Abstract
Biological differences between men and women contribute to many sex-specific illnesses and disorders. Historically, it was argued that such differences were largely, if not exclusively, due to gonadal hormone secretions. However, emerging research has shown that some differences are mediated by mechanisms other than the action of these hormone secretions and in particular by products of genes located on the X and Y chromosomes, which we refer to as direct genetic effects. This paper reviews the evidence for direct genetic effects in behavioral and brain sex differences. We highlight the `four core genotypes' model and sex differences in the midbrain dopaminergic system, specifically focusing on the role of Sry. We also discuss novel research being done on unique populations including people attracted to the same sex and people with a cross-gender identity. As science continues to advance our understanding of biological sex differences, a new field is emerging that is aimed at better addressing the needs of both sexes: gender-based biology and medicine. Ultimately, the study of the biological basis for sex differences will improve healthcare for both men and women.
Keywords: Sexual differentiation, brain anatomy, sex differences, sexual orientation, gender identity, sex chromosomes, SRY, dopamine, behavior
1 INTRODUCTION
Men and women are different in many ways. These differences include both biological phenotypes [e.g. 1] and psychological traits [e.g. 2]. Some of these differences are influenced by environmental factors [3; 4]. Yet, there are fundamental differences between the sexes that are rooted in biology.
Of particular interest are sex differences that have been identified in the brain. Although the brains of men and women are highly similar, they show consistent differences that have important implications for each sex. That is, brain sex differences uniquely affect biochemical processes, may contribute to the susceptibility to specific diseases, and may influence specific behaviors. Such biological differences should never be used to justify discrimination or sexism. However, we believe that a thorough understanding of these differences can inform researchers and clinicians so that they can better address important issues. Two examples include how genetic sex can lead to differences between the sexes in the etiology and the progression of disease and how differences in neural development may result in differences in cognition and behavior.
In this paper, we will review sex differences in brain and behavior that are not due to the action of hormones secreted by the gonads—which has been the dominant mechanism associated with such differences—but from what we term `direct genetic effects.' These are effects that arise from the expression of X and Y genes within non-gonadal cells and result in sex differences in the functions of those cells. First, we will highlight some sex differences at the biological level and at the psychological level. Then, we will review the `classic' view that dominated the field of sex differences—that most sex differences, especially those concerned with reproductive physiology and behavior, were due to the action of hormones produced by the gonads. Next, we will present the emerging view that `direct genetic effects' play an important role as well. Finally, we will discuss novel approaches to studying sex differences by focusing on unique groups of individuals: people with sex-chromosome variations (e.g., Klinefelter's Syndrome and Turner Syndrome), people with genetic mutations in the sexual development pathway, people with an atypical sexual-orientation, and people who experience a cross-gender identity.
2 BIOLOGICAL SEX DIFFERENCES
There are many biological differences between males and females that are beyond the obvious differences at a gross, macro level (e.g., height, weight, and external genitalia). Specifically, there are several important physiological differences that have critical implications including the susceptibility to different diseases and the ability to metabolize different medications. In this section we will highlight some sex differences in neuroanatomy and neurochemistry.
2.1 Neuroanatomy
The two sexes have similar but not identical brains. Most brain studies have focused on gross manifestations of these differences—namely the size of specific regions or nuclei. Yet, there is mounting evidence of sex differences at a finer level including differences in synaptic patterns [5; 6] and neuronal density [7; 8; 9]. It is beyond the scope of this article to provide a comprehensive review of all known neuoranatomical differences. We have provided notable sex differences in the rat brain in Table 1. There are also excellent resources for those who are interested in delving deeper into this topic [10; 11; 12].
Table 1.
Selected neuroanatomical sex differences in the rat.
| Structure/Region | Known roles | Sex difference | Basis of difference |
|---|---|---|---|
| Sexually dimorphic nucleus of the Preoptic Area (SDN-POA) | The POA is implicated in the regulation of male copulatory behavior [14]. Lesions of the SDN alone slow acquisition of this behavior. Potential human equivalent is INAH-3 [18]. | 2.6 times larger in males [19]. | Perinatal aromatized androgen decreases neuronal apoptotic rates in males [20]. |
| Anteroventral Periventricular Nucleus (AVPV) | Involved in regulating the luteinizing hormone surge in females [20] and male copulatory behavior [21]. | 2.2 times larger in females with a higher cell density [22]. | Degeneration of cells in this region is greater in males [23] due to prenatal action of androgen |
| Bed Nucleus of Stria Terminalis (BNST) | Plays a role in the control of male sexual behavior [24], release of gonadotropin [25], and modulation of stress [26; 27]. | The principal nucleus (BNSTp) is larger in volume in males [28]. | The larger volume in males is due to sexually different apoptotic rates caused by testosterone [29]. |
| Corpus Callosum | Conducts information between the two halves of the cortex [30]. | Larger in neonatal males [31]. | Organizational effects of testosterone lead to masculinization while feminization appears to be dependent on estrogens [32; 33]. |
| Arcuate Nucleus (ARC) | Helps regulate the estrus cycle [34], appetite and body weight [35]. | Neurokin-B neurons innervate capillary vessels in the ventromedial ARC in post-pubertal males only [6]. | Dihydrotestosterone is responsible for the masculine projection pattern [36]. |
| Amygdala | Strongly associated with emotion, decision-making and Pavlovian conditioning [37]. | Adult males have a larger medial nucleus than adult females [38]. | Treatment of females with estradiol masculinizes this nucleus [38]. |
| The posterodorsal aspect of the medial amydala is 65% larger in males [39]. | Activational effects of circulating androgens accounts for the larger region in males [40]. | ||
| Cerebral cortex | Connected to a wide range of processes from memory [41] to language [42] to emotional processing [43]. | Right posterior cortex is thicker than left but only in males [44]. | Gonadal hormones play a role in maintaining the sex difference (ovariectomy masculinizes the cortex of females) [44]. |
| Ventromedial Hypothalamic Nucleus (VMN) | Involved in the control of lordosis, mounting, and norepinephrine release [45]. High concentrations of steroid receptor mRNA have been observed in the ventrolateral VMN [46]. |
Females have less synapses in the ventrolateral VMN compared to males [8]. | Organizational effects of aromatized testosterone appear to be crucial in establishing the masculine trait [47]. |
| Substantia nigra pars compacta | Made up almost entirely of dopaminergic neurons. Dopamine is involved in control of motor activity [48]. |
Females have 20% fewer dopaminergic neurons [49]. | A genetic component has been demonstrated in mice [50]. |
*Note: This table highlights some prominent sex differences in the rat brain but it is by no means exhaustive. Conflicting evidence concerning the examples reported here (particularly in the SDN-POA) exist, and the interpretation of the data is often more complicated than this summary implies.
We have chosen to focus on neuroanatomical differences in the rat because the biological significance and origins of these differences are much clearer than in humans. Neuroanatomical differences in humans are also well-studied although ethical reasons preclude the experimental manipulations that have led to the findings detailed in Table 1. This significantly limits the conclusions that can be drawn from any observations made in humans.
Although these neuroanatomical differences are intriguing, most are limited because the practical or functional significance of these findings are unknown. Discovering the significance of these differences is often difficult, even in rodents. de Vries and Sodersten have eloquently outlined the challenges facing researchers who want to understand the link between sex differences in structure and behavior [13]. A highly relevant case study highlighted in their review concerns the sexually dimorphic nucleus of the preoptic area (SDN-POA). The preoptic area (POA) has been implicated in the regulation of male copulatory behavior [14], but the link (if any) between the sex difference in SDN-POA size and behavior remains elusive. Masculinizing the size of the SDN-POA in female rats does not result in a corresponding masculinization and defeminization of behavior [15]. Instead, the SDN-POA may be related to inhibition of female sexual behaviors [16; 17], which might not have been an obvious hypothesis given what was known about the POA previously. As science and technology continue to advance, we will eventually know how to make sense of the mounting evidence of sex differences in the brain. For now, it is reasonable to suspect that such differences may help account for observed sex differences in behavior, neurological diseases, and cognitive abilities. SDN-POA) exist, and the interpretation of the data is often more complicated than this summary implies.
2.2 Neurochemistry
Males and females exhibit different patterns of transmitting, regulating, and processing biomolecules. Table 2 presents some of the neurochemical sex differences that have been identified. As a specific example, we focus below on the monoaminergic system, which has been implicated in several neurological diseases and mental disorders that differentially affect men and women.
Table 2.
Selected neurochemical sex differences in the brain.
| Neurochemical system/pathway | Known roles | Species | Selected sex differences |
|---|---|---|---|
| Cathecolamines (also see Figure 1) | Involved in the control of a variety of processes including reproduction and sexual behavior [51; 52], respiration [53], and stress responses [54]. | Rat | Male have higher norepinephrine (NE) levels in the amygdala and hypothalamus at day 25. Direction of this sex difference is reversed at day 300 [62]. |
| In response to chronic physical stress, dopamine (DA) activity is upregulated only in males whereas NE activity is increased only in females [58]. | |||
| Human | Women appear to be more dependent than men on NE for long-term emotional memory formation [63]. | ||
| Serotonin | Modulates a wide variety of processes including mood, aggression, perception, reward, and attention [64]. | Rat and human | Sex differences in the serotonergic system are found at multiple levels [65; 66; 67; 68; 69]. See Figure 2 for an illustration of some of these differences. |
| Aromatase | Plays a key role in sexual differentiation of the brain by converting testosterone to 17β-estradiol[70]. | Rat | Aromatase activity is higher in males than females in many regions including the anterior hypothalamus, BNST and POA [71]. |
| Only males experience spikes in the expression of brain-specific and total aromatase during embryonic development and shortly after [72]. | |||
| Vasopressin (VP) | VP in the central nervous system (CNS) has been linked to learning, memory and motor behavior [73]. It has also been connected to the control of social behaviors such as pair-bonding, parenting and aggression [74]. | Rat | The number of vasopressin-positive cells is 2 to 3 times higher in males than in females [75]. |
| Vasopressin-positive projections are also 2 to 3 times denser in males [75]. | |||
| Intrahypothalamic release of VP due to an increase of plasma osmolality is higher in females. [76] | |||
| Human | Some studies have found that plasma VP concentrations are higher in men than in women [73]. | ||
| Cholinergic system | The cholinergic system helps regulate the sleep-wake cycle and modulates synaptic plasticity implicated in memory, learning, and development [77; 78]. Sex differences are found at many points in the cholinergic system [reviewed in 73]. | Rat | Levels of acetylcholine (ACh) are higher in females, regardless of estrous cycle, than in males [79]. The maximal level of Ach in females was found at proestrus. |
| The binding affinity of muscarinic Ach receptors is lower in females than in males [80]. Estrogens appear to modulate the binding activity of these receptors [81]. | |||
| Human | Men are more sensitive to cholinergic stimulation than women [82]. | ||
| Opioid system | Opioids are a class of chemical for which receptors are found throughout the CNS [83; 84]. Opioids exert an analgesic effect and also play a role in stress response and reproduction [85]. |
Rat and mouse | Generally, μ and κ class opioids seem more effective in males than females although in some cases the effectiveness is equal [86]. In a minority of cases, they are more effective in females. |
| Human | μ-opioids appear more effective in women than in men [86]. | ||
| μ-opioids show significantly higher binding potential in women in the amygdala, thalamus and the cerebellum [87]. The sex difference in the first two regions is reversed after menopause. |
Monoamines are a class of small-molecule neurotransmitters that are involved in the control of a variety of processes including reproduction and sexual behavior [51; 52], respiration [53], and stress responses [54]. Monoamines have also been implicated in numerous mental disorders, including ones that differentially affect men and women [55; 56]. Likewise, sex differences in the monoaminergic systems in the rat are well-documented. Reisert and Pilgrim provided a comprehensive review of arguments for the genetic bases of these differences [57].
Monoamines are subdivided into two groups—catecholamines and indolamines—based on their molecular structure. The main catecholamines are dopamine (DA), norepinephrine (NE) and epinephrine, which are synthesized from the amino acid tyrosine. Figure 1 highlights some of the known sex differences of the dopaminergic system. Regulation of dopamine can potentially control the levels of the other two catecholamines as they are derived from dopamine.
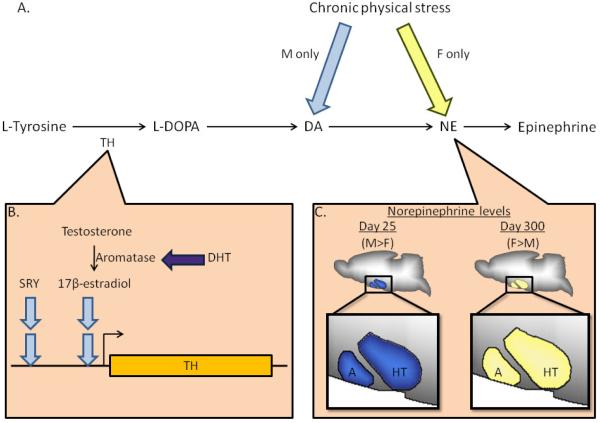
Figure 1.
The catecholaminergic pathway is sexually differentiated TH: Tyrosine hydroxylase, L-DOPA: L-dihydroxyphenylalanine, NE: norepinephrine. (A) Chronic physical stress results in sexually dimorphic responses. Dopamine (DA) activity is upregulated exclusively in males flight blue arrow) while norepinephrine activity is upregulated exclusively in females (yellow arrow) [58]. Only males experience impaired memory. (B) Control of TH expression differs between the sexes. SRY, the testis determining gene, which is not found in females, directly regulates TH expression in males [49; 270]. 17β-estradiol increases TH expression only in males flight blue arrows) [353]. Aromatase activity is more responsive to dihydrotestosterone (DHT) in males than in females (dark blue arrow) [354]. (C) Male rats have higher NE levels than female ones in the amygdala (A) and hypothalamus (HT) early in life [62]. When the rats reach day 300, the direction of this difference is reversed.
Catecholamines are released by the adrenal glands usually in response to stress, which affects males and females differently. For instance, chronic physical stress impairs memory in male rats only [58]. The sexes also show differing neurochemical responses: Dopamine activity is upregulated in males only whereas norepinephrine is upregulated in females only (Figure 1A). Sex differences have also been found in the regulation and modification of dopamine (see Figures 1B and 1C). Specifically, the enzyme tyrosine hydroxylase (TH), which is involved in dopamine synthesis [59], is regulated by Sry—the male sex determination gene—which is not present in females. Additionally, levels of norepinehrine in the amygdala differ between the sexes as a result of age. Thus, it is likely that brain catecholaminergic responses to stress might also differ between the sexes.
Another monoamine is serotonin, which is an indolamine. Unlike catecholamines, serotonin is derived from the amino acid tryptophan. The serotonergic system shows sex differences (Figure 2), though many of these differences remain unlinked to behavioral differences between men and women. Nevertheless, differences in this system likely have consequences given the link between serotonin and numerous mental disorders [60; 61].
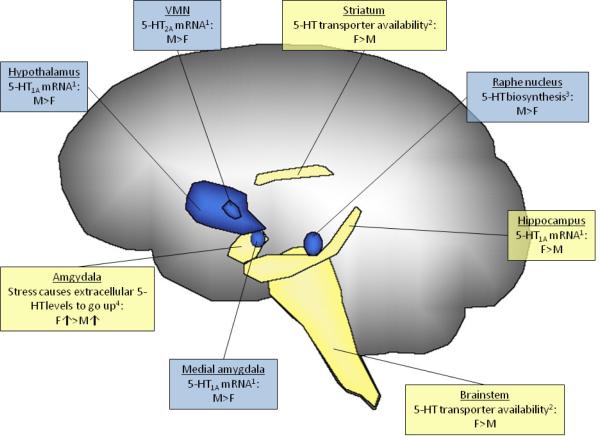
Figure 2.
Serotonin (5-HT) is sexually differentiated on multiple levels. In addition to the differences illustrated above, some of the loci that influence 5-HT levels in the blood are also sexually dimorphic [66]. References: 1 - [67], 2 - [68], 3 - [65], 4 - [69].
3 PSYCHOLOGICAL AND BEHAVIORAL SEX DIFFERENCES
In addition to biological differences, men and women differ in many psychological and behavioral aspects. For instance, men perform better on specific visuospatial aspects (e.g., mental rotation) compared to women; and women perform better on specific verbal tasks (e.g., verbal fluency) compared to men [88]. Furthermore, there is a large sex difference in sexual interests and behaviors, such as interest in casual sex, interest in multiple sex partners, and interest in visual-sexual stimuli (e.g., pornography) [89; 90]. Other examples are summarized in Table 3.
