Abstract
During Drosophila oogenesis, basally localized F-actin bundles in the follicle cells covering the egg chamber drive its elongation along the anterior–posterior axis. The basal F-actin of the follicle cell is an attractive system for the genetic analysis of the regulation of the actin cytoskeleton, and results obtained in this system are likely to be broadly applicable in understanding tissue remodeling. Mutations in a number of genes, including that encoding the p21-activated kinase Pak, have been shown to disrupt organization of the basal F-actin and in turn affect egg chamber elongation. pak mutant egg chambers have disorganized F-actin distribution and remain spherical due to a failure to elongate. In a genetic screen to identify modifiers of the pak rounded egg chamber phenotype several second chromosome deficiencies were identified as suppressors. One suppressing deficiency removes the rho1 locus, and we determined using several rho1 alleles that removal of a single copy of rho1 can suppress the pak phenotype. Reduction of any component of the Rho1-activated actomyosin contractility pathway suppresses pak oogenesis defects, suggesting that Pak counteracts Rho1 signaling. There is ectopic myosin light chain phosphorylation in pak mutant follicle cell clones in elongating egg chambers, probably due at least in part to mislocalization of RhoGEF2, an activator of the Rho1 pathway. In early egg chambers, pak mutant follicle cells have reduced levels of myosin phosphorylation and we conclude that Pak both promotes and restricts myosin light chain phosphorylation in a temporally distinct manner during oogenesis.
EPITHELIAL morphogenesis relies heavily on the dynamic nature of the actin cytoskeleton to facilitate changes in cell shape. These changes occur in response to a variety of signaling cues, including those activating members of the Rho family of small GTPases, which includes Rho, Rac, and Cdc42 (Van Aelst and Symons 2002). These proteins participate in a variety of cellular processes, many of which depend on the ability of the Rho GTPases to regulate and reorganize the actin and microtubule cytoskeletons (Bishop and Hall 2000). Crosstalk occurs between the Rho, Rac, and Cdc42 signaling pathways and, in particular, numerous groups have reported antagonism between Rac/Cdc42 signaling and Rho signaling in cell culture (Kozma et al. 1997; van Leeuwen et al. 1997, 1999; Sander et al. 1999; Sanders et al. 1999; Wahl et al. 2000; Zondag et al. 2000; Tsuji et al. 2002; Nimnual et al. 2003; Sugimoto et al. 2003; Wang et al. 2003; Xu et al. 2003; Seasholtz et al. 2004; Salhia et al. 2005; Rosenfeldt et al. 2006; Wildenberg et al. 2006; Bustos et al. 2008; Wu et al. 2009). This antagonism is conserved in Drosophila, where Rac and Rho have opposing roles in organizing the somatic support cells in the testes germ cell microenvironment and Cdc42 antagonizes Rho at adherens junctions in epithelial cells of the pupal eye (Sarkar et al. 2007; Warner and Longmore 2009). The crosstalk between the Rho family pathways involves upstream regulators of the small GTPases as well as downstream effectors. The group I Pak proteins are some of the best characterized effectors for Rac and Cdc42 and are activated by small GTPase binding to a Cdc42/Rac-binding (CRIB) domain overlapping an autoinhibitory domain (AID) (Bokoch 2003).
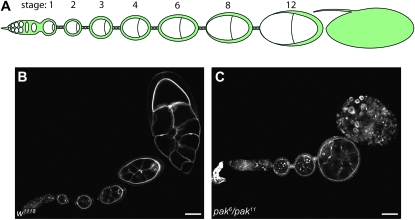
We previously showed that Pak, a group I Pak protein, participates in development of the follicular epithelium (FE) surrounding the Drosophila egg chamber through regulation of the actin cytoskeleton and apicobasal polarity (Conder et al. 2007; Bahri et al. 2010). In middle-stage egg chambers, the F-actin network polarizes to the basal end of the follicle cells where it forms bundles of filaments aligned perpendicularly to the anterior–posterior (A–P) axis of the egg chamber (Gutzeit 1990, 1991; Gutzeit and Haas-Assenbaum 1991). The role of the F-actin bundles during egg chamber elongation in middle-staged egg chambers has been characterized through analysis of mutants affecting these bundles and has led to a model in which the polarized actin bundles act as a “molecular corset” to promote elongation of the egg chambers along the A–P axis through actomyosin contractility along the basal surface of the FE (Gutzeit et al. 1991; Duffy et al. 1998; Bateman et al. 2001; Frydman and Spradling 2001; Deng et al. 2003; Conder et al. 2007; Mirouse et al. 2009; Viktorinova et al. 2009). The egg chambers continue to elongate as they age through stage 14 and develop into mature eggs (Figure 1B).
Figure 1.—
Summary of Drosophila oogenesis. Drosophila oogenesis begins at the anterior-most end of an ovariole in the germarium where two germ-line stem cells divide asymmetrically to produce daughter cysts. These cysts undergo four rounds of incomplete mitosis to produce a 16-cell cyst that is then encapsulated by follicle cells arising from 2 follicle stem cells. The follicle cells continue to divide until stage 6. Prior to stage 6 egg chambers remain spherical; beginning at stage 6, egg chamber elongation occurs along the anterior–posterior axis, giving rise to elongated mature eggs. (A) Schematic diagram of a single ovariole. (B) Wild-type ovariole stained with phalloidin revealing elongation of egg chambers as they age. (C) pak mutant ovariole showing failure of egg chamber elongation and degradation of oldest chamber. Bar: 50 μm.
Trans-heterozygous pak individuals can survive to adulthood but are female sterile and exhibit a number of defects in oogenesis (Hing et al. 1999; Conder et al. 2007). pak mutant egg chambers are spherical as they fail to elongate along the A–P axis and they do not develop past stage 10 and therefore never produce mature eggs (Figure 1C). The inability of pak mutant egg chambers to elongate is likely due to disruptions of the basal F-actin, which is less dense, disorganized, and no longer polarized perpendicularly to the A–P axis (Conder et al. 2007). The F-actin architecture in pak mutant egg chambers does not have a distinct orientation as seen in wild-type egg chambers and is highly disrupted.
It has been noted that the basal F-actin bundles in the FE are similar to the stress fibers of mammalian cultured cells, and the FE provides an attractive system for the genetic analysis of the signaling events regulating the formation of parallel actin bundles (Bateman et al. 2001; Baum and Perrimon 2001). Stress fibers consist of 10–30 bundled actin filaments, the formation of which is regulated by the Rho family small GTPase RhoA through the Rock-Rok-Rho kinase family of serine/threonine kinases (hereafter referred to as Rok) and the Diaphanous-related formin, mDia1 (Chrzanowska-Wodnicka and Burridge 1996; Leung et al. 1996; Watanabe et al. 1997; 1999; Pellegrin and Mellor 2007). Rok promotes stress fiber formation and actomyosin contractility by directly phosphorylating myosin light chain (MLC) and phosphorylating the regulatory myosin binding subunit of MLC phosphatase, inhibiting the phosphatase activity (Amano et al. 1996; Kimura et al. 1996; Kawano et al. 1999; Totsukawa et al. 2000). Rok also phosphorylates LIM kinase, which in turn phosphorylates cofilin, inactivating its actin-depolymerizing function (Ohashi et al. 2000; Sumi et al. 2001). This signaling network regulating actomyosin contractility is conserved in Drosophila, where it has a number of roles in development (reviewed in Settleman 2001).
In this study we investigated the involvement of Pak during egg chamber elongation by screening for second chromosome deficiencies uncovering loci that genetically interact with pak. Here we show that removal of one copy of the rho1 locus is sufficient to suppress the pak rounded egg chamber phenotype and that reduction in any component of the Rho1-activated actomyosin contractility pathway suppresses the pak egg chamber elongation defect. Furthermore, we show that in rescued egg chambers the disorganized arrangement of the basal F-actin is restored back to the characteristic polarized F-actin arrangement. Pak does not appear to act at the level of Rho1 activation in its antagonistic interaction with Rho1 signaling as we have not been able to discern a change in the levels of GTP-bound Rho1 with loss of Pak. However, Pak is required for localization of the upstream activator of the Rho1 pathway, RhoGEF2, and pak mutant follicle cells in elongating egg chambers show ectopic myosin phosphorylation, indicating that Pak may regulate the Rho1 pathway at the level of myosin contractility. Interestingly, Pak is required for myosin light chain phosphorylation in early egg chambers, indicating that Pak's role in the regulation of myosin contractility varies during oogenesis.
