Abstract
The emergence of multigene families has been hypothesized as a major contributor to the evolution of complex traits and speciation. To help understand how such multigene families arose and diverged during plant evolution, we examined the phylogenetic relationships of F-Box (FBX) genes, one of the largest and most polymorphic superfamilies known in the plant kingdom. FBX proteins comprise the target recognition subunit of SCF-type ubiquitin-protein ligases, where they individually recruit specific substrates for ubiquitylation. Through the extensive analysis of 10,811 FBX loci from 18 plant species, ranging from the alga Chlamydomonas reinhardtii to numerous monocots and eudicots, we discovered strikingly diverse evolutionary histories. The number of FBX loci varies widely and appears independent of the growth habit and life cycle of land plants, with a little as 198 predicted for Carica papaya to as many as 1350 predicted for Arabidopsis lyrata. This number differs substantially even among closely related species, with evidence for extensive gains/losses. Despite this extraordinary inter-species variation, one subset of FBX genes was conserved among most species examined. Together with evidence of strong purifying selection and expression, the ligases synthesized from these conserved loci likely direct essential ubiquitylation events. Another subset was much more lineage specific, showed more relaxed purifying selection, and was enriched in loci with little or no evidence of expression, suggesting that they either control more limited, species-specific processes or arose from genomic drift and thus may provide reservoirs for evolutionary innovation. Numerous FBX loci were also predicted to be pseudogenes with their numbers tightly correlated with the total number of FBX genes in each species. Taken together, it appears that the FBX superfamily has independently undergone substantial birth/death in many plant lineages, with its size and rapid evolution potentially reflecting a central role for ubiquitylation in driving plant fitness.
Introduction
The emergence and expansion of gene families and subsequent functional divergence among family members have long been hypothesized to be the principal driving forces in the adaptation of organisms to different environments [1]. Following their initial appearance, gene paralogs can subfunctionalize to extend and diversify their biological roles or can neo-functionalize to generate new traits. Family members can also acquire detrimental mutations that marginalize their activities or become inactivated, leading to the creation of pseudogenes. Some families, such as those encoding transcription factors, tend to have high rates of duplication (birth) but low rates of pseudogenization and loss (death) [2], [3]. At the other extreme, families with high birth and death rates have been found, including those encoding in animals the G protein-coupled chemosensory receptors, which detect food, ordants and pheromones [4], the immunoglobulins which form the primary immune defense system of vertebrates [5], and the nucleotide-binding site–leucine-rich repeat (NBS-LRR) receptors that help plants defend against pathogens, which are themselves under intense selection pressure to avoid detection [6]. Plants in particular appear to have exploited multiple gene families to adapt numerous developmental and physiological processes. These families arose, expanded, and diversified via whole genome duplications (polyploidy), which are common in many plant lineages, more restricted segmental duplications, highly specific tandem duplications and transposon-mediated events, and even exon shuffling among individual genes [7], [8], [9], [10], [11]. For example, it has been estimated that 65% of the Arabidopsis thaliana genes have closely related paralogs, with 17% of them generated from recent tandem duplications [7].
To help understand how divergent plant gene families evolved, we studied the expansion, evolutionary selection, and functional correlations of the F-Box (FBX) superfamily, which represents one of the largest and most diverse gene families in the plant kingdom [10], [12], [13], [14], [15]. FBX proteins comprise the target recognition subunit of SCF-type ubiquitin (Ub)-protein ligases (or E3s). They are defined by a signature ∼40-amino-acid FBX domain (FBXD) at their N-termini. The FBXD forms a compact trihelical structure that promotes docking with a SKP1 protein bridge, which helps associate the FBX protein with the rest of the ligase complex containing the Cullin-1 scaffold and RBX1 proteins [16], [17]. C-terminal to the FBXD is a variable recruitment module that binds to and delivers appropriate substrates for ubiquitylation. Some well-characterized recruitment modules include leucine-rich (LRR), kelch, WD-40 and tetratricopeptide repeats (TPR), Tubby, armadillo and lectin-related, which provide interaction surfaces for specific substrates [12], [15]. Often the ubiquitylated target is subsequently degraded by the 26S proteasome but non-proteolytic consequences have also been described [18].
A. thaliana alone was reported to contain approximately 700 FBX genes [12], [15], [19], [20], with this extraordinary number providing one of the first indications as to the importance of SCF E3s in the control of plant protein abundance. By comparison, only 20, 27, and 69 FBX proteins are encoded by the yeast (Saccharomyces cerevisiae), Drosophila melanogaster, and human genomes, respectively [21]. Such a pervasive role has also been supported by genetic studies linking specific plant FBX proteins to numerous processes, including hormone perception and signaling, stress protection, chromatin remodeling, homeosis, circadian rhythms, self incompatibility, and the defense against pathogens [17], [18], [22]. A connection of plant FBX proteins and other E3 components to the adaptive evolution of plant innate immunity has also been proposed [14], [23].
The sheer number of FBX genes in plants combined with evidence for extensive domain shuffling and polymorphisms and an atomic scale appreciation of their corresponding protein activities (e.g., [24], [25]) makes them excellent candidates to understand how plant gene families arose and diversified. Gagne et al. [12] provide the first detailed phylogenetic description of FBX genes using A. thaliana as the example. In addition to revealing the magnitude of the plant superfamily and the inclusion of numerous target-recruitment modules, they also detected frequent duplications of specific subsets along with potential domain shuffling between FBXD subtypes and the various recruitment modules. Subsequent phylogenetic analyses with Oryza sativa (rice), Populus trichocarpa (poplar), Vitis vinifera (grape), and Carica papaya (papaya) also identified large complements of FBX genes, but found substantial between species variation, with 680, 320, 159, and 139 loci estimated in each species, respectively [10], [15], [19]. High FBX gene numbers in herbaceous O. sativa and A. thaliana compared to the perennials P. trichocarpa and V. vinifera raised the possibility that the large FBX gene superfamilies in annuals reflected selection pressures to conform their ephemeral life cycles to a single growing season [19]. In addition to a set common to most of these species which likely directs conserved ubiquitylation reactions [15], [19], Xu et al. [15] also detected a more rapidly evolving set in A. thaliana which appears to have arisen by unusually frequent adjustments of exon/intron boundaries and frameshift mutations. Unfortunately, these limited phylogenetic studies: (i) failed to account for the likely presence of pseudogenes which may be common to the FBX superfamily [3], [26]; (ii) included very few plant species; (iii) did not infer orthologous relationships necessary to assess patterns of FBX gene gains and losses; and (iv) often failed to exhaustively search for all potential FBX loci, leaving their final gene numbers and conclusions uncertain.
Here, we studied the evolutionary history of plant FBX genes by identifying members of this superfamily in 18 plant species, ranging from the unicellular green alga (Chlamydomonas reinhardtii) and the seedless plants Physcomitrella patens (moss) and Selaginella moellendorffii (lycopod) to 15 monocot and eudicot species (both annual and perennial). Our data confirm that the number of FBX loci varies widely among plant species but shows little correlation with their genome size, growth habit, or annual/perennial life cycles. Moreover, the number of FBX loci can vary substantially even among closely related species, indicating that significant lineage/species-specific gene gains/losses occurred. A subset of highly expressed FBX proteins was detected in nearly all terrestrial species examined, suggesting that they perform functions essential to life on land. In addition, we discovered a substantial and likely non-functional collection of highly polymorphic, lineage/species-specific FBX genes and pseudogenes, strongly suggesting that genomic drift contributed to plant FBX protein diversification.
Results
Comprehensive Identification of FBX Genes Reveals Dramatically Varied Gene Numbers in the Plant Kingdom
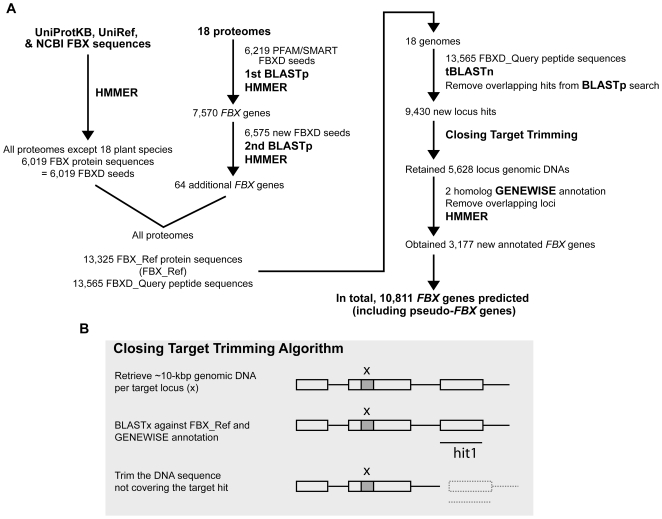
To better understand the organization and evolutionary history of the FBX superfamily in the plant kingdom, we initiated a comprehensive examination of the corresponding genes from all 18 complete or nearly complete plant genomic sequences available as of January 10, 2010 in Phytozome V5 (http://www.phytozome.net/). This database included a wide range of species, thus allowing us to analyze the ∼450 million year evolutionary ascent of multicellular land plants as they evolved presumably from single-cell aquatic progenitors (Table S1). To exhaustively query these genomes, we built a comprehensive FBX gene annotation pipeline and compiled two extensive collections of FBX protein sequences from both plant and non-plant sources (Figure 1). The first collection contained 13,565 FBXD peptide sequences (FBXD_Query, File S1), which was used to initially identify potential FBX genes in each genome. The second collection, containing 13,325 full-length FBX protein sequences (FBX_Ref, File S2), was then employed to develop transcript models and to predict coding sequences in previously non-annotated genes (we designated a gene non-annotated if it was not present previously in the corresponding proteome database). The FBXD_Query collection was used to exhaustively search the 18 unmasked plant genomes for FBXD regions by tBLASTn using an E-value cut off ≤1, Table S2) [27]. Preliminary studies with the A. thaliana superfamily found that this E-value was necessary to include all known FBX loci characterized either genetically or by interactions studies (see below and Tables S3 and S4). In addition to finding most, if not all, previously annotated FBXD-encoding loci [10], [12], [14], [15], [19], [20], we discovered 9,430 new regions, each of which may be part of a previously non-annotated FBX locus (Figure 1A, Table S2).
Figure 1. Pipeline for the comprehensive annotation of FBX genes in 18 plant species.
(A) Procedures of FBX gene identification from 18 plant genomes. The initial FBXD query collection from PFAM and SMART was used to find the FBX proteins by BLASTp searches and HMMER3 predictions of each plant proteome and then incorporated with the FBX proteins collected from the other plant and non-plant proteomes to create two comprehensive sequence collections, FBX_Query and FBX_Ref. FBX_Query was used to search each genome for FBXD regions by tBLASTn and the non-annotated regions surrounding the FBXD were re-annotated by the Closing Target Trimming (CTT) algorithm and the similarity-based annotation program GENEWISE. To avoid the bias, at least two reference sequences from FBX_Ref collection were used to predict the transcript model of each non-annotated potential FBX locus. Only when both sequences predicted the same transcript model was the coding sequence generated with the best GENEWISE score accepted as the final annotation. (B) Schematic description of the CTT algorithm. The “x” indicates a target FBXD region identified from tBLASTn search. “Hit1” indicates the top hit from a BLASTx search using the ∼10-kbp genomic DNA sequence as query against FBX_Ref. The process was iterated for up to 6 times for each non-annotated potential FBX locus.
Initial studies showed that the coding region (plus intervening introns) of most annotated plant FBX genes is less then 5 kbp (95.3%), with a few between 5 kbp and 10 kbp (3.8%), and almost none over 10 kbp (1%) (Figure S1). Because our initial approach would only identify regions with high sequence similarity but not the full-length coding sequence, we retrieved a 10-kbp window surrounding each new FBXD region to obtain a more complete FBX gene model, using the sequence similarity-based predictions of GENEWISE with the FBX_Ref collection serving as the scaffolds. Because plant FBX genes frequently appear in tandem (e.g., [12]), some of these 10-kpb genomic windows likely include multiple FBX loci. To overcome this complication, we developed the Closing Target Trimming (CTT) algorithm to computationally trim the transcriptional unit of each FBX gene in the window and separate it from adjacent FBX loci (Figure 1B). For final annotation of each transcriptional unit (protein-coding gene or pseudogene), we used the two or three top-scored (bit-score value) protein reference sequences in the GENEWISE prediction, obtained by first querying the FBX_Ref collection using BLASTx (E-value ≤1e-5) [27]. From preliminary analysis of 47 well-characterized A. thaliana FBX genes (Table S3), we found that two or more references were required for unbiased annotations. Thus, only when at least two reference sequences predicted the same transcript model (protein coding versus pseudogene) did we conclude that a good model was generated.