Table 3.
Sex differences in behavioral traits in humans.
| Trait | Sex Bias | Evidence for the role of hormones | Evidence for the role of genetic factors | Other factors affecting sex differences in behavior |
|---|---|---|---|---|
| Cognition | Men do better at spatial tasks [94] and mathematical problem solving [95]. Women do better on verbal fluency, articulation, and verbal memory tests [12]. | Prenatal hormone effects shown from studies of CAH, Turner's and androgen insensitivity syndromes [96] | No reliable evidence for the effect of sex chromosome genes proven from studies of Turner's and XX males [97] | Greater brain asymmetry in men for both verbal and non-verbal tasks [98; 99] |
| Play behavior-movement | There are sex differences in choice of toys, gender of the play partner, social play [100] and movement [101; 102; 103] | Testosterone influences juvenile play [104] Prenatal androgen levels affect play behavior and movement [105; 106] |
Genetics sex seems to affect play behavior more than prenatal hormone exposure [104] | Parents and other socializing agents (i.e. peers, community, and child's own cognitive processes) [107] Developmental experience [108], visual information [109] affect movement organization |
| Language | Women perform better on episodic memory [110] and verbal fluency tasks, men are better at visuospatial processing [111; 112; 113] Greater dependence of females on declarative memory and males on procedural memory [114; 115] |
Estrogen influences word and declarative memory abilities in women [116; 117; 118; 119; 120; 121; 122; 123; 124] Testosterone influences word memory in men [125] Prenatal testosterone levels relate to language processing in girls [126] |
Single nucleotide polymorphisms in the gene, brain derived neurotrophic factor (BDNF) affecting BDNF secretion rates, partly accounting for greater dependence of females on declarative memory and the sex differences observed in language-related tasks [127] | Greater degrees of left hemispheric lateralization of brain for language in males and the bilateral language processing in females [128] Faster development of hippocampal brain regions in girls, activation of certain brain regions such as hippocampus and parahippocampal gyrus [129; 130] |
| Aggression | Foul language, imitation of aggressive models, violence and physical aggression more common in males [131] | Estradiol and progesterone influencing the serotonergic system [132; 133] Weak association between testosterone and aggression in both sexes [134; 135] High testosterone levels leading to increased verbal aggression and impulsivity in women [136; 137] |
Association between serotonin transporter gene polymorphisms and greater impulsivity in males but not females [138] Polymorphisms in monoamine oxidase-A (MAOA) gene associated with antisocial personality disorder and aggression in males [139] |
Low self-control, high impulsivity and negative emotionality [140] Sex-specific disparities in the neural circuitry of impulse control and emotion regulation, as well as serotonergic systems [141] Larger orbitofrontal cortexes in women [142] |
Some contend that these differences are due to social systems and gender socialization [cf. 91; 92; 93] . Nevertheless, biological traits likely contribute to many sex differences. Thus, a thorough understanding of the main determinants involved in expression of such sex differences can help us better explain the relationship between brain, behavior, and environment. In addition, it allows us to determine how one's sex potentially influences the risk of developing disorders that manifest and progress differently in men and women. Such knowledge can better inform the treatment of these diseases. Tables 3 and 4 illustrate several factors (e.g. hormones and genes) that may be causally linked to expression of sex differences in behavior and disease, respectively.
Table 4.
Sex differences in neurological disease.
| Disease | Sex Bias | Evidence for the role of hormones | Evidence for the role of genetics | Other factors affecting sex differences in disease |
|---|---|---|---|---|
| Alzheimer's Disease (AD) | Women demonstrate higher AD prevalence at older ages [143; 144]. | Gonadal hormones implicated in gender-related cognitive deficits of AD but the interaction is complex [145] | APOE allele type [146; 147] (i.e. Less and slower rate of amyloid plaque formation in men due to APOE ε2 [148]) | Greater degeneration in areas of orbitofrontal cortex, middle and posterior cingulate cortex, hypothalamus, and mammilary bodies in men, and anterior thalamic in women [149]. |
| Parkinson's disease (PD) | Overrepresented in males [150; 151] Age at onset is later in women [152]. Pathological symptoms of PD differ among males and females [153; 154; 155] |
Most women manifest PD after menopause [156] Estrogen affecting BDNF secretion [157] Early life estrogen decline seems to be more important [158; 159; 160] |
Linkage to X chromosome markers in 362 families, and to Xq28 in 443 discordant sibling pairs [161; 162] Val66met polymorphism in BDNF in women [163] |
Environmental factors [164] Anatomical and structural differences in dopaminergic systems among males and females [107] |
| Autism | There is a high male to female ratio in the prevalence of autism [165] | Gonadal hormones affecting oxytocin (OT) and arginine vasopressin (AVP) receptors [166; 167] | Single nucleotide polymorphisms in the OT receptor in the Chinese Han [168] and American Caucasian population [169], SNPs in the vasopressin receptor (V1aR) gene [170; 171] X-chromosome has effects on cognition and social aspects [172; 173] |
Alterations in oxytocin or arginine vasopressin activity, and differential processing of the oxytocin precursor [174; 175; 176] |
| Addiction | Drug addiction more frequent in men [12] Higher relapse rates, faster progression of compulsive drug abuse and dependence have in women [177; 178]) |
Estradiol levels correlate with drug induced reinforcing behavior whereas progesterone levels are negatively associated with addiction [179; 180; 181] | Genes encoded on sex chromosomes can affect sex-related differences in addiction (the four core genotype mice) [182] | Neuroanatomical differences in motivation systems among males and females [107] Sex-related alterations in the cortico-limbic-striatal system that mediates reward processing [183] |
| Depression | Women are twice as likely as men to develop depression during reproductive years [184] | Low estrogen levels in female rats mediated by influences on neurotransmitter levels [185] Low testosterone levels associate with risk for depression in young and middle aged-men [186; 187] |
Heritability rates estimated to be 70% [188] Polymorphisms in serotonin gene, estrogen receptor 1 (ESR1) polymorphism in the presence of Val/Val genotype of the Val158Met polymorphism in the Catechol-O-methyl transferase (COMT) gene, longer CA repeats of human estrogen receptor 2 (ESR2), short CAG repeats in androgen receptor gene [189] |
Maladaptive coping, pessimism, dependency, low self- esteem, victimization, sexual abuse, comorbid anxiety disorder more common in depressed women [190] Early life events increase depression rates in adult women [191] |
| Anxiety disorders | The rate of anxiety disorders is higher in females [55]. The high comorbidity of these disorders with major depression helps account for the sex difference in depression [192]. | States of anxiety and panic have been reported to be affected by the menstrual cycle and pregnancy, implicating a role for estrogen and progesterone [55]. Pregnancy and lactation seem to alter brain neurochemical system that affect anxiety and fear [193]. |
TheVal158alleleof COMT is associated with panic disorder in Caucasian women but not men [194]. In Asians, Met158 is associated with panic disorder in women but not men [194]. 5HTTLPR is a polymorphism associated with anxiety in humans. The orthologous polymorphism in rhesus macaques interacts with early adversity in a sexually dimorphic manner [195]. |
Animal studies indicate females undergo less neurobiological changes in response to stress compared to males [193]. It is speculated that this indicates increased adaptability in males and hence lower prevalence of affective illness [193]. |
| Schizophrenia | more common in men than in women [196] Age at onset is later in women, another smaller peak of onset during peri- and post-menopause [196; 197] Pathological symptoms of schizophrenia differ among males and females (males experience more negative symptoms, greater decrease in emotion expression and recognition,, greater paranoid delusions in women) [198] Lower chances of full recovery, and a poorer prognosis in men [196; 197] Anatomical brain differences between male and female patients |
This disease is not common before adolescence and puberty [199] Male schizophrenics have higher levels of Luteinizing Hormone (LH) and testosterone than healthy subjects, and female schizophrenics higher levels of LH and lower levels of estrogen [200] |
Eight ultra-rare variants in eight distinct miRNA genes in 4% of analyzed males with schizophrenia [201] Relatives of females with schizophrenia demonstrate higher levels of the psychotic forms whereas relatives of schizophrenic men express lower rates of psychosis suggesting the presence of genetic heterogeneity [202] Higher rate of CAG repeat expansions among families of female patients and not male patients [203] |
Anatomical and structural brain differences among males and females [198] Higher cortical levels in males as compared to females according to some studies [198] Higher sensitivity of the dopamine system in men as compared to women (Normal males produce more striatal dopamine in response to an amphetamine challenge as compared to females) [204] |
4 THE CLASSICAL VIEW ON SEX DIFFERENCES
Researchers have examined what contributes to the differences we see between males and females. Certainly for humans, social environments influence some of these differences. For instance, social stratifications (e.g., social class and the distribution of social power) and social rules (e.g., customs and traditions) may affect the ability for people to access educational resources or to engage in certain behaviors [205; 206]. However, social factors alone do not contribute to all differences seen between males and females—especially regarding biological differences [207].
The life sciences have elucidated many factors that contribute to sex differences. In this section, we briefly review the classical view that gonadal hormones contribute to most, if not all, sex differences after gonadal differentiation. We will then present some findings that have challenged this view.
4.1 The Role of Gonadal Hormones
Sexual development in mammals can be divided into two main components: sex determination and sex differentiation [208]. `Sex determination' is the process by which the bipotential gonad develops into either a testis or an ovary, which depends exclusively on genetics. `Sex differentiation' is the development of other internal reproductive structures, the external genitalia, and non-gonadal sex differences. Unlike sex determination, sex differentiation is driven by gonadal hormones. It was widely believed that sex differences that emerged after sex determination were largely due to the actions of gonadal hormones. Examples of this pervasive view include writings from Lillie in 1939 (“[T]he mechanism of sex differentiation is taken over by extracellular agents, the male and female hormones.” [209]), Jost in 1970 (“The developmental analysis of the body sex characteristics reveals a hormonal control.” [210], Morris et al. in 2004 (“[A] single factor—the steroid hormone testosterone— accounts for most, and perhaps all, of the known sex differences…”[14]) and Zhao et al. in 2010 (“[T]he sexual phenotype of individuals is dependent on the gonad…” [211]). We will use the term `classical view' to refer to this hypothesis.
The classical view was based on decades of compelling research demonstrating the organizational and activation effects of gonadal hormones in vertebrates [212; 213]. `Organizational effects' refer to the permanent, irreversible changes during development that organize the body in either a male- or female-typical pattern. For instance, the neonatal surge of testosterone in male rodents leads to life-long changes in the synaptic pattern of the ventrolateral ventromedial hypothalamic nucleus [47]. `Activational effects' refer to the short-term changes that occur in the body depending on the presence or absence of specific hormones. An example of this is the requirement for the presence of both estrogen and progesterone to induce or “activate” lordosis in female rats [214].
Recently, it was found that gonadal hormones might not be the sole contributor to male- and female-typical development. Genes encoded on the sex chromosomes that directly act on the brain to influence neural developmental and sex-specific behaviors have been identified—an example of what we describe as direct genetic effects [215; 216]. When we use this term, we refer to effects arising from the expression of X and Y genes within non-gonadal cells that result in sex differences in the functions of those cells or target cells. Such direct genetic actions are wide-ranging and can include effects of locally produced hormones or other non-hormonal messenger molecules. For example, sex differences arising in the brain from differential paracrine secretion of neurosteroids would be considered a direct genetic effect. The commonality among these actions is that they are not dependent on mediation by hormones secreted by the gonads. In many cases, the identity of the messenger molecules have yet to be identified. This review will now focus on examples in which sex differences in behaviors are unlikely to be influenced by only the action of gonadal hormonal secretions and may in fact be due to direct genetic effects.
4.2 Exceptions to the Classical View
The idea that factors other than the gonadal hormone milieu could account for sex differences first gained credence from research performed on the zebra finch. In zebra finches, males exhibit courtship behaviors that are unique to their sex. Specifically, they possess the ability to sing a distinct courtship song. This male-specific ability has been attributed to several brain regions that are larger in males compared to females [217; 218]. Given the hypothesis that such differences must have been the result of sex-specific hormones, several researchers unsuccessfully attempted to alter the courtship behavior of finches by manipulating hormone levels [219]. For example, it was shown that castrated male zebra finches were not significantly different from intact male zebra finches in terms of song development [220]. Furthermore, female zebra finches that developed testes continued to develop feminine song circuitry and did not exhibit masculine song behavior [221; 222].
Several other experimental manipulations led researchers to question the role of hormones. For instance, Jacobs et al. treated female zebra finches with estrogen at the beginning of hatching given that estrogen induces male sexual differentiation in the zebra finch neural song system [223]. Interestingly, estrogen treatment was not able to cause full masculinization of the neural circuitry of the zebra finch song system (the song circuitry was still smaller compared to control males) [224; 225] and supraphysiological doses of estrogen were required for full masculinization [226]. Similarly, it was shown that inhibiting the action of estrogen by using aromatase blockers in males did not completely prevent the male differentiation pathway [217; 227; 228; 229; 230].
The discovery of a rare type of zebra finch provided further support for a new hypothesis regarding sexual differentiation: The bilateral gynandromorphic finch has male-typical phenotypes on one half of the body (e.g., plumage, testis, and song circuitry) and female-typical phenotypes on the other half of the body. Each half of such finches is either entirely genetically male or genetically female. Thus, each side contains the sex-specific genes necessary for the development of the corresponding sex-specific traits. In this model, while the gonadal hormonal actions in producing sex differences in the brain cannot be completely ruled out (both sides of the neural song system were larger than that of normal females), their influences cannot fully explain the differences observed between the left and right sides of the brain. Given this explanation, the most reasonable theory is that endogenous genetic differences in the brain cells themselves can also contribute to the unequal differentiation of the two sides producing sex differences through their local action within the brain [231].
Recent work on gynandromorphic chickens strengthens the case that the classical view largely does not apply to sexual differentiation in birds. Zhao et al. showed that the `sex identity' (or the expression of sex-specific phenotypes) of somatic cells in birds is determined by the sex chromosome complement of those cells and not the gonadal hormonal environment [211; 231]. In mammals, transplantation of somatic cells from one sex into the gonad of the other sex reverses the sex identity of the donor somatic cells. For example, XX cells can develop into functioning Sertoli cells while XY cells can become functioning granulosa cells [232; 233]. However, this is not the case in the chicken as male donor cells introduced into the developing ovary continued to express a male-specific marker and were excluded from `functional' structures of the host gonad. The host and donor somatic cells were exposed to the same hormones, but they responded differently based on their respective sex chromosome complement.
A second exception to the classical view that we highlight below concerns the development of the tammar wallaby. As with brain development, gonadal hormones drive the sex-specific development of the external genitalia in most mammals. Specifically, androgens promote the development of male genitalia. However, the formation of reproductive structures in the tammar wallaby appears to be independent of gonadal hormone control and is solely due to the effect of sex chromosome complement.
The tammar wallaby is a marsupial that is much smaller than the kangaroo. During fetal development, the production of testosterone, which would typically masculinize mammalian fetuses, does not occur in these marsupials until about the fourth or fifth day after birth [234; 235; 236; 237]. Yet, signs of sex-specific reproductive structures (e.g., scrotum, mammary gland, and pouch formation) can be observed as early as several days before birth. In mammals, the development of male-specific structures, is thought to be completely dependent on the action of androgens [208]. Experiments that increased or decreased the action of testosterone or estrogen in the tammar wallaby had no significant effect on the development of the external genitalia [238; 239]. This suggested that such differences were not under gonadal hormone control.
A case similar to the gynandromorphic zebra finch has also been reported in tammar wallabies: This consists of wallabies that are XX on one side of the body and XY on the other side of the body. Such wallabies develop a hemipouch on the XX side and a hemiscrotum on the XY side even after exposure to circulating gonadal hormones [226; 240; 241]. As with the zebra finch, such cases challenged the view that all sex differences were due to hormones produced by the gonads.
5 AN EXPANDED VIEW ON SEX DIFFERENCES
In light of scientific findings, such as with the zebra finch and the tammar wallaby presented above, the field of sex differences has now come to encompass studies that examine gonadal hormone as well as genetic origins of these differences. One of the most significant challenges in studying the establishment of sex differences in animal models has been the difficulty in separating gonadal sex from chromosomal sex. These two parameters almost always correlate in an animal.