MATERIALS AND METHODS
Fly stocks:
pak6 and pak11 flies were from H. Hing, pak14FRT82B flies from B. Dickson, rho11B flies from S. Parkhurst, traffic-jam Gal4 flies from G. Tanentzapf and D. Godt, and PKNG58AeGFP/TM3 flies from A. Jacinto. All other stocks were obtained from the Bloomington Drosophila Stock Center. A w1118 stock was used as a wild-type control in this study. All stocks were crossed and maintained at 25° unless otherwise noted. In the genetic screen, pak mutant flies were unambiguously identified by their crumpled, droopy wings and uncoordinated behavior.
Clonal analysis:
pak somatic clones were induced using the FLP/FRT method (Xu and Rubin 1993). To induce pak loss-of-function clones using hs-FLP, third instar larvae from the appropriate crosses were heat-shocked at 37° for 2 hr for 3 consecutive days. Female progeny of the genotype hsFLP; pak14FRT82B/UbiGFP FRT82B were grown on media containing yeast for 2–3 days to allow for optimal development and maturation of ovaries.
Immunostaining and fluorescence microscopy:
Ovary dissection, fixation, and staining were performed as previously described (Verheyen and Cooley 1994). To visualize F-actin the egg chambers were incubated with 1:1,000 FITC- or TRITC-conjugated phalloidin (Sigma, St. Louis) for 30 min with rotation. The antibodies used were mouse anti-GFP (1:500) (Sigma), mouse anti-Rho1 (1:50) (Magie et al. 2002), rabbit anti-RhoGEF2 (1:100) (Rogers et al. 2004), and mouse anti-phospho-MLC (Ser19, corresponding to Ser21 in Drosophila) (1:20) (Cell Signaling). Ovaries were visualized and images acquired using a Zeiss (Carl Zeiss, Thornwood, NY) LSM410 laser scanning confocal microscope, using Plan-Neofluar 25×/0.80 or Plan-apochromat 63×/1.40 oil lenses. All images were processed using Adobe Photoshop CS4.
Rho-GTP activity assay:
We used a pull-down assay to quantitate GTP-Rho1 levels, using the Rho-binding domain (RBD) of rhotekin or mDia (Kimura et al. 2000). Ovaries were dissected out from wild-type and pak mutant flies and flash frozen using liquid nitrogen. Fifty microliters of ovarian tissues was collected for each sample and homogenized in 500 μl of IP Buffer I [475 mm Tris HCl, pH 8.0, 0.5% Triton X-100, 1 complete protease inhibitor tablet per 50 ml (Roche)]. The samples were then centrifuged for 10 min at 4°. The supernatant was removed from the debris and 5% of this was kept for the lysate lane on the gel. The rest of the lysate was incubated with purified GST-mDia-RBD or GST-rhotekin-RBD bound to Glutathione Sepharose 4B beads (GE Healthcare) overnight at 4°. The beads were then centrifuged briefly, supernatants removed, and beads washed three times with IP Buffer II (50% 1 m NaCl, 505 IP Buffer I). These beads were then resuspended in SDS-PAGE sample buffer, boiled for 10 min, run out on an SDS-PAGE gel together with the lysate sample, and subjected to Western blot analysis using anti-Rho1 antibodies to determine the total amount of Rho and anti-GST antibodies (Cell Signaling Technologies) to determine the amount of GTP-bound Rho in the lysates. pGEX-mDia-RBD and pGEX-rhotekin-RBD plasmids were gifts from S. Narumiya. The assay was repeated several times for each RBD, and results were analyzed by performing densitometry using Adobe Photoshop CS4 as described (http://www.lukemiller.org/journal/2007/08/quantifying-western-blots-without.html). GTP-Rho1 levels were normalized against levels of GST-RBD and then compared to total Rho1 levels.
Measuring egg chamber length:
Egg chamber measurements were acquired with Improvision OpenLab Version 5.5.0 software, using a QImaging Retiga EXi camera mounted on a Zeiss Axioplan 2 microscope.
RESULTS
A deficiency screen to identify second-site modifiers of pak mutant oogenesis defects:
In an effort to characterize the role of Pak during egg chamber elongation we carried out a genetic deficiency screen of the second chromosome to identify modifiers of the pak elongation phenotype. For this screen we used flies, which we refer to as pak mutants, trans-heterozygous for the pak6 and pak11 alleles, which both encode a truncated Pak protein with no kinase domain (Hing et al. 1999). We tested 104 deficiencies spanning the second chromosome by comparing egg chambers from females of the genotype Df(2)/+; pak6/pak11 [where Df(2) denotes any given deficiency on the second chromosome] to egg chambers from pak6/pak11 females, with a focus on looking for elongation in the egg chambers of Df(2)-bearing flies.
In the screen we generated flies that were heterozygous for each individual deficiency in the pak mutant background by crossing pak6 females to males carrying the second chromosome deficiency. The male progeny of this cross were then crossed to females carrying the pak11 allele. As a control, in tandem we made pak mutant flies heterozygous for the second chromosome balancers from each deficiency strain and found no effects on the egg chamber elongation defect. We originally intended to use pak allele stocks doubly balanced for the second and third chromosomes in these experiments so that we could follow all chromosomes, but this was not possible due to the poor health of the stocks. In any case, we could unambiguously identify pak mutant females in our crosses by their characteristic crumpled, droopy wings and uncoordinated behavior (Hing et al. 1999) (none of the deficiencies we tested suppressed these phenotypes). Half of the pak mutant females would be heterozygous for a second chromosome deficiency and might show suppression of oogenesis defects. We aged pak females on yeasted media for several days to allow for sufficient ovary development and then dissected out their ovaries. In our dissections we looked for pak females with ovaries larger than typical, as these individuals likely contained suppressing deficiencies, and assessed their ovarioles using phalloidin staining. All putative suppressors were rechecked by repeating the cross with the deficiency stock.
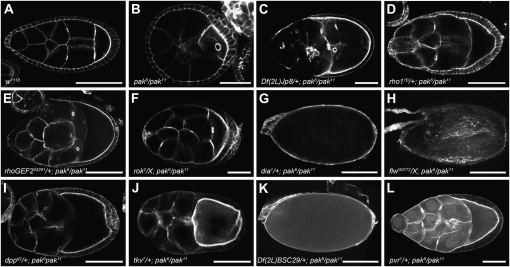
From our screen of the second chromosome we identified 8 deficiencies of 104 that were able to partially suppress the pak elongation defect when made heterozygous in the pak mutant background (Table 1). Given that previous studies in mammalian cell culture have indicated that Pak can have an antagonistic affect on RhoA signaling, and the central role of RhoA signaling in stress fiber formation, we focused our attention on one deficiency, Df(2R)Jp8, that removes cytological region 52F5–53A1 and that fails to complement alleles of the rho1 locus, which encodes the Drosophila ortholog of RhoA (Sanders et al. 1999; Halsell et al. 2000; Rosenfeldt et al. 2006). pak mutant flies heterozygous for Df(2R)Jp8 were healthier than flies that were solely mutant for pak as they survived for a longer period of time, living for 5–6 days compared to the 2- or 3-day life span typical of pak mutant flies. The ovaries of Df(2R)Jp8/+; pak6/pak11 female flies were notably larger than pak6/pak11 mutant ovaries and contained elongated egg chambers and egg chambers that were older than stage 10, including mature eggs (compare Figure 2C to 2B, and data not shown). We quantified the suppression by comparing the lengths of stage 9 egg chambers from the two genotypes and found that Df(2R)Jp8/+; pak6/pak11 individuals had significantly longer chambers than the pak6/pak11 females (Table 2).
TABLE 1.
Second chromosome deficiencies that suppress the pak6/pak11 rounded egg chamber phenotype
| Deficiency name | Region removed by deficiency | Suppressing gene(s) in deficiency |
|---|---|---|
| Df(2L)dpp[d14] | 22E4–F2; 22F3–23A1 | dpp |
| Df(2L)BSC28 | 23C5–D1; 23E2 | mad |
| Df(2L)BSC111 | 28F5; 39B1 | pvr |
| Df(2L)BSC32 | 32A1–2; 32C5–D1 | — |
| Df(2R)Np5 | 44F10; 45D9–E1 | wun |
| Df(2R)BSC29 | 45D3–4; 45F2–6 | wun; wun2 |
| Df(2R)Jp8 | 52F5–9; 52F10–53A1 | rho1 |
| Df(2R)14H10W-35 | 54E5–7; 55B5–7 | — |
—, candidate genes have not yet been identified.
