University). |
| psn | P{GD4624}v43082 | RNAi | Fused | E | VDRC |
| psn | P{GD4624}v43083 | RNAi | Fused | E | VDRC |
| Su(H) | Su(H)NIG.3497R-1 | RNAi | 3/4− | E | NIG |
| Su(H) | Su(H)NIG.3497R-3 | RNAi | 3/4− | E | NIG |
| E(spl) | Df(3R)Espl3 | Del | ND | E | BDSC |
| E(spl) | Df(3R)BSC751 | Del | ND | E | BDSC |
| E(spl) | Df(3R)Espl1 | Del | ND | E | BDSC |
| E(spl) | Df(3R)BSC495 | Del | ND | E | BDSC |
| E(spl) | Df(3R)Exel6204 | Del | ND | E | BDSC |
| E(spl) | UAS-HLH-m-Δ | Ect exp | 3/4++ | S | BDSC |
| E(spl) | UAS-HLH-m8 | Ect exp | 3/4++ | S | BDSC |
| vn | vnNIG.10491R-1 | RNAi | 3/4− | E | NIG |
| Su(dx) | Su(dx)NIG.4244R-1 | RNAi | ACV− | S | NIG |
Deletions, mutations, and RNAi transgenes are indicated as Del, Mut, and RNAi, respectively. Wing phenotypes with ptcGAL4 and ptcGAL4 smo RNAi at 29° are described. Del, deletion; Mut, hypomorphic mutation; Ect exp, ectopic expression; NC, no change; ND, not determined; 3/4−, reduction in distance between veins 3 and 4; 3/4++, increased distance between veins 3 and 4; Fused, fusion of veins 3 and 4; E, enhanced; S, suppressed; L, lethal; VT, vein thickening; BDSC, Bloomington Drosophila Stock Center (USA); NIG, National Institute of Genetics (Mishima, Japan); VDRC, Vienna Drosophila RNAi Center.
Deficiency screen:
The screen used the Bloomington Drosophila Stock Center collection of X chromosome deficiencies, which cumulatively delete >95% of the euchromatin. As diagrammed below, we screened F2's, first crossing each deficiency to FM6 Bar1 (B1), followed by a cross to ptcGAL4 smo RNAi (pWIZ-smo2B) at 29°. Wings from female smo RNAi flies carrying deficiency chromosomes (B+) were compared to wings from sibling female control flies carrying FM6, B1. Enhancement or suppression of the ptcGAL4, smo RNAi wing phenotype was scored in the proximal half of the wing in close proximity to the anterior crossvein by assessing the distance between veins 3 and 4. This region of the wing was sensitive to dosage of the Hh pathway regulators smo, hh, en, ptc, and mts (Casso et al. 2008; Jia et al. 2009), but neither the distance between veins 3 and 4 in the distal part of the wing nor ectopic venation phenotypes consistently correlated with changes in pathway activity. In all genetic interactions reported here, >90% of the wings scored with the indicated phenotype, and each cross was scored at least twice. (Secondary phenotypes such as vein thickness, notching, delta formation, and distance between veins 3 and 4 in the distal wing blade did not correlate with the state of Hh signaling in our assays. One exception is wing notching when ptcGAL4 and RNAi alleles were used, as noted in the results.)
Mutant and clonal analysis:
Negatively marked mutant clones were made by heat-shocking larvae for 30–45 min at 37° 2–3 days after egg laying. Genotypes of heat-shocked larvae were hsflp; Su(H)del47, FRT40A/Ubi-GFP(S65T)nls2L, FRT40A for Su(H) clones, and N55e11 FRT18A/arm-lacZ.VMM1 FRT18A; and MKRS, P{hsFLP}86E for N clones. Temperature-sensitive Nl1N-ts1 larvae were raised at 17° and were shifted to 30° for 24 hr for loss-of-function assays. Assays for vn function were with vn1/vnC221 larvae and pupae.
Immunohistochemistry:
The following antisera were used: Ptc mouse monoclonal, 1:300 (Capdevila et al. 1994); Ci, rat monoclonal 2A1, 1:2000 (Motzny and Holmgren 1995); LacZ, rabbit polyclonal, 1:5000 (Cappel/MP Biomedicals, Solon, OH); N, mouse monoclonal C17.9C6 that recognizes the N intracellular domain, 1:100 (Fehon et al. 1990); and Dl, mouse monoclonal C594.9B that recognizes the Dl extracellular domain, 1:50 (McGlinn et al. 2005).
RESULTS
N is a strong enhancer of smo RNAi:
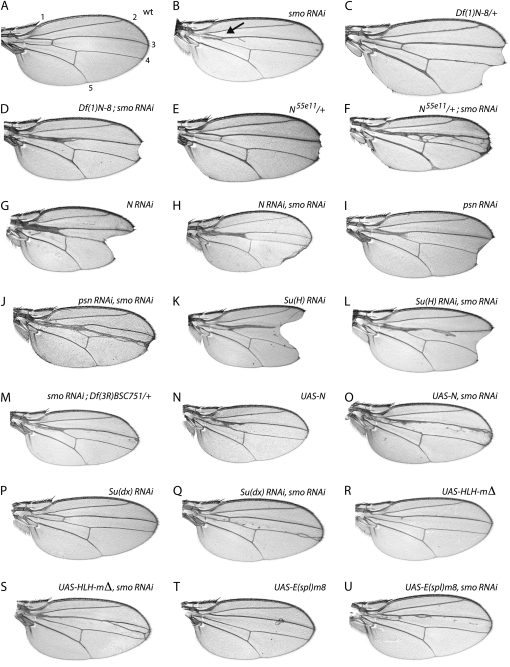
The distance between wing veins 3 and 4 is sensitive to the state of Hh signaling, and it increases or decreases if levels of the Hh pathway components Hh, Ptc, Fu, Cos2, or Ci change. In our screen, Smo function was reduced by expression of smo RNAi at the AP organizer, resulting in a clear and reproducible decrease in the 3–4 intervein region (Figure 1, A and B). We tested 90 strains carrying X chromosome deletions that together remove >95% of the X euchromatin. Two regions, 14C-D and 3C2-D, enhanced the smo RNAi phenotype. Df(1)FDD-0024486 (14C4-D1) and Df(1)FDD-0230908 (14C6-E1) refined the region that enhanced the phenotype to 14C6-D1. The 3C2-D region deficiencies Df(1)RR62 (3C-D), Df(1)N-8 (3C2-3E5), and Df(1)N-264-105 (3C6-3D5) yielded the strongest enhancement of smo RNAi of any of the >300 deficiencies tested in our screening (Figure 1, A–D, and data not shown). Table 1 lists the 14C-D and 3C2-D deficiencies, as well as relevant mutant genes and RNAi alleles that enhanced or suppressed smo RNAi. This report focuses on the 3C2-D region.
Figure 1.—
N function is required for Hh-dependent patterning of the wing. (A) Control (w−). (B) FM6/+; ptcGAL4 smo RNAi. (C) Df(1)N-8/+; note wing-margin notching. (D) Df(1)N-8/+; ptcGAL4 smo RNAi; note vein 3–4 thickening and fusion. (E) N55e11/+ (N null); note notching at margin and veins 3 and 5 thickening. (F) N55e11/+; ptcGAL4 smo RNAi. (G) ptcGAL4 N RNAi (NIG.3936R-2). (H) ptcGAL4 N RNAi smo RNAi. (I) ptcGAL4 psn RNAi (P{GD4624}v43082). (J) ptcGAL4 psn RNAi smo RNAi. (K) ptcGAL4 Su(H) RNAi (NIG.3497R-3). (L) ptcGAL4 Su(H) RNAi smo RNAi. (M) ptcGAL4 smo RNAi; E(Spl)/+. (N) ptcGAL4 UAS-N. (O) ptcGAL4 UAS-N smo RNAi. (P) ptcGAL4 Su(dx) RNAi (NIG.4244R-1). (Q) ptcGAL4 Su(dx) RNAi smo RNAi. (R) ptcGAL4 UAS-HLH-mΔ. (S) ptcGAL4 UAS-HLH-mΔ smo RNAi. (T) ptcGAL4 UAS-E(spl)m8. (U) ptcGAL4 UAS-E(spl)m8 smo RNAi.