In the following section, we highlight the `four core genotypes' model, which has proven to be a powerful tool in teasing out the effects of gonadal versus chromosomal sex and enabling researchers to overcome this confound. We then discuss in-depth sexual differentiation and sex differences in the midbrain dopaminergic system, focusing specifically on the role of Sry. We discuss the implications that these differences may have on the development of this system as well as neurological health implications.
5.1 The `Four Core Genotypes' Model
A 2×2 mouse-model was developed to separate the effects of gonadal sex from chromosomal sex. This model, known as the `four core genotypes' (FCG), allows researchers to establish the relative contribution of sex chromosomes and hormones in sexual differentiation as well as the interaction between the two. Arnold and Chen recently reviewed this model [242]. Here we highlight some of the model's basic concepts.
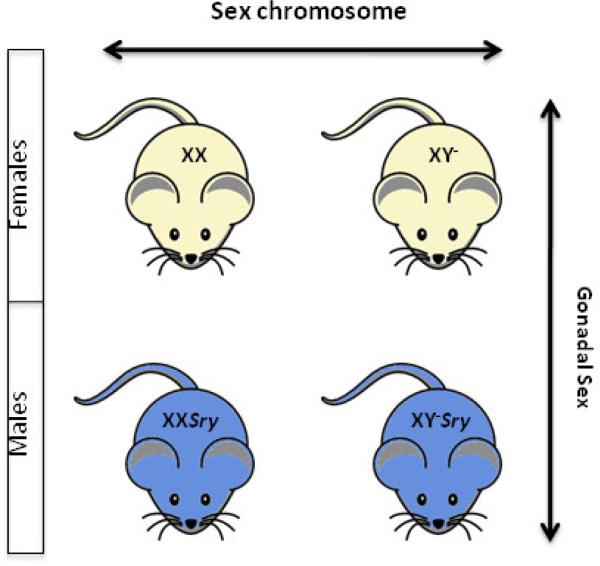
Figure 3 depicts the effect of the presence or absence of Sry—a 12kb region on the Y chromosome that is responsible for testis determination—using the FCG model. An XY mouse should develop testes; however, if Sry is deleted from the Y chromosome (symbolized by Y−) then the mouse will develop ovaries [243]. If the Sry gene is inserted into any chromosome of an XX mouse (symbolized by XXSry), then the mouse will develop testes. Finally, if Sry is deleted from the Y chromosome of an XY mouse and then inserted into one of its autosomes (symbolized by XY−Sry), then it will develop testes.
Figure 3.
2×2 comparison in the four core genotypes model. In this comparison, the factors are gonadal sex and sex chromosome complement.
XY−Sry mice are fully fertile because the presence of Sry promotes testes development. XXSry mice lack some of the genes required for sperm production, which are found on the Y chromosome [244], and therefore do not appear to be fertile. However, they have small testes and are fully masculinized in terms of measures of male copulatory behavior, social exploration behavior, and sexually dimorphic neuroanatomical structures in the septum, and lumbar spinal cord.
XY−Sry mice can be mated with XX females to produce the four types of offspring (XX, XY−, XXSry, and XY−Sry) that can then be used to assess the impact of a mouse's chromosomal and gonadal sex on different phenotypes. That is, if there is a difference between mice that carry the Sry gene (i.e., XXSry and XY−Sry) versus those that do not (i.e., XX and XY−), then the observed difference can be attributed to the gonadal type and/or presence of Sry. On the other hand, if there is a difference between mice that have the Y chromosome (i.e., XY− and XY−Sry) versus those that do not (i.e., XX and XXSry), then the observed difference can be attributed to complement of sex chromosomes (XX versus XY).
5.1.1 Limitations of the FCG model
Some possible limits to the FCG model have been suggested. One possible limit is that the size, morphology, and function of the gonads are not exactly the same in XX and XY mice of the same gonadal type (e.g. XXsry vs. XY−sry). Consequently, the level of gonadal hormone secretions in FCG mice may differ during critical periods of development—a confound that has yet to be investigated. Yet, numerous phenotypes that are responsive to the organizational effects of gonadal hormones (including sexually dimorphic brain structures) do not differ in XX and XY mice of same gonadal type [37,57,80,42], indicating that XX and XY mice of the same sex are likely experiencing similar levels of gonadal secretions. For example, measurements of circulating testosterone in XX and XY males found no difference in testosterone levels between the groups [245].
A second limit relates to the biochemical and molecular environment. That is, one cannot rule out the effect of prenatal hormonal secretions, the influence of adult circulating hormones produced by the gonads or other tissues, acute fluctuations in hormonal levels, and the influence of the Sry transgene (i.e., the potentially higher expression-level of the Sry transgene in XY−Sry animals versus XY mice). For example, the Sry transgene could hasten the early stages of testis organogenesis in XY−Sry males. Furthermore, several phenotypes have been found to differ between XY and XY−Sry males. However, it is not known whether these differences are caused by the effect of Sry on androgen production or by some other mechanisms that are not mediated through the action of gonadal hormones.
To address these limits, it is best to rule out the effect of circulating gonadal hormones. An effective approach would be to first gonadectomize the mice followed by an administration of equivalent doses of gonadal steroid hormones. This is particularly important in the case of XY− females since their level of ovarian steroid hormones differ from that in the XX wild type females [245]. Nevertheless, a major limitation still remains: It will not be obvious whether the sex difference attributed to the complement of sex chromosomes within cells is caused by (a) gene or genes encoded on the Y chromosome; (b) higher dosage of X genes particularly the ones that escape X inactivation in XX animals [246]; or (c) the paternal imprint of the genes encoded on the X chromosome in XX animals, which changes the expression of these genes to exhibit a female-specific pattern [219; 247]. If one determines that the sex difference in phenotype is due to the sex chromosome complement, then the next step would be to discover the nature of the gene or genes involved and identify whether those genes are encoded on the X or Y chromosome and how and where they mediate their role [216].
Notwithstanding these potential limitations, a variety of sex differences have been examined using the FCG model. We review three of these.
5.1.2 Lateral septum
One clear example of the role of sex-chromosome genes in brain phenotypes can be found in the lateral septum. The lateral septum is part of the limbic system and is involved in stress-related behaviors. This nucleus is denser in male brains compared to female brains. However, it was found that the vasopressin fiber density was greater in the lateral septum of XY−Sry and XY− mice compared to XX and XXSry mice [215]. In addition, an examination of vasopressin fiber densities in animals with the same sex chromosome complement indicated a role for the action of gonadal steroid hormones. No interaction was observed between gonadal sex and sex chromosomes [216].
5.1.3 Addiction
On average, women use addictive drugs at lower levels than men, but women become addicted to drugs more rapidly than men [248]. Based on the FCG model, Quinn et al. showed that this difference could be attributed to the differences in the complement of the sex chromosomes and not to the gonadal secretions and/or the expression of the Sry gene. XX mice developed habitual behavior more rapidly than the XY animals independent of their gonadal phenotype and even after gonadectomy. This implies that neither gonadal sex nor circulating steroid hormones exert major effects on the development of habit-driven behavior in mice [182].
5.1.4 Aggression
Males typically exhibit more aggressive behaviors compared to females [249; 250; 251]. Recent reports have shown that aggression latencies are strongly influenced by the simultaneous action of gonadal hormones and sex chromosomes. Using the four core genotypes model, it was found that a significant interaction exists between the two variables. In this model, the XX females appeared to be slower at displaying aggressive behavior on their first encounter with an intruder compared to animals in all other groups [215].
5.2 Direct Role of Sry in Brain Sex Differences
Sex differences in the brain may contribute to some of the psychological and behavioral differences we observe between the sexes. Furthermore, they may influence the susceptibility to different diseases. For instance, Parkinson's disease—a neurodegenerative disease that impairs motor function and speech—affects more men than women. Research has established a link between Parkinson's disease and a loss of dopaminergic neurons in the substantia nigra [252]. Such losses disrupt dopamine pathways, which leads to many of the symptoms associated with Parkinson's disease.
Robust sex differences have been observed in the development, activity, and number of dopaminergic neurons. The data described below represents a clear example of a sex difference in the brain that has a strong genetic component.
5.2.1 Dopaminergic neurons in rodents
Sex differences in dopaminergic neurons have been found prior to exposure to gonadal steroid hormones. During in utero development, rat embryos are exposed to a plasma surge of hormones around embryonic day 17 or 18 (E17 or E18). Yet, as early as E14, dissociated cell cultures of dopaminergic neurons obtained from male and female rat brainstems were found to be fundamentally different in their morphology and function prior to exposure to gonadal steroid hormones [57]. Furthermore, females had higher numbers of dopaminergic, tyrosine hydroxylase-immunoreactive (TH-ir) cells in the midbrain; and their mesencepahlic and diencepahlic neurons produced more dopamine when compared to males. On the other hand, soma measurements of diencephalic neurons from male cultures contained larger dopaminergic neurons. Although it is difficult to make accurate measurements of hormonal levels in the embryonic brain, it is unlikely that there is a huge sex difference due to gonadal hormone exposure at this stage as the rat gonad only begins to differentiate at this point. Therefore, this suggests a contribution of sex chromosome complement and/or sex-specific gene expression.
These differences are not altered even when gonadal hormone levels are manipulated. Specifically, treatment with estradiol and testosterone does not eliminate the observed sex differences in number, size, or function of the dopaminergic cells. Similar findings were later replicated in a study using mesencephalic cultures from the NMRI strain of mice [253]. Collectively, these observations strongly support the idea that some of the sex-specific properties of the dopaminergic neurons appear to be under the control of non-hormonal mechanisms.
5.2.2 The Y chromosome's role in dopaminergic neuron development
A study utilizing the four core genotype model further strengthened the case that a genetic component largely accounts for these sex differences. Carruth et al. cultured mesencephalic neurons from E14 animals representing each of the groups from the four core genotypes [50]. Cultures from XY− and XY−Sry animals developed significantly more TH-ir neurons compared to the XX and XXSry animals. However, gonadal sex did have a small effect: Animals that had Sry (and hence testes) were associated with a higher number TH-ir cells compared to those without Sry. Due to the design of the four core genotypes model, it is difficult to separate the direct effects of Sry from its indirect effects on dopaminergic neurons or their precursors (e.g., through testis determination and the subsequent hormonal secretions).
The data pertaining to sex differences in dopaminergic neuron development show sex differences in distinct directions and so are difficult to interpret. In cultures from E14 rats and NMRI mice, the sex difference is the reverse of what was seen with the four core genotypes. However, rather than invalidating the findings, the conflicting information highlights the complex interactions between genetics and gonadal hormones in leading to the sex differences that are observed. First, the differences between data from NMRI mice and the four core genotypes may be attributable to strain differences. Data from the former study indicate that genetic background can significantly affect whether a sex difference is observed [253]. Carruth et al. had outbred their mice onto the MF1 background [50]. In regards to the differences seen between cultures from rats and the four core genotypes, one possible explanation is that both androgens and the Y chromosome are needed to lead to the number of dopaminergic neurons being higher in males. Support for this hypothesis comes from our own studies where we see that the number of dopaminergic neurons in the rat substantia nigra is higher in adult males [49]. Additionally, the finding that the presence of testis was associated with a higher number of these neurons fits with our hypothesis. There are also important distinctions in the timing of the cultures in relation to gonadal development: while both studies cultured E14 neurons, the bipotential gonad has already differentiated into a testis to a larger extent in the mice [254; 255] than in the rat at this gestational stage [256]. As such, the hormonal environment from which these cultures were derived may not be the same, which could account for some of the disparity in the direction of the sex difference. An elaboration of the hypothesis presented above is that it is not just the testes and androgens that are essential but also Sry, the gene that initiates testicular development. Using a rat model, our laboratory has found evidence that this may be the case and showed that Sry has a direct effect on the expression of TH in the substantia nigra [49].
5.2.3 Sry is a direct effector of TH expression
Sry is the gene on the Y chromosome that directs the bipotential mammalian gonad to develop as testes—hence, its name: Sex determining region on Y. Sry is the founding member of the Sox family of proteins, which play a major role in a wide range of biological processes such as neurogenesis, hematopoiesis, and neural crest development [257]. Sry contains a high mobility group (HMG) box domain and shows little conservation from mouse to human outside this stretch of about 80 amino acids [258].
The HMG box forms a domain that induces a sharp bend in the DNA [259]. It is proposed that this bending of the DNA enhances recruitment of specific transcriptional factors. In line with this hypothesis, Sry has two nuclear localization signals within the HMG domain [260] and its ability to activate transcription in vitro has been demonstrated [261].
Recently, researchers have used genome-wide surveys to identify targets of Sry in the gonad. The most widely known target of Sry is Sox9 [262]. Two other notable targets of Sry are Cbln4 [263], which codes for the cerebellin precursor; and MAO A, which codes for monoamine oxidase A [264]. Wu and colleagues also found that MAO A was upregulated by Sry in the BE(2)C neuroblastoma cell line suggesting that MAO A may be a neural Sry target [264].
Most studies on Sry expression have focused on the gonad and Sry's subsequent effects on sex determination and differentiation [265]. In the developing mouse embryo, Sry is expressed between E 10.5 and E 12.5 in the developing genital ridge, prior to overt testis differentiation [266]. Until recently, it was thought that Sry had no role other than sex determination. However, Sry expression has been found in numerous tissues outside of the testis (see below) and this expression in the adult male rat is now known to have biologically significant effects. Sry's crucial role in the regulation of the catecholaminergic system is one of the best examples of a direct genetic regulator of a trait that differs between the sexes.
5.2.4 SRY in the brain
Clépet et al. were the first to perform a survey of SRY expression in human tissue outside of the gonads [267]. In fetal tissue, SRY was seen in the brain, adrenal, heart, and pancreas. In adults, transcription was detected in the kidney, heart, and liver. This study also showed that SRY was expressed in the teratocarcinoma cell line NT2/D1, which was derived from adult male tissue and which can be used as a model for dopaminergic neurons. When NT2/D1 was induced to differentiate into neurons by retinoic acid, SRY expression remained.
SRY expression in the human adult brain was not surveyed until 1998. Mayer, Lahr et al. showed that SRY mRNA was present in the hypothalamus, frontal, and temporal cortex of only the adult male [268]. Sry mRNA is also found in the adult male mouse brain where it can be detected in the midbrain (including the substantia nigra) and hypothalamus in all developmental stages [269].
5.2.5 SRY and the regulation of TH expression
Sry has a biologically significant role in the brain in at least one instance—the regulation of tyrosine hydroxylase (TH) [49; 270]. In a 2004 study, Milsted, Serova et al. found that Sry is a regulator of TH gene transcription [270]. The study looked at Sry's role in relation to TH in both the brain and the adrenal medulla. They demonstrated that Sry and TH mRNA were co-localized in the locus coeruleus, substantia nigra, and ventral tegmental area of the male rat (Figure 4A). They then used a luciferase reporter assay to show that Sry's ability to upregulate TH expression is dependent on the AP1 binding sites in the promoter of TH.
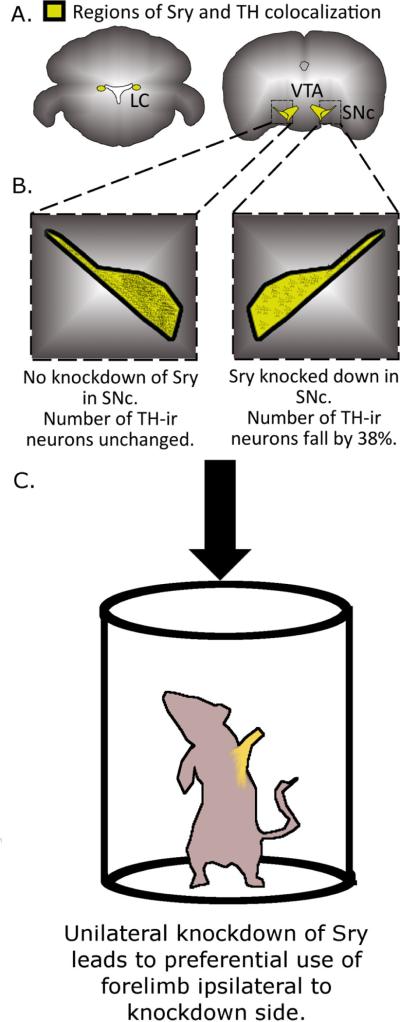
Figure 4.