Figure 2.—
Suppressors of pak rounded egg chamber phenotype. Stage 8 or older egg chambers or eggs stained with phalloidin are shown. Genotypes are shown at the bottom. (A) Wild-type egg chamber elongated along the A–P axis. (B) Spherical pak mutant egg chamber. (C–G) Heterozygosity for various components of the Rho1-activated actomyosin contractility signaling pathway suppresses the pak rounded egg chamber phenotype. (H) Heterozygosity for an allele of the MLC phosphatase flw suppresses the pak elongation defect, allowing development of a mature egg. (I–L) Heterozygosity for candidate genes identified in a screen suppresses the pak rounded egg chamber phenotype. Note that panels are not all to the same scale. Bars: 100 μm in A, D, E, I, and J; 50 μm in B, C, F, and L; and 150 μm in G, H, and K.
TABLE 2.
Quantification of stage 9 egg chamber length
| Genotype | EC length (μm) | SD | n |
|---|---|---|---|
| pak6/pak11 | 150.758 | 19.8 | 20 |
| w1118 | 218.527 | 33.9 | 21 |
| rhoGEF204291/+; pak6/pak11 | 209.975 | 28.6 | 25 |
| Df(2R)Jp8/+; pak6/pak11 | 207.072 | 30 | 19 |
| rho11B/+; pak6/pak11 | 204.717 | 19.8 | 21 |
| sqh2/X; pak6/pak11 | 187.292 | 20.1 | 20 |
| dia1/+; pak6/pak11 | 175.085 | 15.3 | 20 |
Egg chambers were measured from the anterior-most end to the apex of the oocyte at the posterior end. In comparison to pak6/pak11, all other genotypes are significantly different with P-values <0.005. All heterozygous mutations in pak6/pak11 individuals are strong alleles with the exception of dia1, which is a hypomorph.
Heterozygosity for components of Rho1 signaling to actomyosin contractility suppresses the pak rounded egg chamber phenotype:
Two hypomorphic rho1 alleles, rho1rev220 and rho1k02107rev5, and a null allele, rho11B, were tested using the same genetic cross described for the deficiency screen (Magie et al. 1999; Magie and Parkhurst 2005; Sanny et al. 2006). All three alleles suppressed the pak mutant phenotype to a similar extent as Df(2R)Jp8, indicating that loss of Rho1 in this deficiency allows it to suppress and that Pak is a negative regulator of the Rho1 signaling pathway during oogenesis (Figure 2D, Tables 2 and 3, and data not shown).
TABLE 3.
Quantitative production of mature eggs
| Genotype | No. ovarioles | Total no. eggs | Ovarioles with eggs (%) |
|---|---|---|---|
| w1118 | nc | nc | 100 |
| pak6/pak11 | 55 | 0 | 0 |
| rho11B/+; pak6/pak11 | 215 | 22 | 10.23 |
| rok2/X; pak6/pak11 | 47 | 4 | 8.55 |
| limk2/X; pak6/pak11 | 94 | 4 | 4.25 |
| rhoGEF204291/+; pak6/pak11 | 430 | 105 | 24.42 |
| dia1/+; pak6/pak11 | 86 | 2 | 2.32 |
| flwG0172/X; pak6/pak11 | 139 | 6 | 4.32 |
| Df(3L)Exel6102, pak6/pak11 | 100 | 0 | 0 |
nc, not counted as each ovariole in the wild-type sample always contained at least one egg. Df(3L)Exel6102 removes the RhoGEF64C locus. All heterozygous mutations in pak6/pak11 individuals are strong alleles with the exception of dia1, which is a hypomorph.
As discussed above, RhoA signals to activate actomyosin contractility and stress fiber formation, and we checked to see if reduction of various components of this signaling cascade would similarly suppress pak mutant oogenesis defects. We obtained alleles of components acting at different points in the signaling network, extending from activation of Rho1 through to myosin at the end of the cascade. The guanine nucleotide exchange factor RhoGEF2 has been shown to participate in actin regulation in other tissues, likely as an activator specifically of Rho1 (Barrett et al. 1997; Hacker and Perrimon 1998; Halsell et al. 2000; Nikolaidou and Barrett 2004; Rogers et al. 2004; Dawes-Hoang et al. 2005; Grosshans et al. 2005; Padash Barmchi et al. 2005; Simoes et al. 2006; Fox and Peifer 2007; Kolsch et al. 2007; Cao et al. 2008; Mulinari et al. 2008). An allele of RhoGEF2 was an effective suppressor, as were alleles of rok, LIM kinase 1 (LIMK1), diaphanous (dia), spaghetti squash (sqh) encoding the regulatory light chain of nonmuscle myosin, and zipper, encoding nonmuscle myosin heavy chain (Young et al. 1993; Castrillon and Wasserman 1994; Edwards and Kiehart 1996; Barrett et al. 1997; Winter et al. 2001; Ang et al. 2006) (Figure 2, E–G, Tables 2 and 3, and data not shown). Suppression of the pak mutant elongation phenotype by removal of any single component of the Rho1-activated actomyosin contractility pathway suggests that Pak regulates this pathway during egg chamber elongation.
Evidence that RhoGEF2 is the major activator of Rho1 in regulation of the basal F-actin in the follicular epithelium:
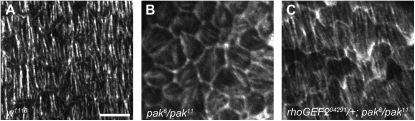
Heterozygosity for RhoGEF2 was as effective as heterozygosity for alleles of Rho1 signaling components in suppressing pak mutant oogenesis defects, and we checked to see if heterozygosity for RhoGEF2 was suppressing the basal actin defects in pak mutant egg chambers. We observed a clear suppression of the disorganized basal F-actin of the pak mutants. As in wild type, the basal F-actin of RhoGEF204291/+; pak6/pak11 egg chambers was arranged in parallel bundles lying perpendicular to the A–P axis, which was significantly different from the basal F-actin of pak mutants (Figure 3). Reduction of other components of the Rho1 pathway similarly rescued the F-actin disruption in pak mutant females (data not shown). Heterozygosity for RhoGEF2 was notably more effective than other Rho pathway components at extending the life span of pak mutant females, with flies surviving ≥2 wk. In addition to RhoGEF2, two other GEFs in Drosophila have been demonstrated to be involved in activating Rho1 signaling to the actin cytoskeleton. Pebble activates Rho1 during cytokinesis and RhoGEF64C is a Rho1 activator participating in formation of the spiracular chamber and in axon attraction (Prokopenko et al. 1999; Bashaw et al. 2001; Somers and Saint 2003; Simoes et al. 2006). Furthermore, RhoGEF64C can promote stress fiber formation in mammalian fibroblasts in a RhoA-dependent manner (Bashaw et al. 2001). Reducing the levels of either of these GEFs using deficiencies or loss-of-function mutations had no effect on pak oogenesis defects (Table 3 and data not shown). We conclude that RhoGEF2 is the major or only GEF regulating Rho1 activation in control of the basal F-actin in the FE.
Figure 3.—
Comparison of the basal F-actin of middle stage egg chambers stained with phalloidin. (A) Basal F-actin of wild-type egg chamber is organized in parallel bundles that are oriented perpendicularly to the A–P axis. (B) Basal F-actin of pak6/pak11 egg chamber displaying disorganized F-actin bundles that have no specific orientation with respect to the A–P axis. (C) Basal F-actin of rhoGEF204291/+; pak6/pak11 egg chamber showing that suppressors of the pak rounded egg chamber phenotype suppress the basal F-actin disorganization. Bar: 25 μm.
Pak does not appear to regulate the levels of activated Rho1 during oogenesis but is required for RhoGEF2 localization:
Mammalian Pak1 is able to interact with the DH–PH domain of the RGS-containing p115-RhoGEF, leading to a disruption in G-protein–coupled receptor-dependent RhoA signaling, thereby implicating Pak in the negative regulation of RhoA signaling events (Rosenfeldt et al. 2006). To determine if such an interaction might be occurring in Drosophila between RhoGEF2 and Pak, we created and used a GST-fusion protein containing the DH–PH domain of RhoGEF2 and attempted to pull down Pak from adult flies, but were not successful (data not shown).