To identify the genes in the 3C-D region whose haplo-insufficiency is responsible for enhancing the smo RNAi wing phenotype, we first tested deletions that partially overlap 3C-D. Neither Df(1)vt (3C2-3C7) or Df(1)w67k30 (3C2-3C6) on the left flank affected the smo RNAi phenotype, nor did Df(1)dm75e19 (3C11-3E4), Df(1)Exel6233 (3D2-3D4), or Df(1)ED6712 (3D3-3F1) on the right flank (data not shown). These results narrow the enhancer at 3C-D to 3C7-3C11 where 13 genes reside. N is one of them. The absence of zygotic N function (i.e., N/N) leads to embryonic lethality and conversion of much of the embryo ectoderm to a neural fate. Reducing N gene copy number [i.e., Df(N)/+] or function (i.e., the amorphic N55e11/+) results in a semi-penetrant notching of the wing margin and an increase in the width of veins 3 and 5 (Figure 1, C and E). Similar phenotypes were observed for the three 3C deletion chromosomes that enhance smo RNAi (Table 1; data not shown), but wings of flies carrying deletions (listed above) that flank the 3C smo RNAi enhancer were not notched at the wing margins, and the widths of their wing veins were not abnormal.
By crossing N mutant and N RNAi alleles with smo RNAi, we tested whether N insufficiency can enhance the smo RNAi phenotype. Strong enhancement similar to phenotypes of the 3C-D deletions was observed with the amorph N55e11 (Figure 1, D and F). Among N hypomorphs that are viable as N/Y males, we found two that strongly enhanced: Nfa-1 and Nnd-0. No enhancement was observed in Nfa-1/+ or Nnd-0/+ females (data not shown). Expression of N RNAi at the AP organizer was also examined. By itself, N RNAi thickened vein 3 and decreased the 3–4 intervein distance, resulting in the fusion of veins 3 and 4 in the proximal portion of the wing (Figure 1G). Although heterozygous N null alleles do not affect veins 3 and 4 in this way, RNAi can reduce target gene expression by more than half (Jacobsen et al. 2006). Co-expression of N RNAi with smo RNAi generated wings with more fusion of veins 3 and 4 than with either RNAi alone (Figure 1H). N RNAi also caused semi-penetrant notching in the wing margin, both alone and in combination with smo RNAi. Growth reductions accompanied by ectopic vein tissue formation have also been observed in N loss-of-function clones between veins 3 and 4 (de Celis and Garcia-Bellido 1994).
Note that smo RNAi not only negatively regulates Hh signaling, but also reduces output from the Hh-dependent ptcGAL4 driver that drives expression of smo RNAi (Casso et al. 2008). Although the consequent negative feedback may have dampened the phenotypic responses that we obtained, this effect may have been advantageous for the screen, both by masking the effects of weak modifiers and by decreasing phenotypic variability. We observed such dampening in flies expressing RNAi directed against N pathway genes such as N, psn, and Su(H) RNAi. The degree of wing “notching” at the DV margin with ptcGAL4 smo RNAi was reduced compared to ptcGAL4> N, psn, or Su(H) RNAi (Figure 1, G–L). There is no evidence that suppressing ptcGAL4 expression compromised our conclusions about the interactions between N pathway genes and smo RNAi.
Loss of genes that activate N signaling enhance the smo RNAi phenotype:
The proteolytic process that activates N signal transduction requires Presenilin (Psn), an intramembrane protease (Struhl and Greenwald 1999; Ye et al. 1999). Without Psn, N cannot be activated, and although loss-of-function mutations (psn/+) or deletions [Df(psn)/+] did not affect the smo RNAi wing phenotype, reducing Psn activity by expressing psn RNAi strongly enhanced it (Figure 1J and data not shown). psn RNAi expression at the AP border also reduced the distance between veins 3 and 4, but not as strongly as it did in combination with smo RNAi (Figure 1, I and J). This result is consistent with similar effects reported previously on the size of the 3–4 intervein region caused by large psn clones (Struhl and Greenwald 1999).
In the absence of N activation, the N co-activator Su(H) functions as a transcriptional repressor and suppresses the expression of N target genes (Furriols and Bray 2001). However, when N is cleaved by Psn, and thus activated, the NICD binds to Su(H) and the resulting protein complex upregulates N pathway target genes (reviewed in Fortini 2009). As was observed with psn, reducing Su(H) function by introduction of mutant alleles did not affect the smo RNAi wing phenotype (data not shown). However, Su(H) RNAi caused a modest reduction in the distance between veins 3 and 4 on its own and strongly enhanced the 3–4 wing-vein fusion caused by smo RNAi (Figure 1, K and L).
The E(spl) gene complex is a transcriptional target of N/Su(H) in many N-dependent cell lineages (De Celis et al. 1996). E(spl) codes for 13 genes, including seven basic helix-loop-helix transcriptional repressors; its genes are clustered in close proximity to one another in region 96F9-10. Df(3R)Espl3 was identified in our original screen as an enhancer of the smo RNAi wing phenotype (Casso et al. 2008). While we were not able to identify a single gene in this region that enhanced smo RNAi [RNAi directed against the E(spl) complex genes HLH-mβ, HLH-mΔ, or HLHm8 did not modify the phenotype (data not shown)], we did identify four smaller chromosomal deletions in this region that did [Df(3R)Exel6204, Df(3R)BSC751, Df(3R)BSC495, Df(3R)Espl1 (Figure 1M and data not shown)]. Each of these interacting deletions removes the entire E(spl) complex, while smaller deletions that removed only part of the E(spl) complex did not interact. The enhancement produced by the E(spl) deletions that we tested was more modest than that seen with N deletions. The groucho (gro) gene is adjacent and distal to the E(spl) complex and is implicated in many E(spl)-mediated processes. No interaction was observed between smo RNAi and the loss-of-function alleles of gro (such as gro1, groC105, and groKG07117) or the deletions of gro that do not remove the whole E(spl) complex [such as Df(3R)P709, Df(3R)Exel6204, and Df(3R)ED6232]. All of the E(spl) deletions that enhanced smo also carry deletions of both the entire E(spl) locus and gro, indicating that gro functions with the E(spl) genes in response to N signaling at the AP border. Although gro has been shown to affect hh and en expression in wing discs (de Celis and Ruiz-Gomez 1995), our data indicate that gro function on its own is not responsible for the interactions of E(spl) deletions with smo RNAi. While the E(spl) genes are targets of N, they are not likely to be the only relevant transcriptional effectors of N signaling (de Celis et al. 1996; Ligoxygakis et al. 1999).