Sry regulates tyrosine hydroxylase (TH) levels and motor behavior. (A) Sry and TH colocalize in the locus coeruleus (LC), ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) [49; 270]. (B) Knockdown of Sry expression in the SNc leads to a reduction in the number of TH-immunoreactive (TH-ir) neurons. Unilateral infusion of antisense oligodeoxynucleotides (ODN) against Sry decreased the number of TH-if neurons by 38% compared to the contralateral side infused with sense ODN [49]. (C) Unilateral downregulation of TH expression by Sry leads to asymmetric limb use. Animals preferentially used the forelimb ipsilateral to the side of the antisense ODN infusion (preferred limb highlighted in yellow) [49].
The in vivo significance of those findings was shown and expanded upon by a study from our laboratory. By in situ hybridization, we were able to determine the spatial distribution of Sry mRNA within the rodent brain [49]. Specific labeling of Sry was observed in the substantia nigra, medial mammillary bodies of the hypothalamus, and the cortex of male rats only. These transcripts were translated and co-localized with the TH protein—all neurons in the substania nigra positive for Sry were also positive for TH. Knocking down Sry expression in the male rat substania nigra led to 38% fewer TH-immunoreactive neurons and introduced a significant asymmetry in limb use where the animals strongly favored the usage of their ipsilateral limbs (Figures 4B and 4C). The reduction in TH-ir neurons was not due to neural degeneration and is most likely due to a reduction in TH expression. There was also a 26% decrease in TH-ir cells in the striatum when Sry expression was knocked down in that region. TH-ir neuron number was not affected in females infused with the Sry antisense cocktail.
The nature of Sry's modulation on TH remains unclear. The results of the study by Milsted, Serova et al. argue that the TH response to Sry is likely an indirect one [270]. Our data indicate that there may be both direct and indirect mechanisms [49].
The identification of a specific function for Sry in the dopaminergic system, specifically, and the brain, generally, is still absent. Additionally, comprehensive temporal and spatial expression studies need to be performed on brain Sry expression. Another largely unanswered question concerns the identity of a female-specific `compensatory' factor for Sry.
We have shown that the attenuation of Sry expression in males results in detrimental motor effects and that females have lower levels of TH neurons [49]. However, female rats do not go through life exhibiting motor dysfunction. The higher susceptibility of men to Parkinson's disease also implies that this factor exists and might have protective effects against the nigrostriatal degeneration that is the hallmark of Parkinson's [252]. Estrogens are a viable candidate for this factor – short-term injections of estradiol benzoate lead to an increase in TH mRNA [271] and ovariectomy results in loss of TH-positive neurons [272].
6 NOVEL APPROACHES TO STUDYING SEX DIFFERENCES
Traditional animal models have played an invaluable role in advancing our understanding of sex differences. In particular, scientists are able to conduct experimental manipulations that would be unethical on human subjects. However, research on specific groups of people has addressed some complex questions. In this section, we focus on research conducted on four such groups: people with sex-chromosome variations, people with genetic mutations of the sexual development pathway, people attracted to the same sex, and those with cross-sex gender identity. For readers interested in learning more about disorders of sex development, several comprehensive resources exist [208; 273].
6.1 Genetic Disorders of the Sex Chromosomes
The most obvious genetic difference between females and males is their sex chromosome complement (i.e., XX and XY). Various human sex chromosome disorders exist, which might be considered a human model for sex chromosome effects similar to the four core genotypes. The most common variants in men involve additional X or Y chromosomes: Klinefelter's Syndrome (47,XXY); and 47,XYY Syndrome. In women, the most common variants entail the addition or absence of X chromosomes including 47,XXX; 48,XXXX; and Turner Syndrome (45,X).
Chromosomal abnormalities can highlight the role that sex chromosomes play in the phenotypic differences typically seen between 46,XY men and 46,XX women. For instance, adolescent girls with Turner Syndrome are more likely to have social difficulties compared to 46,XX girls [274], which may be partly related to facial and emotional-processing impairments [275]. Furthermore, 46,XX girls score better than boys on tests of social cognitive skills [276]. The fact that both 46,XY boys and 45,X girls experience more social adjustment problems compared to 46,XX girls suggests the presence of a genetic locus involved in social cognitive skills on the X chromosome. Data from Skuse et al. [277], suggest that this locus may be subject to imprinting. Significant differences between 45,XpO Turner-syndrome girls (in which the X was of paternal origin) and 45,XmO girls (in which the X was maternally derived) in terms of social skills have been reported. 45,XpO had superior social competence and better social skills than 45,XmO girls suggesting that the genes in this locus are expressed only from the paternal X. This could potentially be one of the reasons why boys are more susceptible to disorders such as autism that affect social adjustment and social skills such as language. In boys the X is only of maternal origin and therefore this locus would be silenced.
In other cases, however, the characteristics of individuals with sex chromosome abnormalities may augment the expected sex difference. On average, men in the general population have better visuospatial skills than women, and women have better verbal skills than men—which suggests that increased dosage of X chromosome genes may contribute to these skills. However, women with Turner Syndrome have impaired visuospatial abilities yet greater language skill compared to control women [278; 279].
The role of the Y chromosome in psychosexual differentiation is still unclear. Work by McCarty et al. indicates that genes on the Y chromosome outside of SRY and the pseudoautosomal region have “no obvious role…on psychosexual differentiation in genetic males [244].” This is difficult to ascertain because (a) the incidence of XY gonadal dysgenesis is extremely rare, estimated to be 1 in 20,000 [280]; and (b) these individuals have been poorly studied in regards to sexual differentiation of the brain.
6.2 Androgen Insensitivity Syndrome
The role of the Androgen Receptor (AR) in brain sexual differentiation has been discussed in patients with Androgen Insensitivity Syndrome (AIS). AIS is an X-linked recessive disorder that is seen in 1 out of 20,400 live male births [281]. There are two forms of AIS: Complete (cAIS) and Partial (pAIS). People with Complete AIS are genetically male (46, XY with undescended testes) but phenotypically female. However, individuals with Partial AIS typically have ambiguous genitalia.
AIS is caused by mutations in the androgen receptor (AR) gene [282]. There is an important difference in the behavioral phenotype between humans and rats with Complete AIS. Humans with Complete AIS are female-typical in their play behavior and sexual orientation [283]. In contrast, XY rats with AR mutations behave sexually like wild-type males and have a male-typical partner preference [283]. The reasons behind this difference remain unclear although the implication is that androgens play an important role in masculinizing the human brain. However, we cannot completely discount the role of estradiol as the expression of aromatase (which converts testosterone to estradiol) is dependent on androgen signaling via AR [284].
Sexual Orientation
Of all behavioral differences between males and females, partner choice is one of the most pronounced. With very few exceptions in the Animal Kingdom, males typically choose females to mate with, and females typically choose males to mate with. Although sexual selection is a driving force of evolution, little is known about the molecular basis of partner preference.
Human sexual orientation is a complex phenotype to study. Part of this difficulty comes from the accurate assessment of sexual orientation [285; 286], especially when researchers depend on self-identification, which may be mediated by numerous social and psychological factors [287; 288; 289]. Nevertheless, most people report primarily opposite-sex or heterosexual attractions. Yet, a significant number of people (approximately 2–6%) report predominantly homosexual attractions [290].
The distribution of sexual behavior differs between men and women. In men, the distribution is largely bimodal [291]. That is, men are either attracted to one sex or the other. Although there is disagreement regarding bisexuality among men [292], physiological research has found that very few men (even those who openly identify as bisexual) show comparable physical attraction to both men and women [293]. The distribution is more complex in women, in which the fraction of women that show exclusive same-sex attraction is lower than men (1–3%), but many more women than men report erotic fantasies towards both sexes [294].
In this section we will highlight some of the biological research that has focused on same-sex attraction. A more thorough review is available for interested readers [295].
6.2.1 Neuroanatomy differences in sexual orientation
Neuroanatomical differences have been reported for three brain regions based on sexual orientation in human males: the arginine vasopressin neuronal population of the suprachiasmatic nucleus, which was larger in gay men than in male and female controls [296]; the third interstitial nucleus of the anterior hypothalamus (INAH-3), which is smaller in gay men and more similar in size to female controls [297]; and the anterior commissure, which is larger in gay men than in control males and females [298]. The most discussed anatomical finding was in INAH-3 [297]. Although subsequent researchers reported inconsistent findings [299], a comparable difference was found in sheep [300].
Approximately 8–10% of the domestic ram population has been found to sexually prefer other males. Unlike other animal models showing atypical sexual behavior, these male-oriented rams mount and ejaculate on other males versus simply exhibiting a passive stance (i.e., lordosis). Consequently, they are an ideal animal model of male homosexuality because their coital behavior is masculine but their sexual partner preference is feminine.
An analogue of the sexually dimorphic nucleus (ovine SDN or oSDN)—a hypothalamic nucleus thought to be involved in mate selection—was identified in the sheep brain [300]. The oSDN was found to be larger in female-oriented rams compared to male-oriented rams (MORs), and ewes; the latter two groups had oSDN's comparable in size. It was hypothesized that the oSDN corresponds with human INAH-3, which suggests that the relevant neuroanatomical pathways are conserved between mammalian species.
6.2.2 The role of prenatal androgens
One of the main hypotheses on the determinants of sexual orientation was that same-sex attraction was the result of atypical sex-hormone levels during gestation. Studies in rodents and ferrets showed that pre- or perinatal hormonal manipulation could lead to changes in partner preference, sexual behavior, and coital performance largely controlled by the hypothalamus [301; 302]. Yet, extending this hypothesis from animal research to humans is difficult in our opinion. Atypical sexual behavior in rodents is hard to equate to human sexuality. For example, the induction of lordosis in male rats does not change their partner preference. Instead, what changes is the rat's entire sexual behavior, which is different from sexual orientation. Rather, an animal that consistently chooses same-sex partners—such as the abovementioned ram whose adult hormone levels are within the male-typical range [303]—would be a better model. Furthermore, the treatment necessary to change the sexual behavior of rodents goes far beyond any naturally occurring variation in androgen levels [304], and as such is unlikely to reflect natural causes of human variation in sexual orientation. Additionally, hormonal manipulations have failed to make male animals mount other males.
Case studies on humans with various genetic defects in the androgen pathway show only limited support for the hypothesis. There are no reports showing an increase in attraction to men in hypovirilized XY individuals relative to the general population. This implies that disruption of the androgen pathway does not have a strong effect on male sexual orientation. The role of androgens in female sexual orientation appears more complex. Women with congenital adrenal hyperplasia (CAH) experience abnormal activity of the embryonic adrenal glands. This leads to a much higher exposure of female fetuses to androgens, greatly exceeding female-typical levels. The exposure is often high enough to cause some degree of genital masculinization. Several studies have found that CAH women reported more same-sex sexual activity and that more self-identified as homosexual compared to the general population, which suggests that typical female sexual development is disrupted by extreme prenatal androgen exposure [305]. It is important to note that while women with CAH reported more gender atypical attitudes, interests, and behavior, the majority still identified as heterosexual. The role of androgens in the sexual orientation of lesbian women who have no genital masculinization is still unclear.
Two studies looked at genetic variation in genes related to the steroid pathway. A candidate gene study on the human androgen receptor gene [306] and one on the aromatase gene (CYP19) [307] found no evidence that variations in these genes play a role in variations in human sexual orientation. A variety of anthropomorphic measures have been used as indirect measures of prenatal androgen exposure, but results have been inconsistent. A recent prospective study showed no correlation between maternal circulating androgen concentration at 18 and 34 weeks of gestation and digit ratio in girls [308]. An in depth discussion of these studies and a speculation on their widely varying results falls outside of the scope of this manuscript. For a review on the often cited 2D:4D finger-length ratio in sexual orientation, see McFadden et al. [309]. Finally, studies retrospectively examining the influence of stressful events during pregnancy have been inconclusive [310; 311]. Therefore we believe that there is little evidence that naturally occurring variations of prenatal circulating gonadal hormones within one sex play a role in determining variants of sexual orientation although diverging views have been expressed on this topic.
6.2.3 The genetics of sexual orientation
Evidence is mounting that there is a strong genetic component influencing sexual orientation. Family studies [312; 313; 314; 315] have found an increased rate of homosexuality among siblings and in the maternal uncles of gay men (a median rate of 9% for brothers of gay men) [316]. Although the concordance rates of homosexuality in monozygotic twins vary depending on ascertainment methods [313; 317; 318; 319], twin studies have found that there is a substantial genetic component in the development of sexual orientation.
There has been limited molecular genetics research. In 1993, Hamer et al. reported that male homosexuality was more often on the mother's side of the family versus the father's side [291]. A linkage scan showed significant linkage of male homosexuality to the X-chromosome region Xq28 [291]. This finding was subsequently replicated by two studies [294; 320] but not by an independent group [321]. However, a meta-analysis of the results across all four studies yielded an estimated level of Xq28 allele sharing between gay brothers of 64% instead of the expected 50% [322]. Nevertheless, the exact gene(s) involved has (have) yet to be identified.
A different method also implicated the role of the X-chromosome. Unlike male cells, female cells contain two X-chromosomes. Consequently, each female cell randomly inactivates one X-chromosome during embryogenesis to create dosage compensation: The inactive chromosome remains inactive in all resulting daughter cells [323]. If inactivation is completely random, this means that in a population of female cells the maternal X will be inactivated in 50% of the cells whereas the paternal X is inactive in the remaining half. If a particular X chromosome, whether maternal or paternal, is inactivated in more than 90% of cells, that individual is considered to be extremely skewed in regards to X-inactivation. Mothers with gay sons were found to have extreme skewing of X-inactivation when compared to mothers with no gay sons: Skewing in mothers with one gay son = 13/97 or 13%; skewing in mothers with two or more gay sons = 10/44 or 23% [324]. This suggested an involvement of the X chromosome in the molecular mechanisms of sexual orientation. Arguably, the effect of the X-chromosome gene(s) or mechanisms that influence sexual orientation in the sons is visible in the blood of their mothers.
A genome-wide linkage scan on gay-brother pairs showed suggestive linkage to loci on chromosome 7 and 8 [325]. A maternal origin effect was found near marker D10S217, located at 10q26, with significant linkage for maternal meioses but no paternal contribution. This result suggested the presence of a maternally-expressed, paternally-silenced imprinted gene for sexual orientation in 10q26. The relatively small sample size (N = 456) likely underpowered this study. However, a larger linkage scan on 1000 homosexual male sibling pairs is currently underway (A.R. Sanders, personal communication).
The presence of a possible imprinted gene on chromosome 10 is particularly interesting. Previously reported evidence of maternal loading of sexual orientation transmission was initially used to implicate the X-chromosome in human sexual orientation, but it could just as well indicate epigenetic factors acting on autosomal genes. A role for imprinted genes in human sexual orientation was hypothesized earlier [326].
One of the most replicated findings in sexual orientation research is known as the `fraternal birth order effect': Each older brother increases the odds of male homosexuality by approximately 33% [327; 328]. This is relative to the baseline frequency of homosexuality, and the odds of being homosexual are about twice as high for the fourth-born son relative to the first-born son. Yet, this finding is not so simple: The effect is only influenced by older brothers born via the same mother, it is not influenced by the number of older sisters, and it only seems to be true for right-handed homosexual men [329]. The dominant hypothesis for this effect, which lacks empirical support, is that each successive male pregnancies increases the mother's immunity against male-specific antigens expressed by the fetus [330; 331], and this immune response affects any subsequent male fetuses. Although this phenomenon does not directly implicate genetics, at the very least it demonstrates a biological basis for human male sexual orientation and suggests the immune system as an alternative, gonadal hormone-independent mechanism through which sex differences can be mediated.
Altogether, there is mounting evidence for a genetic role of human sexual orientation. The overwhelming dominance of heterosexual behavior in the animal kingdom points at a tight molecular regulation of this trait.
6.3 Gender Identity
Gender identity, or our sense of maleness or femaleness, plays an important role throughout our development affecting both our sense of self and our relationships [295; 332; 333]. Our gender identity and the roles ascribed to that gender are heavily influenced by social factors [e.g. 334]. Most people adopt a gender identity congruent with the sex assigned at birth, which remains constant throughout life [206]. The best approach to study the biological basis of gender identity is to study individuals who develop a cross-gender identity—in particular transsexuals.
