Abstract
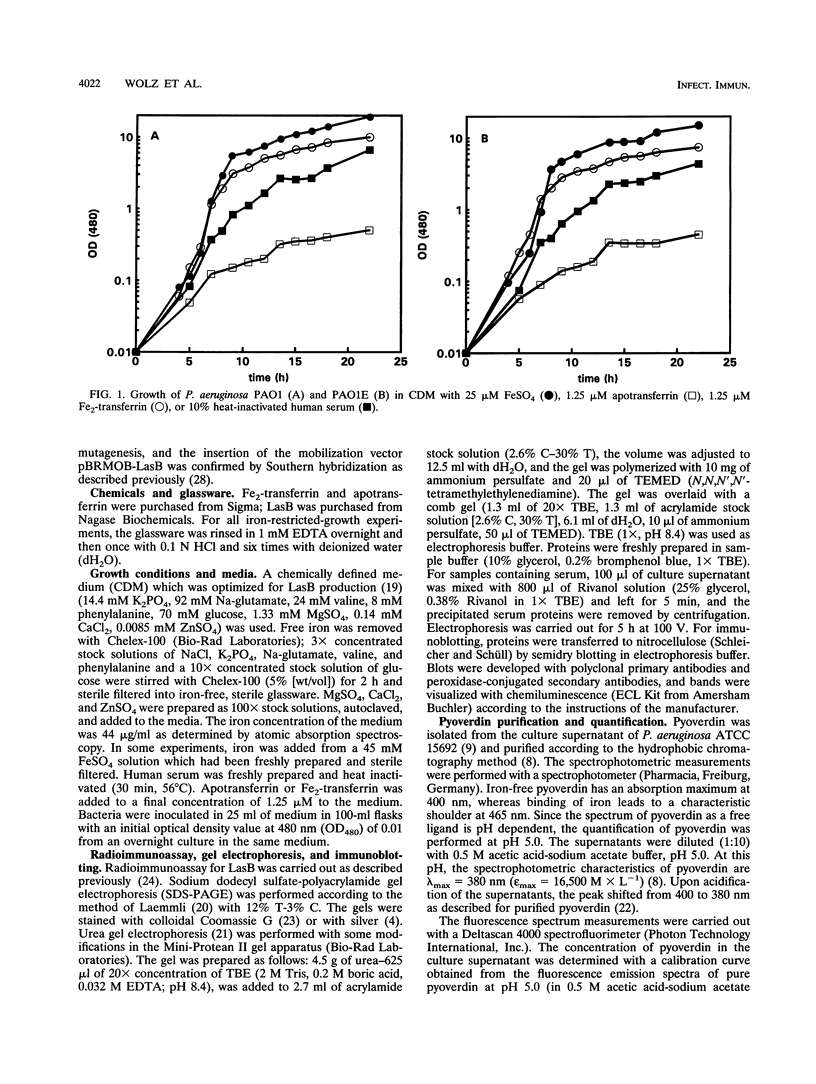
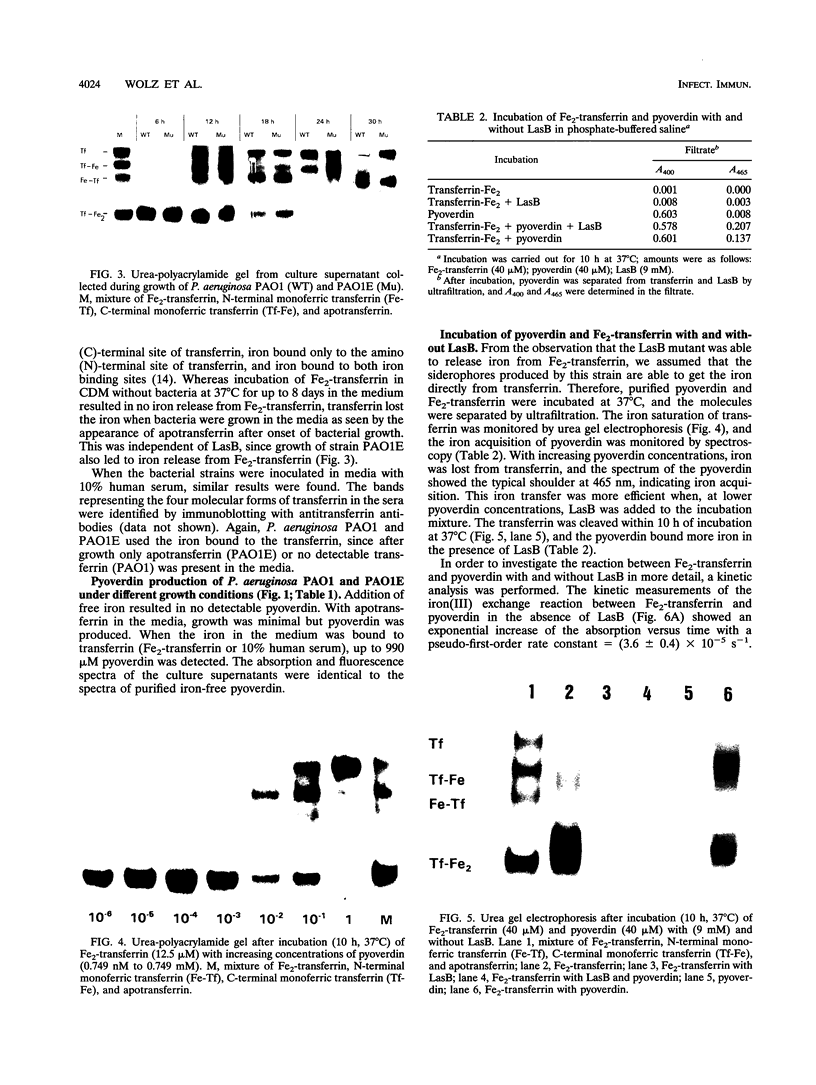
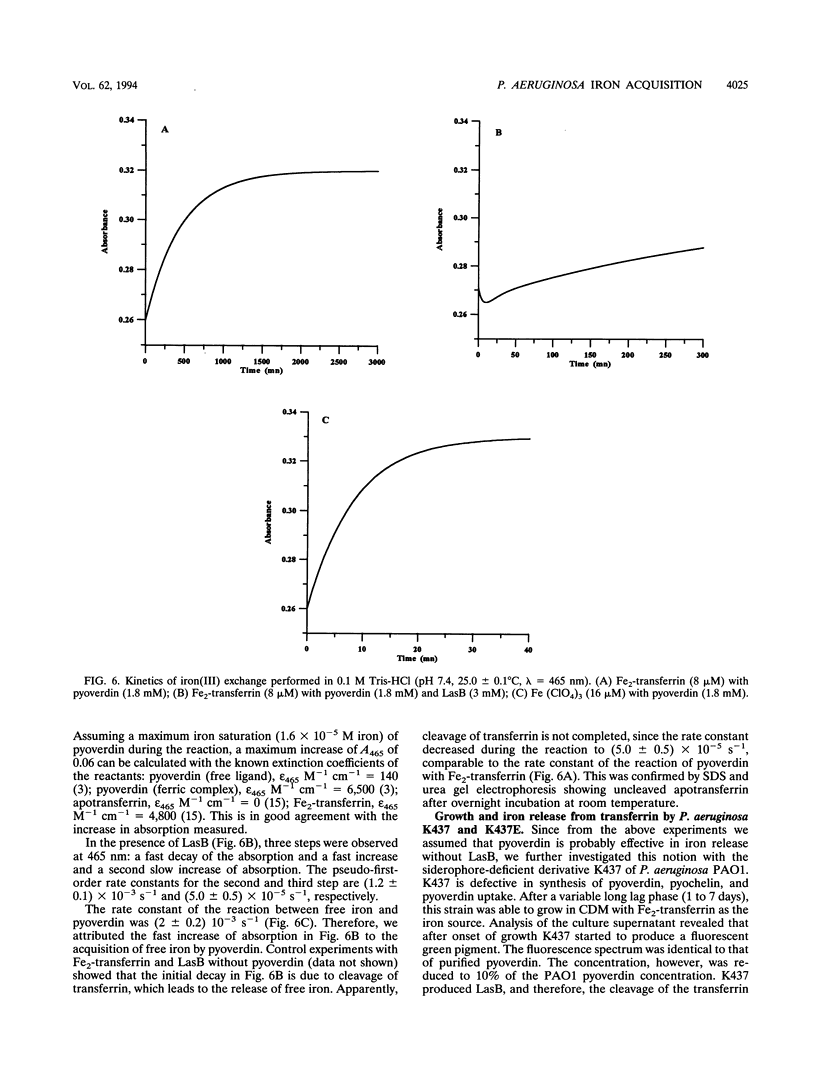
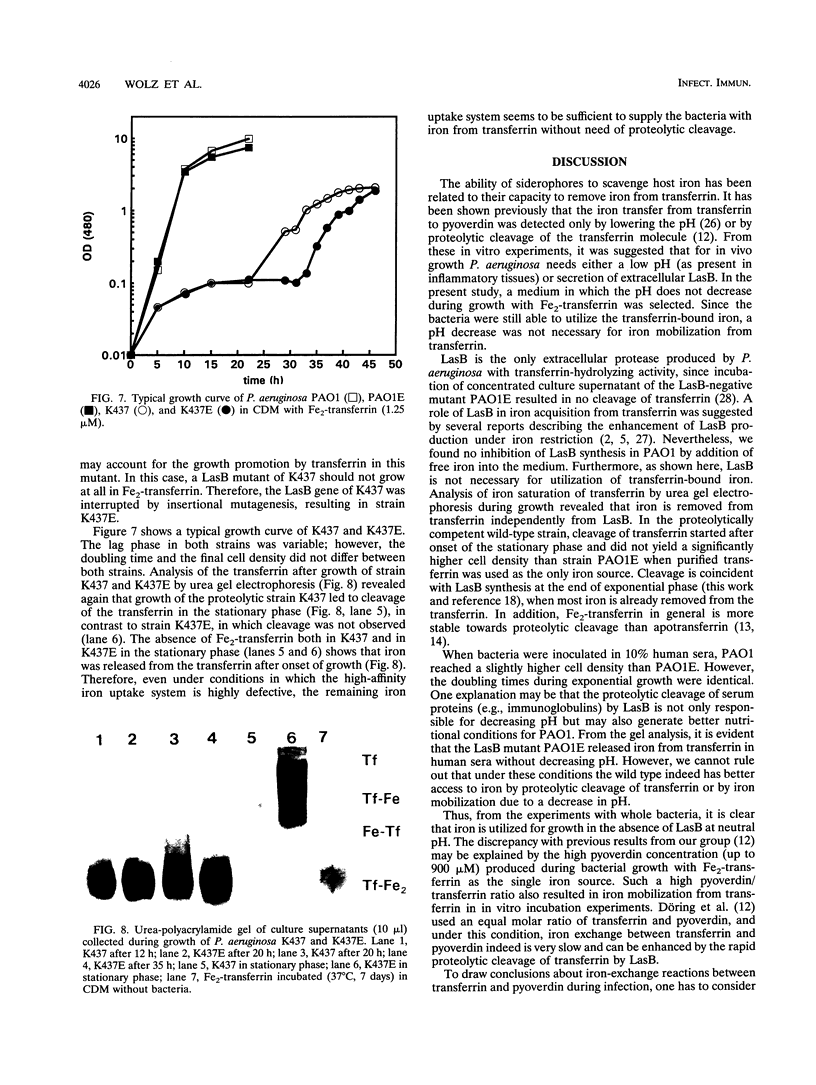
Pseudomonas aeruginosa produces the siderophores pyoverdin and pyochelin as well as receptors for siderophores in response to iron deprivation. Previously, it has been shown in vitro that at neutral pH purified pyoverdin acquires iron from transferrin only in the presence of P. aeruginosa elastase (LasB), which proteolytically degrades transferrin. We constructed a LasB-negative mutant, PAO1E, by insertional mutagenesis to investigate whether this mutant differs in growth from the parental strain PAO1 in an iron-depleted medium supplemented with transferrin or human serum. PAO1 and PAO1E did not differ in growth with 1.25 microM Fe2-transferrin as the only iron source. Urea gel electrophoresis indicated iron release from intact transferrin during the logarithmic growth phase of PAO1 and PAO1E. A total of 333 microM LasB was synthesized from PAO1 after onset of stationary-phase growth. Quantification of pyoverdin by spectroscopy revealed that up to 900 microM pyroverdin was produced during growth of the strains in medium supplemented with Fe2-transferrin or 10% human serum. Incubation of Fe2-transferrin and