Ectopic activation of the N pathway suppresses the smo RNAi phenotype:
If the function of the AP organizer is dependent on N function, then increasing the level of N activation might be expected to suppress smo RNAi phenotypes. Since levels of N protein correlate with N pathway activity, and since ectopic expression of N activates N signaling (Doherty et al. 1996), we increased N expression at the wing AP organizer to test this prediction. Whereas expression of activated N (NICD) in the ptc expression domain was lethal under our assay conditions, expression of full-length wild-type N yielded viable adult flies. Wings from these ptcGAL4 UAS-N flies were almost normal, with only minor defects in the anterior crossvein and mild thickening of vein 3 attributable to overexpression of N (Figure 1N). ptcGAL4 UAS-N smo RNAi flies also had wings with a fairly normal interval between veins 3 and 4 and very modest thickening of vein 3 (Figure 1O), indicating that ectopic N suppresses smo RNAi. Among the known genes that regulate the levels of N, we tested Suppressor of deltex [Su(dx)], since its reduced function can lead to increased N signaling (Fostier et al. 1998). Su(dx) is an E3 ubiquitin ligase that targets nonactivated N to late endosomes and lysosomes and limits the amount of N available for activation (Wilkin et al. 2004). In our system, neither hemizygosity at the Su(dx) locus (22C1) nor Su(dx) RNAi changed wing morphology significantly, but Su(dx) RNAi strongly suppressed smo RNAi, producing wings with only minor vein defects (Figure 1, P and Q).
The N pathway activates expression of NICD/Su(H) target genes such as those in the E(spl) complex. To mimic ectopic N signaling at the AP organizer, we expressed individual E(spl) complex genes by themselves or with smo RNAi. Ectopic expression of either E(spl)m8 or HLH-mΔ prevented normal formation of the anterior crossvein when expressed with ptcGAL4; ectopic expression of HLH-mΔ also increased the 3–4 intervein distance (Figure 1, R and T), mimicking the effect of ectopic Hh signaling at the AP organizer (Johnson et al. 2000). E(spl)m8 and HLH-mΔ strongly suppressed the smo RNAi phenotype (Figure 1, S and U), while ectopic expression of other E(spl) complex genes that we tested did not modify smo RNAi [e.g., E(spl)-mβ, HLHm5, m4.A (data not shown)]. Although HLH-mΔ is not known to be expressed in this region of the wing, the suppression of smo RNAi that we observed may derive from a functional redundancy with other E(spl) genes.
N signaling is activated at the wing-disc AP organizer:
The genetic interactions between smo and components of the N pathway suggest that N signaling is activated at the AP organizer. To assess N signaling directly, we monitored N pathway activity with a lacZ reporter that responds to N pathway activation, and since N levels correlate with activation of the pathway, we also monitored levels of N protein by immunohistochemistry. The wing develops as an epithelial sheet in a region of the wing disc called the wing pouch. At the end of the third larval instar, the wing pouch begins to evert, and as metamorphosis progresses in the early pupa, the wing pouch elongates and flattens, bringing the dorsal (D) and ventral (V) cells together and creating an edge (or margin) at the DV boundary.
The N-inducible lacZ reporter that we used [Su(H)lacZ] has an Hsp70 minimal promoter, three copies of a grainy head DNA-binding element (included to increase expression in wing discs), and two Su(H) DNA-binding sites from the E(spl)m8 gene (Furriols and Bray 2001). In late third instar wing discs, Su(H)lacZ activity has a complex pattern that includes a prominent stripe along the DV boundary of the wing pouch (Figure 2A and Furriols and Bray 2001). This DV expression is consistent with the requirement for N activation to form the wing margin. Another element of its expression pattern is a one- to two-cell-wide stripe perpendicular to the DV boundary and parallel to or coincident with the AP organizer.
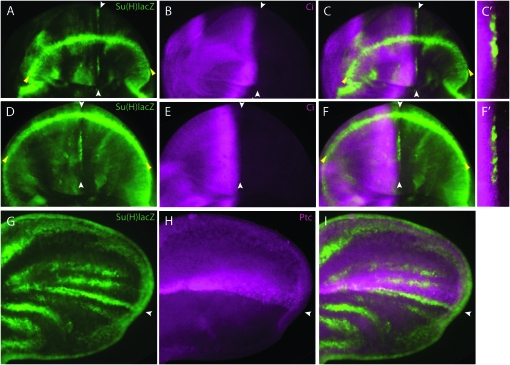
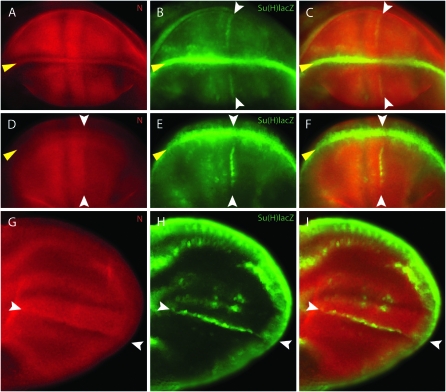
Figure 2.—
N signaling at the AP organizer. Three stages of wing development: (A–C) late third instar disc; (D–F) early pupal wing disc when the dorsal and ventral surfaces of the everting disc are coming into apposition (∼3 hr after pupariation); (G–I) early pupal wing (∼9–12 hr after pupariation). (A, D, and G) Su(H)lacZ expression (green) indicates N signaling both at the DV border (yellow arrowheads) and at the AP organizer (white arrowheads). The DV boundary is in the middle of the wing pouch (A), near the edge in a partially everted disc (D), and at the edge of the pupal wing (G). As development advances, Su(H)lacZ expression increases at the AP border (white arrowheads) and decreases at the DV border (yellow arrowheads). (B, E, and H) Ci expression (magenta) marks anterior cells; the posterior extent of Ci expression marks the AP border. (C, F, and I) Merged images show that Su(H)lacZ expression at the AP organizer is in Ci-expressing anterior cells; Ci staining is enhanced in enlarged views (C′ and F′) to show the position of the AP border and the coincidence of Ci and LacZ expression. (A–F) Orientation: anterior is left and ventral is up; (G–I) anterior is up and distal is right.
To determine the position of the AP stripe of lacZ expression relative to the AP border, we stained late larval wing discs with antibodies directed against LacZ and Ci. Ci is specifically expressed in all cells of the anterior compartment and is most abundant in a band of four to seven cells that are one to two cells from the anterior side of the AP border (Figure 9A). LacZ staining was juxtaposed and posterior to this band of high Ci expression (Figure 2, A–C) and coincident with the posterior edge of the anterior compartment (Figure 2C′). This indicated that Su(H)lacZ is expressed on the anterior side of the AP border, and staining these discs with Ptc (another anterior cell marker) confirmed this location (data not shown). In late larval stages and during early pupal development, Su(H)lacZ expression at the DV margin is stronger than at the AP organizer (Figure 2A). In pupariating wings, the strength of the AP stripe increases in relative intensity, while remaining coincident with the AP organizer (Figure 2, D–I). When the pupal wing has fully everted, a number of stripes of lacZ expression parallel to the AP border are visible; the strongest of these stripes is at the AP border where its posterior limit coincides with Ci (Figure 2, G–I).
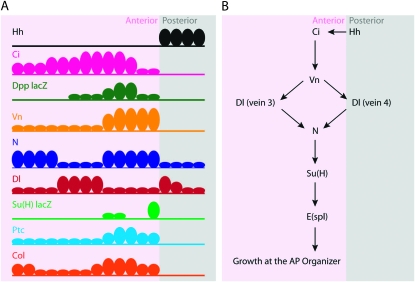
Figure 9.—
Expression of key genes at the AP organizer and a model for N activation by Hh. (A) Ovals represent the width of expression domains in cell diameters perpendicular to the AP axis, and oval sizes reflect expression levels. Expression domains were measured in late third instar wing discs in the ventral compartment intermediate between the edge of the wing pouch and the DV border. Antibodies were used except where lacZ insertions are indicated. (B) Hh-dependent activation of vn at the AP organizer is required for the expression of Dl in vein 3 and vein 4 stripes. N activation induces cell proliferation within the organizer at least in part through the activation of the E(spl) genes.
We observed similar AP expression of the N-responsive E(spl)m-α-GFP reporter (Figure 3, A–C), which carries a 1004-bp genomic DNA fragment from the m-α gene to direct the expression of GFP (Castro et al. 2005). In this case, however, expression of the m-αreporter was not observed at the AP organizer until pupariation, possibly reflecting differences between timing of expression of different E(spl) genes.