To further confirm that our new annotation strategy with CTT would correctly predict transcript models, we re-annotated each A. thaliana and O. sativa FBX locus predicted from the original proteomes from its 10-kbp flanking genomic DNA sequence, which was retrieved as a putative non-annotated FBX loci described above. The result showed that 89.3% (634/710) and 93.9% (750/799) of the originally annotated FBX loci from A. thaliana and O. sativa, respectively, were correctly re-annotated. These new annotations closely matched the original protein-coding genes (sequence identity ∼99% for both A. thaliana and O. sativa). In addition, we discovered a large collection of FBX pseudogenes (sequence identity ∼95% (A. thaliana) and ∼94% (O. sativa)), which were originally annotated as protein-coding loci. In most cases, the coding sequence models of these pseudogenes were shorter than predicted previously due to the presence of frameshifts or premature stop codons (Figure S2, File S3 and S4).
After CTT and sequence similarity-based annotations combined with HMMER3 predictions (E-value <1) using the PFAM database [28] (Version 24.0, released in October 2009, 11,912 protein families), we discovered a collection of 3,177 new FBX loci. When combined with 7,634 loci identified from the previously annotated proteomes, a comprehensive collection of 10,811 FBX genes was generated that encompassed all 18 plant species (Figure 1, Tables 1 and S2, Files S5 and S6). To support the depth of the collection, we searched it for all A. thaliana FBX proteins previously demonstrated to interact with their SKP1 protein bridges (designated ASK1-19), or defined genetically to participate in the Ub-26S proteasome system (UPS); all 86 were found (Tables S3 and S4z).
Table 1. Predictions of FBX gene numbers in the 18 plant species.
| Species1 | Genome size(Mb) | TotalFBX(genes) | LTSP | STSP | FBX Pseudogenes | |||
| genes | % of total | genes | % of total | genes | % of total | |||
| Al | 206.7 | 1350 | 219 | 16.2 | 761 | 56.4 | 370 | 27.4 |
| At | 119.7 | 897 | 191 | 21.3 | 507 | 56.5 | 199 | 22.2 |
| Bd | 271.9 | 998 | 177 | 17.7 | 509 | 51.0 | 312 | 31.3 |
| Cp | 331.3 | 198 | 124 | 62.6 | 35 | 17.7 | 39 | 19.7 |
| Cr | 112.3 | 88 | 9 | 10.2 | 74 | 84.1 | 5 | 5.7 |
| Cs | 203.1 | 207 | 152 | 73.4 | 46 | 22.2 | 9 | 4.3 |
| Gm | 973.3 | 702 | 332 | 47.3 | 208 | 29.6 | 162 | 23.1 |
| Me | 416.7 | 323 | 230 | 71.2 | 75 | 23.2 | 18 | 5.6 |
| Mg | 321.7 | 903 | 459 | 50.8 | 292 | 32.3 | 152 | 16.8 |
| Mt | 307.5 | 1148 | 391 | 34.1 | 517 | 45.0 | 240 | 20.9 |
| Os | 372.3 | 971 | 247 | 25.4 | 517 | 53.2 | 207 | 21.3 |
| Pp | 480 | 258 | 117 | 45.3 | 124 | 48.1 | 17 | 6.6 |
| Pt | 417.1 | 425 | 280 | 65.9 | 107 | 25.2 | 38 | 8.9 |
| Rc | 350.6 | 250 | 158 | 63.2 | 57 | 22.8 | 35 | 14 |
| Sb | 738.5 | 817 | 202 | 24.7 | 472 | 57.8 | 143 | 17.5 |
| Sm | 212.8 | 544 | 136 | 25.0 | 324 | 59.6 | 84 | 15.4 |
| Vv | 497.5 | 315 | 202 | 64.1 | 76 | 24.1 | 37 | 11.7 |
| Zm | 2061 | 417 | 175 | 42.0 | 173 | 41.5 | 69 | 16.5 |
Species abbreviations: Al, Arabidopsis lyrata; At, Arabidopsis thaliana; Bd, Brachypodium distachyon; Cp, Carica papaya; Cr, Chlamydomonas reinhardtii; Cs, Cucumis sativus; Gm, Glycine max; Me, Manihot esculenta; Mt, Medicago truncatula; Mg, Mimulus guttatus; Os, Oryza sativa; Pp, Physcomitrella patens; Pt, Populus trichocarpa; Rc, Ricinus communis; Sm, Selaginella moellendorffii; Sb, Sorghum bicolor; Vv, Vitis vinifera; Zm, Zea mays.
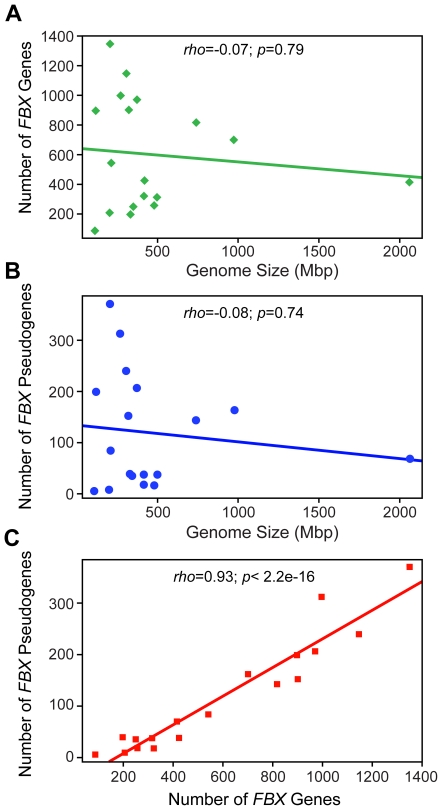
Similar to earlier more-limited studies [10], [15], [19], we found widely different numbers of total FBX genes among the 18 plant species (Table 1). Surprisingly, the gene numbers did not correlate well with the developmental complexity, growth habit, or even evolutionary distance between species. The number of FBX loci also showed no correlation with genome size with some of the smaller plant genomes having larger numbers (e.g., A. lyrata (Figure 2A)). Clearly, the unicellular alga C. reinhardtii has the lowest number of FBX loci (88), implying that expansion of the superfamily was connected in part to the evolution of multi-cellularity and/or terrestrial life. However, S. moellendorffii, a seedless vascular plant, contains substantially more FBX genes than the seed plants, C. papaya, Cucumis sativus (cucumber), and Ricinus communis (castor bean) (544 versus 198/207/255), suggesting that increasing FBX gene numbers are not related to land plant evolution nor the appearance of more sophisticated seed plants. Similarly, two annual herbaceous plants, C. sativus and R. communis, contain lower numbers of FBX genes than the perennial woody plant P. trichocarpa (207/250 versus 425), which does not support the hypothesis that annual species contain more FBX loci than perennial species [19]. Perennial herbaceous plants could have less (903 in Mimulus guttatus or monkey flower) or more (1350 in A. lyrata) FBX genes than annual herbaceous plants (971 in O. sativa and 998 in Brachypodium distachyon or purple false brome). Even closely related species such as Zea mays (corn)/Sorghum bicolor (split ∼12 million-years ago (Mya) [29]), Glycine max (soybean)/Medicago truncatula (barrel medic) (split ∼50 Mya [30]), and A. thaliana/A. lyrata (split ∼5 Mya [31]) have widely different gene numbers (417 versus 817, 702 versus 1148, and 897 versus 1350, respectively), indicating that FBX gene birth/death has been rapid.
Figure 2. Relationship between the number of total FBX genes, FBX pseudogenes and genome size for 18 plant species.
(A,B) The number of total FBX genes (A) and the number of FBX pseudogenes (B) were plotted against the genome size of each species. (C) The number of FBX pseudogenes was plotted against the number of total FBX genes in each species. The Spearman rank correlation coefficients (rho), the corresponding p values, and the linear model-fitted trend lines are shown.
Evidence for Rapid Lineage/Species-Specific Birth/Death of FBX Genes
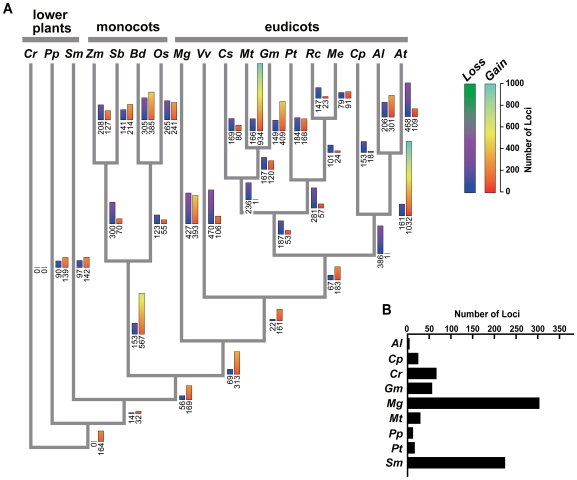
To determine if both birth and death occurred in the FBX superfamily among the 18 plant species, we built phylogenetic trees based on the FBX protein sequences. Because of the size and complexity of the entire 10,811-member collection, we first grouped the sequences into 351 clusters using MCL [32] from the all-against-all BLASTp search result (E-value <1e-5) [27]. Here, full-length protein sequences were used instead of just the ∼40-amino-acid FBXD to improve accuracy. Forty-seven FBX clusters assembled with 734 total sequences contained proteins from only a single species. These unique sequences likely reflect birth of new FBX genes in that species and were removed from the phylogenetic analysis (Figure 3B). Maximum likelihood trees were generated with each of the remaining clusters and the estimates for FBX gene birth and death along each branch of the 18 species phylogeny were inferred based on reconciliation of the gene and species trees (see Materials and Methods).
Figure 3. The gain/loss analysis of FBX genes during the evolution of 18 plant species.
(A) The gain and loss of FBX genes in each species and species split nodes. The phylogeny of each species and the taxonomic group designations were adopted from the Angiosperm Phylogeny Website (http://www.mobot.org/MOBOT/research/APweb/). The heatmap color bars at each branch/node represent the predicted number of genes gained (right) or lost (left), respectively. The actual numbers of gains/losses are indicated below each bar. The full names of the species along with their abbreviations are as listed in Table 1. (B) Species-specific generation of FBX genes in each species.
Based on the tree-reconciled results, we detected several independent and dramatic FBX gene birth/death events during plant evolution. We saw a net positive gain of FBX loci during the steady expansion of the superfamily early in land plant evolution (Figure 3A). In multiple other branches, there were high gene birth rates accompanied by low death rates. Examples include the appearance of the Arabidopsis genus (161 losses and 1032 gains), and the possible speciation of M. truncatula (166 losses and 934 gains) (Figure 3A). However, net gain was not the predominant pattern in the recent history (<450 Mya) of FBX gene evolution. In nine of the 15 lineages leading to extant flowering plant species (A. thaliana, C. papaya, C. sativus, M. guttatus, O. sativa, P. trichocarpa, R. communis, V. vinifera, and Z. mays), there was significantly more gene death relative to gene birth. These patterns together with the finding that FBX gene birth and death in general do not correlated with life history traits or evolutionary distance of the species, indicate that the process(es) which caused the dramatic size differences among FBX gene superfamilies are highly lineage specific.
Expansion of FBX Genes Correlates with a High Frequency of Gene Inactivation
The large variations in total FBX gene numbers and dramatic birth/death in the 18 plant species implied that some loci might be inactive, in part by accumulating deleterious coding region mutations [4]. In accord, we found, while predicting the transcript models of the previously non-annotated FBX genes, a substantial number of putative pseudogenes containing frameshifts or premature stop codons. To find the full complement, we retrieved the full-length genomic DNA sequence for each previously annotated FBX gene and reexamined by GENEWISE its transcript model. Here, two or three top-scored non-self FBX protein sequences were used as references instead of its own protein sequence to avoid bias. When the two lists were combined, we found surprisingly varied numbers of likely FBX pseudogenes in the 18 plant species (Table 1). For example, both A. lyrata and B. distachyon contain over 300 predicted pseudogenes while C. sativus, Manihot esculenta (cassava), and P. patens contain less than 20. The total numbers of pseudogenes in the 18 plant species correlated poorly with the genome size of the plant but had a strikingly tight correlation with the total number of FBX loci (Figure 2B,C). The latter connection implies that the FBX superfamily has undergone substantial death in species with high numbers of FBX loci.