Zhou et al. were the first to describe a sex difference in the central subdivision bed nucleus of the stria terminalis (BSTc) in humans and a potential biological marker for gender identity [335]. The type and direction of the sex difference mirrored that of the rat: the volume of the BSTc is larger in men than in women. The study also found that the BSTc of male-to-female (MtF) transsexuals is female-sized but the interpretation of this finding is complicated. The MtF subjects used in the study had all received estrogen therapy so it remains unclear if the sex difference is related to gender identity or hormonal exposure since estrogens can modify the structure of the brain. A second confound is the relatively small size of the sample pool as the authors were only able to gain access to tissue from six MtF transsexuals.
The literature on the genetic basis of transsexualism is extremely limited. Although there are reports of families where several members identify as transsexuals [336], such reports are rare. There are few twin case studies, and they have reported differing concordance rates for transsexualism [337; 338; 339; 340]. Since no systematic twin study has been reported, it is impossible to separate genetic from environmental influences. Consequently, there is no clear support for a genetic basis of transsexualism at this point.
A number of chromosomal abnormalities have been reported in transsexuals [341; 342; 343; 344]. In all cases, sex chromosomes were involved. The most common association was with disomy-Y (47,XYY). However, because of the relatively high frequency of sex chromosome aneuploidy (1 in 900 males for XYY; [345]) a statistically significant association with transsexualism has not been shown.
A small number of candidate genes have been studied for transsexualism. A recent study looked at a polymorphism in the gene coding for 5-alpha reductase and found no assocation in a sample of MtF and female-to-male (FtM) transsexuals [346]. The same group found a significant association between a single nucleotide polymorphism in the CYP17 gene (which encodes the 17α-hydroxylase enzyme) in FtM transsexuals but not MtF transsexuals [347]. However, their sample size was small and they reported a significant difference in allele distribution between male and female controls as well, shedding doubts on these results.
A small study of MtF transsexuals in a Swedish population studied repeat sequences in or near the androgen receptor gene, the estrogen receptor beta gene, and the aromatase gene. They found an association between MtF transsexualism and a dinucleotide CA polymorphism in the estrogen receptor beta (ERb) gene [348]. However, a larger study of MtF transsexuals failed to replicate the ERb results and instead found a significant association with the androgen receptor repeat[349]. This, too, was not replicated by a subsequent research team [350]. Overall, the significance of these genetics studies remains unclear.
7 CONCLUSION
There are many differences between men and women. In this review, we have focused on brain sex differences because of the role that they play in people's health and behavior. Historically, it was believed that such differences were solely due to gonadal hormone secretions. Yet, emerging research is also implicating direct genetic effects.
The next challenge will be to first elucidate the molecular mechanisms by which these direct genetic effects on sex differences arise. One way to address this question will be to manipulate gene dosage in specific tissues and at specific times of development by using targeted approaches in genetically modified animals. Another critical issue will be to understand how compensation mechanisms between the sexes operate. de Vries proposed that observed molecular sex differences may compensate for other sex differences rather than generating differences between males and females themselves [351]. It will be crucial to tease out whether small imbalances influence specific differences in neuropsychological function. Finally, it will be essential to translate our knowledge of sex differences to improve the quality of medical and psychological care.
As science continues to advance our understanding of sex differences, a new field is emerging focused on better addressing the needs of men and women: gender-based biology and medicine. The ultimate aim of this field is to translate scientific data into practical applications that are effective for each sex [352]. From tailoring preventive screenings to treating sex-specific illnesses, this field recognizes that “one-size-fits-all” healthcare has its limits. Rather, our biological sex is an important variable that must be considered in our mental and physical health.
Acknowledgements
This work was funded in part by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Levant RF, Hall RJ, Williams CM, Hasan NT. Gender Differences in Alexithymia. Psychol Men Masc. 2009;10:190–203. [Google Scholar]
- [2].Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 2009;29:14265–70. doi: 10.1523/JNEUROSCI.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Addis ME, Mansfield AK, Syzdek MR. Is “Masculinity” a Problem?: Framing the Effects of Gendered Social Learning in Men. Psychology of Men & Masculinity. 2010;11:77–90. [Google Scholar]
- [4].Woody S, McLean PD, Taylor S, Koch WJ. Treatment of major depression in the context of panic disorder. J Affect Disord. 1999;53:163–74. doi: 10.1016/s0165-0327(98)00117-7. [DOI] [PubMed] [Google Scholar]
- [5].Greenough WT, Carter CS, Steerman C, DeVoogd TJ. Sex differences in dentritic patterns in hamster preoptic area. Brain Res. 1977;126:63–72. doi: 10.1016/0006-8993(77)90215-3. [DOI] [PubMed] [Google Scholar]
- [6].Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141:1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- [7].Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral Asymmetry and the Effects of Sex and Handedness on Brain Structure: A Voxel-Based Morphometric Analysis of 465 Normal Adult Human Brains. Neurolmage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- [8].Matsumoto A, Arai Y. Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986;42:232–6. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- [9].Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15:3418–28. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hines M. Brain gender. Oxford University Press, Oxford; New York: 2004. pp. 191–197. [Google Scholar]
- [11].Einstein G. Sex and the brain. MIT Press; Cambridge, Mass.: 2007. [Google Scholar]
- [12].Becker JB. Sex differences in the brain : from genes to behavior. Oxford University Press, Oxford; New York: 2008. [Google Scholar]
- [13].de Vries GJ, Sodersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55:589–96. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- [15].Ito S, Murakami S, Yamanouchi K, Arai Y. Prenatal androgen exposure, preoptic area and reproductive functions in the female rat. Brain Dev. 1986;8:463–8. doi: 10.1016/s0387-7604(86)80070-5. [DOI] [PubMed] [Google Scholar]
- [16].Powers B, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175:1003–5. doi: 10.1126/science.175.4025.1003. [DOI] [PubMed] [Google Scholar]
- [17].Hennessey AC, Wallen K, Edwards DA. Preoptic lesions increase the display of lordosis by male rats. Brain Res. 1986;370:21–8. doi: 10.1016/0006-8993(86)91100-5. [DOI] [PubMed] [Google Scholar]
- [18].Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. The Journal of Comparative Neurology. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- [20].Tsukahara Shinji. Sex differences and roles of sex steroids in apoptosis of sexually dimorphic nuclei of preoptic area in postnatal rats. Journal of Neuroendocrinology. 2009;9999 doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- [21].Rhees RW, Al-Saleh HN, Kinghorn EW, Fleming DE, Lephart ED. Relationship between sexual behavior and sexually dimorphic structures in the anterior hypothalamus in control and prenatally stressed male rats. Brain Research Bulletin. 1999;50:193–199. doi: 10.1016/s0361-9230(99)00191-4. [DOI] [PubMed] [Google Scholar]
- [22].Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982;212:118–30. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- [23].Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neuroscience Letters. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- [24].Emery DE, Sachs BD. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav. 1976;17:803–6. doi: 10.1016/0031-9384(76)90044-5. [DOI] [PubMed] [Google Scholar]
- [25].Beltramino C, Taleisnik S. Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinology. 1980;30:238–42. doi: 10.1159/000123007. [DOI] [PubMed] [Google Scholar]
- [26].Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- [27].Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–8. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- [28].del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987;429:295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- [29].Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101:13666–71. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sperry R. Some Effects of Disconnecting the Cerebral Hemispheres. Science. 1982;217:1223–1226. doi: 10.1126/science.7112125. [DOI] [PubMed] [Google Scholar]
- [31].Zimmerberg B, Scalzi LV. Commissural size in neonatal rats: effects of sex and prenatal alcohol exposure. Int J Dev Neurosci. 1989;7:81–6. doi: 10.1016/0736-5748(89)90046-4. [DOI] [PubMed] [Google Scholar]
- [32].Fitch RH, Cowell PE, Schrott LM, Denenberg VH. Corpus callosum: ovarian hormones and feminization. Brain Res. 1991;542:313–7. doi: 10.1016/0006-8993(91)91584-n. [DOI] [PubMed] [Google Scholar]
- [33].Fitch RH, Berrebi AS, Cowell PE, Schrott LM, Denenberg VH. Corpus callosum: effects of neonatal hormones on sexual dimorphism in the rat. Brain Res. 1990;515:111–6. doi: 10.1016/0006-8993(90)90584-x. [DOI] [PubMed] [Google Scholar]
- [34].Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord. 2007;8:21–9. doi: 10.1007/s11154-007-9032-6. [DOI] [PubMed] [Google Scholar]
- [35].Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiology & Behavior. 2007;92:263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- [36].Ciofi P, Lapirot OC, Tramu G. An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience. 2007;146:630–642. doi: 10.1016/j.neuroscience.2007.02.028. [DOI] [PubMed] [Google Scholar]
- [37].Seymour B, Dolan R. Emotion, Decision Making, and the Amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- [38].Mizukami S, Nishizuka M, Arai Y. Sexual difference in nuclear volume and its ontogeny in the rat amygdala. Exp Neurol. 1983;79:569–75. doi: 10.1016/0014-4886(83)90235-2. [DOI] [PubMed] [Google Scholar]
- [39].Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–6. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- [40].Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Badre D, Wagner AD. Semantic Retrieval, Mnemonic Control, and Prefrontal Cortex. Behav Cogn Neurosci Rev. 2002;1:206–218. doi: 10.1177/1534582302001003002. [DOI] [PubMed] [Google Scholar]
- [42].Ben Shalom D, Poeppel D. Functional Anatomic Models of Language: Assembling the Pieces. Neuroscientist. 2008;14:119–127. doi: 10.1177/1073858407305726. [DOI] [PubMed] [Google Scholar]
- [43].Ochsner KN. The Social-Emotional Processing Stream: Five Core Constructs and Their Translational Potential for Schizophrenia and Beyond. Biological Psychiatry. 2008;64:48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Diamond MC, Dowling GA, Johnson RE. Morphologic cerebral cortical asymmetry in male and female rats. Exp Neurol. 1981;71:261–8. doi: 10.1016/0014-4886(81)90087-x. [DOI] [PubMed] [Google Scholar]
- [45].Etgen AM, Morales JC. Somatosensory Stimuli Evoke Norepinephrine Release in the Anterior Ventromedial Hypothalamus of Sexually Receptive Female Rats. Journal of Neuroendocrinology. 2002;14:213–218. doi: 10.1046/j.0007-1331.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- [46].Simerly LWSRB, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. The Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- [47].Pozzo MLD, Aoki A. Stereological analysis of the hypothalamic ventromedial nucleus. II. Hormone-induced changes in the synaptogenic pattern. Brain Res Dev Brain Res. 1991;61:189–96. doi: 10.1016/0165-3806(91)90131-2. [DOI] [PubMed] [Google Scholar]
- [48].Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10:107–20. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16:415–20. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- [50].Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. NatNeurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- [51].Kordon C, Glowinski J. Role of hypothalamic monoaminergic neurones in the gonadotrophin release-regulating mechanisms. Neuropharmacology. 1972;11:153–62. doi: 10.1016/0028-3908(72)90088-3. [DOI] [PubMed] [Google Scholar]
- [52].Kalra SP, Kalra PS. Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev. 1983;4:311–51. doi: 10.1210/edrv-4-4-311. [DOI] [PubMed] [Google Scholar]
- [53].Gargaglioni LH, Bícego KC, Branco LGS. Brain monoaminergic neurons and ventilatory control in vertebrates. Respiratory Physiology & Neurobiology. 2008 doi: 10.1016/j.resp.2008.04.017. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- [54].Joca S.m.R.L.o., Ferreira F.R.r., Guimarães FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- [55].Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154:1641–7. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- [56].Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–8. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Reisert I, Pilgrim C. Sexual differentiation of monoaminergic neurons - genetic or epigenetic? Trends in Neurosciences. 1991;14:468–473. doi: 10.1016/0166-2236(91)90047-x. [DOI] [PubMed] [Google Scholar]
- [58].Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–16. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- [59].Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the Rate-Limiting Step in Norepinephrine Biosynthesis in the Perfused Guinea-Pig Heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- [60].Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: review. Mol Psychiatry. 2003;8:951–73. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- [61].Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- [62].Siddiqui A, Shah BH. Neonatal androgen manipulation differentially affects the development of monoamine systems in rat cerebral cortex, amygdala and hypothalamus. Brain Res Dev Brain Res. 1997;98:247–52. doi: 10.1016/s0165-3806(96)00171-x. [DOI] [PubMed] [Google Scholar]
- [63].van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JPA, Rombouts SARB. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neurolmage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- [64].Berger M, Gray JA, Roth BL. The Expanded Biology of Serotonin. Annual Review of Medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Weiss LA, Abney M, Cook EH, Jr, Ober C. Sex-Specific Genetic Architecture of Whole Blood Serotonin Levels. The American Journal of Human Genetics. 2005;76:33–41. doi: 10.1086/426697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–9. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
- [68].Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O'Malley S, Innis RB. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–84. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- [69].Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci. 2006;24:3245–54. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- [70].Naftolin F. Brain aromatization of androgens. J Reprod Med. 1994;39:257–61. [PubMed] [Google Scholar]
- [71].Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–7. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- [72].Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Developmental Brain Research. 2005;155:107–116. doi: 10.1016/j.devbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- [73].Rhodes ME, Rubin RT. Functional sex differences ([`]sexual diergism') of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Research Reviews. 1999;30:135–152. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- [74].Holmes MM, Goldman BD, Goldman SL, Seney ML, Forger NG. Neuroendocrinology and sexual differentiation in eusocial mammals. Frontiers in Neuroendocrinology. 2009;30:519–533. doi: 10.1016/j.yfrne.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behavioural Brain Research. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- [76].Ota M, Crofton JT, Share L. Hemorrhage-induced vasopressin release in the paraventricular nucleus measured by in vivo microdialysis. Brain Research. 1994;658:49–54. doi: 10.1016/s0006-8993(09)90009-9. [DOI] [PubMed] [Google Scholar]