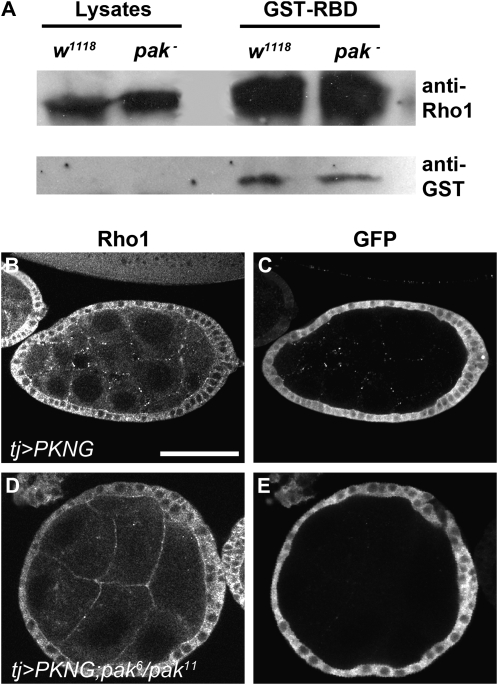
Although we did not find a direct interaction between Pak and RhoGEF2, we were still interested in determining whether Pak was regulating the activity of RhoGEF2 during oogenesis and consequently the activation of Rho1. We assessed the levels of active, GTP-bound Rho1 in ovarian tissue lysates collected from wild-type flies and pak mutant flies, using a pull-down assay (Kimura et al. 2000). GST fusions of the RBD of mDia or Rhotekin were used to pull down GTP-Rho1 from the lysates, and levels of GTP-Rho1 were compared to the total levels of Rho1 (Figure 4A and data not shown). We did not detect a significant increase in GTP-Rho1 levels in pak mutant lysates.
Figure 4.—
Pak does not appear to regulate the levels of activated Rho1. (A) Rho activity assay. Using a fusion protein composed of the Rho-binding domain (RBD) of the Rho1-binding protein Rhotekin fused to GST in a GST pull-down assay allowed for the detection of GTP-bound Rho1 in wild-type and pak mutant ovarian tissue lysates. Shown is a representative SDS-PAGE gel Western blotted with anti-Rho1 antibody. The left side shows lanes containing equal volumes of ovarian tissue lysates from wild-type and pak mutant flies. The lanes on the right side show equal volumes of ovarian tissue lysates that were passed through columns of GST-Rhotekin-RBD Sepharose beads and precipitated beads run on a gel. Incubation of these same lanes with anti-GST antibodies revealed amounts of GST-Rhotekin-RBD in each lane. Intensity of Rho1 pull-down bands was normalized against intensity of GST bands and compared to total Rho1 input. (B–E) GFP-based in vivo reporter to detect subcellular changes in activated Rho1 levels. The follicle cell-specific driver tj-Gal4 was used to express UAS-PKNG58AeGFP in a wild-type background (B and C) or a pak mutant background (D and E). Anti-Rho1 antibody shows the level and distribution of total Rho1 whereas anti-GFP antibody shows the level and distribution of activated Rho1. Bar: 50 μm.
If Pak were regulating Rho1 activation only regionally in the ovary, this might not be detectable using the pull-down assay, and we visualized Rho1 activation in situ in the ovary using a transgenic GFP-based reporter, PKNG58AeGFP, that binds to GTP-Rho1 and results in an intense GFP localization wherever GTP-Rho1 accumulates (Simoes et al. 2006). We expressed the reporter in the follicular epithelium of wild-type and pak mutant embryos using 198Y-GAL4, 185Y-GAL4, or traffic jam (tj)-GAL4 drivers (Manseau et al. 1997; Hayashi et al. 2002; Li et al. 2003; Tanentzapf et al. 2007) and detected GTP-Rho1 with anti-GFP antibody and total Rho1 levels with anti-Rho1 antibody. As Pak becomes localized basally when the basal F-actin begins to polarize, it could be negatively regulating Rho1 activation only at this end of the follicle cells. To see whether this was the case we looked for an increase in GTP-Rho1 at the basal end of follicle cells in pak mutant egg chambers but saw no obvious difference compared to wild type (Conder et al. 2007) (Figure 4, B–E, and data not shown).
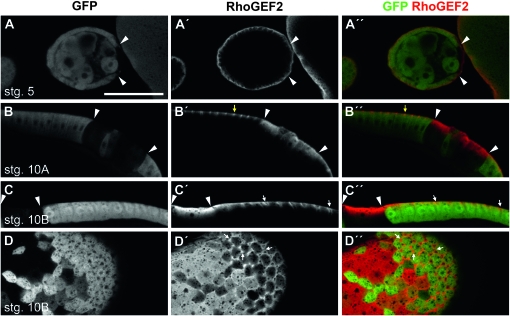
To explore further the relationship between Pak and RhoGEF2 we looked at RhoGEF2 distribution in wild-type and pak mutant follicle cells. We used an available FRT-recombined allele, pak14 (Newsome et al. 2000), which encodes a protein two amino acids shorter than that encoded by pak6, to make follicle cell clones (FCC) lacking pak (marked by the absence of GFP), and assessed the distribution of RhoGEF2 using an anti-RhoGEF2 antibody. In wild-type egg chambers RhoGEF2 was enriched throughout oogenesis to the basal end of follicle cells, including at the basolateral domain between follicle cells in stage 10B egg chambers, which parts as follicle cells flatten to accommodate growth of the oocyte (Figure 5, A′–D′) (Schotman et al. 2008). During early oogenesis loss of Pak had little or no effect on RhoGEF2 (Figure 5A″), whereas pak14 FCCs in stage 10A or older egg chambers showed delocalization of RhoGEF2 such that it was no longer basally restricted or highly enriched at the basolateral junction as seen in the neighboring wild-type cells, but rather was distributed throughout the cell (Figure 5, B″–D″).
Figure 5.—
RhoGEF2 is basally localized in the follicular epithelium and its localization is regulated by Pak. (A–D) Anti-GFP. (A′–D′) Anti-RhoGEF2. (A″–D″) Merge. FCC are distinguished by a lack of GFP staining. Arrowheads mark some clone boundaries. (A–A″) pak14 FCC in a stage 5 egg chamber showing that the basal localization of RhoGEF2 is slightly reduced. (B–B″) pak14 FCC in columnar cells of a stage 10A egg chamber showing that the localization of RhoGEF2 is no longer restricted to the basal end of follicle cells in the absence of Pak. Yellow arrows mark basal punctate localization of RhoGEF2 in a wild-type cell. (C–C″) pak14 FCC in a stage 10B egg chamber showing ectopic RhoGEF2 distribution throughout cells. (D–D″) pak14 FCC imaged at the basal surface of a stage 10B egg chamber showing that RhoGEF2 accumulation at the points of basal membrane separation is lost in the absence of Pak. White arrows mark obvious sites of basal membrane separation in wild-type tissue. Bar: 50 μm.
Pak regulates the phosphorylation of the nonmuscle myosin regulatory light chain in follicle cells:
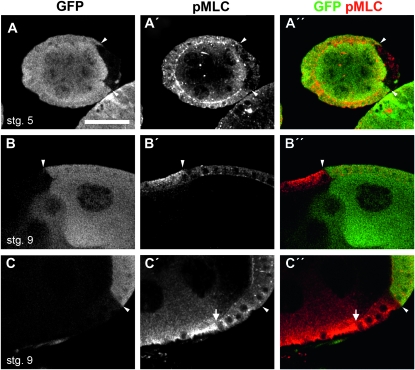
A major output of RhoA signaling is the phosphorylation of the nonmuscle MLC. Mammalian Pak1 phosphorylates and inhibits the activity of myosin light chain kinase (MLCK), leading to a reduction in phosphorylation of MLC (Sanders et al. 1999). To determine if loss of Pak affected MLC phosphorylation we created pak14 FCC and assessed the levels of phospho-MLC (pMLC). In wild-type egg chambers pMLC is largely restricted to the apical end of follicle cells from stages 3 to 6 but can also be seen at the lateral membrane. During and following stage 7 pMLC also accumulates at the basal end of follicle cells around the time that the parallel actin bundles emerge and can be detected with an antibody against human pMLC (Wang and Riechmann 2007). Staining with this anti-pMLC antibody revealed that pak14 mutant cells in early egg chambers had a loss of pMLC with respect to the neighboring wild-type cells (Figure 6, A–A″). pak14 FCCs in older, elongating egg chambers showed an ectopic distribution of pMLC throughout the cytoplasm of the follicle cells compared to their wild-type neighbors (Figure 6, B–B″). However, in follicle cells that had begun their cuboidal to columnar transition and were positioned over the oocyte, pak14 FCCs did not show ectopic pMLC (Figure 6, C–C″).
