Abstract
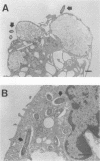
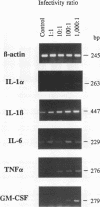
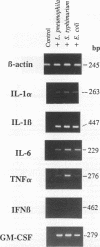
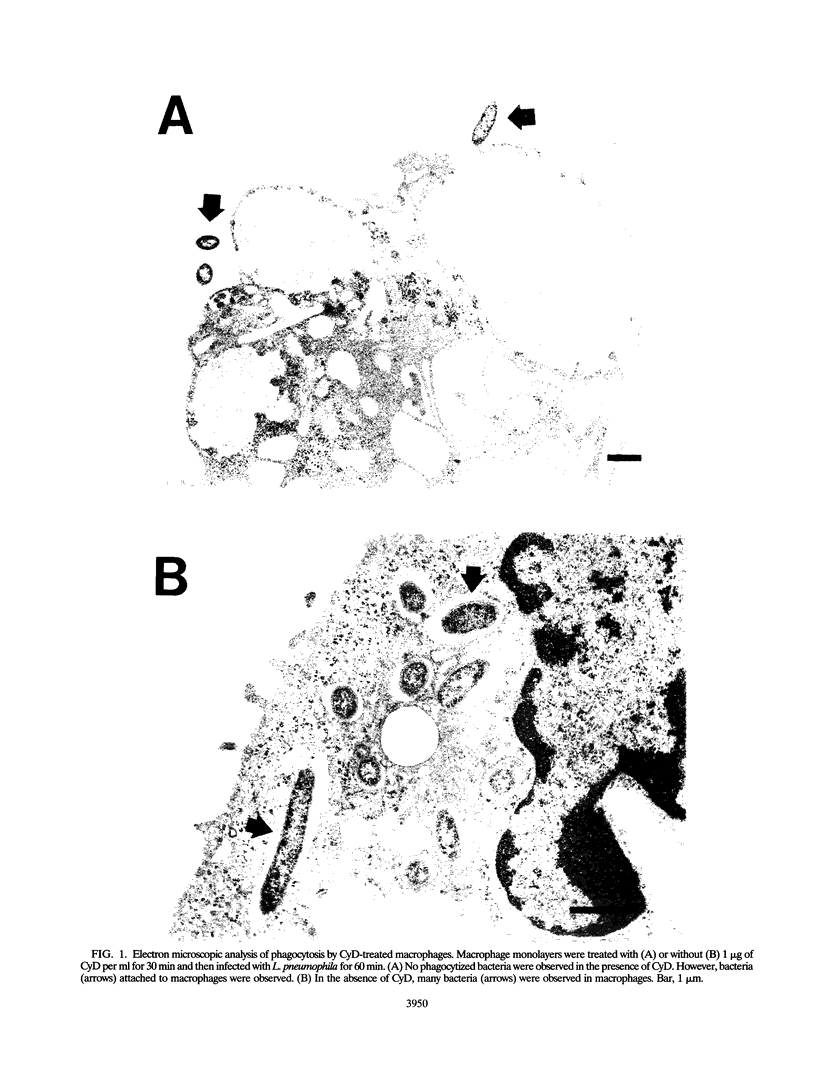
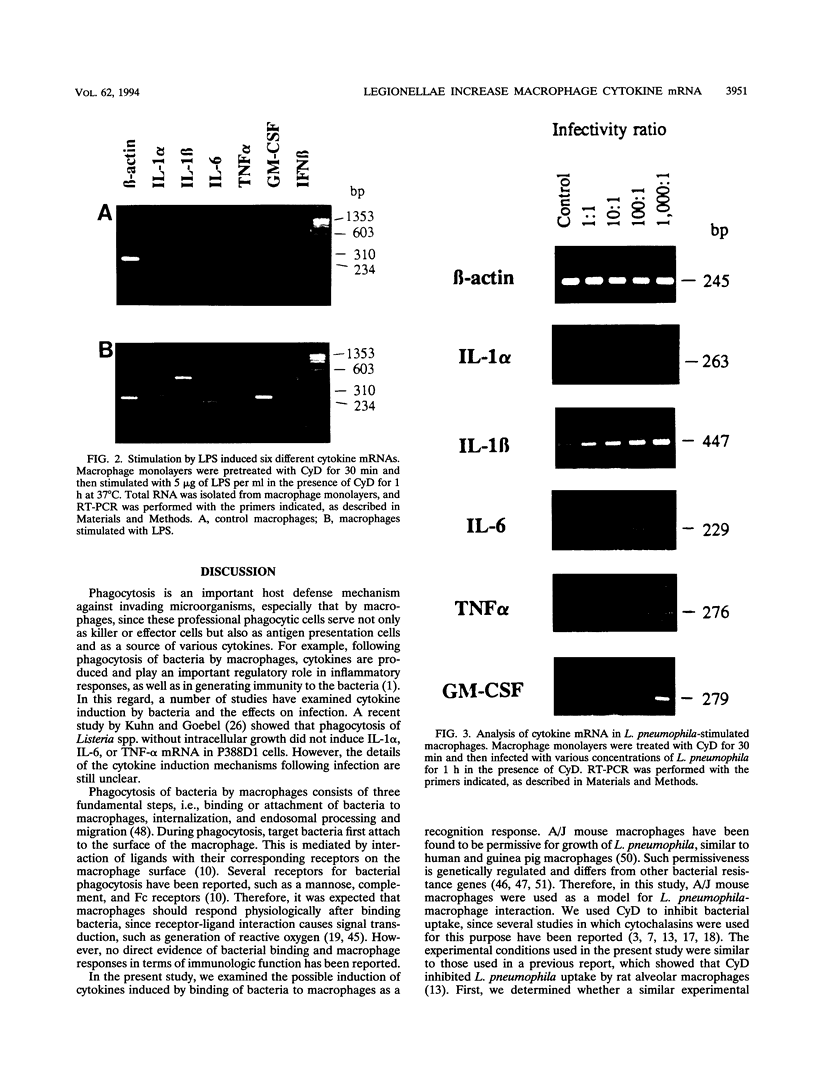
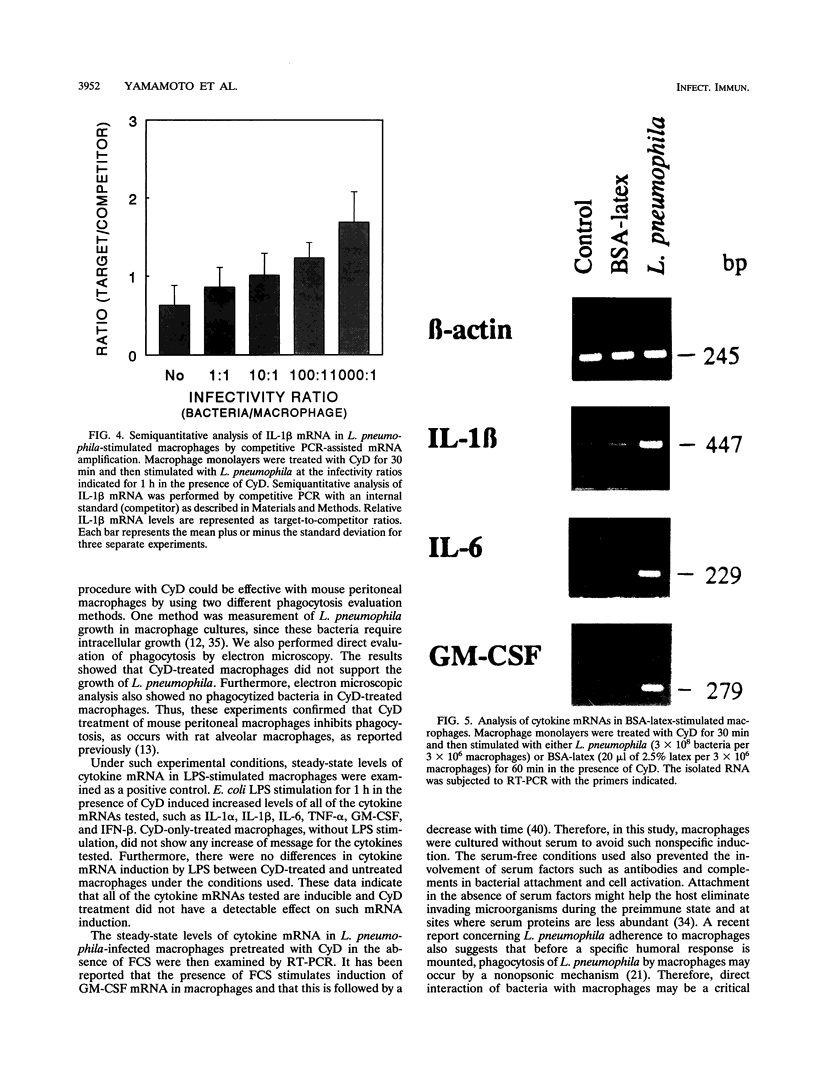
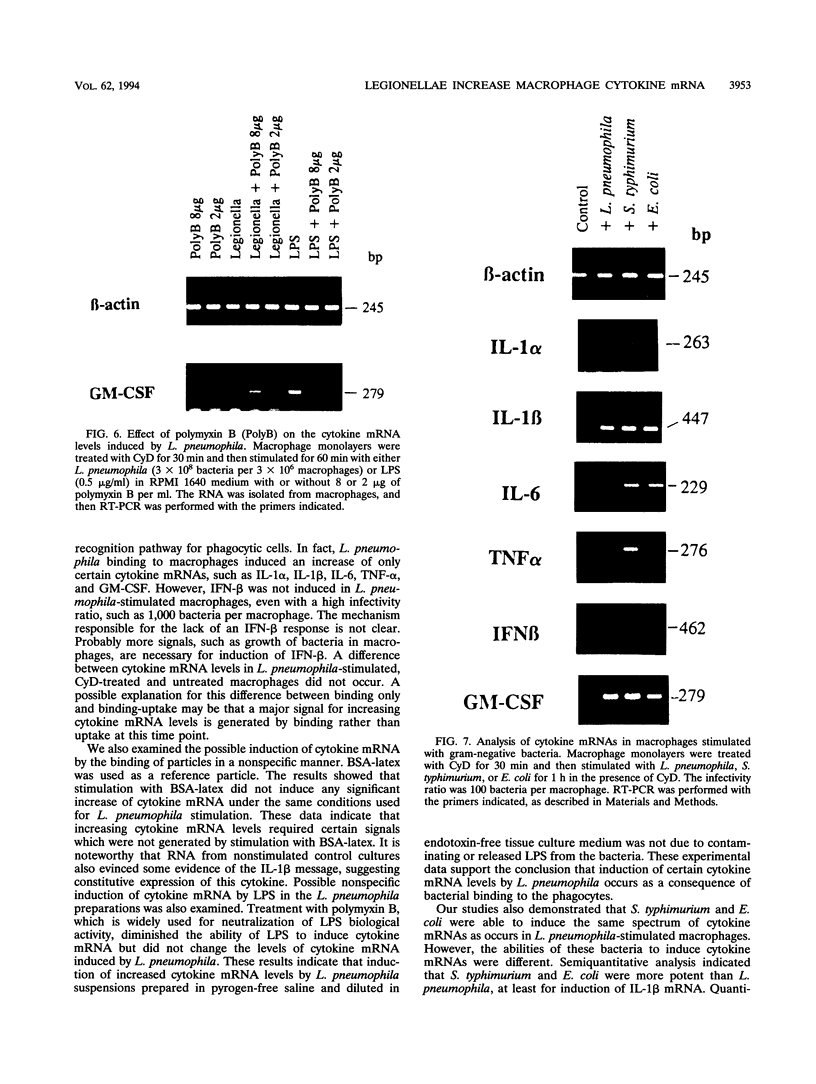
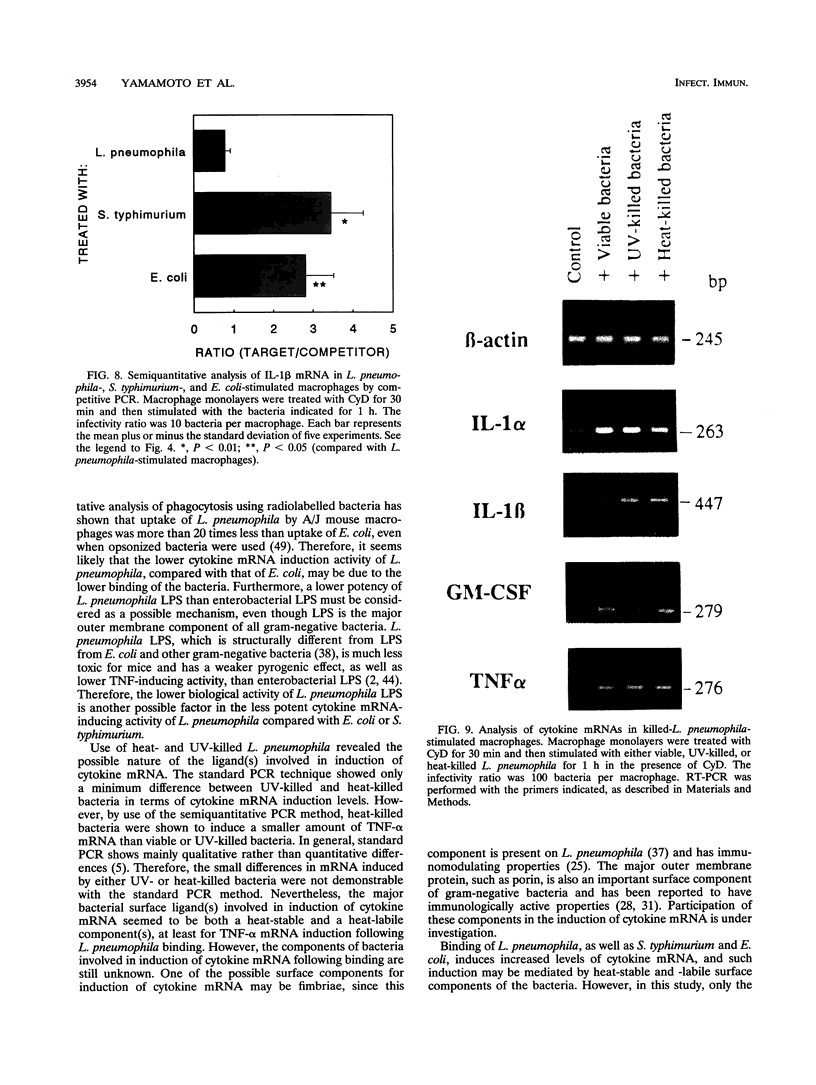
Infection of macrophages with Legionella pneumophila induces formation of interleukin 1 beta (IL-1 beta), but the molecular basis of this is not understood. Binding of bacteria to macrophage surfaces is the first step in an infection process. Therefore, we examined whether this step was sufficient to increase the cellular level of mRNAs for IL-1 beta and other cytokines. To assess the effect of binding of L. pneumophila on the steady-state levels of cytokine mRNAs, cultures of thioglycolate-elicited macrophages from L. pneumophila-susceptible A/J mice were treated with cytochalasin D and infected with L. pneumophila and the total RNA was extracted for analysis by reverse transcription-PCR with primers for IL-1 alpha, IL-1 beta, IL-6, tumor necrosis factor alpha, granulocyte macrophage colony-stimulating factor, and beta interferon (IFN-beta). L. pneumophila treatment increased the cellular steady-state mRNA levels of all cytokines except IFN-beta. To determine the specificity of this effect, macrophage cultures were treated with cytochalasin D and either bacterial lipopolysaccharide, bovine serum albumin-sensitized latex, Salmonella typhimurium, or Escherichia coli. Lipopolysaccharide treatment increased all mRNAs, bovine serum albumin-sensitized latex had no significant effect, and treatment