Varied Distributions of FBX Protein Subfamilies within the Plant Kingdom
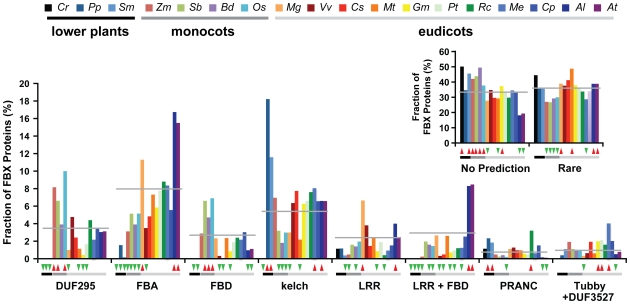
Prior studies with A. thaliana, P. trichocarpa and O. sativa suggested that the FBX superfamily has not changed uniformly in plants but with a lineage-specific emphases on particular types of C-terminal recruitment modules (e.g., LRR, kelch repeat, and FBX-associated (FBA)) [15]. To provide further support for such uneven gains/losses, we determined the number of predicted FBX proteins containing the various recruitment modules in each species, using HMMER3 to detect known domains from the PFAM database. If two or more modules were predicted, the FBX protein was sorted according to the combination. Based on the above criterion, we classified the FBX protein collection from the 18 species into 3,269 groups, which either contained no recognizable modules (3,589 total), modules/combinations found rarely (3,678 total), or modules/combinations found significantly more frequently (≥10 representatives, 3,544 total) (Fischer's exact test, p<0.05). The latter group encompassed 32 different modules/combinations, including the FBA, kelch repeat, DUF295, FBD, LRR and Tubby+DUF3527 modules (Table S5). Interestingly, the predicted FBX pseudogenes were selectively enriched in the group without any predicted recruitment modules (Fischer's exact test, p<0.05), suggesting that many of the corresponding proteins lost their ability to bind targets even if expressed (Table S6). Such loss is consistent with the fact that pseudogenes are no longer under selection pressure, with many becoming fragmented as compared to functional genes [3]. In contrast, the remaining FBX loci without the hallmarks of pseudogenization were enriched in coding regions for known recruitment modules (Fischer's exact test, p<0.05), including 10 well-characterized interaction domains that could participate in target recognition (Table S6).
When we compared the FBX locus numbers in the no recognizable, infrequent and frequent categories among the 18 plant species, we identified numerous species that are selectively enriched/depleted in specific recruitment modules/combinations (Figure 4 and Table S7). As examples, C. reinhardtii and the seedless plants P. patens and S. moellendorffii do not encode FBX proteins with obvious DUF295, FBD, or LRR-FBD modules even though FBX proteins with these modules are common in seed plants. Conversely, P. patens and S. moellendorffii are enriched in FBX proteins with kelch repeat or PRANC modules. A. thaliana and A. lyrata are enriched in FBX proteins with LRR+FBD and the FBA modules, whereas most monocots are depleted in these subtypes. Overall, module enrichment/depletion did not agree with the evolutionary tree of the represented plants, in line with the seemingly heterogeneous birth/death rates of the entire FBX gene superfamily.
Figure 4. The enrichment/depletion of various C-terminal substrate-recognition modules in the collection of FBX proteins from each species.
The red and green-inverted triangles indicate the significant enrichment and depletion of FBX proteins (p<0.05, Fisher's exact test), respectively, for each species as compared with the average number (indicated by a horizontal grey line) of FBX proteins containing the same module from all 18 plant species.
Evidence for Three Subgroups of FBX Genes with Distinct Evolutionary Histories
The functional importance of SCF E3s in plants [17], [18] combined with the heterogeneous FBX gene number and module distributions implied that multiple subgroups exist. One group may have been retained during plant (or even eukaryote) evolution and thus likely participates in conserved, possibly essential processes, whereas another could have appeared more recently and likely participates in more species-specific events or is inactive. To help identify members of these two groups, we assigned each FBX gene (protein coding and pseudogene) in the 18 species to orthologous groups (OGs) using OrthoMcl [33]. Those FBX genes which were not assigned to any OG were defined as “orphan”, while the remaining were grouped into 18 bins depending on whether a homolog could be found in its own genome (potential paralogs with absence of obvious orthologs in the other species, bin 1) or in the 17 other plant species in the collection (potential orthologs, bins 2–18).
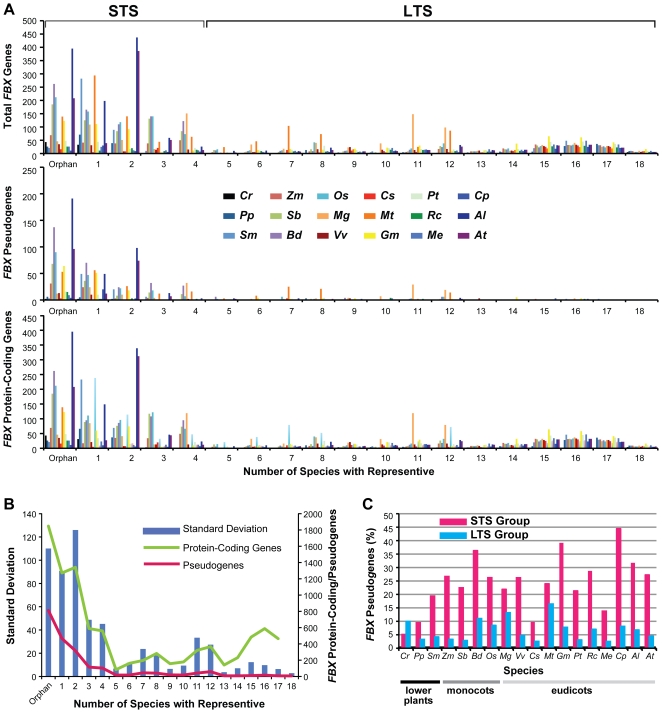
As can be seen in Figure 5A,B, two separate OG collections emerged depending on the number of species included. The larger collection included the orphan genes, numerous loci in bin 1 which appear to be species specific, and loci in bins 2–4 which have orthologs in only 1–3 of the 17 other species. We designated this collection as small taxonomic scale (STS) to reflect their limited distributions, with the logical inference that they are either inactive loci or perform functions limited to few plant species. In fact, most of the pseudogenes are in the STS collection (Figure 5C). The confinement of most FBX pseudogenes to a single or few species implies that they either were formed relatively recently or represent ancient loci that have diverged by neutral change beyond recognition. Whereas land plants have 18–60% of their FBX genes in the STS collection, C. reinhardtii has a substantially higher percentage (84% of total). This increase may reflect methodological issues in generating OG relationships with such a divergent plant species, or the possibility that many of the C. reinhardtii FBX loci have either algal-specific functions, have functions in common within land plants but have significantly diverged sequences, or have diverged enough to become inactive.
Figure 5. The divergent distribution of FBX gene numbers at different conservation levels.
(A) The numbers of total FBX genes (top panel), FBX pseudogenes (middle panel), and FBX protein-coding genes (bottom panel) from each species were plotted against the number of species represented in the FBX orthologous groups (OGs). “Orphan” indicates the genes without any orthologs. “1–18” denotes the numbers of species in an OG. STS, small taxonomic scale; LTS, large taxonomic scale. (B) The distribution of standard deviations of FBX gene numbers, total protein-coding FBX gene numbers, and total FBX pseudogenes from 17 plant species (Cr is not included) at different conservation levels. (C) The percentage of FBX pseudogenes with low (STS) or high (LTS) conservation levels in each of the 18 plant species.
The second smaller OG collection included members in bins 5–18, which we have designated large taxonomic scale (LTS) to reflect their wider distributions among the plants. The LTS collection has fewer predicted pseudogenes as compared to the STS collection and three potentially distinct subsets of protein-coding genes (Figure 5A,B). The largest subset includes members in bins 14–18, which based on their broad distribution, likely encode highly conserved and ancient FBX proteins that perform tasks essential to all plants, or even to eukaryotes in general. In support, a number of well-characterized FBX proteins from A. thaliana are in these bins, including TIR1, COI1, SLY1, LKP2, FKF1, ZTL, and EBF1/2 (Table S3). Two more restricted subsets, centered at bins 7–8 and 10–12, include FBX genes with partially limited phylogenetic distributions. By checking the OGs from these more restricted bins, we found that the species represented were not limited to eudicots, monocots, or lower plants separately but in various combinations, suggesting that their emergence was not caused by species sampling biases (e.g., 11 eudicots versus 4 monocots). For example, analysis of all 34 OGs centered at bins 10–12, revealed only 5 that comprised a FBX gene subgroup which was enriched in eudicots (10 of the 11 species) but absent in the monocots and lower plant representatives. The remaining 29 OGs contained species in various combinations, suggesting they experienced independent lineage-specific losses. The only notable exception to the LTS/STS grouping was C. reinhardtii, which was calculated to have more pseudogenes in its LTS collection on a percentage basis than STS. We do not consider this deviation significant given the fact that this alga has few total FBX loci (88), and very few pseudogenes (5) and members of the LTS collection (9) (Table 1).
Based on this OG analysis, we divided the FBX loci into three broad groups, pseudogenes, and protein-coding genes in the STS (STSP) and LTS (LTSP) groups. We then examined separately their evolutionary selection (purifying or relaxed), mechanism of duplication (e.g., segmental versus tandem duplications), and their expression levels/patterns, which are all potential indicators of functionality and evolutionary history.
Differential Selection Pressures on the LTSP, STSP and FBX Pseudogene Loci
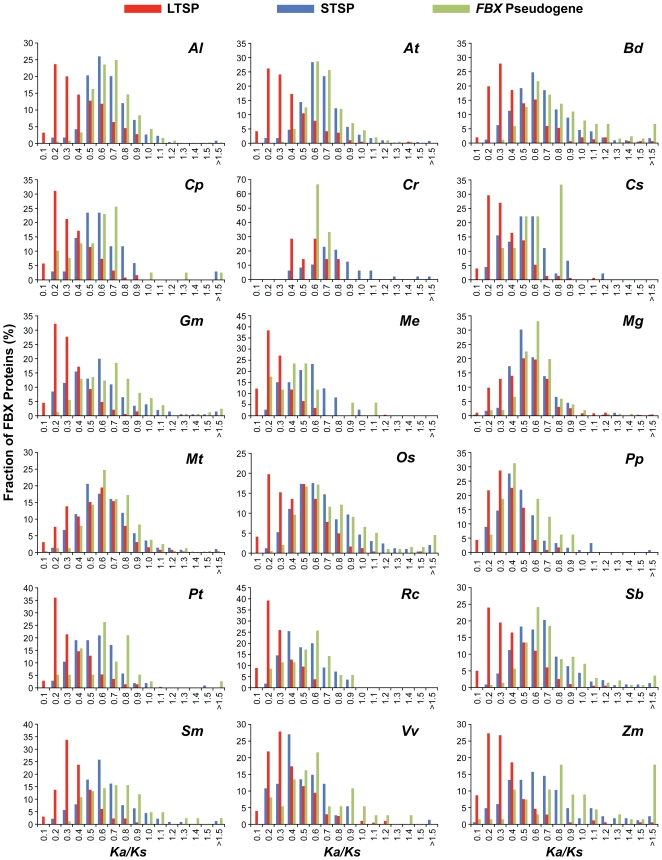
The ratio of non-synonymous (Ka) to synonymous (Ks) nucleotide substitution rates allows assessing the nature of gene selection in which Ka/Ks values well below 1 are indicative of functional genes under purifying selection, whereas Ka/Ks values approaching 1 are indicative of non-functional genes experiencing relaxed selection and pseudogenes undergoing neutral change. Using the Ka/Ks estimation method of Goldman and Yang [34], we found that the LTSP group of FBX loci is enriched for genes undergoing stronger purifying selection whereas the STSP and pseudogene groups are enriched for loci experiencing more relaxed selection or neutral change. This difference between LTSP and STSP/pseudogenes, as observed in the frequency distributions of the Ka/Ks values (Figure 6, Table S8), was obvious for almost all plant species tested with significant purifying selection evident for the LTSP genes in 16 of the 18 species (Wilcoxon rank sum test, p<0.001). The differences in frequency distributions were statistically less significant for the remaining two species, C. reinhardtii and P. patens, even though a slight separation of the Ka/Ks values could be seen between the LTSP and STSP/pseudogene groups (Figure 6, Table S8).
Figure 6. The distributions of Ka/Ks values for the LTSP, STSP and FBX pseudogenes in each of 18 plant species.
The Ka/Ks value for each full-length sequence was calculated by comparing it to the MRCA transcript sequence using the method of Goldman and Yang [34].
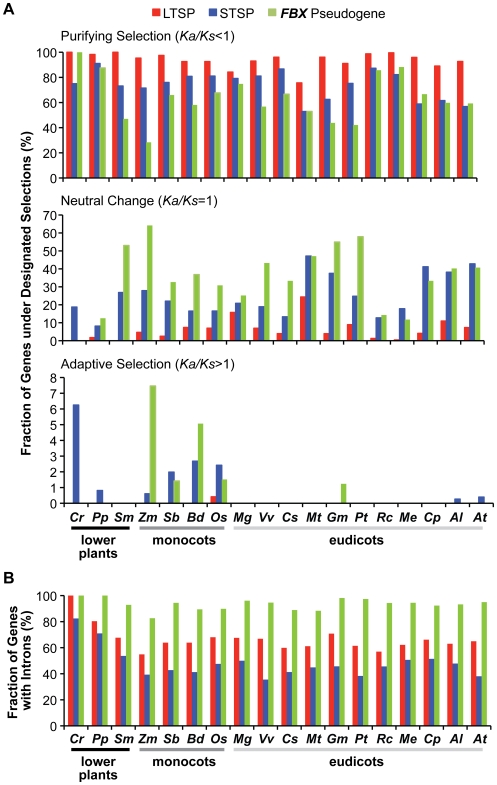
The overlapping Ka/Ks profiles of STSP and FBX pseudogene groups predicted that a substantial number of STSP genes experienced the same neutral change constraints as FBX pseudogenes. To further validate this scenario, we applied the Ka/Ks ratio test of Nekrutenko et al. [35] to compare the LTSP, STSP and FBX pseudogene groups for the enrichment of loci with more purifying or more relaxed/neutral Ka/Ks values (at 5% significance level), respectively. As seen in Figure 7A, both the STSP and FBX pseudogene groups had lower percentages of genes with purifying Ka/Ks values but contained much higher percentages of genes with relaxed/neutral Ka/Ks values as compared to the LTSP group for almost all 18 species. Some species displayed especially strong differences with few LTSP genes containing neutral values (e.g., S. moellendorffii, R. communis, and M. esculenta). M. guttatus was the only exception; here, the percentages of genes with neutral Ka/Ks values were near equal for the LTSP and STSP/pseudogene groups (Figure 7A). It is also noteworthy that we detected a small collection of mainly STSP and predicted FBX pseudogenes with Ka/Ks values exceeding 1.4, which could be indicative of adaptive selection (Figure 6). Most of these loci were from C. reinhardtii and the sampled monocot species (e.g., Z. mays (Figure 7A)). The reason(s) behind this anomaly are unknown but could imply that some monocot FBX loci are under strong positive selection, i.e., accumulating non-synonymous substitutions faster than synonymous substitutions.