Figure 6.—
Pak regulates phosphorylation of MLC during development of follicular epithelium. (A–C) Anti-GFP. (A′–C′) Anti-pMLC. (A″–C″) Merge. FCC are distinguished by a lack of GFP staining. Arrowheads mark some clone boundaries. (A–A″) pak14 FCC in a stage 5 egg chamber showing a loss of pMLC staining from the apical and lateral membranes. (B–B″) pak14 FCCs in main-body follicle cells of stage 9 egg chamber showing ectopic pMLC in the absence of Pak. (C–C″) Stage 9 egg chamber in which the pak14 mutant clone contains both main-body follicle cells and follicle cells over the oocyte. The arrow denotes the junction between these two cell types. In mutant follicle cells that are over the oocyte, pMLC levels are slightly reduced at the apical membrane but are otherwise unaffected. However, in the mutant main-body follicle cells there is ectopic pMLC. Bar: 25 μm.
To determine if the ectopic pMLC in pak mutant follicle cells was due to a failure to negatively regulate MLCK, we tested to see if reducing MLCK function in pak mutant flies would suppress the egg chamber elongation defect. Three Drosophila loci have been identified encoding members of the Titin/MLCK family, Stretchin-MLCK, bent, and CG1776, although the products of these genes have yet to be tested with regard to effects on MLC (Champagne et al. 2000). We saw no suppression of oogenesis defects in pak mutants made heterozygous for alleles of these genes (data not shown).
The loss of pMLC in pak mutant FCCs in early egg chambers indicates that during oogenesis Pak positively contributes to phosphorylation of MLC prior to its role as a negative regulator. A role for Pak in driving MLC phosphorylation is further supported by a genetic interaction between pak and an allele of flapwing (flw), encoding the MLC phosphatase PP1β (Vereshchagina et al. 2004). Flw suppresses basolateral MLC phosphorylation in the follicular epithelium and we therefore tested for a genetic interaction with pak (Vereshchagina et al. 2004; Wang and Riechmann 2007). Heterozygosity for flw suppressed the egg chamber elongation defect of pak mutant flies and allowed the development of mature eggs (Figure 2H, Table 3).
Dpp pathway and other signaling components identified as pak interactors in the suppressor screen:
In addition to identifying the Rho1 pathway members as Pak interactors, we have evidence that other signaling proteins interact with Pak in regulating egg chamber elongation. Given the recent demonstration of a link between Decapentaplegic (Dpp) signaling and the Rho1 pathway, we were interested that two of the deficiencies identified as suppressors in our screen deleted genes encoding components of the Dpp pathway (Widmann and Dahmann 2009). Df(2L)dpp[d14] removes dpp, and Df(2L)BSC28 removes mothers against Dpp (mad), an R-Smad mediating Dpp signaling to the nucleus (Segal and Gelbart 1985; Parks et al. 2004) (Table 1). To determine whether pak had an antagonistic relationship with the Dpp pathway, we made pak mutants heterozygous for alleles of dpp, mad, and thickveins (tkv), encoding a type I Dpp receptor, and found that removing a single copy of any of these Dpp signaling components was sufficient to suppress the pak mutant elongation defect (Figure 2, I and J, and data not shown).
We identified a pair of overlapping deficiencies, Df(2R)Np5 and Df(2R)BSC29, in the screen that removes wunen (wun), which encodes a phosphatidic acid phosphatase involved in germ cell migration that also interacts with Rho1 signaling (Zhang et al. 1996, 1997; Parks et al. 2004; Gregory et al. 2007) (Table 1, Figure 2K, and data not shown). Df(2R)BSC29 also removes the related gene wun2, which works together with wun in regulating germ cell migration (Starz-Gaiano et al. 2001), and we found that heterozygosity for alleles of either wun or wun2 suppressed pak elongation defects (data not shown).
PDGF- and VEGF-receptor related (Pvr) is disrupted in Df(2L)BSC111 and encodes a receptor tyrosine kinase guiding migration of the border cells, a subset of the follicle cells (Duchek et al. 2001) (Table 1). Given that Pvr regulates the actin cytoskeleton in a Rac-dependent manner, it is a suitable candidate for a Pak-interacting protein and we determined that heterozygosity for a Pvr allele suppressed pak elongation defects (Figure 2L).
DISCUSSION
The follicular epithelium as a system for studying stress fibers and actomyosin contractility:
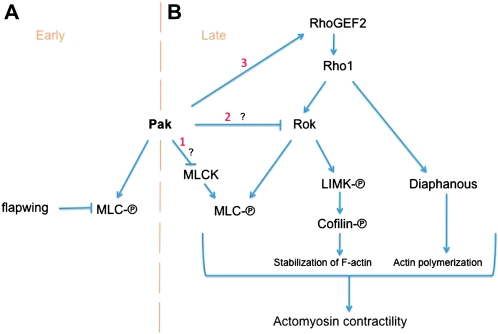
Our study establishes the basal F-actin of the follicular epithelium as an attractive system for the genetic analysis of the signaling pathways regulating the formation of stress fiber-like structures. The actin bundles in the follicle cells appear to be similar to the ventral stress fibers of nonmotile cultured cells, for which one model of stress fiber formation is that it is driven by bundling of actin filaments by actomyosin contractility (Chrzanowska-Wodnicka and Burridge 1996; Pellegrin and Mellor 2007). Consistent with this model, our results indicate that the major cause of basal F-actin disruption in pak mutant cells is misregulated actomyosin contractility that can be suppressed by reduction of the Rho1 pathway. We found that Pak regulates pMLC distribution during oogenesis, at first being required for pMLC and later restricting where it is present. Such conflicting roles for Pak have been reported in isolation in mammalian cell culture studies, but our results are the first to show that they can be temporally separated during development of an epithelial cell (see model in Figure 7). Paks from diverse species can function as MLCKs (Ramos et al. 1997; Chew et al. 1998; Zeng et al. 2000; Bisson et al. 2003; Zhang et al. 2005; Loo and Balasubramanian 2008; Szczepanowska et al. 2006), and such an activity for Pak is indicated in early stage egg chambers, where Pak's MLCK function is opposed by the Flw MLC phosphatase. Later in oogenesis, around the time of egg chamber elongation, Pak restricts the distribution of MLC phosphorylation and comes into conflict with the Rho1/Rok pathway.
Figure 7.—
Rho1-mediated actomyosin contractility during egg chamber elongation and model for Pak's role. Pak functions to both promote and restrict MLC phosphorylation. (A) During early oogenesis Pak acts as a positive contributor to MLC phosphorylation, probably functioning as an MLCK. This function is inhibited by the MLC phosphatase Flw. (B) During later stages of oogenesis, Pak restricts MLC phosphorylation, counteracting the Rho1 actomyosin contractility pathway. Consistent with the literature on mammalian Pak, this could be occurring through negative regulation of an MLCK (1). Another possibility is that Pak negatively regulates Rok (2). Pak likely also controls the distribution of MLC phosphorylation by regulating RhoGEF2 localization (3).
There are a number of ways that Pak could impinge on the Rok pathway, with one being at the level of RhoGEF2 at the top of the pathway (Figure 7). Pak is required for the basal localization of RhoGEF2, and the mislocalized RhoGEF2 seen in pak mutant clones could at least in part be responsible for the ectopic pMLC seen in older egg chambers. A protein similar to RhoGEF2 in mammals, P115-RhoGEF, appears to be negatively regulated by Pak binding to its DH–PH domain but we have been unable to find a similar physical interaction between Pak and the RhoGEF2 DH–PH, nor have we detected an effect of Pak on Rho1-GTP levels, although it is possible that there could be an effect not detectable by our assays (Rosenfeldt et al. 2006). A recent study showed that the PDZ domain of RhoGEF2 is required for its localization at the furrow canal during cellularization (Wenzl et al. 2010). Furthermore, the novel protein Slam, which complexes with the RhoGEF2 PDZ domain, is required for RhoGEF2 localization during cellularization, and it will be of interest to determine if Pak regulation of RhoGEF2 localization in the follicular epithelium involves the PDZ domain and/or Slam (Wenzl et al. 2010). Another possibility is that Pak regulates RhoGEF2 through a trimeric G-protein interaction. RhoGEF2 is a member of the RGS-containing family of GEFs that interact with the activated Gα subunits of trimeric G proteins through their RGS domain (reviewed in Sternweis et al. 2007) and members of the Pak family bind the Gβγ subunit complex through a motif conserved in Drosophila Pak (Leeuw et al. 1998; Leberer et al. 2000).
