Abstract
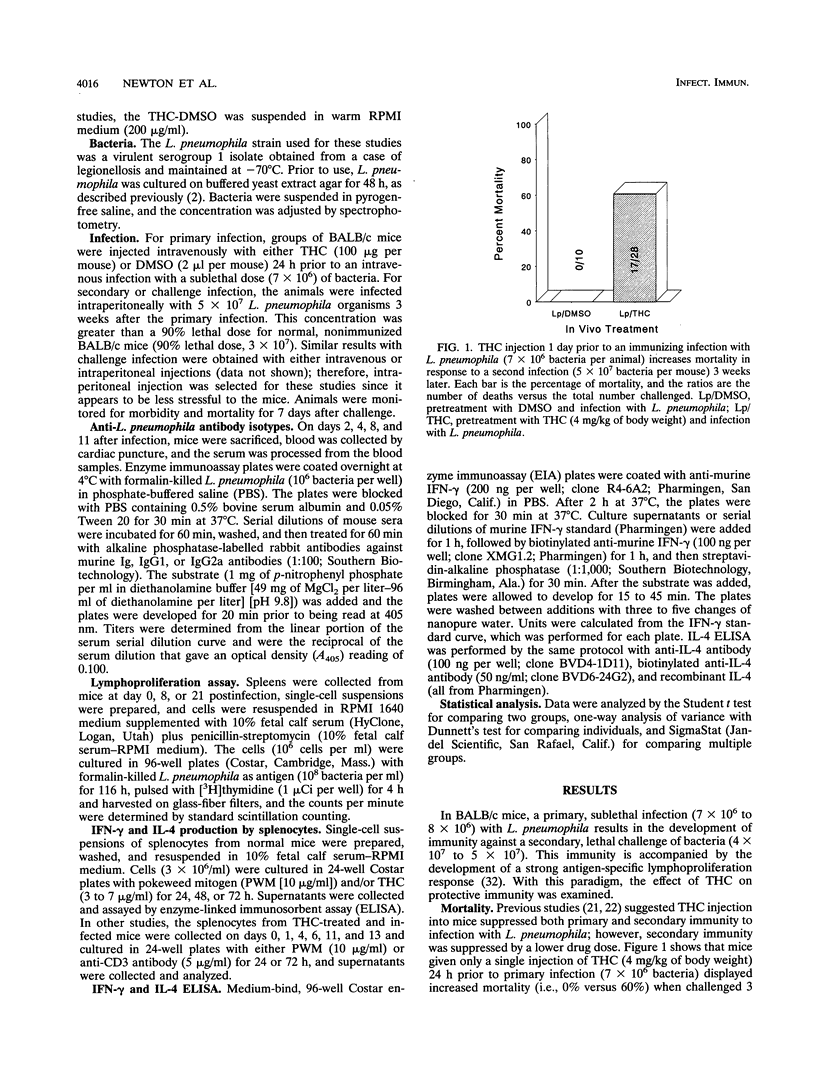
Resistance to infection with Legionella pneumophila is primarily dependent upon cell-mediated immunity rather than humoral immunity. Recent evidence suggests that activation of cell-mediated immunity depends on Th1 cells and activation of humoral immunity depends on Th2 cells. In this report, delta 9-tetrahydrocannabinol (THC), the major psychoactive cannabinoid of marijuana and an immunomodulator, suppressed development of secondary immunity to L. pneumophila, which correlated with a reduction in Th1 activity. BALB/c mice, infected with a primary sublethal dose of L. pneumophila, developed resistance to a larger challenge infection 3 to 4 weeks later. However, intravenous injection of THC (4 mg/kg of body weight) 1 day prior to primary infection resulted in increased mortality after the challenge infection. The level of anti-L. pneumophila antibodies in serum increased in both THC-treated and control mice; however, in the THC group IgG1 antibodies which are stimulated by Th2 cells were elevated while Th1-regulated, IgG2a antibodies were depressed. Furthermore, cultured splenocytes from THC-treated mice had less L. pneumophila-specific lymphoproliferation, indicating a deficiency in cell-mediated immunity. Normal mouse splenocytes treated in vitro with THC and pokeweed mitogen showed suppressed production of gamma interferon, a cytokine associated with Th1 cells, but increased production of interleukin 4, a cytokine produced by Th2 cells. Splenocytes from THC-treated mice, stimulated in vitro with either pokeweed mitogen or anti-CD3 antibodies, also produced less gamma interferon, indicating less Th1 activity in these mice. These results suggest that THC decreases the development of anti-L. pneumophila immunity by causing a change in the balance of Th1 and Th2 activities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashfaq M. K., Watson E. S., elSohly H. N. The effect of subacute marijuana smoke inhalation on experimentally induced dermonecrosis by S. aureus infection. Immunopharmacol Immunotoxicol. 1987;9(2-3):319–331. doi: 10.3109/08923978709035217. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Friedman H., Stewart W. E., 2nd, Klein T. W., Djeu J. Y. Role of gamma interferon in induction of natural killer activity by Legionella pneumophila in vitro and in an experimental murine infection model. Infect Immun. 1988 May;56(5):1187–1193. doi: 10.1128/iai.56.5.1187-1193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., Newton C., Klein T. W., Stewart W. E., 2nd, Friedman H. In vitro and in vivo suppressive effects of delta-9-tetrahydrocannabinol on interferon production by murine spleen cells. Int J Immunopharmacol. 1986;8(7):819–824. doi: 10.1016/0192-0561(86)90020-2. [DOI] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with Legionella pneumophila membranes induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. Protective immunity independent of the major secretory protein of Legionella pneumophila. J Clin Invest. 1991 Mar;87(3):1054–1059. doi: 10.1172/JCI115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Bradley S. G., Munson A. E., Dewey W. L., Harris L. S. Enhanced susceptibility of mice to combinations of delta 9-tetrahydrocannabinol and live or killed gram-negative bacteria. Infect Immun. 1977 Aug;17(2):325–329. doi: 10.1128/iai.17.2.325-329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- Clerici M., Wynn T. A., Berzofsky J. A., Blatt S. P., Hendrix C. W., Sher A., Coffman R. L., Shearer G. M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994 Feb;93(2):768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Fitch F. W., McKisic M. D., Lancki D. W., Gajewski T. F. Differential regulation of murine T lymphocyte subsets. Annu Rev Immunol. 1993;11:29–48. doi: 10.1146/annurev.iy.11.040193.000333. [DOI] [PubMed] [Google Scholar]