Figure 7. Comparisons of evolutionary selections and intronization among the LTSP, STSP and FBX pseudogene loci in each species.
(A) The percentage of FBX genes under purifying selection (top panel), neutral change (middle panel), and adaptive selection (bottom panel) in each subgroup. (B) The percentage of FBX genes containing at least one intron in each subgroup.
As an alternative, we used the method of Nei and Gojobori [36] to calculate Ka/Ks values, which applies p-distance for sequence distance calculations. Similar differences in Ka/Ks frequency distributions were observed for the LTSP, STSP and FBX pseudogenes among the 18 species (Table S9), further supporting the different selection pressures among the three groups. The Ka/Ks comparisons discussed above were based on the complete FBX gene. To test whether the FBXDs and the remainder of the FBX coding regions (ΔFBXDs) might be under different selection pressures, we also compared the Ka/Ks distributions for the FBXD and ΔFBXD regions separately. Like the full-length FBX Ka/Ks distributions, those for the FBXD and ΔFBXD sequences differed between the LTSP and STSP/pseudogene groups, suggesting that the FBXD and recruitment modules were under similar purifying and relaxed/neutral change pressures, respectively. The differences between the groups were less for the FBXD comparisons, likely reflecting the fact that the FBXDs must retain their interactions with a limited set of SKP1 proteins to remain functional (Figures S3, S4, and Table S9). Taken together, it appears that the LTSP genes are under stronger purifying selection while STSP genes and FBX pseudogenes are under more relaxed selection. The relatively reduced selection constraints of STSP members are consistent with a lesser functional importance and suggest that many are progressing toward becoming pseudogenes.
STSP and FBX Pseudogenes Used Similar Amplification Mechanisms
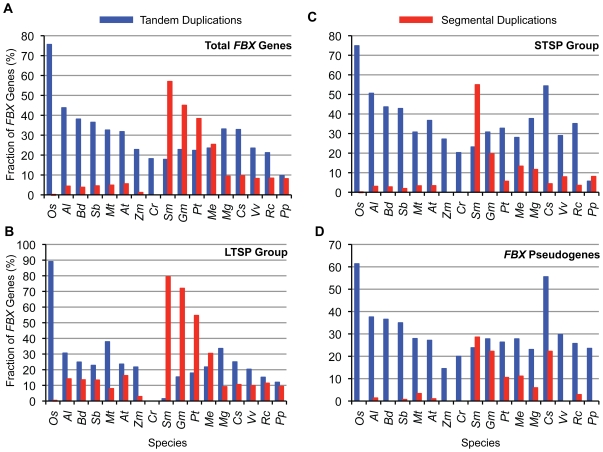
Further evidence that STSP and FBX pseudogenes are evolutionarily related was provided by examining their amplification patterns and chromosomal locations. Previous studies with A. thaliana and O. sativa suggested that a large number of FBX genes evolved recently by tandem duplications [12], [15], [19]. To examine if this mechanism was used more universally, we determined by DAGchainer [37] the number of LTSP, STSP and FBX pseudogene loci among 17 of the plant species which were derived from tandem or segmental duplications (File S7). (C. papaya was excluded due to the absence of gene position information at the time of the analysis). Segmental duplications included those events generated via whole genome amplifications (polyploidy) as well as the duplication of smaller chromosomal segments, while tandem duplications were defined as events that generated paralogous genes in proximal genomic regions [11]. The remaining loci were either singletons or duplicates of unclear origins (e.g., retrotransposition).
Based on the relative contributions of tandem versus segmental duplications to the FBX gene repertoire, we found three different FBX gene expansion patterns (Figure 8A). One pattern, evident in A. thaliana, A. lyrata, B. distachyon, C. reinhardtii, M. truncatula, O. sativa, S. bicolor, and Z. mays, predicted that tandem duplications played the main role while segmental duplication played only a minor role during the FBX gene expansion. The most obvious examples are C. reinhardtii and O. sativa where none of their entire collection of FBX loci arose from segmental duplications while most arose from tandem duplications. The second pattern, evident in S. moellendorffii, G. max, P. trichocarpa, and M. esculenta, predicted that both segmental and tandem duplications contributed significantly to FBX gene expansion with segmental events playing the dominant role in forming the LTSP group (Figure 8A–C). The third pattern was intermediate of the first two and was exemplified by C. sativus, M. guttatus, P. patens, R. communis, and V. vinifera. None of these three patterns was confined to a particular plant group (algae, lower plants, eudicots and monocots), implying that the relative importance of duplication mechanisms, like other traits of FBX loci, differed among the plant lineages.
Figure 8. The distributions of tandem and segmental duplicated FBX genes in each of 18 plant species.
(A) Total FBX genes. (B) STSP genes. (C) LTSP genes. (D) FBX pseudogenes.
When analyzing the duplication patterns of the LTSP, STSP and FBX pseudogene groups separately, it became obvious that the STSP and FBX pseudogene loci relied more heavily on tandem duplications for their birth as compared to the LTSP group (Figure 8B–D). Species with especially strong bias for tandem versus segmental events include B. distachyon, C. reinhardtii, O. sativa, P. patens, V. vinifera and Z. mays, which have very few or no STSP/pseudogene loci potentially generated by segmental events (Figure 8D)). Pseudogenes are typically more enriched relative to active genes in the heterochromatic and generally transcriptionally silent regions near centromeres [38]. Using A. thaliana as an example, we accordingly found that the distributions of STSP and FBX pseudogene members are more clustered and extend closer to the centromeres than are LTSP genes, especially for chromosomes 2, 3 and 5 (Figure S5).
We also examined whether the sizes of the STSP and LTSP groups correlated with genome size by a Spearman rank correlation coefficient analysis of all the 18 plant species. No significant correlations were observed (LTSP, rho = 0.3, p = 0.3 and STSP, rho = −0.1, p = 0.7). In contrast to a previous hypothesis that herbaceous annual plants contain more FBX genes than woody perennial plants [19], we detected no such connection. Using the two-sided Kolmogorov-Smirnov test, we found that the plants analyzed here (Table S1) did not cluster significantly based on growth habit when either the total FBX (p = 0.1), LTSP (p = 0.6), STSP (p = 0.04), or FBX pseudogene (p = 0.04) groups were examined separately.
The FBX pseudogenes could have arisen from gene duplication events followed by inactivation or by retrotransposition using FBX mRNAs as templates, which would generate a priori pseudogenes without introns or native promoters. Analysis of human pseudogene birth found that both mechanisms were involved with the paucity of obvious introns implying that retrotransposition played the dominant role (e.g., [39]). For the 18 plant species tested here, we found the opposite effect, that the exonic regions of most FBX pseudogenes were interrupted by intron-like sequences. In fact, a greater percentage of FBX pseudogenes were affected by intron-like sequences and contained higher numbers of these inserts than both LTSP and STSP genes (Figure 7B). These intervening sequences could represent ancestral introns, thus arguing against a role for retrotransposition in FBX pseudogene expansion. Alternatively, some intron-containing pseudogenes may still have arisen by retrotransposition with the intron-free coding regions then frequently acquiring intron-like insertions during their initial inactivation and/or continued degeneration. The fact that members of the STSP group, which could represent non-functional loci in the process of pseudogenization, have comparably fewer introns would support the latter scenario.
A Substantial Number of STSP and FBX Pseudogene Loci are Predicted to be Less or Non-Functional
To test whether a substantial number of STSP loci are less or non-functional, we compared the expression patterns of members from LTSP, STSP and FBX pseudogene groups in each plant species, using the availability of expressed sequence tags (ESTs) to estimate transcriptional activity. In 15 of the 18 species, significantly higher numbers of expressed FBX genes were from the LTSP group (Fischer's exact test, p<0.001, Table 2). Conversely, for 14 of the 18 species, loci with no evidence of expression were statistically enriched in the STSP and FBX pseudogene groups (p<0.001, Table 2), suggesting that many of the loci in these two groups are transcriptionally inactive and thus not likely functional.
Table 2. Comparison of FBX gene expression among LTSP genes, STSP genes, and FBX pseudogenes.
| Species1 | LTSP | STSP | FBX Pseudogene | Fisher's exact test | |||||
| W_est | Wo_est | W_est | Wo_est | W_est | Wo_est | LTSP>STSP | LTSP>Pseudo | STSP>Pseudo | |
| Al | 116 | 103 | 49 | 712 | 10 | 360 | <2.2E-16* | <2.2E-16* | 4.4E-03 |
| At | 161 | 30 | 172 | 335 | 42 | 157 | <2.2E-16* | <2.2E-16* | 4.7E-04* |
| Bd | 140 | 37 | 218 | 291 | 107 | 205 | <2.2E-16* | <2.2E-16* | 9.1E-03 |
| Cp | 54 | 70 | 4 | 31 | 2 | 37 | 2.5E-04* | 1.9E-06* | 2.9E-01 |
| Cr | 1 | 8 | 13 | 61 | 1 | 4 | 8.3E-01 | 8.9E-01 | 7.9E-01 |
| Cs | 17 | 135 | 1 | 45 | 0 | 9 | 4.8E-02 | 3.6E-01 | 8.4E-01 |
| Gm | 273 | 59 | 39 | 169 | 27 | 135 | <2.2E-16* | <2.2E-16* | 3.5E-01 |
| Me | 76 | 154 | 6 | 69 | 2 | 16 | 4.9E-06* | 4.1E-02 | 8.2E-01 |
| Mg | 135 | 324 | 37 | 255 | 11 | 141 | 3.6E-08* | 1.6E-09* | 5.3E-02 |
| Mt | 134 | 257 | 68 | 449 | 14 | 226 | 3.5E-14* | <2.2E-16* | 1.3E-03 |
| Os | 183 | 64 | 204 | 313 | 42 | 165 | <2.2E-16* | <2.2E-16* | 3.5E-07* |
| Pp | 73 | 44 | 60 | 64 | 7 | 10 | 2.0E-02 | 8.2E-02 | 3.8E-01 |
| Pt | 95 | 185 | 14 | 93 | 2 | 36 | 1.8E-05* | 7.2E-05* | 1.5E-01 |
| Rc | 69 | 89 | 2 | 55 | 4 | 31 | 1.1E-09* | 1.8E-04* | 9.7E-01 |
| Sb | 107 | 95 | 123 | 349 | 14 | 129 | 2.4E-11* | <2.2E-16* | 1.2E-05* |
| Sm | 63 | 73 | 66 | 258 | 8 | 76 | 3.0E-08* | 2.9E-09* | 1.3E-02 |
| Vv | 95 | 107 | 15 | 61 | 7 | 30 | 1.8E-05* | 9.7E-04* | 5.7E-01 |
| Zm | 166 | 9 | 121 | 52 | 28 | 41 | 2.8E-10* | <2.2E-16* | 2.4E-05* |
*p<0.001.
See Table 1 for species abbreviations.
As a further test of this hypothesis, we reviewed the literature for available genetic and biochemical data on FBX genes in A. thaliana, whose genome is the best interrogated in plants, and compared the results generated for the LTSP, STSP and FBX pseudogene loci. To date, the functions of 47 A. thaliana FBX genes have been characterized; most come from the LTSP group (39 of the 191 members) with few from the STSP group (8 of 507 members) and none from the FBX pseudogene group (0 of 199 members) (Tables 2 and S3). Because functional studies might be biased by researcher's interests, we also examined the collection of A. thaliana FBX proteins basically chosen at random for biochemical studies, including representatives tested for SKP1/ASK interaction (e.g., [12], [20]) (Table S4). Of the 72 FBX proteins confirmed to associate with SKP1/ASK proteins, 45 were from the LTSP group, 16 were from the STSP group, and none were from the FBX pseudogene group, again a significant enrichment for LTSP members (Fisher's exact test, p<2.2e-16). The substantial enrichment of expressed and functional FBX loci in the LTSP group further supports the notion that they are more functionally important than members of STSP and FBX pseudogene groups.
