Abstract
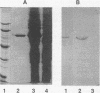
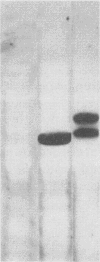
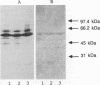
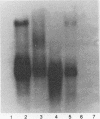
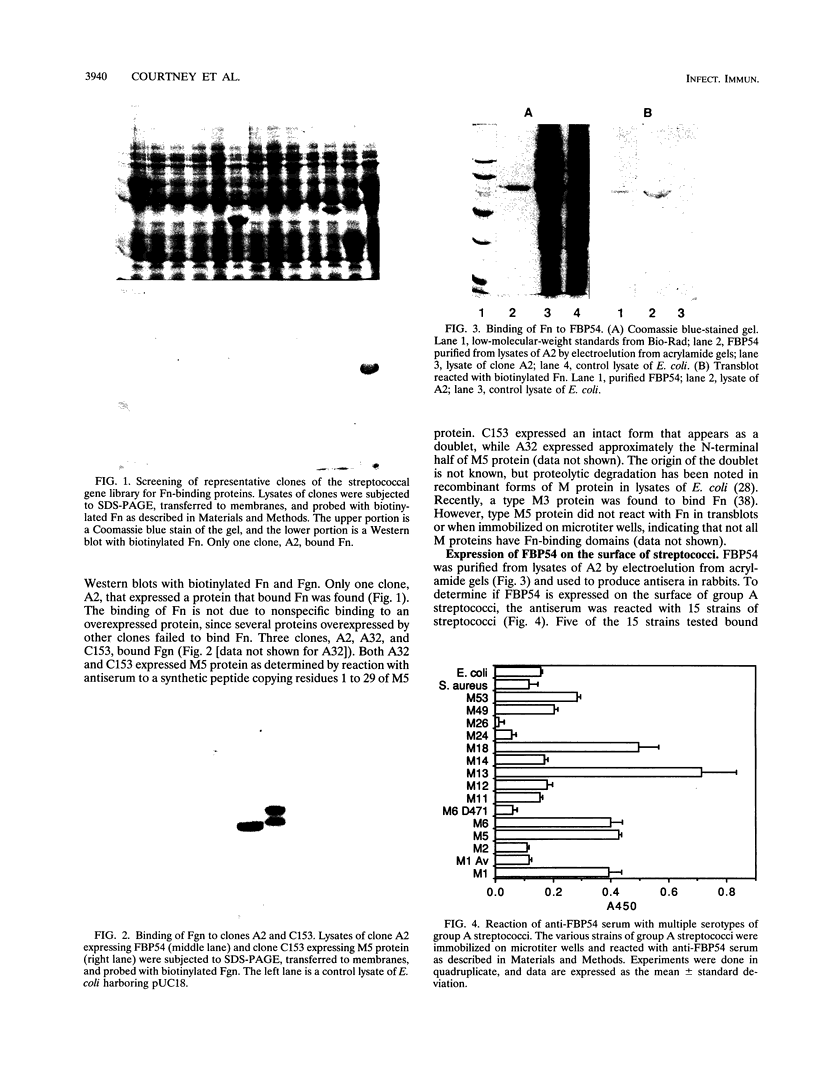
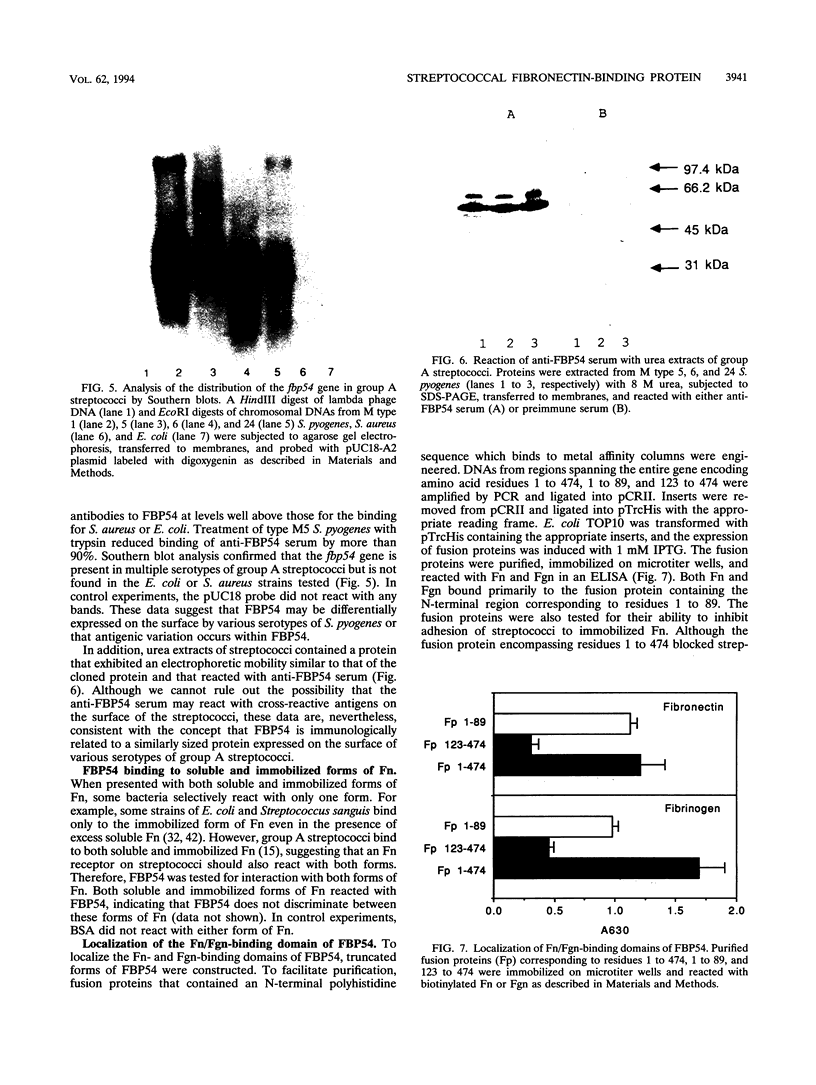
Lipoteichoic acid and several streptococcal proteins have been reported to bind fibronectin (Fn) or fibrinogen (Fgn), which may serve as host receptors. We searched for such proteins by screening a library of genes from M type 5 group A streptococci cloned into Escherichia coli. Lysates of clones were probed with biotinylated Fn and biotinylated Fgn. One clone expressed a 54-kDa protein that reacted with Fn and Fgn. The protein, termed FBP54, was purified and used to immunize rabbits. Anti-FBP54 serum reacted with purified, recombinant FBP54 and with a protein of similar electrophoretic mobility in extracts of M type 5, 6, and 24 streptococci. Anti-FBP54 serum also reacted with 5 of 15 strains of intact, live streptococci, suggesting that FBP54 may be a surface antigen. Southern blot analysis confirmed that the gene is found in group A streptococci but not in Staphylococcus aureus or E. coli. The cloned gene was sequenced and contained an open reading frame encoding a protein with a calculated molecular weight of 54,186. Partial amino acid sequencing of purified FBP54 confirmed that this open reading frame encoded the protein. As determined by utilizing fusion proteins containing truncated forms of FBP54, the primary Fn/Fgn-binding domain appears to be contained in residues 1 to 89. These data suggest that FBP54 may be a surface protein of streptococci that reacts with both Fn and Fgn and therefore may participate in the adhesion of group A streptococci to host cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada K. M. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985 Apr 10;260(7):4492–4500. [PubMed] [Google Scholar]
- Bayer E. A., Skutelsky E., Wilchek M. The avidin-biotin complex in affinity cytochemistry. Methods Enzymol. 1979;62:308–315. doi: 10.1016/0076-6879(79)62235-8. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Courtney H. S. Bacterial adherence: the attachment of group A streptococci to mucosal surfaces. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S475–S481. doi: 10.1093/clinids/9.supplement_5.s475. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Fischetti V. A. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988 Oct;56(10):2666–2672. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronze M. S., Courtney H. S., Dale J. B. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J Immunol. 1992 Feb 1;148(3):888–893. [PubMed] [Google Scholar]
- Buckland R., Wild F. Leucine zipper motif extends. Nature. 1989 Apr 13;338(6216):547–547. doi: 10.1038/338547a0. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Genetic manipulation of pathogenic streptococci. Methods Enzymol. 1991;204:556–586. doi: 10.1016/0076-6879(91)04028-m. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Stephens D. S., Olsén A., Scott J. R. Role of M protein in adherence of group A streptococci. Infect Immun. 1991 May;59(5):1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal G. S., Valentin-Weigand P., Timmis K. N. Bacterial infection of wounds: fibronectin-mediated adherence group A and C streptococci to fibrin thrombi in vitro. Infect Immun. 