with S. typhimurium or E. coli increased all mRNAs except that of IFN-beta. These results suggested that the binding of gram-negative bacteria to the macrophage surface was sufficient to induce a unique pattern of cytokine mRNAs. Additional studies that examined the characteristics of the bacterial ligands involved indicated involvement of both heat-labile and heat-stable surface ligands.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Bromander A., Holmgren J., Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991 May 1;146(9):2908–2914. [PubMed] [Google Scholar]
- Chakravarti B., Chakravarti D. N. Phagocytosis: an overview. Pathol Immunopathol Res. 1987;6(5-6):316–342. doi: 10.1159/000157062. [DOI] [PubMed] [Google Scholar]
- Elliott J. A., Winn W. C., Jr Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect Immun. 1986 Jan;51(1):31–36. doi: 10.1128/iai.51.1.31-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Friedman H., Widen R., Klein T., Searls L., Cabrian K. Legionella pneumophila-induced blastogenesis of murine lymphoid cells in vitro. Infect Immun. 1984 Jan;43(1):314–319. doi: 10.1128/iai.43.1.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann L. K., Johnson W. Adherence of Legionella pneumophila to guinea pig peritoneal macrophages, J774 mouse macrophages, and undifferentiated U937 human monocytes: role of Fc and complement receptors. Infect Immun. 1992 Dec;60(12):5212–5218. doi: 10.1128/iai.60.12.5212-5218.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., White J. D., Shirey F. G., McGann V. G., Berendt R. F., Larson E. W., Hedlund K. W. In vitro responses of guinea pig peritoneal macrophages to Legionella pneumophila. Infect Immun. 