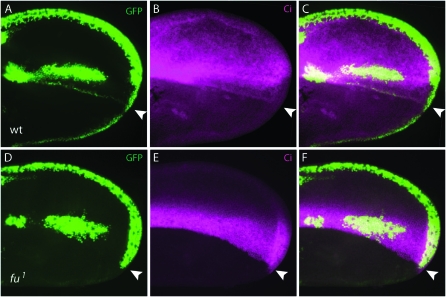
Figure 3.—
Expression of the N reporter E(spl)m-α-GFP at the AP organizer in pupal wings. (A and D) E(spl)m-α-GFP expression (green). (B and E) Ci expression (magenta). (C and F) A merge of GFP and Ci. (A–C) A control wing. (D–F) A fu1 wing. The position of the AP border where Ci expression ends is indicated by a white arrowhead.
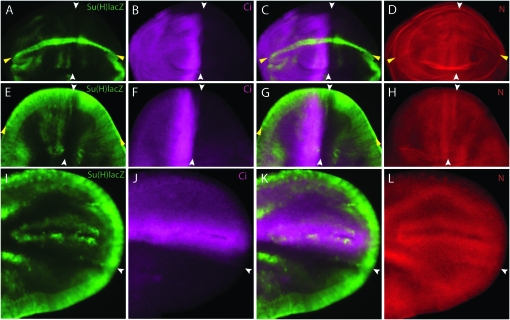
Since N signaling can increase N levels, and N signaling can be enhanced or ectopically activated by increasing N expression (Huppert et al. 1997), the levels of N protein are expected to be elevated at sites of N activation. We therefore probed wing discs and pupal wings for expression of Su(H)lacZ and N. High levels of staining with anti-N antibody were detected coincident with sites of Su(H)lacZ expression. For example, high N protein levels were present along the DV margin of the wing pouch in both late larval and early pupal stages (Figure 4, A–F). N protein levels were also high in the anterior compartment in a band approximately five cells wide at the AP organizer, including the stripe of LacZ-expressing cells along the posterior side of the N stripe (Figure 4, A–F, white arrowheads; Figure 9A). During pupal wing development when Su(H)lacZ expression increases, a stripe of high N protein expression was coincident with LacZ expression at the AP organizer (Figure 4, G–I). Our results are consistent with previously described N expression patterns in wing discs and pupal wings (Fehon et al. 1991; Kooh et al. 1993; de Celis et al. 1997). Although the three readouts that we used show that N signaling is activated in the AP organizer, they do not establish if there are cells that do not activate N signaling, and finer resolution characterization of N signaling awaits better probes of N activation.
Figure 4.—
N protein levels are elevated at the AP organizer. (A–C) Late third instar wing disc. (D–F) Pupal wing disc. (G–I) Early pupal wing. (A, D, and G) Expression of N protein (red) is high at both the AP (white arrows) and the DV (yellow arrows) border regions. (B, E, and H) Expression of Su(H)lacZ (green) indicates activation of N signaling at both DV and AP borders. (C, F, and I) Merged images show that Su(H)lacZ expression at the AP border is in cells with high levels of N protein.
Hh is required for N activation at the AP organizer:
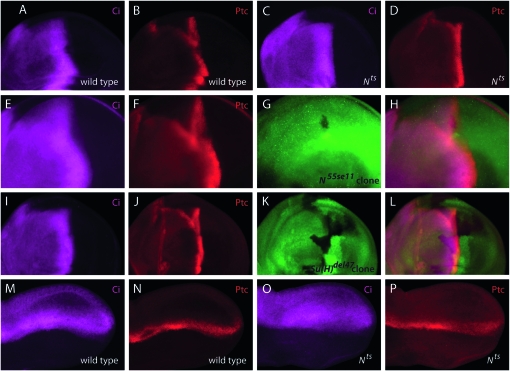
The genetic interactions between the N and Hh pathways at the AP organizer do not make it clear whether these pathways function independently or whether one is necessary for the other. To investigate their relationship further, we examined Ptc and Ci expression in (and adjacent to) clones of N55e11, Su(H)del47, Dl, and Ser. We also examined Ptc and Ci expression in Nl1N-ts1 wing discs and pupal wings at permissive and nonpermissive temperatures (Figure 5, A–D and M–P). Clones of particular interest were those on the anterior side of the AP compartment border that included the stripes of Ptc and Ci expression, as well as clones in the posterior compartment that abutted the AP border (Figure 5, E–L, and data not shown). No defects in Ptc or Ci expression were apparent under any of these conditions of N pathway perturbation: these experiments suggest that N signaling is not required for the activation of Hh target gene expression at the AP organizer (Glise et al. 2002).
Figure 5.—
Disruption of N signaling does not alter Hh signaling. Ci (magenta) and Ptc (red) expression in third instar wing discs (A–L) and pupal wings (M–P). Clones are negatively marked by lack of GFP or lacZ expression (green). (A and B) w− wing discs. (C and D) Wing discs from Nl1N-ts1 larvae shifted to 30° (restrictive temperature) for 24 hr. (E–H) Clone of null allele N55e11 within the AP organizer. (I–L) Ventral clone in the posterior compartment and a dorsal clone in the anterior compartment of null allele Su(H)del47. (H and L) Merged images showing the positions of these clones in relation to Ptc and Ci expression. (M–P) Expression of Ptc and Ci in w− and temperature-shifted Nts pupal wings. (M and N) w− pupal wings. Note that at this stage dorsal and ventral surfaces of the wing are not apposed to each other, resulting in the apparent splitting of the Pct and Ci stripes in the proximal region (left) of the wing. (O and P) Nl1N-ts1 pupal wings shifted to 30° for 24 hr.
To monitor the N pathway in conditions with compromised Hh signaling, we first focused on the Hh target gene vn. vn is expressed in a stripe of anterior cells at the AP border (Wessells et al. 1999) (Figure 9A), and in the third instar, the N ligand Dl is expressed in a pair of stripes near the AP border (Figure 6A and Figure 9A). These Dl stripes mark the primordia that will produce vein 3 (in the anterior compartment) and vein 4 (in the posterior compartment; Figure 6, B–D; Figure 9A). The position of the Dl vein 4 stripe is posterior to the AP border. During pupariation, N is activated in a subset of cells in the vein 4 stripe that differentiate into a vein (De Celis et al. 1997; Huppert et al. 1997). We note that, in contrast to the posterior position of Dl vein 4, the location of the stripes of Su(H)lacZ and elevated N that we describe above are anterior of the border and therefore not associated with the differentiation of vein and pro-vein tissue within vein 4.
Figure 6.—
Dl expression in the vein 4 primordium is in the posterior compartment. (A) A wild-type wing imaginal disc showing stripes of Dl expression in veins 3, 4, and 5 primordia. (B–D) High magnification of the AP organizer region in the dorsal wing pouch. Veins 3 and 4 stripes of Dl expression (red) flank the AP border indicated by arrowheads. Expression of Ci (green) marks the cells of the anterior compartment. The Dl vein 3 stripe is anterior of the border, while the vein 4 stripe is posterior and directly adjacent to the border. (A and B–D are from two different discs.)