purified pyoverdin in concentrations similar to those found in the culture supernatant resulted in release iron from transferrin after 10 h at 37 degrees C. However, LasB significantly enhanced the rate constant for iron acquisition of pyoverdin from transferrin. We conclude that P. aeruginosa can use transferrin as an iron source without further need of LasB or pH changes. This is further supported by experiments with P. aeruginosa K437, which has a defective iron uptake system, and its LasB-negative mutant, K437E. Though K437 and K437E did not differ in growth with Fe2-transferrin as the only iron source, their growth was significantly reduced relative to that of PAO1 and PAO1E.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankenbauer R., Sriyosachati S., Cox C. D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985 Jul;49(1):132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik M. J., Storey D. G. Zinc and iron regulate translation of the gene encoding Pseudomonas aeruginosa elastase. Mol Microbiol. 1992 Feb;6(3):337–344. doi: 10.1111/j.1365-2958.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Dalhoff A., Vogel O., Brunner H., Dröge U., Botzenhart K. In vivo activity of proteases of Pseudomonas aeruginosa in a rat model. J Infect Dis. 1984 Apr;149(4):532–537. doi: 10.1093/infdis/149.4.532. [DOI] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K., Flehmig B., Høiby N., Hofmann A. Proteases of Pseudomonas aeruginosa in patients with cystic fibrosis. J Infect Dis. 1983 Apr;147(4):744–750. doi: 10.1093/infdis/147.4.744. [DOI] [PubMed] [Google Scholar]
- Döring G., Pfestorf M., Botzenhart K., Abdallah M. A. Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect Immun. 1988 Jan;56(1):291–293. doi: 10.1128/iai.56.1.291-293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza I., Brock J. H. The effect of trypsin digestion on the structure and iron-donating properties of transferrins from several species. Biochim Biophys Acta. 1980 Apr 25;622(2):297–307. doi: 10.1016/0005-2795(80)90040-9. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams J. Studies of the binding of different iron donors to human serum transferrin and isolation of iron-binding fragments from the N- and C-terminal regions of the protein. Biochem J. 1978 Aug 1;173(2):543–552. doi: 10.1042/bj1730543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B., Kraut J., Marks J., Zanker S. C., Castignetti D. Siderophore presence in sputa of cystic fibrosis patients. Infect Immun. 