1981 Mar;31(3):1209–1213. doi: 10.1128/iai.31.3.1209-1213.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G. Cytochalasin B. Dissociation of pinocytosis and phagocytosis by peritoneal macrophages. Exp Eye Res. 1973 Apr;79(1):73–78. [PubMed] [Google Scholar]
- Klein T. W., Yamamoto Y., Wilson S., Newton C., Friedman H. Legionella pneumophila infection and cytokine production. Adv Exp Med Biol. 1992;319:97–104. doi: 10.1007/978-1-4615-3434-1_11. [DOI] [PubMed] [Google Scholar]
- Kreft B., Bohnet S., Carstensen O., Hacker J., Marre R. Differential expression of interleukin-6, intracellular adhesion molecule 1, and major histocompatibility complex class II molecules in renal carcinoma cells stimulated with S fimbriae of uropathogenic Escherichia coli. Infect Immun. 1993 Jul;61(7):3060–3063. doi: 10.1128/iai.61.7.3060-3063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Induction of cytokines in phagocytic mammalian cells infected with virulent and avirulent Listeria strains. Infect Immun. 1994 Feb;62(2):348–356. doi: 10.1128/iai.62.2.348-356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby D. A., Fortier A. H., Crawford R. M., Schreiber R. D., Nacy C. A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992 Jan;60(1):84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K., Arai T. Protective immunities induced by porins from mutant strains of Salmonella typhimurium. Microbiol Immunol. 1990;34(11):917–927. doi: 10.1111/j.1348-0421.1990.tb01070.x. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Muthukkumar S., Muthukkaruppan V. R. Mechanism of protective immunity induced by porin-lipopolysaccharide against murine salmonellosis. Infect Immun. 