Lineage/Species-Specifically Expanded FBX Genes are not Significantly Linked to Stress/Pathogen Responses in A. thaliana
Previous work proposed that a subfamily of A. thaliana FBX genes (mostly from STSP and FBX pseudogene groups base on our analysis (Figure 5)) evolved by positive selection (Ka/Ks >1), with variations in a collection of amino acids in the recruitment module potentially playing an important role in innate immunity and stress responses [14]. However, our data imply that most STSP and FBX pseudogene members are under relaxed selection or neutral change and not under positive selection (Figures 6, 7, S3, and S4; Tables S8 and S9).
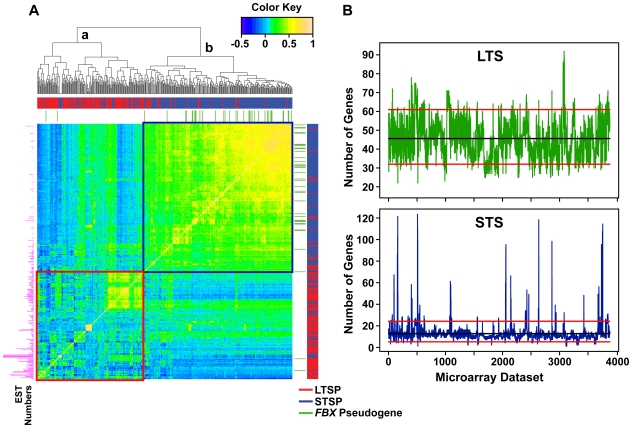
To explore a possible connection between A. thaliana STSP and FBX pseudogenes and the stress/pathogen responses, we examined the expression correlations of all A. thaliana FBX genes in the full compilation of expression microarray datasets available at NASCArrays. These experiments integrated 396 of the 897 potential FBX loci, 146 from LTSP group, 213 from STSP group, and 37 from the FBX pseudogene group. Interestingly, pairwise correlations revealed two large clusters with related expression profiles (Figure 9A). One displayed divergent expression patterns and was strikingly enriched for LTSP members (cluster a, average Pearson's correlation coefficient = 0.1), in agreement with their predicted or known roles in numerous unrelated processes in plants (Figure 9B and Table S10). In contrast, a second cluster displayed highly correlated co-expression and was significantly enriched in STSP and FBX pseudogene members (cluster b, average Pearson's correlation coefficient = 0.4). To eliminate the possibility that the tight correlation in cluster b was caused by low expression of its members, we further compared the expression correlations between the cluster b FBX genes and 28 non-plant internal controls (median microarray value ≤ median microarray value of cluster b FBX genes). If random weak signals above noise generated the tight correlations, the internal controls would have been intermingled with cluster b FBX genes. Instead, clear differential correlation patterns between these two group genes were resolved (Figure S6), suggesting that the transcriptional patterns of cluster b FBX genes might be equally unregulated or regulated similarly.
Figure 9. Expression correlation test and functional predictions of A. thaliana FBX genes.
(A) The expression correlations of 395 FBX genes. The Pearson's correlation coefficients of ∼4,000 microarray datasets for each FBX gene were calculated pairwise. The dendogram at the top of the panel shows the hierarchical clustering of 395 FBX genes based on the dissimilarities of Pearson's correlation coefficients. The bar codes indicated the distributions of LTSP FBX genes (red color), STSP FBX genes (blue color), and FBX pseudogenes (green color). The horizontal histogram (magenta color) on the left shows the EST numbers for each FBX gene. The heatmap color key, indicating the Pearson's correlation coefficient values, is shown on the right top corner of the panel. The correlations of cluster-a genes are highlighted with a red box and the correlations of cluster-b genes are highlighted with a blue box. (B) Enrichment assay of expressed LTS (top panel) and STS (bottom panel) FBX genes in each of the 3,868 different microarray datasets. The black line in each panel indicates the mean numbers of expressed FBX genes. Experiments above the top or below the bottom red line in each panel represent the datasets which significantly increased or decreased overall FBX gene expression frequency, respectively (Fisher's exact test, p<0.05). The statistically significant enrichment of expressed FBX genes in a microarray dataset, which could infer the function(s) of FBX genes, and the experimental condition examined by the microarray for both expressed LTS and STS FBX genes are summarized in Table S10.
When comparing the expression enrichment of LTSP versus STSP members in A. thaliana, the expression patterns of STSP members did not specifically correlate with exposure to almost all stresses tested (heat, cold, oxidative, nitrogen/carbon/potassium starvation, salinity, heavy metals, high light, wounding, etc,), programmed cell death, or with invasion by a number of pathogens (Figure 9B and Table S10). In contrast, the best specific correlations of STSP FBX loci to gene expression were for microarray examinations of embryo and pollen development and the response to phosphate starvation (Table 3). Collectively, the expression studies failed to detect a connection between STSP and FBX pseudogenes and plant stress/pathogen defense.
Table 3. The only three microarray datasets showing the higher enrichment of expressed STS FBX genes than that of LTS FBX genes from 3,868 microarray datasets (p<0.05).
| Group | Slide_name | Number of genes | p-value | Experiment ID | Category |
| LTS | Lindsey_1-1_globular-apical_Rep1_ATH1 | |||||||||||||||||||||| 45/104 | 2.7E-02 | 55 | development_embryo |
| STS | Lindsey_1-1_globular-apical_Rep1_ATH1 | ||||||||||||||||||||||||| 50/193 | |||
| LTS | Broadley_1-2_A2-Bo-P-phosphate-starved_Rep1_ATH1 | ||||||||||||||||||||||||| 51/98 | 4.5E-02 | 121 | nutrient _phosphate |
| STS | Broadley_1-2_A2-Bo-P-phosphate-starved_Rep1_ATH1 | |||||||||||||||||||||||||||||| 61/182 | |||
| LTS | Honys_BCP1_SLD | |||||||||||||||||||||| 44/105 | 4.7E-02 | 48 | development _pollen |
| STS | Honys_BCP1_SLD | ||||||||||||||||||||||||| 51/192 |
p-value was calculated by comparing pairwise the expressed gene numbers of LTS and STS genes using Fisher's exact test. The numerator shows the expressed gene numbers and the denominator shows the non-expressed gene numbers in each group. The bar codes display the numbers of expressed genes. The title for each experiment is shown in Table S10.
Discussion
Plant genomes have undergone a wide array of molecular events that have enlarged or contracted their sizes, which in turn have diversified the size and complexity of various multi-gene families. Despite extensive gene duplication events, the great majority of plant gene families contain only a handful of members [11]. At the other extreme, a small number of gene families have expanded considerably to now include several hundreds to even >1,000 members [7], [12], [40]. Among the significantly expanded families, the FBX superfamily has arguably experienced the most dramatic changes in which a large number of lineage/species-specific gains and losses have occurred as land plants evolved from single-cell aquatic species to advanced angiosperms. These dramatic changes in FBX gene family content even occurred among closely related species. As illustrations, A. thaliana and A. lyrata which diverged only 5 Mya, differ by 453 FBX loci, with A. thaliana acquiring 109 loci and losing 468 loci as compared to their MRCA. G. max and M. truncatula, which split ∼50 Mya differ by 446 FBX genes with M. truncatula acquiring 934 loci and losing 166 loci since the divergence of these two species. Similarly high rates of FBX gene birth and death were also observed by Thomas [14] in the nematodes Caenorhabditis elegans, C. briggsae and C. remanei, which were estimated to have diverged between ∼20–50 [41] and 100 Mya [42], suggesting that such rapid gene gains and losses may be a common feature of this superfamily.
Key to our approach was the development of a pipeline to comprehensively search plant genomes for all FBX loci, using a deep list of FBXD sequences as the initial queries, which was followed up by a second search with a large collection of previously annotated FBX gene sequences. This strategy identified numerous FBX loci that were not yet annotated, which concomitantly expanded the FBX gene superfamilies substantially over those previously reported. For example, the A. thaliana FBX superfamily increased from 694 [12] to 897 loci, the O. sativa superfamily increased from 779 [15] to 971 loci, and the P. trichocarpa superfamily increased from 337 [15] to 425 loci, as compared to the previous most complete annotations. The depth of the collection was supported by subsequent analysis of the A. thaliana superfamily. Here, we found that all previously described FBX proteins, either studied genetically or by interaction assays with SKP1, were in our complete list (Tables S3 and S4), as opposed to prior studies where some notable representatives were missing (e.g., COI1 [19]).
Our analyses revealed that several search parameters can significantly affect FBX gene predictions. First, the detection of FBXD sequences using HMMER is sensitive to the E-value cutoff (Tables S3 and S4). Our observations that all SKP1-interacting FBX proteins from A. thaliana contain an FBXD with an E-value cutoff ≤1 (Table S4), while more stringent E-value cutoffs (≤0.01) exclude some well-known FBX proteins (e.g., A. thaliana AFB4/5 [43], MAX2/ORE9 [44], [45], and COI1 [24], [46]), demonstrate that an E-value cutoff near 1 may be necessary to avoid false predictions but still generate a comprehensive collection. The use of a HMMER E-value cutoff of ≤0.01 by Yang et al. [19] may explain why they reported fewer FBX genes in A thaliana, O. sativa and P. trichocarpa (660, 680 and 320, respectively) and missed functionally important FBX proteins in A. thaliana as compared to our study. Conversely, Xu et al. [15] used an E-value cutoff ≤10, which could have falsely identified some loci.
Second, we found that the genomic coding region of most FBX genes is larger than 1 kbp (Figure S1). Consequently, using just 1-kbp genomic regions as done previously [14], likely predict poor transcript models with high frequency. Here, we retrieved a ∼10-kbp region for each locus, based on the likely upper size limit of most FBX loci, to help ensure that the most complete transcript sequences would be annotated by sequence similarity-based GENEWISE predictions. Third, because many FBX genes arose by tandem duplications, the building of both transcript models and coding sequence annotations by GENEWISE can be challenged by adjacent FBX gene sequences, which will induce GENEWISE to overlook the neighboring FBX loci. The CTT algorithm developed in this work helped overcome this hurdle by allowing us to find and separate these tandem FBX genes by iterative trimming of a 10-kbp window. Our discovery of a significant number of previously non-annotated FBX loci by CTT suggests that it could help the comprehensive annotation of other superfamilies enriched in tandem duplicates.
Domain analysis of the FBX protein collection detected a wide array of C-terminal recruitment modules linked to the N-terminal FBXD. Asymmetric partitioning of the various modules among the different plant lineages was surprisingly variable (e.g., C. reinhardtii and the lower plants P. patens and S. moellendorffii lack FBX proteins with the DUF295 domain and the LRR-FBD modules). Such differential enrichments/depletions could reflect the targeting of lineage/species-specific substrates or convergent evolution in which the various plant lineages adopted different substrate recruitment modules in FBX proteins to recognize the same substrate. Examples of the former could include exogenous proteins derived from pathogens or endogenous proteins required for self-recognition during pollination, which has been shown in Solanaceae, Plantaginaceae, and Rosaceae species to involve polymorphic collections of FBX proteins [47], [48]. As an aside, possible convergent evolution could challenge attempts to predict target/E3 interactions based on the paired arrangements known in other species.
Based on the evolutionary history and species distributions, we divided the FBX superfamily into three groups, LTSP, STSP and FBX pseudogene. It is likely that the LTSP and the STSP/pseudogene collections correspond to the more “stable/conserved” and more “unstable/divergent” clusters described by Thomas [14] and Xu et al. [15], respectively, from more limited analyses of plant FBX genes. The wide distribution of LSTP group members implies that they direct essential ubiquitylation processes in plants. In accord, the LTSP group has experienced the strongest purifying selection among the three groups, shows the highest and most diverse expression patterns, and includes most A. thaliana FBX loci previously demonstrated to control key processes in plant biology (Tables S3 and S4). One collection of LTSP genes in particular was found in most, if not all, land plants (OG bins 14–18 Figure 5) with relatively similar numbers present in each of the 17 land plant species examined (ranging from 81 to 211 members, median = 105±32). We predict that these FBX proteins assemble SCF E3 complexes critical to a core set of ancient ubiquitylation events. In this core set, only a handful has demonstrated functions (e.g., TIR1/ABF1-5, COI1, EBF1/2, FBL17, SLY1, and ZTL (Table S3)). Consequently, focused reverse genetic analyses on these widely distributed, but as yet uncharacterized, LTSP members will likely provide important insights into other processes common in land plants that rely on ubiquitylation as a key component. Using A. thaliana and O. sativa as illustrations, this would concentrate genetic efforts to 104 and 107 LTSP loci, instead of the 897 and 971 total FBX loci present in each species, respectively.