1990 Sep;58(9):3015–3019. doi: 10.1128/iai.58.9.3015-3019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S., Hasty D. L., Dale J. B., Poirier T. P. A 28-kilodalton fibronectin-binding protein of group A streptococci. Curr Microbiol. 1992 Nov;25(5):245–250. doi: 10.1007/BF01575856. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Ofek I., Simpson W. A., Hasty D. L., Beachey E. H. Binding of Streptococcus pyogenes to soluble and insoluble fibronectin. Infect Immun. 1986 Sep;53(3):454–459. doi: 10.1128/iai.53.3.454-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S., Simpson W. A., Beachey E. H. Binding of streptococcal lipoteichoic acid to fatty acid-binding sites on human plasma fibronectin. J Bacteriol. 1983 Feb;153(2):763–770. doi: 10.1128/jb.153.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S., Stanislawski L., Ofek I., Simpson W. A., Hasty D. L., Beachey E. H. Localization of a lipoteichoic acid binding site to a 24-kilodalton NH2-terminal fragment of fibronectin. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S360–S362. doi: 10.1093/cid/10.supplement_2.s360. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., von Hunolstein C., Dale J. B., Bronze M. S., Beachey E. H., Hasty D. L. Lipoteichoic acid and M protein: dual adhesins of group A streptococci. Microb Pathog. 1992 Mar;12(3):199–208. doi: 10.1016/0882-4010(92)90054-r. [DOI] [PubMed] [Google Scholar]
- Dale J. B., Baird R. W., Courtney H. S., Hasty D. L., Bronze M. S. Passive protection of mice against group A streptococcal pharyngeal infection by lipoteichoic acid. J Infect Dis. 1994 Feb;169(2):319–323. doi: 10.1093/infdis/169.2.319. [DOI] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Localization of protective epitopes of the amino terminus of type 5 streptococcal M protein. J Exp Med. 1986 May 1;163(5):1191–1202. doi: 10.1084/jem.163.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Horwitz P. A., Caparon M. G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992 Dec;60(12):5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Ofek I., Courtney H. S., Doyle R. J. Multiple adhesins of streptococci. Infect Immun. 1992 Jun;60(6):2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Abraham S., Caparon M., Falk P., St Geme J. W., 3rd, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993 Jun 4;73(5):887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- Kehoe M. A., Poirier T. P., Beachey E. H., Timmis K. N. Cloning and genetic analysis of serotype 5 M protein determinant of group A streptococci: evidence for multiple copies of the M5 determinant in the Streptococcus pyogenes genome. Infect Immun. 1985 Apr;48(1):190–197. doi: 10.1128/iai.48.1.190-197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect Immun. 1985 Oct;50(1):77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- LeBoeuf R. D., Raja R. H., Fuller G. M., Weigel P. H. Human fibrinogen specifically binds hyaluronic acid. J Biol Chem. 1986 Sep 25;261(27):12586–12592. [PubMed] [Google Scholar]