1993 Jul;61(7):3017–3025. doi: 10.1128/iai.61.7.3017-3025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992 Feb;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman L., Maluszynska G., Magnusson K. E., Stendahl O. Surface interaction between bacteria and phagocytic cells. Prog Drug Res. 1988;32:131–147. doi: 10.1007/978-3-0348-9154-7_4. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Whisenand J., McIntosh A. T. Effects of cytochalasin B on actin and myosin association with particle binding sites in mouse macrophages: implications with regard to the mechanism of action of the cytochalasins. J Cell Biol. 1981 Nov;91(2 Pt 1):373–384. doi: 10.1083/jcb.91.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers F. G., Greaves P. W., Macrae A. D. Flagella and fimbriae on Legionella organisms. Lancet. 1979 Oct 6;2(8145):753–754. doi: 10.1016/s0140-6736(79)90691-3. [DOI] [PubMed] [Google Scholar]
- Sonesson A., Jantzen E., Bryn K., Larsson L., Eng J. Chemical composition of a lipopolysaccharide from Legionella pneumophila. Arch Microbiol. 1989;153(1):72–78. doi: 10.1007/BF00277544. [DOI] [PubMed] [Google Scholar]
- Spilberg I., Mehta J. Chemotactic factor receptor modulation and cytoskeletal structures. Infect Immun. 1981 Apr;32(1):268–272. doi: 10.1128/iai.32.1.268-272.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. The involvement of TNF, IL-1 and IL-6 in the immune response to protozoan parasites. Immunol Today. 1991 Mar;12(3):A13–A16. doi: 10.1016/S0167-5699(05)80005-2. [DOI] [PubMed] [Google Scholar]