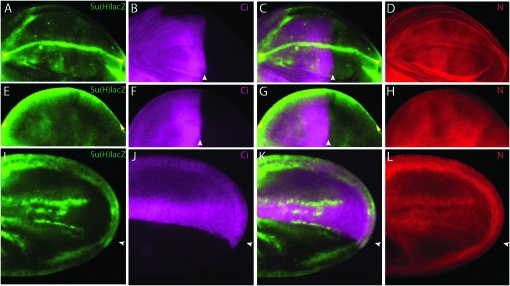
Expression of Dl in these regions is dependent on the EGF ligand Vn. When vn function was reduced (as in vn1/vnC221), the vein 4 stripe of Dl expression was absent in larval discs and the vein 3 stripe of Dl was reduced (Biehs et al. 1998 and data not shown). Moreover, expression of Su(H)lacZ at the AP organizer in vn1/vnC221 third instar and pupal wing discs was almost undetectable (Figure 7, A–C and E–G), expression in pupal wings was severely reduced compared to wild type (Figure 7, I–K), the AP band of N expression was weaker and narrower, and no peak of expression was present at the AP border (Figure 7, D, H, and L).
Figure 7.—
N activation in the AP organizer depends on vn. N activation in a vn1/vnC221 mutant (A–D) late third instar wing disc. (E–H) Pupal disc. (I–L) Early pupal wing. Relative to wild type (Figure 4), expression of Su(H)lacZ (green) and N (red) is reduced at the AP organizer (white arrowheads), whose position is marked by Ci (magenta). DV boundary is indicated by yellow arrowheads.
We also tested the Hh dependence on N signaling by examining fu mutants, since Fu is required for Hh signaling at the AP organizer. We monitored the Su(H)lacZ and E(spl)m-α-GFP reporters as well as N expression. In contrast to wild type (Figures 2 and 3), fu41 larval and pupal discs had no visible AP stripe of expression (Figure 8, A–C and E–G), and although Su(H)lacZ expression was active in the AP border stripe in fu41 pupal wings, its activity was much lower than that of wild type (compare Figure 2, G–I, and Figure 8, I–K). Similar results were obtained with fu1 and fu54 mutants (data not shown). Assays of N expression gave results that are consistent with the Su(H)lacZ reporter: N expression was not elevated at the AP organizer in fu41 wing discs or pupal wings (compare Figure 4 and Figure 8, D and H), and only a thin, weak stripe of elevated N expression was present at the AP organizer in pupal wings (Figure 8L). As described above, E(spl)m-α-GFP expression was present at the AP organizer in pupal wings (Figure 3, A–C), but was not observed in either larval or pupal discs. We did not detect E(spl)m-α-GFP expression in fu1 pupal wings (Figure 3, D–F).
Figure 8.—
N signaling and expression at the AP organizer depend on fu. Three stages of wing development: (A–D) late third instar disc; (E–H) early pupal wing disc when the dorsal and ventral surfaces of the everting disc are coming into apposition (∼3 hr after pupariation); and (I–L) early pupal wing (∼9–12 hr after pupariation). (A, E, and I) Su(H)lacZ expression (green) indicates N signaling both at the DV border (yellow arrowheads) and at the AP organizer (white arrowheads). The DV boundary is in the middle of the wing pouch (A), partially everted in E, and at the edge of the pupal wing (I). As development advances, Su(H)lacZ expression increases at the AP border (white arrowheads) and decreases at the DV border (yellow arrowheads). (B, F, and J) Ci expression (magenta) marks anterior cells; the posterior extent of Ci expression marks the AP border. (C, F, and I) Merged images show that Su(H)lacZ expression at the AP organizer is in Ci-expressing anterior cells. (A–F) Orientation: anterior is left and ventral is up. (I–L) Anterior is up and distal is right.
The domains of Dl and N expression correlate with the AP organizer and the AP compartment border (see Figure 9A). The Dl vein 3 stripe is produced by cells close to the AP border that express the high levels of Ci protein, the Dl vein 4 stripe is expressed in posterior cells that are immediately juxtaposed to the AP border, and N is expressed abundantly in a band of five cells on the anterior side of the border between the Dl stripes. These expression data are consistent with the notion that Hh signaling regulates expression of Dl and N at the AP organizer.
DISCUSSION
A new role for N signaling in the wing disc:
In this article, we show activation of N signaling at the wing AP organizer by defining with cellular resolution the expression patterns of N protein and N pathway reporters in relation to the AP organizer and show dependence on Hh signaling. We also show strong interactions between hh- and N-signaling pathways and confirm that the activation of N signaling is necessary for the normal growth of the AP organizer. Our work uncovers a previously unknown activity of the Hh pathway in mitogenesis at the AP organizer: the activation of N signaling. Our results are surprising in that they show that the roles of N signaling in the growth of the wing are not limited to the function of the DV organizer and a general growth-promoting function in the wing: N signaling also induces growth downstream of hh at the AP organizer.
N is essential for the cells that give rise to the DV margin, veins, and sensory organs of the wing, and its expression is elevated in the progenitors that produce these structures (Fehon et al. 1991). The DV margin progenitors, which transect the wing disc in a band that is orthogonal to the Hh-dependent AP organizer, express wg in response to N (Rulifson and Blair 1995). These wg-expressing cells function as a DV organizer (Diaz-Benjumea and Cohen 1995), and several lines of evidence suggest that the AP and DV organizers function independently: Hh signaling along the AP axis is not N-dependent, N signaling along the DV axis is not hh-dependent, and targets regulated by the AP and DV organizers are not the same. The findings that we report here show that, separately from its roles elsewhere in the wing disc, N signaling has an essential mitogenic role in the cells of the AP organizer region.
While N can stimulate growth by inducing expression of wg (as it does in the DV organizer), hyper-activation of N signaling near the AP border of the wing pouch causes overgrowth that is independent of wg (Speicher et al. 1994; Doherty et al. 1996; Go et al. 1998). wg is not normally expressed along the AP axis, but we found that N signaling is activated at the AP compartment border in late third instar discs, pupal discs, and pupal wings. Through vn expression, Hh signaling at the AP compartment border increases expression of Dl flanking the organizer, and Hh signaling activates N in the 3–4 intervein region. While we have not directly investigated a role for Ser at the AP organizer, Ser expression in the wing disc is very similar to that of Dl, with high levels of Ser in the vein 3 and 4 primordia as well as along the DV border (Speicher et al. 1994; de Celis and Bray 2000; De Celis 2003). Our results show that growth of the 3–4 intervein region, long known to be dependent on Hh, is also dependent on Hh-induced activation of N.
Expression of N pathway reporters and components and genetic interactions support this model of regulation of the intervein region. The reporters Su(H)lacZ and E(spl)m-α-GFP express at the AP border in a Hh-dependent manner (Figures 2 and 3). Elevated levels of N protein expression on the anterior side of the AP border require Vn signaling (Figures 4 and 7). This N region is flanked by Dl expression in the vein 3 and vein 4 primordia; Dl expression is known to be dependent upon expression of the Hh target vn (Biehs et al. 1998). Genetic interactions between smo RNAi and N and between smo RNAi and N pathway components [e.g., the Psn intramembrane protease, which activates N; the Su(H) transcriptional co-activator; the Su(dx) E3 ubiquitin ligase, which monitors levels of N protein; and the E(spl) complex of N transcriptional targets] also indicate a functional link between the Hh and N systems (Figure 1).
Our model for the role of N in the 3–4 intervein region is consistent with previous reports of expression patterns of the E(spl) genes E(spl)m8 (Furriols and Bray 2001), M-β (Nellesen et al. 1999), and M-α (Castro et al. 2005). Ectopic expression of HLH-mΔ and m8 rescues smo RNAi. Although HLH-mΔ does not appear to be expressed in the AP organizer in a wild-type wing because the E(spl) genes are thought to have partially overlapping functions, the fact that mΔ phenocopies the rescue by m8 reinforces our conclusion that the function of the E(spl) genes is critical to inducing growth at the AP organizer. Importantly, our findings show that the cells that activate N are the anterior cells of the AP organizer and are not associated with development of veins in pupal wings. Vein 4 develops within the posterior compartment and in many cases has posterior cells between it and the AP border. Since we never observed activation of these reporters extending into posterior territory, their expression correlates better with the position of the AP organizer than with vein/intervein territories at the stages that we examined. It should be noted that no single readout currently available marks all tissues in which N is activated. The E(spl) genes, for example, express in a variety of spatial and temporal patterns in response to N, and these patterns are only partially overlapping (Nellesen et al. 1999). We therefore do not exclude the possibility that N signaling is also activated along the stripe of Dl expression in the vein 3 primordium or that signaling could be occurring in the entire broad stripe of elevated N expression in the AP organizer. We were not able to see changes in proliferation using a direct readout such as phosphohistone staining of mitotic cells to visualize increases or decreases in growth at the AP organizer. These proliferation assays mark cell cycle progression at a single time point in fixed tissues, and the changes that we see in the adult wing could be due to one or two fewer cell division cycles occurring over the course of days of development.