1991 Nov;59(11):3997–4000. doi: 10.1128/iai.59.11.3997-4000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Fecycz I. T., Campbell J. N. Nutritional factors controlling exocellular protease production by Pseudomonas aeruginosa. J Bacteriol. 1980 Nov;144(2):844–847. doi: 10.1128/jb.144.2.844-847.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Obernesser H. J., Döring G. Extracellular toxins of Pseudomonas aeruginosa. IV. Radioimmunoassay for detection of elastase. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):248–256. [PubMed] [Google Scholar]
- Sriyosachati S., Cox C. D. Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect Immun. 1986 Jun;52(3):885–891. doi: 10.1128/iai.52.3.885-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. G., Ujack E. E., Rabin H. R. Population transcript accumulation of Pseudomonas aeruginosa exotoxin A and elastase in sputa from patients with cystic fibrosis. Infect Immun. 1992 Nov;60(11):4687–4694. doi: 10.1128/iai.60.11.4687-4694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolz C., Hellstern E., Haug M., Galloway D. R., Vasil M. L., Döring G. Pseudomonas aeruginosa LasB mutant constructed by insertional mutagenesis reveals elastolytic activity due to alkaline proteinase and the LasA fragment. Mol Microbiol. 1991 Sep;5(9):2125–2131. doi: 10.1111/j.1365-2958.1991.tb02142.x. [DOI] [PubMed] [Google Scholar]