- Lowrance J. H., Hasty D. L., Simpson W. A. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect Immun. 1988 Sep;56(9):2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992 Aug 1;176(2):415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B. A., Davison V. E., Ramsay M. A. Fibrinogen-mediated adherence of group A Streptococcus to influenza A virus-infected cell cultures. Infect Immun. 1982 Nov;38(2):513–520. doi: 10.1128/iai.38.2.513-520.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K. H., Köhler W. T-Proteine des Streptococcus pyogenes IV. Mitteilung: Isolierung von T1-Protein über Affinitätschromatographie an immobilisiertem Fibrinogen. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(4):449–456. doi: 10.1016/s0176-6724(84)80021-8. [DOI] [PubMed] [Google Scholar]
- Schmidt K. H., Mann K., Cooney J., Köhler W. Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immunol Med Microbiol. 1993 Aug;7(2):135–143. doi: 10.1111/j.1574-695X.1993.tb00392.x. [DOI] [PubMed] [Google Scholar]
- Sela S., Aviv A., Tovi A., Burstein I., Caparon M. G., Hanski E. Protein F: an adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol Microbiol. 1993 Dec;10(5):1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Signäs C., Raucci G., Jönsson K., Lindgren P. E., Anantharamaiah G. M., Hök M., Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(2):699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Courtney H. S., Ofek I. Interactions of fibronectin with streptococci: the role of fibronectin as a receptor for Streptococcus pyogenes. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S351–S359. doi: 10.1093/clinids/9.supplement_4.s351. [DOI] [PubMed] [Google Scholar]
- Sokurenko E. V., Courtney H. S., Abraham S. N., Klemm P., Hasty D. L. Functional heterogeneity of type 1 fimbriae of Escherichia coli. Infect Immun. 1992 Nov;60(11):4709–4719. doi: 10.1128/iai.60.11.4709-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg L., O'Toole P., Lindahl G. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol Microbiol. 1992 May;6(9):1185–1194. doi: 10.1111/j.1365-2958.1992.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talay S. R., Valentin-Weigand P., Jerlström P. G., Timmis K. N., Chhatwal G. S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992 Sep;60(9):3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Weigand P., Grulich-Henn J., Chhatwal G. S., Müller-Berghaus G., Blobel H., Preissner K. T. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin). Infect Immun. 1988 Nov;56(11):2851–2855. doi: 10.1128/iai.56.11.2851-2855.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waine G. J., Becker M., Yang W., Kalinna B., McManus D. P. Cloning, molecular characterization, and functional activity of Schistosoma japonicum glyceraldehyde-3-phosphate dehydrogenase, a putative vaccine candidate against schistosomiasis japonica. Infect Immun. 1993 Nov;61(11):4716–4723. doi: 10.1128/iai.61.11.4716-4723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. R., Stinson M. W. M protein mediates streptococcal adhesion to HEp-2 cells. Infect Immun. 1994 Feb;62(2):442–448. doi: 10.1128/iai.62.2.442-448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Briles D. E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992 Jan;174(2):601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K. Genetics of adhesive fimbriae of intestinal Escherichia coli. Curr Top Microbiol Immunol. 1990;151:29–53. doi: 10.1007/978-3-642-74703-8_2. [DOI] [PubMed] [Google Scholar]