Figure 9A summarizes expression patterns of Hh and N pathway genes that we obtained by recording the levels of expression and the positions of expressing cells relative to the AP compartment border. Figure 9A depicts the spatial relationship between Hh, which is expressed by posterior cells, and its targets Ci, Ptc, Dpp, Col, and Vn. It similarly depicts the spatial relationship between N, Dl, and Su(H)lacZ expression. Our findings indicate a link between the Hh and N pathways and suggest a model in which the domain of N activation at the AP border [manifested by Su(H)lacZ expression] is a consequence both of flanking cells that express high levels of Dl and of Hh signaling (Figure 9B). Our proposed role for Hh signaling is multifaceted: Hh is required for vn expression, which is itself required for high levels of Dl expression in the vein 3 stripe (data not shown) and the vein 4 stripe (Biehs et al. 1998 and data not shown) and for N expression at the AP organizer. Although we have not directly tested whether Dl expression in veins 3 and 4 activates N signaling, vn function is necessary for N activation (Figure 7), and the reciprocal relationship between cells expressing high levels of Dl and neighboring cells expressing high levels of N is well established.
Interactions between the N and Hh pathways:
Interactions between the Sonic hedgehog (SHH) and N signaling pathways have been identified previously in vertebrates. Particularly noteworthy for their relevance to the interactions that we found in the Drosophila wing disc are the increased expression of the Serrate-related N ligand, Jagged 1, in the mouse Gli3Xt mutant (McGlinn et al. 2005); reduced expression of Jagged1 and Notch2 in the cerebella of mice with reduced SHH signaling (Dakubo et al. 2006); regulation of the Delta-related ligand, DNER, by SHH in Purkinje neurons and fetal prostate (Dahmane and Ruiz i Altaba 1999; Wallace 1999; Tohgo et al. 2006; Yu et al. 2009); activation of N signaling in neuroblastomas in Ptch+/− mice with elevated SHH signaling (Dakubo et al. 2006); and Notch2 overexpression in mice carrying an activated allele of smo (Hallahan et al. 2004). These studies establish a positive effect of SHH signaling on the N pathway, consistent with our data.
In Drosophila, there have been several reports of interactions between the N and Hh pathways. In the wing pouch, for example, expression levels of the Hh targets ptc, ci, col, and en are markedly lower at the intersection of the AP and DV borders than elsewhere in the AP organizer. This repression is mediated by wg (Glise et al. 2002). In addition, N and col function together to determine the position of wing veins 3 and 4 (Crozatier et al. 2003). However, loss of function of either col or vn did not show interactions with smo RNAi (data not shown).
Hh, N, and proliferation at the AP organizer:
N functions in two types of settings (reviewed by Fortini 2009). One is associated with binary fate choices; it involves adjacent cells that adopt either of two fates on the basis of the activation of N signaling in one cell and inactivation in the other. In these settings, activation of N not only induces differentiation in a designated cell, but also blocks activation of N in the neighbors. The second type of setting does not induce a binary fate choice, but instead activates the pathway at the junction of two distinct cell types. N pathway activation at the DV border in the wing is one example (Furriols and Bray 2001); in this setting, N is activated in a band that straddles the DV border and the N ligands Dl and Ser signal from adjacent domains from either the dorsal (i.e., Dl) or the ventral (i.e., Ser) side. Activation of N in the 3–4 intervein region at the AP border appears to be of this second type: it occurs adjacent to regions of elevated Dl expression at the apposition of anterior and posterior cell types. There is no apparent binary fate choice in this region of the wing.
In ways that are not understood well, development of the 3–4 intervein region is controlled differently from other regions of the wing pouch. Whereas Hh induces expression of Dpp, and Dpp orchestrates proliferation and patterning of wing pouch cells generally, Dpp does not have the same role in the 3–4 intervein cells. For these cells, Hh appears to control proliferation and patterning directly (Mullor et al. 1997; Strigini and Cohen 1997). For example, the lateral regions of wings that develop from discs with compromised Dpp function are reduced, but their central regions, between veins 3 and 4, are essentially normal (de Celis et al. 1996; de Celis 2003). Downregulation of Dpp activity and repression of expression of the Dpp receptor appears to be the basis for this insensitivity (Tanimoto et al. 2000). In contrast, partial impairment of Hh signal transduction that is insufficient to reduce Dpp function, such as in fu mutants or in the smo RNAi genotypes that we characterized, results in wings that are normal in size and pattern except for a small or absent 3–4 intervein region. Since the 3–4 intervein cells divide one to two times in the early pupa during disc eversion and wing formation (Schubiger and Palka 1987; Buttitta et al. 2007), the direct role of Hh in regulating these cells may be specific to this post-larval period. N signaling has a well-described mitogenic function in the wing. Ectopic signaling causes hyper-proliferation, while clones that impair the activation of the pathway reduce growth (de Celis and Garcia-Bellido 1994; Speicher et al. 1994; Doherty et al. 1996; Klein et al. 1997; Struhl and Greenwald 1999; Baonza and Garcia-Bellido 2000; Lawrence et al. 2000). Our findings indicate that Hh regulates proliferation of cells in the 3–4 intervein region at least in part by activating N signal transduction.
The idea that our model promotes is that Hh-dependent activation of N at the AP organizer is stage- and position-specific. This model is consistent with the complex pattern of N expression and activation in the wing, since different pathways may regulate N in different locations (Figures 4, 7, and 8). It is also consistent with the proposed role of N regulating the width and position of veins 3 and 4 (Crozatier et al. 2003), since the processes that establish the veins and control proliferation of the intervein cells need not be the same, even if they are interdependent. The temporal specificity that we describe represents an example of how complex patterns are generated with a limited number of signaling pathways—in this case by using N signaling for different outcomes at different times and in different places. Throughout larval development, Dpp regulates proliferation and patterning in the wing disc. In the pupal wing, Dpp takes on a new instructive vein-positioning function (Ralston and Blair 2005). There is no evidence that Hh regulates Dpp in the pupal wing, and moreover, the cells that had produced Dpp at the AP organizer no longer do so and no longer function as a AP organizers. Our data show that N also takes on a new role during late larval and pupal stages: functioning at the AP organizer to regulate growth in response to Hh signaling.
Acknowledgments
The X chromosome deficiency kit and other stocks were kindly provided by the Bloomington Drosophila Stock Center (Bloomington, IN). We thank the following for generously providing stocks: Susan Younger, Nikolay Ninov, Carla Pratt, Kat Millen, Sarah Bray, Anette Preiss, Vikram Ranade, Richard Mann, Gary Struhl, Benjamin Ohlstein, Raghavendra Chavourkar, Suchandra Ghosh, Uptal Banerjee, Nicholas Baker, Mark Fortini, Ariel Altaras, Celeste Berg, Karen Schulze, Hugo Bellen, Sui Zhang, and Jim Posakony. We thank the following individuals for helpful comments in the course of this research: Susan Younger, Brenda Ng, Sougata Roy, Weitao Chen, Zehra Murthy, Carla Pratt, and Songmei Liu. We thank Prashanth Rao and Stacey Ogden for critically reading this manuscript. This work was supported by a grant from the National Institutes of Health (GM77407) to T.B.K.