Another route by which Pak could be restricting pMLC distribution is through regulation of a MLCK cooperating with Rok. Work on mammalian Pak has demonstrated that Pak can negatively regulate the activity of MLCK, thus reducing the level of MLC phosphorylation, and we considered three potential MLCKs as candidate Pak targets (Sanders et al. 1999). Alleles of these genes did not suppress pak oogenesis defects, suggesting either that they are not regulated by pak during oogenesis or that more than one is being regulated by Pak. Another possibility is that Pak is directly regulating Rok in some manner to restrict the output of this pathway. Interestingly, in the columnar epithelial cells over the occyte in late egg chambers, Pak does not regulate MLC phosphorylation and this may be to allow the extensive actomyosin contractility likely to be required to shape these cells.
Finally, we have not eliminated the possibility that Pak could be regulating pMLC levels simply by controlling the overall amount of MLC, but this seems unlikely given the considerable evidence that vertebrate Pak regulates MLC phosphorylation.
Confirmation of RhoGEF2 as the major activator of Rho1 in epithelia:
Our finding that RhoGEF2 is a basally localized regulator of actomyosin contractility in the follicular epithelium is consistent with numerous previous studies indicating that RhoGEF2 is the major activator of Rho1 during epithelial morphogenesis (Barrett et al. 1997; Hacker and Perrimon 1998; Halsell et al. 2000; Bayer et al. 2003; Nikolaidou and Barrett 2004; Dawes-Hoang et al. 2005; Padash Barmchi et al. 2005; Simoes et al. 2006; Fox and Peifer 2007; Kolsch et al. 2007; Mulinari et al. 2008). As mentioned above, two other RhoGEFs known to regulate actin, Pebble and RhoGEF64C, did not affect the pak mutant egg chamber phenotype. We tested deficiencies and/or alleles disrupting 20 other predicted RhoGEFs for the ability to suppress the dpak mutant egg chamber phenotype and found that none were effective (S. Vlachos, unpublished observations). Similarly, a recent study tested predicted RhoGEFs as Rho1 regulators in driving epithelial morphogenesis during imaginal disc morphogenesis and concluded that RhoGEF2 is a key regulator (Patch et al. 2009). Many of the RhoGEFs have not been characterized functionally, although some have been shown to be GEFs for GTPases other than Rho1 and to function in nonepithelial cells such as neurons.
RhoGEF2 is enriched at the basal end of the follicle cells throughout oogenesis including the points of basal membrane separation between follicle cells that occurs during follicle cell flattening in late stage egg chambers (Schotman et al. 2008). Recently, it was shown that the Rho1 actomyosin contractility pathway is required for this separation between follicle cells at the basal membrane and presumably this signaling is activated by RhoGEF2 (Schotman et al. 2009).
RhoGEF2 alleles are much more effective than alleles of other Rho1 pathway components at extending the life span of pak mutant females, implying that RhoGEF2 may have roles independent of the Rho1 actomyosin contractility pathway that could be regulated by Pak. There is evidence that RhoGEFs have functions distinct from small GTPase activation; for example, Pebble has a Rho1-independent role in mesoderm migration (Schumacher et al. 2004; Rossman et al. 2005).
Candidate regulators of the Rho pathway identified in a screen:
In addition to the Rho pathway, we uncovered an antagonistic relationship between Pak and the Dpp pathway in the regulation of egg chamber elongation. A recent study of the Drosophila wing disc demonstrated that Dpp signaling regulates the subcellular distribution of Rho1 activity and MLC phosphorylation in epithelial cells (Widmann and Dahmann 2009). If this link between pathways also occurs in the follicular epithelium, it may be that loss of Dpp is suppressing the pak mutant phenotype through disruption of Rho1 signaling. Another possibility is that Dpp regulation of the actin filament cross-linking protein α-actinin in the follicular epithelium is relevant (Wahlstrom et al. 2006).
The ability of wun and wun2 alleles to suppress the pak egg chamber elongation defect might also be due to downregulation of the Rho1 pathway, as wun was picked up in an overexpression screen for suppressors of impaired Rho1 signaling (Gregory et al. 2007). Wun and Wun2 belong to a family of lipid phosphate phosphatases that regulate the levels of lipids involved in signaling including lysophosphatidic acid, which is an important activator of the RhoA pathway (Moolenaar et al. 2004; Pyne et al. 2004).
Acknowledgments
We thank R. Conder for input and comments during the early stages of this work; B. Dickson, D. Godt, H. Hing, A. Jacinto, S. Parkhurst, and G. Tanentzapf for fly stocks; S. Rogers for the anti-RhoGEF2 antibody; S. Narumiya for the pGEX-mDia1 (RBD) and pGEX-rhotekin (RBD) constructs; N. Hawkins and E. Verheyen for advice and discussions; and E. Verheyen for comments on the manuscript. This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada.
Note added in proof: Another group recently demonstrated that the Rho1-Rok pathway is required for actomyosin contractility in the follicular epithelium (L. He, X. Wang, H. L. Tang and D. J. Montell, 2010 Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 12: 1133–1142).
References
- Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara et al., 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271 20246–20249. [DOI] [PubMed] [Google Scholar]
- Ang, L. H., W. Chen, Y. Yao, R. Ozawa, E. Tao et al., 2006. Lim kinase regulates the development of olfactory and neuromuscular synapses. Dev. Biol. 293 178–190. [DOI] [PubMed] [Google Scholar]
- Bahri, S., S. Wang, R. Conder, J. Choy, S. Vlachos et al., 2010. The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development 137 2023–2032. [DOI] [PubMed] [Google Scholar]
- Barrett, K., M. Leptin and J. Settleman, 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91 905–915. [DOI] [PubMed] [Google Scholar]
- Bashaw, G. J., H. Hu, C. D. Nobes and C. S. Goodman, 2001. A novel Dbl family RhoGEF promotes Rho-dependent axon attraction to the central nervous system midline in Drosophila and overcomes Robo repulsion. J. Cell Biol. 155 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman, J., R. S. Reddy, H. Saito and D. Van Vactor, 2001. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr. Biol. 11 1317–1327. [DOI] [PubMed] [Google Scholar]
- Baum, B., and N. Perrimon, 2001. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat. Cell Biol. 3 883–890. [DOI] [PubMed] [Google Scholar]
- Bayer, C. A., S. R. Halsell, J. W. Fristrom, D. P. Kiehart and L. von Kalm, 2003. Genetic interactions between the RhoA and Stubble-stubbloid loci suggest a role for a type II transmembrane serine protease in intracellular signaling during Drosophila imaginal disc morphogenesis. Genetics 165 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A. L., and A. Hall, 2000. Rho GTPases and their effector proteins. Biochem. J. 348 241–255. [PMC free article] [PubMed] [Google Scholar]
- Bisson, N., N. Islam, L. Poitras, S. Jean, A. Bresnick et al., 2003. The catalytic domain of xPAK1 is sufficient to induce myosin II dependent in vivo cell fragmentation independently of other apoptotic events. Dev. Biol. 263 264–281. [DOI] [PubMed] [Google Scholar]
- Bokoch, G. M., 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72 743–781. [DOI] [PubMed] [Google Scholar]
- Bustos, R. I., M. A. Forget, J. E. Settleman and S. H. Hansen, 2008. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr. Biol. 18 1606–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J., R. Albertson, B. Riggs, C. M. Field and W. Sullivan, 2008. Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J. Cell Biol. 182 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon, D. H., and S. A. Wasserman, 1994. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 120 3367–3377. [DOI] [PubMed] [Google Scholar]
- Champagne, M. B., K. A. Edwards, H. P. Erickson and D. P. Kiehart, 2000. Drosophila stretchin-MLCK is a novel member of the Titin/Myosin light chain kinase family. J. Mol. Biol. 300 759–777. [DOI] [PubMed] [Google Scholar]
- Chew, T. L., R. A. Masaracchia, Z. M. Goeckeler and R. B. Wysolmerski, 1998. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J. Muscle Res. Cell Motil. 19 839–854. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M., and K. Burridge, 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conder, R., H. Yu, B. Zahedi and N. Harden, 2007. The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev. Biol. 305 470–482. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang, R. E., K. M. Parmar, A. E. Christiansen, C. B. Phelps, A. H. Brand et al., 2005. folded gastrulation, cell shape change and the control of myosin localization. Development 132 4165–4178. [DOI] [PubMed] [Google Scholar]