- Friedman H., Widen R., Klein T., Searls L., Cabrian K. Legionella pneumophila-induced blastogenesis of murine lymphoid cells in vitro. Infect Immun. 1984 Jan;43(1):314–319. doi: 10.1128/iai.43.1.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Horwitz M. A. Cell-mediated immunity in Legionnaires' disease. J Clin Invest. 1983 Jun;71(6):1686–1697. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981 Feb 1;153(2):386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Klein T. W., Newton C., Friedman H. Inhibition of natural killer cell function by marijuana components. J Toxicol Environ Health. 1987;20(4):321–332. doi: 10.1080/15287398709530986. [DOI] [PubMed] [Google Scholar]
- Klein T. W., Newton C., Friedman H. Resistance to Legionella pneumophila suppressed by the marijuana component, tetrahydrocannabinol. J Infect Dis. 1994 May;169(5):1177–1179. doi: 10.1093/infdis/169.5.1177-a. [DOI] [PubMed] [Google Scholar]
- Klein T. W., Newton C., Widen R., Friedman H. Delta 9-tetrahydrocannabinol injection induces cytokine-mediated mortality of mice infected with Legionella pneumophila. J Pharmacol Exp Ther. 1993 Nov;267(2):635–640. [PubMed] [Google Scholar]
- Lichtman A. H., Chin J., Schmidt J. A., Abbas A. K. Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9699–9703. doi: 10.1073/pnas.85.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R. Cellular effects of cannabinoids. Pharmacol Rev. 1986 Mar;38(1):45–74. [PubMed] [Google Scholar]
- Mills K. H., Barnard A., Watkins J., Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993 Feb;61(2):399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Klykken P. C., Smith S. H., Harris L. S., Munson A. E. Effects of cannabinoids on host resistance to Listeria monocytogenes and herpes simplex virus. Infect Immun. 1979 Mar;23(3):670–674. doi: 10.1128/iai.23.3.670-674.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L., Troutt A. B., McLeod K. S., Kelso A., Handman E., Aebischer T. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immun. 1993 Aug;61(8):3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nahas G. G., Morishima A., Desoize B. Effects of cannabinoids on macromolecular synthesis and replication of cultured lymphocytes. Fed Proc. 1977 Apr;36(5):1748–1752. [PubMed] [Google Scholar]
- Orme I. M., Roberts A. D., Griffin J. P., Abrams J. S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993 Jul 1;151(1):518–525. [PubMed] [Google Scholar]
- Scharton T. M., Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993 Aug 1;178(2):567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991 Nov 1;147(9):3149–3155. [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]
- Winn W. C., Jr Legionnaires disease: historical perspective. Clin Microbiol Rev. 1988 Jan;1(1):60–81. doi: 10.1128/cmr.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C., Friedman H. Differing macrophage and lymphocyte roles in resistance to Legionella pneumophila infection. J Immunol. 1992 Jan 15;148(2):584–589. [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]