References
- Amin, A., Y. Li and R. Finkelstein, 1999. Hedgehog activates the EGF receptor pathway during Drosophila head development. Development 126 2623–2630. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc, P., F. A. Ramirez-Weber, M. P. Laget, C. Schwartz and T. B. Kornberg, 1997. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89 1043–1053. [DOI] [PubMed] [Google Scholar]
- Baonza, A., and A. Garcia-Bellido, 2000. Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc. Natl. Acad. Sci. USA 97 2609–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler, K., and G. Struhl, 1994. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368 208–214. [DOI] [PubMed] [Google Scholar]
- Biehs, B., M. A. Sturtevant and E. Bier, 1998. Boundaries in the Drosophila wing imaginal disc organize vein-specific genetic programs. Development 125 4245–4257. [DOI] [PubMed] [Google Scholar]
- Buttitta, L. A., A. J. Katzaroff, C. L. Perez, A. de la Cruz and B. A. Edgar, 2007. A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev. Cell 12 631–643. [DOI] [PubMed] [Google Scholar]
- Capdevila, J., F. Pariente, J. Sampedro, J. L. Alonso and I. Guerrero, 1994. Subcellular localization of the segment polarity protein patched suggests an interaction with the wingless reception complex in Drosophila embryos. Development 120 987–998. [DOI] [PubMed] [Google Scholar]
- Casso, D. J., S. Liu, D. D. Iwaki, S. K. Ogden and T. B. Kornberg, 2008. A screen for modifiers of hedgehog signaling in Drosophila melanogaster identifies swm and mts. Genetics 178 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, B., S. Barolo, A. M. Bailey and J. W. Posakony, 2005. Lateral inhibition in proneural clusters: cis-regulatory logic and default repression by Suppressor of Hairless. Development 132 3333–3344. [DOI] [PubMed] [Google Scholar]
- Chen, Y., and G. Struhl, 1996. Dual roles for patched in sequestering and transducing Hedgehog. Cell 87 553–563. [DOI] [PubMed] [Google Scholar]
- Crozatier, M., B. Glise, V. Khemici and A. Vincent, 2003. Vein-positioning in the Drosophila wing in response to Hh: new roles of Notch signaling. Mech. Dev. 120 529–535. [DOI] [PubMed] [Google Scholar]
- Dahmane, N., and A. Ruiz i Altaba, 1999. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126 3089–3100. [DOI] [PubMed] [Google Scholar]
- Dakubo, G. D., C. J. Mazerolle and V. A. Wallace, 2006. Expression of Notch and Wnt pathway components and activation of Notch signaling in medulloblastomas from heterozygous patched mice. J. Neurooncol. 79 221–227. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., 2003. Pattern formation in the Drosophila wing: the development of the veins. Bioessays 25 443–451. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., and S. J. Bray, 2000. The Abruptex domain of Notch regulates negative interactions between Notch, its ligands and Fringe. Development 127 1291–1302. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., and A. Garcia-Bellido, 1994. Roles of the Notch gene in Drosophila wing morphogenesis. Mech. Dev. 46 109–122. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., and M. Ruiz-Gomez, 1995. groucho and hedgehog regulate engrailed expression in the anterior compartment of the Drosophila wing. Development 121 3467–3476. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., J. de Celis, P. Ligoxygakis, A. Preiss, C. Delidakis et al., 1996. Functional relationships between Notch, Su(H) and the bHLH genes of the E(spl) complex: the E(spl) genes mediate only a subset of Notch activities during imaginal development. Development 122 2719–2728. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., S. Bray and A. Garcia-Bellido, 1997. Notch signalling regulates veinlet expression and establishes boundaries between veins and interveins in the Drosophila wing. Development 124 1919–1928. [DOI] [PubMed] [Google Scholar]
- Delidakis, C., and S. Artavanis-Tsakonas, 1992. The Enhancer of split [E(spl)] locus of Drosophila encodes seven independent helix-loop-helix proteins. Proc. Natl. Acad. Sci. USA 89 8731–8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delidakis, C., A. Preiss, D. A. Hartley and S. Artavanis-Tsakonas, 1991. Two genetically and molecularly distinct functions involved in early neurogenesis reside within the Enhancer of split locus of Drosophila melanogaster. Genetics 129 803–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Benjumea, F. J., and S. M. Cohen, 1995. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121 4215–4225. [DOI] [PubMed] [Google Scholar]
- Doherty, D., G. Feger, S. Younger-Shepherd, L. Y. Jan and Y. N. Jan, 1996. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 10 421–434. [DOI] [PubMed] [Google Scholar]
- Fehon, R. G., P. J. Kooh, I. Rebay, C. L. Regan, T. Xu et al., 1990. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous gene in Drosophila. Cell 61 523–534. [DOI] [PubMed] [Google Scholar]
- Fehon, R. G., K. Johansen, I. Rebay and S. Artavanis-Tsakonas, 1991. Complex cellular and subcellular regulation of notch expression during embryonic and imaginal development of Drosophila: implications for notch function. J. Cell Biol. 113 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini, M. E., 2009. Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell 16 633–647. [DOI] [PubMed] [Google Scholar]
- Fostier, M., D. A. Evans, S. Artavanis-Tsakonas and M. Baron, 1998. Genetic characterization of the Drosophila melanogaster Suppressor of deltex gene: a regulator of notch signaling. Genetics 150 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols, M., and S. Bray, 2001. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr. Biol. 11 60–64. [DOI] [PubMed] [Google Scholar]
- Glise, B., D. L. Jones and P. W. Ingham, 2002. Notch and Wingless modulate the response of cells to Hedgehog signalling in the Drosophila wing. Dev. Biol. 248 93–106. [DOI] [PubMed] [Google Scholar]
- Go, M. J., D. S. Eastman and S. Artavanis-Tsakonas, 1998. Cell proliferation control by Notch signaling in Drosophila development. Development 125 2031–2040. [DOI] [PubMed] [Google Scholar]
- Hallahan, A. R., J. I. Pritchard, S. Hansen, M. Benson, J. Stoeck et al., 2004. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 64 7794–7800. [DOI] [PubMed] [Google Scholar]
- Huppert, S. S., T. L. Jacobsen and M. A. Muskavitch, 1997. Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development 124 3283–3291. [DOI] [PubMed] [Google Scholar]
- Hurlbut, G. D., M. W. Kankel, R. J. Lake and S. Artavanis-Tsakonas, 2007. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 19 166–175. [DOI] [PubMed] [Google Scholar]
- Jacobsen, T. L., D. Cain, L. Paul, S. Justiniano, A. Alli et al., 2006. Functional analysis of genes differentially expressed in the Drosophila wing disc: role of transcripts enriched in the wing region. Genetics 174 1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings, B., A. Preiss, C. Delidakis and S. Bray, 1994. The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development 120 3537–3548. [DOI] [PubMed] [Google Scholar]
- Jia, H., Y. Liu, W. Yan and J. Jia, 2009. PP4 and PP2A regulate Hedgehog signaling by controlling Smo and Ci phosphorylation. Development 136 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. L., L. Milenkovic and M. P. Scott, 2000. In vivo functions of the patched protein: requirement of the C terminus for target gene inactivation but not Hedgehog sequestration. Mol. Cell 6 467–478. [DOI] [PubMed] [Google Scholar]