The expansion of the FBX superfamily was significantly correlated with a substantial gain in the number of putative pseudogenes as identified by the presence of frameshifts and premature stop codons, and lack of expression. For example, we predict that A. lyrata, B. distachyon, and M. truncatula, which have some of the largest FBX gene superfamilies, have at least 370, 312 and 240 FBX pseudogenes, whereas C. communis, P. patens, and M. esculenta, which have some of the smallest FBX gene superfamilies, have as few as 9, 17 and 18, respectively. Although the number of FBX pseudogenes we identified is likely an underestimate, either because many FBX pseudogenes may be too divergent for FBXD detection or appear by sequence comparisons to be intact, our findings are consistent with the global analysis of pseudogenes in A. thaliana and O. sativa [3]. A previous FBX gene phylogeny studies did not account for pseudogenes [15], but noted that a significant number of annotated A. thaliana FBX genes contain mutations, which affected the C-terminal ends of the encoded proteins. While they hypothesized that the frameshift defects altered the recruitment modules to potentially increase target diversity for the resulting SCF complex, our findings that many of these frame-shifted genes likely evolved neutrally and are not expressed suggest that they are pseudogenes. Furthermore, the significant enrichment in intron-like insertions and the absence of known target recruitment modules at the C-termini of the encoded proteins predict that few of these FBX loci direct the assembly of functional SCF E3s complexes even if translated.
In addition to FBX, a number of other plant gene families are significantly overpopulated with pseudogenes (e.g., receptor-like kinases, cytochrome P450 reductases, NBS-LRR, and cellulose synthase [3]). Several pseudogene-rich families have connections to environmental responses with the implication that their rapid turnover is driven by the selection pressure imposed by the environmental conditions. However, several features set the FBX superfamily apart from these other pseudogene-rich families, suggesting that the FBX superfamily is not under the same evolutionary constraints. First, except for the receptor-like kinases [40], the FBX superfamily is much larger. Second, the birth and death rates of FBX loci are much more variable than essentially all other plant families including receptor-like kinases [11], [40]. And third, whereas members of many pseudogene-rich families tend to be transcriptionally up-regulated during environmental stress, the FBX superfamily is overall not responsive to either biotic or abiotic challenges [49].
Although members of the STSP group do not have obvious premature stop codons or frameshifts, they share several features in common with FBX pseudogenes. Like FBX pseudogenes, members of the STSP group have experienced more relaxed purifying selection pressures and have weaker and more correlated expression patterns, with the group mostly devoid of FBX loci with demonstrated functional importance, implying that many are still transcriptionally active but in the process of pseudogenization. While a prior study suggested that a subgroup of A. thaliana FBX genes, which appear related to our STSP group, are important for pathogen defense potentially by degrading pathogen proteins entering the plant host via bacterial type III secretion systems [14], our correlation analysis of STSP expression patterns do not support such a role. However, it could be argued that low-level constitutive expression is what one might expect for FBX genes involved in defense, given that the host can predict neither the time nor the place of the infection.
Although more limited in scope, a surprisingly similar evolutionary pattern was predicted for the SKP1-binding partners of FBX proteins in A. thaliana and O. sativa. In both species, a subset of slowly evolving SKP1 loci with strong expression and confirmed biological importance were identified along with a more diverse, faster evolving subset with weak or limited expression [50], [51]; attributes remarkably similar to the LTSP and STSP FBX gene groups identified here. Such connections suggest that the plant SKP1 family diversified in concert with the FBX superfamily possibly to maintain the necessary expression patterns and protein-protein interactions. Similarly, analysis of the BTB family of substrate recruitment modules in A, thaliana, O sativa, and a few other plant species detected a subfamily of MATH-BTB proteins that underwent substantial expansion in the monocot lineages with evidence for diversifying selection and pseudogenization, suggesting that this E3 family is also under unusual selection pressures [23]. Taken together, these phylogenetic studies suggest that diversification Ub ligases in particular, and the UPS in general, may be strongly encouraged in plants.
Contrary to expectations, we found little correlation in FBX gene numbers to the growth habit (herbaceous versus woody), life cycle (annual versus perennial), or evolutionary history of land plants. In fact, when plotted against the lineage tree of the 18 plant species examined, dramatic differences in the numbers of gene gains and losses were found in all lineages, suggesting the most members of the FBX superfamily were affected by rapid and multiple lineage-specific birth/death processes even between closely related species. The evolutionary patterns of FBX genes have striking similarities to the chemosensory receptor genes in vertebrates and insects, which also vary extensively in the numbers of functional genes and pseudogenes both within and between related species [4], [52], [53]. From these and other observations, Nei [54] proposed an evolution model called genomic drift to explain these seemly random changes in gene numbers. This genomic drift hypothesis postulates that, similar to genetic drift at the population level, neutral evolution generates at random widely different gene family sizes among species. While variations in sequence and copy number for drifting genes within a family may be largely inconsequential within populations thus enabling gene loss (death), some loci will become fixed if they help acclimate the individuals to the new niches and habitats [4].
Xu et al. [15] first speculated that genomic drift might play an important role in plant FBX gene evolution. Our more comprehensive analyses provide additional support. Included are the extraordinarily high and seemingly random birth/death rates of FBX genes among plant species, and the identification of numerous loci (STSP and FBX pseudogene groups) which are under more relaxed selection/evolution and appear to be transcriptionally inactive; all signatures of genomic drift [4], [54]. The recent sequencing of numerous interbreeding A. thaliana ecotypes suggests that such genomic drift of FBX loci can also be seen at the population level [13]. Along with the NBS-LRR gene family involved in pathogen defense, the FBX superfamily appears to be one of the most highly polymorphic gene families among the ecotypes. Under the assumption that the FBX superfamily (as possibly its SKP1 partners) is affected by genomic drift, we speculate that a substantial pool of FBX loci are not functional but can act as seeds to generate new FBX protein functions. The more relaxed selection or neutral change of this diverse collection of seeds could randomly generate new SCF E3s with different expression patterns, altered binding affinities for existing targets, or with new target specificities. Although it is likely that most of these FBX loci eventually become pseudogenes, some could be selected for and retained to improve plant fitness to an ever changing environment through trial and error. One possible example of this success is the STSP pair ETP1/2 from A. thaliana. These FBX proteins participate in ethylene perception by helping remove the central regulator EIN2 [55]. While EIN2 is widely distributed among land plants, obvious ETP1/2 orthologs can only be found in A. lyrata among the 17 other plant species examined here, suggesting that ETP1/2 arose as lineage-specific modulators of ethylene signaling.
This pattern of genomic drift evolution has two important implications for the FBX gene superfamily. First, it suggests that that adaptive evolution of FBX genes occurs very frequently, more so than most gene families in plants. How the underpinning rapid birth/death mechanism(s) are selectively applied to FBX loci are unclear. Second, such an adaptive response may be relatively transient given the high birth and death rates in this family where most members do not persist over a long time. Nonetheless, many FBX loci have clearly survived over the course of plant evolution, especially members of the LTSP group. So, while genomic drift may have contributed to the initial emergence of new LTSP loci in general, strong selection pressures would promote their long-term retention. Contemporary examples of this selective retention could include the family of TIR1/ABF FBX proteins in A. thaliana and other plants which can detect natural and synthetic auxins and their AUX/IAA protein targets with different affinities [56], and the A. thaliana EBF1 and 2 FBX proteins whose differential expression patterns provide a mechanism to temporally regulate the abundance of the EIN3/EIL1 transcription factors during ethylene signaling [57]. If genomic drift is in action, its effect would dramatically reduce the final count of truly functional FBX genes in plants, but on the contrary identify an important reservoir exploited by the UPS to improve plant fitness.
Materials and Methods
Creation of the FBXD_Query and FBX_Ref Collections
The initial FBXD query collection of 6219 non-redundant FBXD peptide sequences was obtained from PFAM (http://PFAM.sanger.ac.uk/) and SMART (http://smart.embl-heidelberg.de/) (as of October, 2009) from both plant and non-plant sources and included the 694 potential A. thaliana FBXDs first described by Gagne et al. [12]. Redundant entries were removed by the CD-HIT program [58]. The initial FBXD query collection was used to screen each of the 18 plant proteomes for previously-annotated proteins bearing FBXD sequences by BLASTp (E-value ≤1) [27]. Additional plant and non-plant FBX protein sequences were retrieved from the protein RefSeqs of NCBI (http://www.ncbi.nlm.nih.gov/refseq), and the UniProtKB and UniRef databases (http://www.uniprot.org). The presence of a FBXD in each protein along with the identification of new FBXD sequences were determined by searching the PFAM database with HMMER3 (http://www.hmmer.org/) using an E-value ≤1 cutoff.
Newly predicted FBXD sequences, which were not part of the PFAM and SMART collections, were then used to search each of the 18 proteomes one more time. In total, 6575 and 0 new FBXD sequences and 7,570 and 64 plant FBX genes were found in the first and second searches, respectively (Figure 1A, Table S1). When combined with all FBXD/FBX protein sequence lists obtained above, we generated two non-redundant sequence collections, called FBXD_Query and FBX_Ref (File S1 and S2).
Similarity-Based Re-annotation of FBX Genes
Via the re-annotation pipeline summarized in Figure 1, we searched each of the 18 unmasked plant genomes for all FBXD regions using the FBXD_Query database as the query. To predict the correct full-length coding sequence of each previously non-annotated FBX locus in each proteome database, a 10-kbp genomic sequence sandwiching the FBXD region was first retrieved and the adjacent sequences not predicted to be transcriptionally connected to the potential FBX gene coding sequence were removed by an iterative application of the Closing Target Trimming algorithm (CTT) (see below). Each trimmed genomic DNA sequence was then used as a query to search FBX_Ref to find homologous FBX protein sequences. The top two (if only two sequences similar to FBXD were found) or top three (if more than two sequences were found) scored (bit-score values) homologous protein sequences were used as references to back search the genomic DNA sequence to predict the transcript model and the correct coding sequence using GENEWISE. The coding sequence predicted with the highest GENEWISE score was taken as the best prediction of the FBX locus. To identify the FBXD region in previously or newly annotated FBX protein sequences, we used HMMER3 to search each protein sequence against PFAM database (E-value <1). Introns within the coding region were detected using BLAT [59] to align the genomic sequence and coding sequence of each FBX gene.
Closing Target Trimming Algorithm (CTT)
To remove coding regions from adjacent genes in the 10-kbp window containing previously non-annotated FBX loci, we applied the CTT algorithm (Figure 1B). In brief, each ∼10-kb genomic DNA sequence was used to search FBX_Ref by BLASTx to identify similar protein sequences (E-value <1e-5) [27]. The top-scored (bit score value) protein sequence was used to search for coding regions with GENEWISE (score ≥50) in the ∼10-kb genomic sequence. If the coding region did not contain the target FBXD sequence, identified by the positions of the two regions in the ∼10-kb genomic, that part of genomic DNA sequence was trimmed. Sequential BLASTx/GENEWISE/trimming was iterated up to six times until a single potential FBX coding region with the best sequence match covered the target FBXD region first identified by tBLASTn. If a potential FBX coding region was not found after six rounds, the locus was omitted from further analysis.
Inferring Birth/Death Events of FBX Genes
Results from an all-against-all BLASTp search (E-value <1e-5) of the full-length FBX protein sequences grouped the 10,811 FBX protein sequences into 351 clusters using MCL (granularity I = 4) [32]. The FBX genes in 47 of the clusters were from only one species; they were counted as gene gains in that species. For the remaining 304 clusters, multiple protein sequence alignments were generated with MUSCLE version 3.5 [60] (maximum iterations = 16). Two large clusters (>1,000 members) were further divided into smaller subclusters based on neighbor-joining trees [61]. If the species number in a cluster/subcluster was less than 4 but larger than 1, the FBX genes in that cluster/subcluster were excluded for further calculation because the minimum number of species for phylogenetic reconstruction required ≥4 species. For the remaining clusters/subclusters, maximum likelihood trees were generated by RAxML (version 7.04) [62] using the PROTGAMMAJTT substitution model and default settings. Gene duplication and loss events were inferred by reconciling the gene tree for each cluster/subcluster with the species tree using Notung (version 2.5) [63]).
FBX Pseudogene Predictions
The genomic DNA sequence of each plant FBX locus was retrieved from the corresponding genomic database and used to search the entire FBX protein reference collection (FBX_Ref) for potential homologs by BLASTx (E-value < 1e-5). The top two (if only two non-self hits were obtained) or top three (if more than two non-self hits were obtained) non-self hits were used as references to re-annotate the transcript model of the FBX locus by GENEWISE. If two reference sequences predicted the presence of either frameshifts or premature stop codons, the locus was classified as a pseudogene. Preliminary studies showed that the application of two references sequences helped reduce false classification of functional FBX genes as pseudogenes.