- Deng, W. M., M. Schneider, R. Frock, C. Castillejo-Lopez, E. A. Gaman et al., 2003. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130 173–184. [DOI] [PubMed] [Google Scholar]
- Duchek, P., K. Somogyi, G. Jekely, S. Beccari and P. Rorth, 2001. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107 17–26. [DOI] [PubMed] [Google Scholar]
- Duffy, J. B., D. A. Harrison and N. Perrimon, 1998. Identifying loci required for follicular patterning using directed mosaics. Development 125 2263–2271. [DOI] [PubMed] [Google Scholar]
- Edwards, K. A., and D. P. Kiehart, 1996. Drosophila nonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development 122 1499–1511. [DOI] [PubMed] [Google Scholar]
- Fox, D. T., and M. Peifer, 2007. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development 134 567–578. [DOI] [PubMed] [Google Scholar]
- Frydman, H. M., and A. C. Spradling, 2001. The receptor-like tyrosine phosphatase Lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development 128 3209–3220. [DOI] [PubMed] [Google Scholar]
- Gregory, S. L., T. Shandala, L. O'Keefe, L. Jones, M. J. Murray et al., 2007. A Drosophila overexpression screen for modifiers of Rho signalling in cytokinesis. Fly 1 13–22. [DOI] [PubMed] [Google Scholar]
- Grosshans, J., C. Wenzl, H. M. Herz, S. Bartoszewski, F. Schnorrer et al., 2005. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development 132 1009–1020. [DOI] [PubMed] [Google Scholar]
- Gutzeit, H. O., 1990. The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur. J. Cell Biol. 53 349–356. [PubMed] [Google Scholar]
- Gutzeit, H. O., 1991. Organization and in vitro activity of microfilament bundles associated with the basement membrane of Drosophila follicles. Acta Histochem. Suppl. 41 201–210. [PubMed] [Google Scholar]
- Gutzeit, H. O., and A. Haas-Assenbaum, 1991. The somatic envelopes around the germ-line cells of polytrophic insect follicles: structural and functional aspects. Tissue Cell 23 853–865. [DOI] [PubMed] [Google Scholar]
- Gutzeit, H. O., W. Eberhardt and E. Gratwohl, 1991. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J. Cell Sci. 100 781–788. [DOI] [PubMed] [Google Scholar]
- Hacker, U., and N. Perrimon, 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsell, S. R., B. I. Chu and D. P. Kiehart, 2000. Genetic analysis demonstrates a direct link between Rho signaling and nonmuscle myosin function during Drosophila morphogenesis. Genetics 155 1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, S., K. Ito, Y. Sado, M. Taniguchi, A. Akimoto et al., 2002. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis 34 58–61. [DOI] [PubMed] [Google Scholar]
- Hing, H., J. Xiao, N. Harden, L. Lim and S. L. Zipursky, 1999. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell 97 853–863. [DOI] [PubMed] [Google Scholar]
- Kawano, Y., Y. Fukata, N. Oshiro, M. Amano, T. Nakamura et al., 1999. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 147 1023–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K., M. Ito, M. Amano, K. Chihara, Y. Fukata et al., 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273 245–248. [DOI] [PubMed] [Google Scholar]
- Kimura, K., T. Tsuji, Y. Takada, T. Miki and S. Narumiya, 2000. Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J. Biol. Chem. 275 17233–17236. [DOI] [PubMed] [Google Scholar]
- Kolsch, V., T. Seher, G. J. Fernandez-Ballester, L. Serrano and M. Leptin, 2007. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315 384–386. [DOI] [PubMed] [Google Scholar]
- Kozma, R., S. Sarner, S. Ahmed and L. Lim, 1997. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell. Biol. 17 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer, E., D. Dignard, D. Y. Thomas and T. Leeuw, 2000. A conserved Gβ binding (GBB) sequence motif in Ste20p/PAK family protein kinases. Biol. Chem. 381 427–431. [DOI] [PubMed] [Google Scholar]
- Leeuw, T., C. Wu, J. D. Schrag, M. Whiteway, D. Y. Thomas et al., 1998. Interaction of a G-protein β-subunit with a conserved sequence in Ste20/PAK family protein kinases. Nature 391 191–195. [DOI] [PubMed] [Google Scholar]
- Leung, T., X. Q. Chen, E. Manser and L. Lim, 1996. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 16 5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. A., J. D. Alls, R. M. Avancini, K. Koo and D. Godt, 2003. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat. Cell Biol. 5 994–1000. [DOI] [PubMed] [Google Scholar]
- Loo, T. H., and M. Balasubramanian, 2008. Schizosaccharomyces pombe Pak-related protein, Pak1p/Orb2p, phosphorylates myosin regulatory light chain to inhibit cytokinesis. J. Cell Biol. 183 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magie, C. R., and S. M. Parkhurst, 2005. Rho1 regulates signaling events required for proper Drosophila embryonic development. Dev. Biol. 278 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magie, C. R., M. R. Meyer, M. S. Gorsuch and S. M. Parkhurst, 1999. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development 126 5353–5364. [DOI] [PubMed] [Google Scholar]
- Magie, C. R., D. Pinto-Santini and S. M. Parkhurst, 2002. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development 129 3771–3782. [DOI] [PubMed] [Google Scholar]
- Manseau, L., A. Baradaran, D. Brower, A. Budhu, F. Elefant et al., 1997. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev. Dyn. 209 310–322. [DOI] [PubMed] [Google Scholar]
- Mirouse, V., C. P. Christoforou, C. Fritsch, D. St Johnston and R. P. Ray, 2009. Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev. Cell 16 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moolenaar, W. H., L. A. van Meeteren and B. N. Giepmans, 2004. The ins and outs of lysophosphatidic acid signaling. BioEssays 26 870–881. [DOI] [PubMed] [Google Scholar]
- Mulinari, S., M. P. Barmchi and U. Hacker, 2008. DRhoGEF2 and diaphanous regulate contractile force during segmental groove morphogenesis in the Drosophila embryo. Mol. Biol. Cell 19 1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome, T. P., S. Schmidt, G. Dietzl, K. Keleman, B. Asling et al., 2000. Trio combines with Dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 101 283–294. [DOI] [PubMed] [Google Scholar]
- Nikolaidou, K. K., and K. Barrett, 2004. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr. Biol. 14 1822–1826. [DOI] [PubMed] [Google Scholar]
- Nimnual, A. S., L. J. Taylor and D. Bar-Sagi, 2003. Redox-dependent downregulation of Rho by Rac. Nat. Cell Biol. 5 236–241. [DOI] [PubMed] [Google Scholar]
- Ohashi, K., K. Nagata, M. Maekawa, T. Ishizaki, S. Narumiya et al., 2000. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J. Biol. Chem. 275 3577–3582. [DOI] [PubMed] [Google Scholar]
- Padash Barmchi, M., S. Rogers and U. Hacker, 2005. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J. Cell Biol. 168 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36 288–292. [DOI] [PubMed] [Google Scholar]
- Patch, K., S. R. Stewart, A. Welch and R. E. Ward, 2009. A second-site noncomplementation screen for modifiers of Rho1 signaling during imaginal disc morphogenesis in Drosophila. PLoS One 4 e7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin, S., and H. Mellor, 2007. Actin stress fibres. J. Cell Sci. 120 3491–3499. [DOI] [PubMed] [Google Scholar]
- Prokopenko, S. N., A. Brumby, L. O'Keefe, L. Prior, Y. He et al., 1999. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 13 2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne, S., K. C. Kong and P. I. Darroch, 2004. Lysophosphatidic acid and sphingosine 1-phosphate biology: the role of lipid phosphate phosphatases. Semin. Cell Dev. Biol. 15 491–501. [DOI] [PubMed] [Google Scholar]
- Ramos, E., R. B. Wysolmerski and R. A. Masaracchia, 1997. Myosin phosphorylation by human cdc42-dependent S6/H4 kinase/gammaPAK from placenta and lymphoid cells. Recept. Signal Transduct. 7 99–110. [PubMed] [Google Scholar]
- Rogers, S. L., U. Wiedemann, U. Hacker, C. Turck and R. D. Vale, 2004. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 14 1827–1833. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt, H., M. D. Castellone, P. A. Randazzo and J. S. Gutkind, 2006. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. J. Mol. Signal. 1 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman, K. L., C. J. Der and J. Sondek, 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell. Biol. 6 167–180. [DOI] [PubMed] [Google Scholar]