- Klambt, C., E. Knust, K. Tietze and J. A. Campos-Ortega, 1989. Closely related transcripts encoded by the neurogenic gene complex enhancer of split of Drosophila melanogaster. EMBO J. 8 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, T., K. Brennan and A. M. Arias, 1997. An intrinsic dominant negative activity of serrate that is modulated during wing development in Drosophila. Dev. Biol. 189 123–134. [DOI] [PubMed] [Google Scholar]
- Knust, E., H. Schrons, F. Grawe and J. A. Campos-Ortega, 1992. Seven genes of the Enhancer of split complex of Drosophila melanogaster encode helix-loop-helix proteins. Genetics 132 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooh, P. J., R. G. Fehon and M. A. Muskavitch, 1993. Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophila development. Development 117 493–507. [DOI] [PubMed] [Google Scholar]
- Lawrence, N., T. Klein, K. Brennan and A. Martinez Arias, 2000. Structural requirements for notch signalling with delta and serrate during the development and patterning of the wing disc of Drosophila. Development 127 3185–3195. [DOI] [PubMed] [Google Scholar]
- Lecuit, T., W. J. Brook, M. Ng, M. Calleja, H. Sun et al., 1996. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381 387–393. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis, P., S. J. Bray, Y. Apidianakis and C. Delidakis, 1999. Ectopic expression of individual E(spl) genes has differential effects on different cell fate decisions and underscores the biphasic requirement for notch activity in wing margin establishment in Drosophila. Development 126 2205–2214. [DOI] [PubMed] [Google Scholar]
- McGlinn, E., K. L. van Bueren, S. Fiorenza, R. Mo, A. M. Poh et al., 2005. Pax9 and Jagged1 act downstream of Gli3 in vertebrate limb development. Mech. Dev. 122 1218–1233. [DOI] [PubMed] [Google Scholar]
- Motzny, C. K., and R. Holmgren, 1995. The Drosophila Cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev. 52 137–150. [DOI] [PubMed] [Google Scholar]
- Mullor, J. L., M. Calleja, J. Capdevila and I. Guerrero, 1997. Hedgehog activity, independent of decapentaplegic, participates in wing disc patterning. Development 124 1227–1237. [DOI] [PubMed] [Google Scholar]
- Nagel, A. C., D. Maier and A. Preiss, 2000. Su(H)-independent activity of hairless during mechano-sensory organ formation in Drosophila. Mech. Dev. 94 3–12. [DOI] [PubMed] [Google Scholar]
- Nellen, D., R. Burke, G. Struhl and K. Basler, 1996. Direct and long-range action of a DPP morphogen gradient. Cell 85 357–368. [DOI] [PubMed] [Google Scholar]
- Nellesen, D. T., E. C. Lai and J. W. Posakony, 1999. Discrete enhancer elements mediate selective responsiveness of enhancer of split complex genes to common transcriptional activators. Dev. Biol. 213 33–53. [DOI] [PubMed] [Google Scholar]
- Nestoras, K., H. Lee and J. Mohler, 1997. Role of knot (kn) in wing patterning in Drosophila. Genetics 147 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, B., 2007. Posttranslational regulation of Cubitus interruptus in Hedgehog signaling in Drosophila. Ph.D. Thesis, University of California, San Francisco.
- Parody, T. R., and M. A. Muskavitch, 1993. The pleiotropic function of Delta during postembryonic development of Drosophila melanogaster. Genetics 135 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston, A., and S. S. Blair, 2005. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev. Biol. 280 187–200. [DOI] [PubMed] [Google Scholar]
- Robbins, D. J., K. E. Nybakken, R. Kobayashi, J. C. Sisson, J. M. Bishop et al., 1997. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90 225–234. [DOI] [PubMed] [Google Scholar]
- Rulifson, E. J., and S. S. Blair, 1995. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 121 2813–2824. [DOI] [PubMed] [Google Scholar]
- Schnepp, B., G. Grumbling, T. Donaldson and A. Simcox, 1996. Vein is a novel component in the Drosophila epidermal growth factor receptor pathway with similarity to the neuregulins. Genes Dev. 10 2302–2313. [DOI] [PubMed] [Google Scholar]
- Schrons, H., E. Knust and J. A. Campos-Ortega, 1992. The Enhancer of split complex and adjacent genes in the 96F region of Drosophila melanogaster are required for segregation of neural and epidermal progenitor cells. Genetics 132 481–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger, M., and J. Palka, 1987. Changing spatial patterns of DNA replication in the developing wing of Drosophila. Dev. Biol. 123 145–153. [DOI] [PubMed] [Google Scholar]
- Shellenbarger, D. L., and J. D. Mohler, 1978. Temperature-sensitive periods and autonomy of pleiotropic effects of l(1)Nts1, a conditional notch lethal in Drosophila. Dev. Biol. 62 432–446. [DOI] [PubMed] [Google Scholar]
- Singson, A., M. W. Leviten, A. G. Bang, X. H. Hua and J. W. Posakony, 1994. Direct downstream targets of proneural activators in the imaginal disc include genes involved in lateral inhibitory signaling. Genes Dev. 8 2058–2071. [DOI] [PubMed] [Google Scholar]
- Speicher, S. A., U. Thomas, U. Hinz and E. Knust, 1994. The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development 120 535–544. [DOI] [PubMed] [Google Scholar]
- Strigini, M., and S. M. Cohen, 1997. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124 4697–4705. [DOI] [PubMed] [Google Scholar]
- Struhl, G., and I. Greenwald, 1999. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398 522–525. [DOI] [PubMed] [Google Scholar]
- Tabata, T., and T. B. Kornberg, 1994. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76 89–102. [DOI] [PubMed] [Google Scholar]
- Tabata, T., C. Schwartz, E. Gustavson, Z. Ali and T. B. Kornberg, 1995. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development 121 3359–3369. [DOI] [PubMed] [Google Scholar]
- Tanimoto, H., S. Itoh, P. ten Dijke and T. Tabata, 2000. Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell 5 59–71. [DOI] [PubMed] [Google Scholar]
- Tohgo, A., M. Eiraku, T. Miyazaki, E. Miura, S. Y. Kawaguchi et al., 2006. Impaired cerebellar functions in mutant mice lacking DNER. Mol. Cell. Neurosci. 31 326–333. [DOI] [PubMed] [Google Scholar]
- Vervoort, M., M. Crozatier, D. Valle and A. Vincent, 1999. The COE transcription factor Collier is a mediator of short-range Hedgehog-induced patterning of the Drosophila wing. Curr. Biol. 9 632–639. [DOI] [PubMed] [Google Scholar]
- Wallace, V. A., 1999. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 9 445–448. [DOI] [PubMed] [Google Scholar]
- Wessells, R. J., G. Grumbling, T. Donaldson, S. H. Wang and A. Simcox, 1999. Tissue-specific regulation of vein/EGF receptor signaling in Drosophila. Dev. Biol. 216 243–259. [DOI] [PubMed] [Google Scholar]
- Wilkin, M. B., A. M. Carbery, M. Fostier, H. Aslam, S. L. Mazaleyrat et al., 2004. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr. Biol. 14 2237–2244. [DOI] [PubMed] [Google Scholar]
- Wilson, C. W., and P. T. Chuang, 2010. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development 137 2079–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach, E., I. Wech and A. Preiss, 1999. The Enhancer of split complex of Drosophila melanogaster harbors three classes of Notch responsive genes. Mech. Dev. 80 171–180. [DOI] [PubMed] [Google Scholar]
- Ye, Y., N. Lukinova and M. E. Fortini, 1999. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature 398 525–529. [DOI] [PubMed] [Google Scholar]
- Yu, M., J. Gipp, J. W. Yoon, P. Iannaccone, D. Walterhouse et al., 2009. Sonic hedgehog-responsive genes in the fetal prostate. J. Biol. Chem. 284 5620–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca, M., K. Basler and G. Struhl, 1995. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development 8 2265–2278. [DOI] [PubMed] [Google Scholar]