FBX Gene Expression and Functional Predictions of A. thaliana FBX Genes
EST databases from each of the 18 plant species were retrieved from NCBI and used to search for representatives of the corresponding species FBX gene collection (last retrieved February 17, 2010). Relative expression was estimated by counting the total number of ESTs for each FBX locus. An EST was considered a true reflection of transcript expression if: (i) it had >95% identity to the query FBX coding region, (ii) at least 75% of the EST or the query sequence was aligned, and if (iii) at least 50 nucleotides of the EST was included in the alignment. Microarray expression data for individual A. thaliana FBX genes (as of January 12, 2010) were retrieved from the Nottingham Arabidopsis Stock Centre's microarray database (NASCArrays) (http://affymetrix.arabidopsis.info/narrays/experimentbrowse.pl), which is Minimum Information About a Microarray Experiment (MIAME) supportive [64]. The pairwise Pearson's correlation coefficient for each gene was calculated based on the ∼4000 microarray experiments and clustered and viewed using heatmap.2 in R. To support the proposed grouping of LTSP, STSP/pseudogene classifications and to predict the general functions of the LTSP and STSP genes, we compared the expression frequencies of each group as calculated by Fisher's exact test based on the number of expressed genes in the microarray relative to the average number of expressed genes. If a statistically significant enrichment of expressed FBX genes was observed in a microarray dataset, the biological functions tested by the microarray was retrieved. If two microarrays were used in the same experiment, only the one with the higher number of expressed FBX genes was analyzed.
Calculation of Ka/Ks Values
To increase the strength of Ka/Ks calculations because many FBX genes appear to have been generated by the recent duplications, we used an alternative method to calculate the MRCAs. Here, the protein sequence of each FBX gene (Files S5) was used as a query to identify the top two non-self hits in the FBX_Ref dataset (File S2) by BLASTp and the corresponding transcript sequences were retrieved from File S8. The Ka/Ks value of the target gene was then calculated by first aligning its protein sequence with the two peptide sequences via T-coffee [65]. From the codon alignment and the guide neighbor-joining tree rooted at the midpoint using Phylip (http://evolution.genetics.washington.edu/phylip.html), the MRCA sequence of the three FBX genes was calculated using PAMP (PAML4 package [66]). The Ka, Ks, and Ka/Ks values of the target FBX gene was obtained by comparing its coding sequence to that of the MRCA using the pairwise model of CodeML (PAML4 package [66]). Sequences that were either too similar (Ks<0.005) or too divergent (Ks>3) were excluded. In total, 10,596 of 10,811 (98%) total FBX genes generated with this three-way comparison had useable Ks values between these extremes (File S9). By the same method, the Ka, Ks, and Ka/Ks values of FBXD, and ΔFBXD regions were generated.
To test whether an FBX gene is under neutral change, we collected the maximum likelihood values ML1 and ML2 from two runs of CodeML with the Ka/Ks ratio of the full-length zcoding sequence fixed at 1 and free, respectively. From these values, we calculated the likelihood ratio (LR) = 2(lnML1-lnML2) as described by Nekrutenko et al. [35] (File S9). For LR values less than 3.84 (X0.05 2), the Ka/Ks value for the FBX gene should approach 1 and thus be indicative of neutral change.
Detection of Tandemly or Segmentally Duplicated FBX Genes
For each of 18 plant species, the entire lists of tandem or segmental duplicated genes (including the new annotated FBX genes obtained in this work) were predicted by DAGchainer [37] with the default settings based on sequence similarities and the genomic position information of annotated genes. FBX genes affected by these duplication mechanisms were then extracted from this list.
Supporting Information
The database resources of 18 plant genome annotations used in this work.
(DOC)
Summary of different FBX gene annotation steps in the 18 plant species.
(DOC)
Functionally characterized FBX genes from A. thaliana .
(DOC)
Biochemical evidence in the literature confirming the interaction between A. thaliana FBX proteins and one or more SKP1/ASK proteins.
(DOC)
The enrichment of various domain (dmn) combinations in the putative substrate recruitment module of FBX proteins. p indicates the enrichment probability of FBX proteins with a specific domain/domain combination as compared to that for the average number of FBX proteins. In total, 3,269 domains/domain combinations (including no prediction group) were identified from 10,811 FBX proteins.
(DOC)
The enrichment comparison of domain (dmn) combinations in the putative substrate recruitment module of the translated protein products from FBX pseudogenes and protein-coding genes. p (left) indicates the domain enrichment probability in the translated protein products from FBX pseudogenes. p (right) indicates the domain enrichment probability in the translated protein products from protein-coding genes.
(DOC)
Fisher's exact test of the enrichment or depletion of domain (dmn) combinations in the putative substrate recruitment module of FBX proteins in each plant species. p (left) indicates the domain enrichment probability in a species. p (right) indicates the domain depletion probability in a species.
(DOC)
Wilcoxon rank sum test (one tailed) of Ka/Ks values of the LTS protein-coding FBX genes (LTSP), STS protein-coding FBX genes (STSP) and FBX pseudogenes (ψ in each plant species.
(DOC)
Wilcoxon rank sum test (one tailed) of Ka/Ks values from FBXD and Δ FBXD identified within the LTS protein-coding FBX gene (LTSP), STS protein-coding FBX gene (STSP), and FBX pseudogene (ψ groups in each of the 18 plant species.
(DOC)
Enrichment in various microarray datasets and functional prediction of expressed FBX genes from LTS and STS groups of A. thaliana .
(DOC)
Size distributions in nucleotides of the complete set of FBX genes predicted from all 18 plant species (see Table S1 for list and abbreviation for each species).
(TIF)
Evaluations of the CTT and the similarity-based FBX gene annotation protocols. A. thaliana (At). O. sativa (Os). “Frame shift” indicates FBX genes containing one or more reading-frame shifts. “Premature Stop” indicates FBX genes containing premature stop codon(s). “Protein” indicates FBX protein-coding genes.
(TIF)
The distributions of FBXD Ka/Ks values of FBX genes from the LTSP, STSP and FBX pseudogene groups in each of 18 plant species. The Ka/Ks value for each FBXD sequence was calculated by comparing FBXD sequence to the MRCA transcript sequence using the method of Goldman and Yang [34].
(TIF)
The distributions of Δ FBXD Ka/Ks values of FBX genes from the LTSP, STSP and FBX pseudogene groups in each of 18 plant species. The Ka/Ks value for each ΔFBXD sequence was calculated by comparing ΔFBXD sequence to the MRCA transcript sequence using the method of Goldman and Yang [34].
(TIF)
The distributions of LTSP, STSP and FBX pseudogene loci along the chromosomes of A. thaliana .
(TIF)
Microarray data correlations of A. thaliana FBX genes from cluster b in Figure 8A , and 28 internal non-plant genes from NASCArrays. The Pearson's correlation coefficients were calculated as described in Figure 8. The dendrogram on the top of the panel showed the hierarchical clustering based on the dissimilarities of Pearson's correlation coefficients. The bar codes indicate the distributions of LTS FBX genes (red color), STS FBX genes (blue color), and the 28 non-plant internal control genes (green color).
(TIF)
The non-redundant peptide sequences from the 13,565 FBXDs in the FBXD_Query collection. The FBXD peptide sequences were collected from three resources: (i) PFAM and SMART, (ii) FBXD peptide sequences of FBX proteins predicted from the 18 plant proteomes, and (iii) FBXD peptide sequences of FBX proteins from the species outside the 18 plant proteomes which were collected from UniProtKB, UniRef, and/or the NCBI reference sequence database RefSeq.
(TXT)
The non-redundant protein sequences from the 13,325 reference FBX genes in the FBX_Ref collection. The FBX protein sequences were collected from two resources: (i) FBX protein sequences predicted from the 18 plant species, and (ii) FBX protein sequences from the species outside the 18 plant species, which were collected from UniProtKB, UniRef, and/or the NCBI reference sequence databases RefSeq.
(TXT)
The comparison between the re-annotated transcript sequence and the original annotated transcript sequence of each FBX gene from A. thaliana using bl2seq. The re-annotated transcript sequences were obtained using CTT and similarity-based annotation pipeline as described in the Materials and Methods.
(TXT)
The comparison between the re-annotated transcript sequence and the original annotated transcript sequence of each FBX gene from O. sativa using bl2seq. The re-annotated transcript sequences were obtained using CTT and the similarity-based annotation pipeline as described in the Materials and Methods.
(TXT)
The protein sequences derived from all 10,811 FBX genes obtained from the 18 plant species. The header for each FBX protein sequence contains two items. The first is a new ID used in this work and the second is a previous ID used in the original annotation of each species. “New_annotated” indicates the FBX genes newly annotated in this work.
(TXT)
The coding sequences of all 10,811 FBX genes obtained from the 18 plant species.
(TXT)
The list of tandem and segmental duplicated FBX genes and pseudogenes in each of the 18 plant species.
(TXT)
The coding sequences of 13,325 reference FBX genes. The coding sequence for each FBX gene was retrieved from NCBI mRNA database, EMBL coding sequence database, and/or the annotation pipeline from the 18 plant species described here.
(TXT)
Ka/Ks and Ks values of FBX genes from the 18 plant species. The Ka/Ks value for each full-length sequence was calculated by comparing it to the MRCA transcript sequence using the method of Goldman and Yang [34].
(TXT)
Acknowledgments
We appreciate the helpful discussions from Aaron Lomax and Marcus Miller (Vierstra lab) and Liang Song (Fedoroff lab). We thank each plant genome-sequencing project for providing the annotation and genome sequences and for the TAIR, NCBI, UniProt, EMBL, PFAM, SMART, Phytozome, and NASCarray databases for their collected resources. We also thank numerous researchers for providing the free open source codes for many of the programs used here.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Science Foundation Arabidopsis 2010 Program (MCB-0115870) to R.D.V and S.-H. Shiu. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.W-H Li. Evolution of duplicate genes and pseudogenes. In: Nei M, Koehm RK, editors. Evolution of genes and proteins. Sunderland, MA: Sinauer Associates, Inc; 1983. pp. 14–37. [Google Scholar]
- 2.Shiu S-H, Shih M-C, Li W-H. Transcription factor families have much higher expansion rates in plants than in animals. Plant Physiol. 2005;139:18–26. doi: 10.1104/pp.105.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou C, Lehti-Shiu MD, Thibaud-Nissen F, Prakash T, Buell CR, et al. Evolutionary and expression signatures of pseudogenes in Arabidopsis and rice. Plant Physiol. 2009;151:3–15. doi: 10.1104/pp.109.140632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 5.Nei M, Gu X, Sitnikova T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci USA. 1997;94:7799–7806. doi: 10.1073/pnas.94.15.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McHale L, Tan X, Koehl P, Michelmore RW. Plant NBS-LRR proteins: adaptable guards. Genome Biol. 2006;7:212. doi: 10.1186/gb-2006-7-4-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 8.Bowers JE, Chapman BA, Rong J, Paterson AH. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003;422:433–438. doi: 10.1038/nature01521. [DOI] [PubMed] [Google Scholar]
- 9.Simillion C, Vandepoele K, Van Montagu MC, Zabeau M, Van de Peer Y. The hidden duplication past of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2002;99:13627–13632. doi: 10.1073/pnas.212522399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, et al. F-Box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007;143:1467–1483. doi: 10.1104/pp.106.091900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanada K, Zou C, Lehti-Shiu MD, Shinozaki K, Shiu S-H. Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol. 2008;148:993–1003. doi: 10.1104/pp.108.122457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD. The F-Box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA. 2002;99:11519–11524. doi: 10.1073/pnas.162339999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark RM, Schweikert G, Toomajian C, Ossowski S, Zeller G, et al. Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science. 2007;317:338–342. doi: 10.1126/science.1138632. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JH. Adaptive evolution in two large families of ubiquitin-ligase adapters in nematodes and plants. Genome Res. 2006;16:1017–1030. doi: 10.1101/gr.5089806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu G, Ma H, Nei M, Kong H. Evolution of F-Box genes in plants: different modes of sequence divergence and their relationships with functional diversification. Proc Natl Acad Sci USA. 2009;106:835–840. doi: 10.1073/pnas.0812043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 17.Hua Z, Vierstra RD. Annu Rev Plant Biol (in press); 2011. The Cullin-RING ubiquitin-protein ligases: the 800-lbs gorillas in plant post-translational regulation. [DOI] [PubMed] [Google Scholar]
- 18.Vierstra RD. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol. 2009;10:385–397. doi: 10.1038/nrm2688. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Kalluri UC, Jawdy S, Gunter LE, Yin T, et al. The F-Box gene family is expanded in herbaceous annual plants relative to woody perennial plants. Plant Physiol. 2008;148:1189–1200. doi: 10.1104/pp.108.121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, et al. Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 2003;34:753–767. doi: 10.1046/j.1365-313x.2003.01768.x. [DOI] [PubMed] [Google Scholar]