- Salhia, B., F. Rutten, M. Nakada, C. Beaudry, M. Berens et al., 2005. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res. 65 8792–8800. [DOI] [PubMed] [Google Scholar]
- Sander, E. E., J. P. ten Klooster, S. van Delft, R. A. van der Kammen and J. G. Collard, 1999. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, L. C., F. Matsumura, G. M. Bokoch and P. de Lanerolle, 1999. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283 2083–2085. [DOI] [PubMed] [Google Scholar]
- Sanny, J., V. Chui, C. Langmann, C. Pereira, B. Zahedi et al., 2006. Drosophila RhoGAP68F is a putative GTPase activating protein for RhoA participating in gastrulation. Dev. Genes Evol. 216 543–550.16609869 [Google Scholar]
- Sarkar, A., N. Parikh, S. A. Hearn, M. T. Fuller, S. I. Tazuke et al., 2007. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr. Biol. 17 1253–1258. [DOI] [PubMed] [Google Scholar]
- Schotman, H., L. Karhinen and C. Rabouille, 2008. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev. Cell 14 171–182. [DOI] [PubMed] [Google Scholar]
- Schotman, H., L. Karhinen and C. Rabouille, 2009. Integrins mediate their unconventional, mechanical-stress-induced secretion via RhoA and PINCH in Drosophila. J. Cell Sci. 122 2662–2672. [DOI] [PubMed] [Google Scholar]
- Schumacher, S., T. Gryzik, S. Tannebaum and H. A. Muller, 2004. The RhoGEF Pebble is required for cell shape changes during cell migration triggered by the Drosophila FGF receptor Heartless. Development 131 2631–2640. [DOI] [PubMed] [Google Scholar]
- Seasholtz, T. M., J. Radeff-Huang, S. A. Sagi, R. Matteo, J. M. Weems et al., 2004. Rho-mediated cytoskeletal rearrangement in response to LPA is functionally antagonized by Rac1 and PIP2. J. Neurochem. 91 501–512. [DOI] [PubMed] [Google Scholar]
- Segal, D., and W. M. Gelbart, 1985. Shortvein, a new component of the decapentaplegic gene complex in Drosophila melanogaster. Genetics 109 119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman, J., 2001. Rac 'n Rho: the music that shapes a developing embryo. Dev. Cell 1 321–331. [DOI] [PubMed] [Google Scholar]
- Simoes, S., B. Denholm, D. Azevedo, S. Sotillos, P. Martin et al., 2006. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development 133 4257–4267. [DOI] [PubMed] [Google Scholar]
- Somers, W. G., and R. Saint, 2003. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4 29–39. [DOI] [PubMed] [Google Scholar]
- Starz-Gaiano, M., N. K. Cho, A. Forbes and R. Lehmann, 2001. Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development 128 983–991. [DOI] [PubMed] [Google Scholar]
- Sternweis, P. C., A. M. Carter, Z. Chen, S. M. Danesh, Y. F. Hsiung et al., 2007. Regulation of Rho guanine nucleotide exchange factors by G proteins. Adv. Protein Chem. 74 189–228. [DOI] [PubMed] [Google Scholar]
- Sugimoto, N., N. Takuwa, H. Okamoto, S. Sakurada and Y. Takuwa, 2003. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol. Cell. Biol. 23 1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi, T., K. Matsumoto and T. Nakamura, 2001. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J. Biol. Chem. 276 670–676. [DOI] [PubMed] [Google Scholar]
- Szczepanowska, J., E. D. Korn and H. Brzeska, 2006. Activation of myosin in HeLa cells causes redistribution of focal adhesions and F-actin from cell center to cell periphery. Cell Motil. Cytoskeleton 63 356–374. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G., D. Devenport, D. Godt and N. H. Brown, 2007. Integrin-dependent anchoring of a stem-cell niche. Nat. Cell Biol. 9 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa, G., Y. Yamakita, S. Yamashiro, D. J. Hartshorne, Y. Sasaki et al., 2000. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 150 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji, T., T. Ishizaki, M. Okamoto, C. Higashida, K. Kimura et al., 2002. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J. Cell Biol. 157 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst, L., and M. Symons, 2002. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 16 1032–1054. [DOI] [PubMed] [Google Scholar]
- van Leeuwen, F. N., H. E. Kain, R. A. Kammen, F. Michiels, O. W. Kranenburg et al., 1997. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 139 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen, F. N., S. van Delft, H. E. Kain, R. A. van der Kammen and J. G. Collard, 1999. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1 242–248. [DOI] [PubMed] [Google Scholar]
- Vereshchagina, N., D. Bennett, B. Szoor, J. Kirchner, S. Gross et al., 2004. The essential role of PP1β in Drosophila is to regulate nonmuscle myosin. Mol. Biol. Cell 15 4395–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen, E., and L. Cooley, 1994. Looking at oogenesis. Methods Cell Biol. 44 545–561. [PubMed] [Google Scholar]
- Viktorinova, I., T. Konig, K. Schlichting and C. Dahmann, 2009. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development 136 4123–4132. [DOI] [PubMed] [Google Scholar]
- Wahl, S., H. Barth, T. Ciossek, K. Aktories and B. K. Mueller, 2000. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom, G., H. L. Norokorpi and T. I. Heino, 2006. Drosophila α-actinin in ovarian follicle cells is regulated by EGFR and Dpp signalling and required for cytoskeletal remodelling. Mech. Dev. 123 801–818. [DOI] [PubMed] [Google Scholar]
- Wang, H. R., Y. Zhang, B. Ozdamar, A. A. Ogunjimi, E. Alexandrova et al., 2003. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302 1775–1779. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and V. Riechmann, 2007. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr. Biol. 17 1349–1355. [DOI] [PubMed] [Google Scholar]
- Warner, S. J., and G. D. Longmore, 2009. Cdc42 antagonizes Rho1 activity at adherens junctions to limit epithelial cell apical tension. J. Cell Biol. 187 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., P. Madaule, T. Reid, T. Ishizaki, G. Watanabe et al., 1997. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16 3044–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., T. Kato, A. Fujita, T. Ishizaki and S. Narumiya, 1999. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1 136–143. [DOI] [PubMed] [Google Scholar]
- Wenzl, C., S. Yan, P. Laupsien and J. Grosshans, 2010. Localization of RhoGEF2 during Drosophila cellularization is developmentally controlled by slam. Mech. Dev. 127 371–384. [DOI] [PubMed] [Google Scholar]
- Widmann, T. J., and C. Dahmann, 2009. Dpp signaling promotes the cuboidal-to-columnar shape transition of Drosophila wing disc epithelia by regulating Rho1. J. Cell Sci. 122 1362–1373. [DOI] [PubMed] [Google Scholar]
- Wildenberg, G. A., M. R. Dohn, R. H. Carnahan, M. A. Davis, N. A. Lobdell et al., 2006. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127 1027–1039. [DOI] [PubMed] [Google Scholar]
- Winter, C. G., B. Wang, A. Ballew, A. Royou, R. Karess et al., 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105 81–91. [DOI] [PubMed] [Google Scholar]
- Wu, Y. I., D. Frey, O. I. Lungu, A. Jaehrig, I. Schlichting et al., 2009. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., F. Wang, A. Van Keymeulen, P. Herzmark, A. Straight et al., 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114 201–214. [DOI] [PubMed] [Google Scholar]
- Xu, T., and G. M. Rubin, 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 1223–1237. [DOI] [PubMed] [Google Scholar]
- Young, P. E., A. M. Richman, A. S. Ketchum and D. P. Kiehart, 1993. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 7 29–41. [DOI] [PubMed] [Google Scholar]
- Zeng, Q., D. Lagunoff, R. Masaracchia, Z. Goeckeler, G. Cote et al., 2000. Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J. Cell Sci. 113 471–482. [DOI] [PubMed] [Google Scholar]
- Zhang, H., D. J. Webb, H. Asmussen, S. Niu and A. F. Horwitz, 2005. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 25 3379–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., J. Zhang, Y. Cheng and K. Howard, 1996. Identification and genetic analysis of wunen, a gene guiding Drosophila melanogaster germ cell migration. Genetics 143 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., J. Zhang, K. J. Purcell, Y. Cheng and K. Howard, 1997. The Drosophila protein Wunen repels migrating germ cells. Nature 385 64–67. [DOI] [PubMed] [Google Scholar]
- Zondag, G. C., E. E. Evers, J. P. ten Klooster, L. Janssen, R. A. van der Kammen et al., 2000. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J. Cell Biol. 149 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]