- [77].Dani JA, Bertrand D. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- [78].Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends in Pharmacological Sciences. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- [79].Hörtnagl H, Hansen L, Kindel G, Schneider B, Tamert AE, Hanin I. Sex differences and estrous cycle-variations in the AF64A-induced cholinergic deficit in the rat hippocampus. Brain Research Bulletin. 1993;31:129–134. doi: 10.1016/0361-9230(93)90019-8. [DOI] [PubMed] [Google Scholar]
- [80].Avissar S, Egozi Y, Sokolovsky M. Studies on Muscarinic Receptors in Mouse and Rat Hypothalamus: A Comparison of Sex and Cyclical Differences. Neuroendocrinology. 1981;32:295–302. doi: 10.1159/000123175. [DOI] [PubMed] [Google Scholar]
- [81].Egozi Y, Avissar S, Sokolovsky M. Muscarinic Mechanisms and Sex Hormone Secretion in Rat Adenohypophysis and Preoptic Area. Neuroendocrinology. 1982;35:93–97. doi: 10.1159/000123361. [DOI] [PubMed] [Google Scholar]
- [82].Rubin RT, Sekula LK, O'Toole S, Rhodes ME, Czambel RK. Pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration in normal women and men. Neuropsychopharmacology. 1999;20:443–46. doi: 10.1016/S0893-133X(98)00077-3. [DOI] [PubMed] [Google Scholar]
- [83].Yaksh TL. Opioid receptor systems and the endorphins: a review of their spinal organization. Journal of Neurosurgery. 1987;67:157–176. doi: 10.3171/jns.1987.67.2.0157. [DOI] [PubMed] [Google Scholar]
- [84].Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends in Neurosciences. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- [85].Tilbrook AJ, Turner AI, Clarke IJ. Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress. 2002;5:83–100. doi: 10.1080/10253890290027912. [DOI] [PubMed] [Google Scholar]
- [86].Craft RM. Sex differences in opioid analgesia: “From mouse to man”. Clinical Journal of Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- [87].Zubieta J-K, Dannals RF, Frost JJ. Gender and Age Influences on Human Brain Mu-Opioid Receptor Binding Measured by PET. Am J Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- [88].Hyde JS. The gender similarities hypothesis. Am Psychol. 2005;60:581–92. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- [89].Lippa RA. Sex differences in sex drive, sociosexuality, and height across 53 nations: testing evolutionary and social structural theories. Arch Sex Behav. 2009;38:631–51. doi: 10.1007/s10508-007-9242-8. [DOI] [PubMed] [Google Scholar]
- [90].Schmitt DP. Sociosexuality from Argentina to Zimbabwe: a 48-nation study of sex, culture, and strategies of human mating. Behav Brain Sci. 2005;28:247–75. doi: 10.1017/s0140525x05000051. discussion 275–311. [DOI] [PubMed] [Google Scholar]
- [91].Sylvester M, Hayes SC. Unpacking Masculinity as a Construct: Ontology, Pragmatism, and an Analysis of Language. Psychology of Men & Masculinity. 2010;11:91–97. [Google Scholar]
- [92].Brooks GR. Despite Problems, “Masculinity” is a Vital Construct. Psychology of Men & Masculinity. 2010;11:107–108. [Google Scholar]
- [93].O'neil JM. Is Criticism of Generic Masculinity, Essentialism, and Positive-Healthy-Masculinity a Problem for the Psychology of Men? Psychology of Men & Masculinity. 2010;11:98–106. [Google Scholar]
- [94].Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117:250–70. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- [95].Benbow CP, Lubinski D, Shea DL, Eftekhari-Sanjani H. Sex differences in mathematical reasoning ability at age 13: their status 20 years later. Psychol Sci. 2000;11:474–80. doi: 10.1111/1467-9280.00291. [DOI] [PubMed] [Google Scholar]
- [96].Halpern DF. Sex Differences in Cognitive Abilities. Lawrence Erlbaum Associates; 2000. pp. 159–163. [Google Scholar]
- [97].Halpern DF. Sex Differences in Cognitive Abilities. Lawrence Erlbaum Associates; 2000. pp. 147–148. [Google Scholar]
- [98].Harshman RA, Hampson E, Berenbaum SA. Individual differences in cognitive abilities and brain organization, Part I: Sex and handedness differences in ability. Can J Psychol. 1983;37:144–92. doi: 10.1037/h0080690. [DOI] [PubMed] [Google Scholar]
- [99].Kimura D, Harshman RA. Sex differences in brain organization for verbal and non-verbal functions. Prog Brain Res. 1984;61:423–41. doi: 10.1016/S0079-6123(08)64452-0. [DOI] [PubMed] [Google Scholar]
- [100].Hines M. Brain gender. Oxford University Press, Oxford; New York: 2004. [Google Scholar]
- [101].Almli CR, Ball RH, Wheeler ME. Human fetal and neonatal movement patterns: Gender differences and fetal-to-neonatal continuity. Dev Psychobiol. 2001;38:252–73. doi: 10.1002/dev.1019. [DOI] [PubMed] [Google Scholar]
- [102].Davies PL, Rose JD. Motor skills of typically developing adolescents: awkwardness or improvement? Phys Occup Ther Pediatr. 2000;20:19–42. [PubMed] [Google Scholar]
- [103].Piek JP, Gasson N, Barrett N, Case I. Limb and gender differences in the development of coordination in early infancy. Hum Mov Sci. 2002;21:621–39. doi: 10.1016/s0167-9457(02)00172-0. [DOI] [PubMed] [Google Scholar]
- [104].Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci. 2009;20:144–8. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nordenstrom A, Servin A, Bohlin G, Larsson A, Wedell A. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2002;87:5119–24. doi: 10.1210/jc.2001-011531. [DOI] [PubMed] [Google Scholar]
- [106].Berenbaum SA, Duck SC, Bryk K. Behavioral effects of prenatal versus postnatal androgen excess in children with 21-hydroxylase-deficient congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85:727–33. doi: 10.1210/jcem.85.2.6397. [DOI] [PubMed] [Google Scholar]
- [107].Becker JB. Sex differences in the brain : from genes to behavior. Oxford University Press, Oxford; New York: 2008. [Google Scholar]
- [108].Hall JA, Kimura D. Sexual orientation and performance on sexually dimorphic motor tasks. Arch Sex Behav. 1995;24:395–407. doi: 10.1007/BF01541855. [DOI] [PubMed] [Google Scholar]
- [109].Tottenham LS, Saucier DM. Throwing accuracy during prism adaptation: male advantage for throwing accuracy is independent of prism adaptation rate. Percept Mot Skills. 2004;98:1449–55. doi: 10.2466/pms.98.3c.1449-1455. [DOI] [PubMed] [Google Scholar]
- [110].Herlitz A, Airaksinen E, Nordstrom E. Sex differences in episodic memory: The impact of verbal and visuospatial ability. Neuropsychology. 1999;13:590–597. doi: 10.1037//0894-4105.13.4.590. [DOI] [PubMed] [Google Scholar]
- [111].Kimura D. Sex, sexual orientation and sex hormones influence human cognitive function. Curr Opin Neurobiol. 1996;6:259–63. doi: 10.1016/s0959-4388(96)80081-x. [DOI] [PubMed] [Google Scholar]
- [112].Lewin C, Wolgers G, Herlitz A. Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology. 2001;15:165–73. doi: 10.1037//0894-4105.15.2.165. [DOI] [PubMed] [Google Scholar]
- [113].Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–51. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- [114].Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 2004;92:231–70. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- [115].Hartshorne JK, Ullman MT. Why girls say `holded' more than boys. Dev Sci. 2006;9:21–32. doi: 10.1111/j.1467-7687.2005.00459.x. [DOI] [PubMed] [Google Scholar]
- [116].Sherwin BB. Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med. 1998;217:17–22. doi: 10.3181/00379727-217-44200. [DOI] [PubMed] [Google Scholar]
- [117].Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- [118].Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–83. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- [119].Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am J Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- [120].Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–39. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- [121].Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–9. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- [122].Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:11412–7. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9:349–54. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- [124].Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- [125].Estabrooke IV, Mordecai K, Maki P, Ullman MT. The effect of sex hormones on language processing. Brain and Language. 2002;83:143–146. [Google Scholar]
- [126].Grimshaw GM, Bryden MP, Finegan JAK. Relations between Prenatal Testosterone and Cerebral Lateralization in Children. Neuropsychology. 1995;9:68–79. [Google Scholar]
- [127].Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- [128].Kansaku K, Yamaura A, Kitazawa S. Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex. 2000;10:866–72. doi: 10.1093/cercor/10.9.866. [DOI] [PubMed] [Google Scholar]
- [129].Pfluger T, Weil S, Weis S, Vollmar C, Heiss D, Egger J, Scheck R, Hahn K. Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Epilepsia. 1999;40:414–23. doi: 10.1111/j.1528-1157.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- [130].Becker JB. Sex differences in the brain : from genes to behavior. Oxford University Press, Oxford; New York: 2008. p. 427. [Google Scholar]
- [131].Bettencourt BA, Miller N. Gender differences in aggression as a function of provocation: A meta-analysis. Psychological Bulletin. 1996;119:422–447. doi: 10.1037/0033-2909.119.3.422. [DOI] [PubMed] [Google Scholar]
- [132].Wihlback AC, Poromaa IS, Bixo M, Allard P, Mjorndal T, Spigset O. Influence of menstrual cycle on platelet serotonin uptake site and serotonin(2A) receptor binding. Psychoneuroendocrinology. 2004;29:757–766. doi: 10.1016/S0306-4530(03)00120-3. [DOI] [PubMed] [Google Scholar]
- [133].Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: Implications for affective regulation. Biological Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- [134].Archer J, Graham-Kevan N, Davies M. Testosterone and aggression: A reanalysis of book, Starzyk, and Quinsey's (2001) study. Aggression and Violent Behavior. 2005;10:241–261. [Google Scholar]
- [135].Book AS, Starzyk KB, Quinsey VL. The relationship between testosterone and aggression: a meta-analysis. Aggression and Violent Behavior. 2001;6:579–599. [Google Scholar]
- [136].Cashdan E. Hormones and competitive aggression in women. Aggressive Behavior. 2003;29:107–115. [Google Scholar]
- [137].von der Pahlen B, Lindman R, Sarkola T, Makisalo H, Eriksson CJP. An exploratory study on self-evaluated aggression and androgens in women. Aggressive Behavior. 2002;28:273–280. [Google Scholar]
- [138].Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Research. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- [139].Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- [140].Lagrange TC, Silverman RA. Low self-control and opportunity: Testing the general theory of crime as an explanation for gender defferences in delinquency. Criminology. 1999;37:41–72. [Google Scholar]
- [141].Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, DeMontigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporolimbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- [143].Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–7. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- [144].Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- [145].Becker JB. Sex differences in the brain : from genes to behavior. Oxford University Press, Oxford; New York: 2008. pp. 442–445. [Google Scholar]
- [146].Kehoe P, Wavrant-De Vrieze F, Crook R, Wu WS, Holmans P, Fenton I, Spurlock G, Norton N, Williams H, Williams N, Lovestone S, Perez-Tur J, Hutton M, Chartier-Harlin MC, Shears S, Roehl K, Booth J, Van Voorst W, Ramie D, Williams J, Goate A, Hardy J, Owen MJ. A full genome scan for late onset Alzheimer's disease. Hum Mol Genet. 1999;8:237–45. doi: 10.1093/hmg/8.2.237. [DOI] [PubMed] [Google Scholar]
- [147].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- [148].Johnson JK, McCleary R, Oshita MH, Cotman CW. Initiation and propagation stages of beta-amyloid are associated with distinctive apolipoprotein E, age, and gender profiles. Brain Res. 1998;798:18–24. doi: 10.1016/s0006-8993(98)00363-1. [DOI] [PubMed] [Google Scholar]
- [149].Callen DJ, Black SE, Caldwell CB, Grady CL. The influence of sex on limbic volume and perfusion in AD. Neurobiol Aging. 2004;25:761–70. doi: 10.1016/j.neurobiolaging.2003.08.011. [DOI] [PubMed] [Google Scholar]
- [150].de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–4. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- [151].Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- [152].Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson's disease. Mov Disord. 2003;18:19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- [153].Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson's disease phenotype. J Neurol. 2005;252:1201–5. doi: 10.1007/s00415-005-0835-7. [DOI] [PubMed] [Google Scholar]
- [154].Fernandez HH, Lapane KL, Ott BR, Friedman JH. Gender differences in the frequency and treatment of behavior problems in Parkinson's disease. SAGE Study Group. Systematic Assessment and Geriatric drug use via Epidemiology. Mov Disord. 2000;15:490–6. [PubMed] [Google Scholar]
- [155].Rojo A, Aguilar M, Garolera MT, Cubo E, Navas I, Quintana S. Depression in Parkinson's disease: clinical correlates and outcome. Parkinsonism Relat Disord. 2003;10:23–8. doi: 10.1016/s1353-8020(03)00067-1. [DOI] [PubMed] [Google Scholar]
- [156].Becker JB. Sex differences in the brain : from genes to behavior. Oxford University Press, Oxford; New York: 2008. p. 458. [Google Scholar]
- [157].Foltynie T, Lewis SG, Goldberg TE, Blackwell AD, Kolachana BS, Weinberger DR, Robbins TW, Barker RA. The BDNF Val66Met polymorphism has a gender specific influence on planning ability in Parkinson's disease. J Neurol. 2005;252:833–8. doi: 10.1007/s00415-005-0756-5. [DOI] [PubMed] [Google Scholar]
- [158].Benedetti MD, Maraganore DM, Bower JH, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Mov Disord. 2001;16:830–7. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- [159].Martignoni E, Nappi RE, Citterio A, Calandrella D, Corengia E, Fignon A, Zangaglia R, Riboldazzi G, Pacchetti C, Nappi G. Parkinson's disease and reproductive life events. Neurol Sci. 2002;23(Suppl 2):S85–6. doi: 10.1007/s100720200082. [DOI] [PubMed] [Google Scholar]
- [160].Ragonese P, D'Amelio M, Salemi G, Aridon P, Gammino M, Epifanio A, Morgante L, Savettieri G. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology. 2004;62:2010–4. doi: 10.1212/wnl.62.11.2010. [DOI] [PubMed] [Google Scholar]
- [161].Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, Pant PV, Frazer KA, Cox DR, Ballinger DG. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005;77:685–93. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Pankratz N, Nichols WC, Uniacke SK, Halter C, Murrell J, Rudolph A, Shults CW, Conneally PM, Foroud T. Genome-wide linkage analysis and evidence of gene-by-gene interactions in a sample of 362 multiplex Parkinson disease families. Hum Mol Genet. 2003;12:2599–608. doi: 10.1093/hmg/ddg270. [DOI] [PubMed] [Google Scholar]
- [163].Hong CJ, Liu HC, Liu TY, Lin CH, Cheng CY, Tsai SJ. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphisms in Parkinson's disease and age of onset. Neurosci Lett. 2003;353:75–7. doi: 10.1016/j.neulet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- [164].Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson's disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- [165].Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–7. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- [166].De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci. 1994;14:1789–94. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wang Z, Bullock NA, De Vries GJ. Sexual differentiation of vasopressin projections of the bed nucleus of the stria terminals and medial amygdaloid nucleus in rats. Endocrinology. 1993;132:2299–306. doi: 10.1210/endo.132.6.8504734. [DOI] [PubMed] [Google Scholar]
- [168].Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–7. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- [169].Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin la receptor (AVPRla) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11:488–94. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- [171].Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–72. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- [172].Marco EJ, Skuse DH. Autism-lessons from the X chromosome. Soc Cogn Affect Neurosci. 2006;1:183–193. doi: 10.1093/scan/nsl028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].van Rijn S, Swaab H, Aleman A, Kahn RS. X Chromosomal effects on social cognitive processing and emotion regulation: A study with Klinefelter men (47,XXY) Schizophr Res. 2006;84:194–203. doi: 10.1016/j.schres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- [174].Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- [175].Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- [177].Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–52. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- [178].Kosten TR, Rounsaville BJ, Kleber HD. Ethnic and gender differences among opiate addicts. Int J Addict. 1985;20:1143–62. doi: 10.3109/10826088509056356. [DOI] [PubMed] [Google Scholar]