- 21.Skaar JR, Pagan JK, Pagano M. SnapShot: F-Box Proteins I. Cell. 2009;137:1160.e1161. doi: 10.1016/j.cell.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 22.Lechner E, Achard P, Vansiri A, Potuschak T, Genschik P. F-Box proteins everywhere. Curr Opin Plant Biol. 2006;9:631–638. doi: 10.1016/j.pbi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Gingerich DJ, Hanada K, Shiu SH, Vierstra RD. Large-scale, lineage-specific expansion of a bric-a-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice. Plant Cell. 2007;19:2329–2348. doi: 10.1105/tpc.107.051300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468:400–405. doi: 10.1038/nature09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 26.Thibaud-Nissen F, Ouyang S, Buell CR. Identification and characterization of pseudogenes in the rice gene complement. BMC Genomics. 2009;10:317. doi: 10.1186/1471-2164-10-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Finn RD, Mistry J, Tate J, Coggill P, Heger A, et al. The PFAM protein families database. Nucleic Acids Res. 2010;38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swigonova Z, Lai J, Ma J, Ramakrishna W, Llaca V, et al. Close split of sorghum and maize genome progenitors. Genome Res. 2004;14:1916–1923. doi: 10.1101/gr.2332504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavin M, Herendeen PS, Wojciechowski MF. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol. 2005;54:575–594. doi: 10.1080/10635150590947131. [DOI] [PubMed] [Google Scholar]
- 31.Koch MA, Haubold B, Mitchell-Olds T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol Biol Evol. 2000;17:1483–1498. doi: 10.1093/oxfordjournals.molbev.a026248. [DOI] [PubMed] [Google Scholar]
- 32.Van Dongen SM. Graph clustering by flow simulation. University of Utrecht. 2000:169. [Google Scholar]
- 33.Chen F, Mackey AJ, Vermunt JK, Roos DS. Assessing performance of orthology detection strategies applied to eukaryotic genomes. PloS ONE. 2007;2:e383. doi: 10.1371/journal.pone.0000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol. 1994;11:725–736. doi: 10.1093/oxfordjournals.molbev.a040153. [DOI] [PubMed] [Google Scholar]
- 35.Nekrutenko A, Makova KD, Li WH. The Ka/Ks ratio test for assessing the protein-coding potential of genomic regions: an empirical and simulation study. Genome Res. 2002;12:198–202. doi: 10.1101/gr.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 37.Haas BJ, Delcher AL, Wortman JR, Salzberg SL. DAGchainer: a tool for mining segmental genome duplications and synteny. Bioinformatics. 2004;20:3643–3646. doi: 10.1093/bioinformatics/bth397. [DOI] [PubMed] [Google Scholar]
- 38.Copenhaver GP, Nickel K, Kuromori T, Benito MI, Kaul S, et al. Genetic definition and sequence analysis of Arabidopsis centromeres. Science. 1999;286:2468–2474. doi: 10.1126/science.286.5449.2468. [DOI] [PubMed] [Google Scholar]
- 39.Torrents D, Suyama M, Zdobnov E, Bork P. A genome-wide survey of human pseudogenes. Genome Res. 2003;13:2559–2567. doi: 10.1101/gr.1455503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehti-Shiu MD, Zou C, Hanada K, Shiu S-H. Evolutionary history and stress regulation of plant receptor-like kinase/pelle genes. Plant Physiol. 2009;150:12–26. doi: 10.1104/pp.108.134353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent WJ, Zahler AM. Conservation, regulation, synteny, and introns in a large-scale C. briggsae-C. elegans genomic alignment. Genome Res. 2000;10:1115–1125. doi: 10.1101/gr.10.8.1115. [DOI] [PubMed] [Google Scholar]
- 42.Hillier LW, Miller RD, Baird SE, Chinwalla A, Fulton LA, et al. Comparison of C. elegans and C. briggsae genome sequences reveals extensive conservation of chromosome organization and synteny. PLoS Biol. 2007;5:e167. doi: 10.1371/journal.pbio.0050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh TA, Neal R, Merlo AO, Honma M, Hicks GR, et al. Mutations in an auxin receptor homolog AFB5 and in SGT1b confer resistance to synthetic picolinate auxins and not to 2,4-dichlorophenoxyacetic acid or indole-3-acetic acid in Arabidopsis. Plant Physiol. 2006;142:542–552. doi: 10.1104/pp.106.085969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stirnberg P, Furner IJ, Ottoline Leyser HM. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007;50:80–94. doi: 10.1111/j.1365-313X.2007.03032.x. [DOI] [PubMed] [Google Scholar]
- 45.Woo HR, Chung KM, Park JH, Oh SA, Ahn T, et al. ORE9, an F-Box protein that regulates leaf senescence in Arabidopsis. Plant Cell. 2001;13:1779–1790. doi: 10.1105/TPC.010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L, Liu F, Lechner E, Genschik P, Crosby WL, et al. The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell. 2002;14:1919–1935. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonneveld T, Tobutt KR, Vaughan SP, Robbins TP. Loss of pollen-S function in two self-compatible selections of Prunus avium is associated with deletion/mutation of an S haplotype-specific F-Box gene. Plant Cell. 2005;17:37–51. doi: 10.1105/tpc.104.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua Z, Meng X, Kao TH. Comparison of Petunia inflata S-locus F-Box protein (Pi SLF) with Pi SLF like proteins reveals its unique function in S-RNase based self-incompatibility. Plant Cell. 2007;19:3593–3609. doi: 10.1105/tpc.107.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou C, Lehti-Shiu MD, Thomashow M, Shiu S-H. Evolution of stress-regulated gene expression in duplicate genes of Arabidopsis thaliana. PLoS Genet. 2009;5:e1000581. doi: 10.1371/journal.pgen.1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong H, Landherr LL, Frohlich MW, Leebens-Mack J, Ma H, et al. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: evidence for multiple mechanisms of rapid gene birth. Plant J. 2007;50:873–885. doi: 10.1111/j.1365-313X.2007.03097.x. [DOI] [PubMed] [Google Scholar]
- 51.Kong H, Leebens-Mack J, Ni W, dePamphilis CW, Ma H. Highly heterogeneous rates of evolution in the SKP1 gene family in plants and animals: functional and evolutionary implications. Molec Biol Evol. 2004;21:117–128. doi: 10.1093/molbev/msh001. [DOI] [PubMed] [Google Scholar]
- 52.Niimura Y, Nei M. Extensive gains and losses of olfactory receptor genes in mammalian evolution. PLoS ONE. 2007;2:e708. doi: 10.1371/journal.pone.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasin Y, Olender T, Khen M, Gonzaga-Jauregui C, Kim PM, et al. High-resolution copy-number variation map reflects human olfactory receptor diversity and evolution. PLoS Genet. 2008;4:e1000249. doi: 10.1371/journal.pgen.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nei M. The new mutation theory of phenotypic evolution. Proc Natl Acad Sci USA. 2007;104:12235–12242. doi: 10.1073/pnas.0703349104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiao H, Chang KN, Yazaki J, Ecker JR. Interplay between ethylene, ETP1/ETP2 F-Box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev. 2009;23:512–521. doi: 10.1101/gad.1765709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, et al. Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci USA. 2009;106:22540–22545. doi: 10.1073/pnas.0911967106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, et al. The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell. 2007;19:509–523. doi: 10.1105/tpc.106.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W, Godzik A. CD-HIT: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 59.Kent WJ. BLAT-the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edgar RC, Batzoglou S. Multiple sequence alignment. Curr Opin Struct Biol. 2006;16:368–373. doi: 10.1016/j.sbi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 62.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 63.Chen K, Durand D, Farach-Colton M. NOTUNG: a program for dating gene duplications and optimizing gene family trees. J Comput Biol. 2000;7:429–447. doi: 10.1089/106652700750050871. [DOI] [PubMed] [Google Scholar]
- 64.Craigon DJ, James N, Okyere J, Higgins J, Jotham J, et al. NASCArrays: a repository for microarray data generated by NASC's transcriptomics service. Nucleic Acids Res. 2004;32:D575–577. doi: 10.1093/nar/gkh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 66.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The database resources of 18 plant genome annotations used in this work.
(DOC)
Summary of different FBX gene annotation steps in the 18 plant species.
(DOC)
Functionally characterized FBX genes from A. thaliana .
(DOC)
Biochemical evidence in the literature confirming the interaction between A. thaliana FBX proteins and one or more SKP1/ASK proteins.
(DOC)
The enrichment of various domain (dmn) combinations in the putative substrate recruitment module of FBX proteins. p indicates the enrichment probability of FBX proteins with a specific domain/domain combination as compared to that for the average number of FBX proteins. In total, 3,269 domains/domain combinations (including no prediction group) were identified from 10,811 FBX proteins.
(DOC)
The enrichment comparison of domain (dmn) combinations in the putative substrate recruitment module of the translated protein products from FBX pseudogenes and protein-coding genes. p (left) indicates the domain enrichment probability in the translated protein products from FBX pseudogenes. p (right) indicates the domain enrichment probability in the translated protein products from protein-coding genes.
(DOC)
Fisher's exact test of the enrichment or depletion of domain (dmn) combinations in the putative substrate recruitment module of FBX proteins in each plant species. p (left) indicates the domain enrichment probability in a species. p (right) indicates the domain depletion probability in a species.
(DOC)
Wilcoxon rank sum test (one tailed) of Ka/Ks values of the LTS protein-coding FBX genes (LTSP), STS protein-coding FBX genes (STSP) and FBX pseudogenes (ψ in each plant species.
(DOC)
Wilcoxon rank sum test (one tailed) of Ka/Ks values from FBXD and Δ FBXD identified within the LTS protein-coding FBX gene (LTSP), STS protein-coding FBX gene (STSP), and FBX pseudogene (ψ groups in each of the 18 plant species.
(DOC)
Enrichment in various microarray datasets and functional prediction of expressed FBX genes from LTS and STS groups of A. thaliana .
(DOC)
Size distributions in nucleotides of the complete set of FBX genes predicted from all 18 plant species (see Table S1 for list and abbreviation for each species).
(TIF)
Evaluations of the CTT and the similarity-based FBX gene annotation protocols. A. thaliana (At). O. sativa (Os). “Frame shift” indicates FBX genes containing one or more reading-frame shifts. “Premature Stop” indicates FBX genes containing premature stop codon(s). “Protein” indicates FBX protein-coding genes.
(TIF)
The distributions of FBXD Ka/Ks values of FBX genes from the LTSP, STSP and FBX pseudogene groups in each of 18 plant species. The Ka/Ks value for each FBXD sequence was calculated by comparing FBXD sequence to the MRCA transcript sequence using the method of Goldman and Yang [34].
(TIF)
The distributions of Δ FBXD Ka/Ks values of FBX genes from the LTSP, STSP and FBX pseudogene groups in each of 18 plant species. The Ka/Ks value for each ΔFBXD sequence was calculated by comparing ΔFBXD sequence to the MRCA transcript sequence using the method of Goldman and Yang [34].
(TIF)
The distributions of LTSP, STSP and FBX pseudogene loci along the chromosomes of A. thaliana .
(TIF)
Microarray data correlations of A. thaliana FBX genes from cluster b in Figure 8A , and 28 internal non-plant genes from NASCArrays. The Pearson's correlation coefficients were calculated as described in Figure 8. The dendrogram on the top of the panel showed the hierarchical clustering based on the dissimilarities of Pearson's correlation coefficients. The bar codes indicate the distributions of LTS FBX genes (red color), STS FBX genes (blue color), and the 28 non-plant internal control genes (green color).
(TIF)
The non-redundant peptide sequences from the 13,565 FBXDs in the FBXD_Query collection. The FBXD peptide sequences were collected from three resources: (i) PFAM and SMART, (ii) FBXD peptide sequences of FBX proteins predicted from the 18 plant proteomes, and (iii) FBXD peptide sequences of FBX proteins from the species outside the 18 plant proteomes which were collected from UniProtKB, UniRef, and/or the NCBI reference sequence database RefSeq.
(TXT)
The non-redundant protein sequences from the 13,325 reference FBX genes in the FBX_Ref collection. The FBX protein sequences were collected from two resources: (i) FBX protein sequences predicted from the 18 plant species, and (ii) FBX protein sequences from the species outside the 18 plant species, which were collected from UniProtKB, UniRef, and/or the NCBI reference sequence databases RefSeq.
(TXT)
The comparison between the re-annotated transcript sequence and the original annotated transcript sequence of each FBX gene from A. thaliana using bl2seq. The re-annotated transcript sequences were obtained using CTT and similarity-based annotation pipeline as described in the Materials and Methods.
(TXT)
The comparison between the re-annotated transcript sequence and the original annotated transcript sequence of each FBX gene from O. sativa using bl2seq. The re-annotated transcript sequences were obtained using CTT and the similarity-based annotation pipeline as described in the Materials and Methods.
(TXT)
The protein sequences derived from all 10,811 FBX genes obtained from the 18 plant species. The header for each FBX protein sequence contains two items. The first is a new ID used in this work and the second is a previous ID used in the original annotation of each species. “New_annotated” indicates the FBX genes newly annotated in this work.
(TXT)
The coding sequences of all 10,811 FBX genes obtained from the 18 plant species.
(TXT)
The list of tandem and segmental duplicated FBX genes and pseudogenes in each of the 18 plant species.
(TXT)
The coding sequences of 13,325 reference FBX genes. The coding sequence for each FBX gene was retrieved from NCBI mRNA database, EMBL coding sequence database, and/or the annotation pipeline from the 18 plant species described here.
(TXT)
Ka/Ks and Ks values of FBX genes from the 18 plant species. The Ka/Ks value for each full-length sequence was calculated by comparing it to the MRCA transcript sequence using the method of Goldman and Yang [34].
(TXT)