- Tsukada H., Kawamura I., Fujimura T., Igarashi K., Arakawa M., Mitsuyama M. Induction of macrophage interleukin-1 production by Listeria monocytogenes hemolysin. Cell Immunol. 1992 Mar;140(1):21–30. doi: 10.1016/0008-8749(92)90173-m. [DOI] [PubMed] [Google Scholar]
- Widen R. H., Newton C. A., Klein T. W., Friedman H. Antibody-mediated enhancement of Legionella pneumophila-induced interleukin 1 activity. Infect Immun. 1993 Oct;61(10):4027–4032. doi: 10.1128/iai.61.10.4027-4032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Moss C. W., Hochstein D. H., Arko R. J., Schalla W. O. "Endotoxicity" of the Legionnaires' disease bacterium. Ann Intern Med. 1979 Apr;90(4):624–627. doi: 10.7326/0003-4819-90-4-624. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Friedman H. Genetic control of macrophage susceptibility to infection by Legionella pneumophila. FEMS Microbiol Immunol. 1992 Feb;4(3):137–145. doi: 10.1111/j.1574-6968.1992.tb04980.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Friedman H. Legionella pneumophila growth in macrophages from susceptible mice is genetically controlled. Proc Soc Exp Biol Med. 1991 Apr;196(4):405–409. doi: 10.3181/00379727-196-43207. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C. A., Widen R., Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988 Feb;56(2):370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Goto Y., Mizuguchi Y., Nomoto K., Skamene E. Genetic control of natural resistance in mouse macrophages regulating intracellular Legionella pneumophila multiplication in vitro. Infect Immun. 1991 Jan;59(1):428–432. doi: 10.1128/iai.59.1.428-432.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]