- [179].White FJ. A behavioral/systems approach to the neuroscience of drug addiction. J Neurosci. 2002;22:3303–5. doi: 10.1523/JNEUROSCI.22-09-03303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Justice AJ, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–15. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- [181].Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- [182].Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. NatNeurosci. 2007;10:1398–400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- [183].Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–30. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- [184].Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- [185].Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986;382:416–21. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- [186].Colangelo LA, Sharp L, Kopp P, Scholtens D, Chiu BC, Liu K, Gapstur SM. Total testosterone, androgen receptor polymorphism, and depressive symptoms in young black and white men: the CARDIA Male Hormone Study. Psychoneuroendocrinology. 2007;32:951–8. doi: 10.1016/j.psyneuen.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Seidman SN, Araujo AB, Roose SP, McKinlay JB. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol Psychiatry. 2001;50:371–6. doi: 10.1016/s0006-3223(01)01148-9. [DOI] [PubMed] [Google Scholar]
- [188].Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of 1-year prevalence of major depression in women. Arch Gen Psychiatry. 1993;50:843–52. doi: 10.1001/archpsyc.1993.01820230009001. [DOI] [PubMed] [Google Scholar]
- [189].Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- [190].Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–55. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- [191].Kessler R, Frank E. Gender and its Effects on Psychopathology. American Psychiatric Press; Washington: 2000. Gender differences in major depression. Epidemiological findings; pp. 61–84. [Google Scholar]
- [192].Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- [193].Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50:534–8. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- [194].Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–45. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- [195].Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101:12358–63. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Jablensky A. Epidemiology of schizophrenia: the global burden of disease and disability. Eur Arch Psychiatry Clin Neurosci. 2000;250:274–85. doi: 10.1007/s004060070002. [DOI] [PubMed] [Google Scholar]
- [197].Preston NJ, Orr KG, Date R, Nolan L, Castle DJ. Gender differences in premorbid adjustment of patients with first episode psychosis. Schizophr Res. 2002;55:285–90. doi: 10.1016/s0920-9964(01)00215-8. [DOI] [PubMed] [Google Scholar]
- [198].Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav. 2006;50:612–22. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- [199].Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- [200].Kulkarni J, de Castella A, Smith D, Taffe J, Keks N, Copolov D. A clinical trial of the effects of estrogen in acutely psychotic women. Schizophr Res. 1996;20:247–52. doi: 10.1016/0920-9964(96)82949-5. [DOI] [PubMed] [Google Scholar]
- [201].Feng J, Sun G, Yan J, Noltner K, Li W, Buzin CH, Longmate J, Heston LL, Rossi J, Sommer SS. Evidence for X-chromosomal schizophrenia associated with microRNA alterations. PLoS One. 2009;4:e6121. doi: 10.1371/journal.pone.0006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Goldstein JM, Faraone SV, Chen WJ, Tsuang MT. Genetic heterogeneity may in part explain sex differences in the familial risk for schizophrenia. Biol Psychiatry. 1995;38:808–13. doi: 10.1016/0006-3223(95)00054-2. [DOI] [PubMed] [Google Scholar]
- [203].Morris AG, Gaitonde E, McKenna PJ, Mollon JD, Hunt DM. CAG repeat expansions and schizophrenia: association with disease in females and with early age-at-onset. Hum Mol Genet. 1995;4:1957–61. doi: 10.1093/hmg/4.10.1957. [DOI] [PubMed] [Google Scholar]
- [204].Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–74. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- [205].Hyde JS. New Directions in the Study of Gender Similarities and Differences. Current Directions in Psychological Science. 2007;16:259–263. [Google Scholar]
- [206].Wood W, Eagly AH. A cross-cultural analysis of the behavior of women and men: implications for the origins of sex differences. Psychol Bull. 2002;128:699–727. doi: 10.1037/0033-2909.128.5.699. [DOI] [PubMed] [Google Scholar]
- [207].Confers JC, Easton JA, Fleischman DS, Goetz CD, Lewis DM, Perilloux C, Buss DM. Evolutionary psychology: Controversies, questions, prospects, and limitations. Am Psychol. 2010;65:110–26. doi: 10.1037/a0018413. [DOI] [PubMed] [Google Scholar]
- [208].Vilain E. Genetics of intersexuality. Journal of Gay & Lesbian Psychotherapy. 2006;10:9–26. [Google Scholar]
- [209].Lillie FR. General biological introduction. Williams and Wilkins Co.; Baltimore: 1939. [Google Scholar]
- [210].Jost A. Hormonal factors in the sex differentiation of the mammalian foetus. Philos Trans R Soc Lond B Biol Sci. 1970;259:119–30. doi: 10.1098/rstb.1970.0052. [DOI] [PubMed] [Google Scholar]
- [211].Zhao D, McBride D, Nandi S, McQueen HA, McGrew MJ, Hocking PM, Lewis PD, Sang HM, Clinton M. Somatic sex identity is cell autonomous in the chicken. Nature. 2010;464:237–42. doi: 10.1038/nature08852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Lillie FR. The Theory of the Free-Martin. Science. 1916;43:611–613. doi: 10.1126/science.43.1113.611. [DOI] [PubMed] [Google Scholar]
- [213].Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- [214].Powers JB. Hormonal control of sexual receptivity during the estrous cycle of the rat. Physiology & Behavior. 1970;5:831–835. doi: 10.1016/0031-9384(70)90167-8. [DOI] [PubMed] [Google Scholar]
- [215].Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–42. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–14. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Horm Behav. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- [218].Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–3. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- [219].Pfaff DW. Hormones, brain, and behavior. Academic Press; Amsterdam; Boston: 2002. [Google Scholar]
- [220].Arnold AP. The effects of castration on song development in zebra finches (Poephila guttata) Journal of Experimental Zoology. 1975;191:261–278. doi: 10.1002/jez.1401910212. [DOI] [PubMed] [Google Scholar]
- [221].Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc Natl Acad Sci U S A. 1996;93:5264–8. doi: 10.1073/pnas.93.11.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Wade J. Zebra finch sexual differentiation: the aromatization hypothesis revisited. Microsc Res Tech. 2001;54:354–63. doi: 10.1002/jemt.1148. [DOI] [PubMed] [Google Scholar]
- [223].Gurney ME, Konishi M. Hormone-Induced Sexual Differentiation of Brain and Behavior in Zebra Finches. Science. 1980;208:1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- [224].Jacobs EC, Grisham W, Arnold AP. Lack of a synergistic effect between estradiol and dihydrotestosterone in the masculinization of the zebra finch song system. J Neurobiol. 1995;27:513–9. doi: 10.1002/neu.480270406. [DOI] [PubMed] [Google Scholar]
- [225].Simpson HB, Vicario DS. Early estrogen treatment of female zebra finches masculinizes the brain pathway for learned vocalizations. J Neurobiol. 1991;22:777–93. doi: 10.1002/neu.480220711. [DOI] [PubMed] [Google Scholar]
- [226].Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [227].Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc Natl Acad Sci U S A. 1999;96:8241–6. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–5. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- [229].Arnold AP. Sexual differentiation of the zebra finch song system: positive evidence, negative evidence, null hypotheses, and a paradigm shift. J Neurobiol. 1997;33:572–84. [PubMed] [Google Scholar]
- [230].Arnold AP, Wade J, Grisham W, Jacobs EC, Campagnoni AT. Sexual differentiation of the brain in songbirds. Dev Neurosci. 1996;18:124–36. doi: 10.1159/000111400. [DOI] [PubMed] [Google Scholar]
- [231].Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci U S A. 2003;100:4873–8. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Patek CE, Kerr JB, Gosden RG, Jones KW, Hardy K, Muggleton-Harris AL, Handyside AH, Whittingham DG, Hooper ML. Sex chimaerism, fertility and sex determination in the mouse. Development. 1991;113:311–325. doi: 10.1242/dev.113.1.311. [DOI] [PubMed] [Google Scholar]
- [233].Burgoyne PS, Buehr M, McLaren A. XY follicle cells in ovaries of XX—XY female mouse chimaeras. Development. 1988;104:683–688. doi: 10.1242/dev.104.4.683. [DOI] [PubMed] [Google Scholar]
- [234].O WS, Short RV, Renfree MB, Shaw G. Primary genetic control of somatic sexual differentiation in a mammal. Nature. 1988;331:716–7. doi: 10.1038/331716a0. [DOI] [PubMed] [Google Scholar]
- [235].Renfree MB, Short RV. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Philos Trans R Soc Lond B Biol Sci. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- [236].Renfree MB, O WS, Short RV, Shaw G. Sexual differentiation of the urogenital system of the fetal and neonatal tammar wallaby, Macropus eugenii. Anat Embryol (Berl) 1996;194:111–34. doi: 10.1007/BF00195006. [DOI] [PubMed] [Google Scholar]
- [237].Wilson JD, George FW, Renfree MB. The endocrine role in mammalian sexual differentiation. Recent Prog Horm Res. 1995;50:349–64. doi: 10.1016/b978-0-12-571150-0.50021-4. [DOI] [PubMed] [Google Scholar]
- [238].Lucas JC, Renfree MB, Shaw G, Butler CM. The influence of the anti-androgen flutamide on early sexual differentiation of the marsupial male. J Reprod Fertil. 1997;109:205–12. doi: 10.1530/jrf.0.1090205. [DOI] [PubMed] [Google Scholar]
- [239].Shaw G, Renfree MB, Short RV, O WS. Experimental manipulation of sexual differentiation in wallaby pouch young treated with exogenous steroids. Development. 1988;104:689–701. doi: 10.1242/dev.104.4.689. [DOI] [PubMed] [Google Scholar]
- [240].Sharman GB. The chromosomal basis of sex differentiation in marsupials. Aust. J. Zool. 1990;37:451–466. [Google Scholar]
- [241].Cooper DW. The evolution of sex determination, sex chromosome dimorphism, and X-inactivation in therian mammals: A comparison of metatherians (marsupials) and eutherians (“placentals”) In: Reed K, Graves JAM, editors. Sex chromosomes and sex-determining genes. Harwood Academic Publishers, Chur; Switzerland: Langhorne, PA: 1993. pp. 183–200. [Google Scholar]
- [242].Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in Neuroendocrinology. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Gubbay J, Koopman P, Collignon J, Burgoyne P, Lovell-Badge R. Normal structure and expression of Zfy genes in XY female mice mutant in Tdy. Development. 1990;109:647–53. doi: 10.1242/dev.109.3.647. [DOI] [PubMed] [Google Scholar]
- [244].McCarty BM, Migeon CJ, Meyer-Bahlburg HF, Zacur H, Wisniewski AB. Medical and psychosexual outcome in women affected by complete gonadal dysgenesis. J Pediatr Endocrinol Metab. 2006;19:873–7. doi: 10.1515/jpem.2006.19.7.873. [DOI] [PubMed] [Google Scholar]
- [245].Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Carrel L, Cottle AA, Goglin KC, Willard HF. A first-generation X-inactivation profile of the human X chromosome. Proc Natl Acad Sci U S A. 1999;96:14440–4. doi: 10.1073/pnas.96.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11:1409–19. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- [248].Hart CL, Smith GD. Alcohol consumption and mortality and hospital admissions in men from the Midspan collaborative cohort study. Addiction. 2008;103:1979–86. doi: 10.1111/j.1360-0443.2008.02373.x. [DOI] [PubMed] [Google Scholar]
- [249].Murphy CM, Taft CT, Eckhardt CI. Anger problem profiles among partner violent men: Differences in clinical presentation and treatment outcome. Journal of Counseling Psychology. 2007;54:189–200. [Google Scholar]
- [250].Cohn AM, Zeicher A, Seibert AL. Labile affect as a risk factor for aggressive behavior in men. Psyc Men Masc. 2008;9:29–39. [Google Scholar]
- [251].Tager D, Good GE, Brammer S. “Walking Over 'Em”: An Exploration of Relations Between Emotion Dysregulation, Masculine Norms, and Intimate Partner Abuse in a Clinical Sample of Men. Psychology of Men & Masculinity. 2010;11:233–239. [Google Scholar]
- [252].Gibb WR. Neuropathology of Parkinson's disease and related syndromes. Neurol Clin. 1992;10:361–76. [PubMed] [Google Scholar]
- [253].Sibug R, Kuppers E, Beyer C, Maxson SC, Pilgrim C, Reisert I. Genotype-dependent sex differentiation of dopaminergic neurons in primary cultures of embryonic mouse brain. Brain Res Dev Brain Res. 1996;93:136–42. doi: 10.1016/0165-3806(96)00024-7. [DOI] [PubMed] [Google Scholar]
- [254].Eicher EM, Washburn LL, Whitney JB, 3rd, Morrow KE. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science. 1982;217:535–7. doi: 10.1126/science.7089579. [DOI] [PubMed] [Google Scholar]
- [255].Koopman P. Gonad development: signals for sex. Curr Biol. 2001;11:R481–3. doi: 10.1016/s0960-9822(01)00287-1. [DOI] [PubMed] [Google Scholar]
- [256].Magre S, Jost A. Sertoli cells and testicular differentiation in the rat fetus. J Electron Microsc Tech. 1991;19:172–88. doi: 10.1002/jemt.1060190205. [DOI] [PubMed] [Google Scholar]
- [257].Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39:2195–214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Whitfield LS, Lovell-Badge R, Goodfellow PN. Rapid sequence evolution of the mammalian sex-determining gene SRY. Nature. 1993;364:713–5. doi: 10.1038/364713a0. [DOI] [PubMed] [Google Scholar]
- [259].Weiss MA. Floppy SOX: mutual induced fit in hmg (high-mobility group) box-DNA recognition. Mol Endocrinol. 2001;15:353–62. doi: 10.1210/mend.15.3.0617. [DOI] [PubMed] [Google Scholar]
- [260].Sudbeck P, Scherer G. Two independent nuclear localization signals are present in the DNA-binding high-mobility group domains of SRY and SOX9. J Biol Chem. 1997;272:27848–52. doi: 10.1074/jbc.272.44.27848. [DOI] [PubMed] [Google Scholar]
- [261].Dubin RA, Ostrer H. Sry is a transcriptional activator. Mol Endocrinol. 1994;8:1182–92. doi: 10.1210/mend.8.9.7838151. [DOI] [PubMed] [Google Scholar]
- [262].Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- [263].Bradford ST, Hiramatsu R, Maddugoda MP, Bernard P, Chaboissier MC, Sinclair A, Schedl A, Harley V, Kanai Y, Koopman P, Wilhelm D. The Cerebellin 4 Precursor Gene Is a Direct Target of SRY and SOX9 in Mice. Biol Reprod. 2009 doi: 10.1095/biolreprod.108.071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Wu JB, Chen K, Li Y, Lau Y-FC, Shih JC. Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB J. 2009 doi: 10.1096/fj.09-139097. fj.09-139097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Taketo T, Lee CH, Zhang J, Li Y, Lee CY, Lau YF. Expression of SRY proteins in both normal and sex-reversed XY fetal mouse gonads. Dev Dyn. 2005;233:612–22. doi: 10.1002/dvdy.20352. [DOI] [PubMed] [Google Scholar]
- [266].Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–14. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- [267].Clepet C, Schafer AJ, Sinclair AH, Palmer MS, Lovell-Badge R, Goodfellow PN. The human SRY transcript. Hum Mol Genet. 1993;2:2007–12. doi: 10.1093/hmg/2.12.2007. [DOI] [PubMed] [Google Scholar]
- [268].Mayer A, Lahr G, Swaab DF, Pilgrim C, Reisert I. The Y-chromosomal genes SRY and ZFY are transcribed in adult human brain. Neurogenetics. 1998;1:281–8. doi: 10.1007/s100480050042. [DOI] [PubMed] [Google Scholar]
- [269].Mayer A, Mosler G, Just W, Pilgrim C, Reisert I. Developmental profile of Sry transcripts in mouse brain. Neurogenetics. 2000;3:25–30. doi: 10.1007/s100480000093. [DOI] [PubMed] [Google Scholar]
- [270].Milsted A, Serova L, Sabban EL, Dunphy G, Turner ME, Ely DL. Regulation of tyrosine hydroxylase gene transcription by Sry. Neurosci Lett. 2004;369:203–7. doi: 10.1016/j.neulet.2004.07.052. [DOI] [PubMed] [Google Scholar]
- [271].Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;1015:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- [272].Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE., Jr. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J Neurosci. 2000;20:8604–9. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Fleming A, Vilain E. The endless quest for sex determination genes. Clin Genet. 2005;67:15–25. doi: 10.1111/j.1399-0004.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- [274].McCauley E, Feuillan P, Kushner H, Ross JL. Psychosocial development in adolescents with Turner syndrome. J Dev Behav Pediatr. 2001;22:360–5. doi: 10.1097/00004703-200112000-00003. [DOI] [PubMed] [Google Scholar]
- [275].Lawrence K, Kuntsi J, Coleman M, Campbell R, Skuse D. Face and emotion recognition deficits in Turner syndrome: a possible role for X-linked genes in amygdala development. Neuropsychology. 2003;17:39–49. [PubMed] [Google Scholar]
- [276].Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leepe r.G., Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs PA. Evidence from Turner's syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387 doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- [277].Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs PA. Evidence from Turner's syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–8. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- [278].Ross JL, Stefanatos GA, Kushner H, Bondy C, Nelson L, Zinn A, Roeltgen D. The Effect of Genetic Differences and Ovarian Failure: Intact Cognitive Function in Adult Women with Premature Ovarian Failure Versus Turner Syndrome. J Clin Endocrinol Metab. 2004;89:1817–1822. doi: 10.1210/jc.2003-031463. [DOI] [PubMed] [Google Scholar]
- [279].Temple CM. Oral fluency and narrative production in children with Turner's syndrome. Neuropsychologia. 2002;40:1419–27. doi: 10.1016/s0028-3932(01)00201-9. [DOI] [PubMed] [Google Scholar]
- [280].Chadwick D, Goode J. The genetics and biology of sex determination. J. Wiley; Chichester, West Sussex, England: New York, NY: 2002. [Google Scholar]
- [281].Poletti A, Negri-Cesi P, Martini L. Reflections on the diseases linked to mutations of the androgen receptor. Endocrine. 2005;28:243–62. doi: 10.1385/ENDO:28:3:243. [DOI] [PubMed] [Google Scholar]
- [282].Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- [283].Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: What we've learned from the testicular feminization mutation. Hormones and Behavior. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) Trends Neurosci. 1998;21:243–9. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- [285].Meyer IH, Wilson PA. Sampling Lesbian, Gay, and Bisexual Populations. Journal of Counseling Psychology. 2009;56:23–31. [Google Scholar]
- [286].Moradi B, Mohr JJ, Worthington RL, Fassinger RE. Counseling Psychology Research on Sexual (Orientation) Minority Issues: Conceptual and Methodological Challenges and Opportunities. Journal of Counseling Psychology. 2009;56:5–22. [Google Scholar]
- [287].Chow PKY, Cheng ST. Shame, Internalized Heterosexism, Lesbian Identity, and Coming Out to Others: A Comparative Study of Lesbians in Mainland China and Hong Kong. Journal of Counseling Psychology. 2010;57:92–104. doi: 10.1037/a0017930. [DOI] [PubMed] [Google Scholar]
- [288].Herek GM, Gillis JR, Cogan JC. Internalized Stigma Among Sexual Minority Adults: Insights from a Social Psychological Perspective. Journal of Counseling Psychology. 2009;56:32–43. [Google Scholar]
- [289].Levitt HM, Ovrebo E, Anderson-Cleveland MB, Leone C, Jeong JY, Arm JR, Bonin BP, Cicala J, Coleman R, Laurie A, Vardaman JM, Home SG. Balancing Dangers: GLBT Experience in a Time of Anti-GLBT Legislation. Journal of Counseling Psychology. 2009;56:67–81. [Google Scholar]
- [290].Diamond M. Homosexuality and bisexuality in different populations. Arch Sex Behav. 1993;22:291–310. doi: 10.1007/BF01542119. [DOI] [PubMed] [Google Scholar]
- [291].Hamer D, Hu S, Magnuson V, Hu N, Pattatucci AM. A linkage between DNA markers on the X chromosome and male sexual orientation. Science. 1993;261:321–327. doi: 10.1126/science.8332896. [DOI] [PubMed] [Google Scholar]
- [292].Worthington RL, Reynolds AL. Within-group differences in sexual orientation and identity. Journal of Counseling Psychology. 2009;56:44–55. [Google Scholar]
- [293].Rieger G, Chivers ML, Bailey JM. Sexual arousal patterns of bisexual men. Psychol Sci. 2005;16:579–84. doi: 10.1111/j.1467-9280.2005.01578.x. [DOI] [PubMed] [Google Scholar]
- [294].Hu S, Pattatucci A, Patterson C, Li L, Fulker D, Cherny S, Kruglyak L, Hamer D. Linkage between sexual orientation and chromosome Xq28 in males but not females. Nature Genetics. 1995;11:248–256. doi: 10.1038/ng1195-248. [DOI] [PubMed] [Google Scholar]
- [295].Sánchez FJ, Bocklandt S, Vilain E. The biology of sexual orientation and gender identity. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Academic Press; San Diego, CA: 2009. pp. 1911–1929. [Google Scholar]
- [296].Swaab DF, Zhou JN, Fodor M, Hofman MA. Sexual differentiation of the human hypothalamus: Differences according to sex, sexual orientation, and transsexuality. In: Lee Ellis, Linda Ebertz., editors. Sexual orientation: Toward biological understanding. Praeger Publishers/Greenwood Publishing Group, Inc.; Westport, CT, US: 1997. pp. 129–150. xxii, 276 pp.SEE BOOK, 1997. [Google Scholar]
- [297].LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- [298].Allen LS, Gorski RA. Sexual orientation and the size of the anterior commissure in the human brain. Proceedings of the National Academy of Sciences of the US. 1992;89:7199–7202. doi: 10.1073/pnas.89.15.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Byne W, Tobet S, Mattiace L, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. The interstitial nuclei of the human anterior hypothalamus: An investigation of variation within sex, sexual orientation and HIV status. Hormones & Behavior. 2001;40:86–92. doi: 10.1006/hbeh.2001.1680. [DOI] [PubMed] [Google Scholar]
- [300].Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–83. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- [301].Dominguez-Salazar E, Portillo W, Baum MJ, Bakker J, Paredes RG. Effect of prenatal androgen receptor antagonist or aromatase inhibitor on sexual behavior, partner preference and neuronal Fos responses to estrous female odors in the rat accessory olfactory system. Physiol Behav. 2002;75:337–46. doi: 10.1016/s0031-9384(01)00674-6. [DOI] [PubMed] [Google Scholar]
- [302].Stockman ER, Callaghan RS, Baum MJ. Effects of neonatal castration and testosterone treatment on sexual partner preference in the ferret. Physiol Behav. 1985;34:409–14. doi: 10.1016/0031-9384(85)90204-5. [DOI] [PubMed] [Google Scholar]
- [303].Roselli CE, Stormshak F. The neurobiology of sexual partner preferences in rams. Horm Behav. 2009;55:611–20. doi: 10.1016/j.yhbeh.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- [305].Hines M, Brook C, Conway GS. Androgen and psychosexual development: core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH) J Sex Res. 2004;41:75–81. doi: 10.1080/00224490409552215. [DOI] [PubMed] [Google Scholar]
- [306].Macke JP, Hu N, Hu S, Bailey M, King VL, Brown T, Hamer D, Nathans J. Sequence variation in the androgen receptor gene is not a common determinant of male sexual orientation. American Journal of Human Genetics. 1993;53:844–52. [PMC free article] [PubMed] [Google Scholar]
- [307].DuPree MG, Mustanski BS, Bocklandt S, Nievergelt C, Hamer DH. A candidate gene study of CYP19 (aromatase) and male sexual orientation. Behav Genet. 2004;34:243–50. doi: 10.1023/B:BEGE.0000017870.77610.52. [DOI] [PubMed] [Google Scholar]
- [308].Hickey M, Doherty DA, Hart R, Norman RJ, Mattes E, Atkinson HC, Sloboda DM. Maternal and umbilical cord androgen concentrations do not predict digit ratio (2D:4D) in girls: A prospective cohort study. Psychoneuroendocrinology. 35:1235–1244. doi: 10.1016/j.psyneuen.2010.02.013. [DOI] [PubMed] [Google Scholar]
- [309].McFadden D, Loehlin JC, Breedlove SM, Lippa RA, Manning JT, Rahman Q. A reanalysis of five studies on sexual orientation and the relative length of the 2nd and 4th fingers (the 2D:4D ratio) Arch Sex Behav. 2005;34:341–56. doi: 10.1007/s10508-005-3123-9. [DOI] [PubMed] [Google Scholar]
- [310].Bailey JM, Willerman L, Parks C. A test of the maternal stress theory of human male homosexuality. Archives of Sexual Behavior. 1991;20:277–293. doi: 10.1007/BF01541847. [DOI] [PubMed] [Google Scholar]
- [311].Ellis L, Cole-Harding S. The effects of prenatal stress, and of prenatal alcohol and nicotine exposure, on human sexual orientation. Physiology & Behavior. 2001;74:213–26. doi: 10.1016/s0031-9384(01)00564-9. [DOI] [PubMed] [Google Scholar]
- [312].Pillard RC, Weinrich JD. Evidence of familial nature of male homosexuality. Archives of General Psychiatry. 1986;43:808–812. doi: 10.1001/archpsyc.1986.01800080094012. [DOI] [PubMed] [Google Scholar]
- [313].Bailey JM, Pillard RC. A genetic study of male sexual orientation. Archives of General Psychiatry. 1991;48:1089–1096. doi: 10.1001/archpsyc.1991.01810360053008. [DOI] [PubMed] [Google Scholar]
- [314].Pattatucci AML, Hamer DH. Development and familiality of sexual orientation in females. Behavior Genetics. 1995;25:407–420. doi: 10.1007/BF02253370. [DOI] [PubMed] [Google Scholar]
- [315].Bailey JM, Pillard RC, Dawood K, Miller MB, Farrer LA, Trivedi S, Murphy RL. A family history study of male sexual orientation using three independent samples. Behavior Genetics. 1999;29:79–86. doi: 10.1023/a:1021652204405. [DOI] [PubMed] [Google Scholar]
- [316].Bailey JM, Pillard RC. Genetics of human sexual orientation. Annual Review of Sex Research. 1995:126–150. [Google Scholar]
- [317].Bailey JM, Dunne MP, Martin NG. Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. Journal of Personality and Social Psychology. 2000;78:524–536. doi: 10.1037//0022-3514.78.3.524. [DOI] [PubMed] [Google Scholar]
- [318].Kendler KS, Thornton LM, Gilman SE, Kessler RC. Sexual Orientation in a U.S. National Sample of Twin and Nontwin Sibling Pairs. The American Journal of Psychiatry. 2000;157:1843–1846. doi: 10.1176/appi.ajp.157.11.1843. [DOI] [PubMed] [Google Scholar]
- [319].Kirk KM, Bailey JM, Dunne MP, Martin NG. Measurement models for sexual orientation in a community twin sample. Behavior Genetics. 2000;30:345–356. doi: 10.1023/a:1026557719181. [DOI] [PubMed] [Google Scholar]
- [320].Sanders AR, Dawood K. Nature Encyclopedia of Life Sciences. Nature Publishing Group; London: 2003. [Google Scholar]
- [321].Rice G, Anderson C, Risch N, Ebers G. Male homosexuality: Absence of linkage to microsatellite markers atXq28. Science. 1999;284:665–667. doi: 10.1126/science.284.5414.665. [DOI] [PubMed] [Google Scholar]
- [322].Hamer D. Genetics and male sexual orientation. Science. 1999;285:803. [Google Scholar]
- [323].Brown CJ, Robinson WP. The causes and consequences of random and non-random X chromosome inactivation in humans. Clin Genet. 2000;58:353–63. doi: 10.1034/j.1399-0004.2000.580504.x. [DOI] [PubMed] [Google Scholar]
- [324].Bocklandt S, Horvath S, Vilain E, Hamer DH. Extreme skewing of X chromosome inactivation in mothers of homosexual men. Hum Genet. 2006;118:691–4. doi: 10.1007/s00439-005-0119-4. [DOI] [PubMed] [Google Scholar]
- [325].Mustanski BS, Dupree MG, Nievergelt CM, Bocklandt S, Schork NJ, Hamer DH. A genomewide scan of male sexual orientation. Hum Genet. 2005;116:272–8. doi: 10.1007/s00439-004-1241-4. [DOI] [PubMed] [Google Scholar]
- [326].Bocklandt S, Hamer DH. Beyond hormones: a novel hypothesis for the biological basis of male sexual orientation. J Endocrinol Invest. 2003;26:8–12. [PubMed] [Google Scholar]
- [327].Blanchard R. Birth order and sibling sex ration in homosexual versus heterosexual males and females. Annual Review of Sex Research. 1997;8:27–67. [PubMed] [Google Scholar]
- [328].Jones MB, Blanchard R. Birth order and male homosexuality: extension of Slater's index. Hum Biol. 1998;70:775–87. [PubMed] [Google Scholar]
- [329].Blanchard R, Cantor JM, Bogaert AF, Breedlove SM, Ellis L. Interaction of fraternal birth order and handedness in the development of male homosexuality. Horm Behav. 2006;49:405–14. doi: 10.1016/j.yhbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [330].Blanchard R, Bogaert AF. Homosexuality in men and number of older brothers. American Journal of Psychiatry. 1996;153:27–31. doi: 10.1176/ajp.153.1.27. [DOI] [PubMed] [Google Scholar]
- [331].Blanchard R, Klassen P. H-Y antigen and homosexuality in men. Journal of Theoretical Biology. 1997;185:373–378. doi: 10.1006/jtbi.1996.0315. [DOI] [PubMed] [Google Scholar]
- [332].Sanchez FJ, Vilain E. Collective Self-Esteem as a Coping Resource for Male-to-Female Transsexuals. Journal of Counseling Psychology. 2009;56:202–209. doi: 10.1037/a0014573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Schweizer K, Brunner F, Schutzmann K, Schonbucher V, Richter-Appelt H. Gender Identity and Coping in Female 46, XY Adults With Androgen Biosynthesis Deficiency (Intersexuality/DSD) Journal of Counseling Psychology. 2009;56:189–201. [Google Scholar]
- [334].Moss-Racusin CA, Phelan JE, Rudman LA. When Men Break the Gender Rules: Status Incongruity and Backlash Against Modest Men. Psychology of Men & Masculinity. 2010;11:140–151. [Google Scholar]
- [335].Zhou JN, Hofman MA, Gooren LJ, Swaab DF. A sex difference in the human brain and its relation to transsexuality. Nature. 1995;378:68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- [336].Green R. Family coocurrence of “gender dysphoria”: Ten siblings or parent-child pairs. Archives of Sexual Behavior. 2000;29:499–507. doi: 10.1023/a:1001947920872. [DOI] [PubMed] [Google Scholar]
- [337].Segal NL. Two monozygotic twin pairs discordant for female-to-male transsexualism. Arch Sex Behav. 2006;35:347–58. doi: 10.1007/s10508-006-9037-3. [DOI] [PubMed] [Google Scholar]
- [338].Garden GM, Rothery DJ. A female monozygotic twin pair discordant for transsexualism. Some theoretical implications. Br J Psychiatry. 1992;161:852–4. doi: 10.1192/bjp.161.6.852. [DOI] [PubMed] [Google Scholar]
- [339].Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand. 1977;56:265–75. doi: 10.1111/j.1600-0447.1977.tb00227.x. [DOI] [PubMed] [Google Scholar]
- [340].Sadeghi M, Fakhrai A. Transsexualism in female monozygotic twins: A case report. Australian and New Zealand Journal of Psychiatry. 2000;34:862–864. doi: 10.1080/j.1440-1614.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- [341].Haberman M, Hollingsworth F, Falek A, Michael RP. Gender identity confusion, schizophrenia and a 47 XYY karyotype: a case report. Psychoneuroendocrinology. 1975;1:207–9. doi: 10.1016/0306-4530(75)90013-x. [DOI] [PubMed] [Google Scholar]
- [342].Buhrich N, Barr R, Lam-Po-Tang PR. Two transsexuals with 47-XYY karyotype. Br J Psychiatry. 1978;133:77–81. doi: 10.1192/bjp.133.1.77. [DOI] [PubMed] [Google Scholar]
- [343].Taneja N, Ammini AC, Mohapatra I, Saxena S, Kucheria K. A transsexual male with 47,XYY karyotype. Br J Psychiatry. 1992;161:698–9. doi: 10.1192/bjp.161.5.698. [DOI] [PubMed] [Google Scholar]
- [344].Snaith RP, Penhale S, Horsfield P. Male-to-female transsexual with XYY karyotype. Lancet. 1991;337:557–8. doi: 10.1016/0140-6736(91)91348-x. [DOI] [PubMed] [Google Scholar]
- [345].Nielsen J, Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Orig Artie Ser. 1990;26:209–23. [PubMed] [Google Scholar]
- [346].Bentz EK, Schneeberger C, Hefler LA, van Trotsenburg M, Kaufmann U, Huber JC, Tempfer CB. A common polymorphism of the SRD5A2 gene and transsexualism. Reprod Sci. 2007;14:705–9. doi: 10.1177/1933719107306230. [DOI] [PubMed] [Google Scholar]
- [347].Bentz EK, Hefler LA, Kaufmann U, Huber JC, Kolbus A, Tempfer CB. A polymorphism of the CYP17 gene related to sex steroid metabolism is associated with female-to-male but not male-to-female transsexualism. Fertil Steril. 2008;90:56–9. doi: 10.1016/j.fertnstert.2007.05.056. [DOI] [PubMed] [Google Scholar]
- [348].Henningsson S, Westberg L, Nilsson S, Lundstrom B, Ekselius L, Bodlund O, Lindstrom E, Hellstrand M, Rosmond R, Eriksson E, Landen M. Sex steroid-related genes and male-to-female transsexualism. Psychoneuroendocrinology. 2005;30:657–64. doi: 10.1016/j.psyneuen.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [349].Hare L, Bernard P, Sanchez FJ, Baird PN, Vilain E, Kennedy T, Harley VR. Androgen receptor repeat length polymorphism associated with male-to-female transsexualism. Biol Psychiatry. 2009;65:93–6. doi: 10.1016/j.biopsych.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Ujike H, Otani K, Nakatsuka M, Ishii K, Sasaki A, Oishi T, Sato T, Okahisa Y, Matsumoto Y, Namba Y, Kimata Y, Kuroda S. Association study of gender identity disorder and sex hormone-related genes. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1241–4. doi: 10.1016/j.pnpbp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- [351].De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–8. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- [352].Institute of Medicine (U.S.) Committee on Understanding the Biology of Sex and Gender Differences. In: Wizemann TM, Pardue ML, editors. Exploring the biological contributions to human health : does sex matter? National Academy Press; Washington, D.C.: 2001. [PubMed] [Google Scholar]
- [353].Raab H, Pilgrim C, Reisert I. Effects of sex and estrogen on tyrosine hydroxylase mRNA in cultured embryonic rat mesencephalon. Brain Res Mol Brain Res. 1995;33:157–64. doi: 10.1016/0169-328x(95)00125-c. [DOI] [PubMed] [Google Scholar]
- [354].Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–7. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]