Abstract
Recent evidence highlights monoamine oxidases (MAO) as another prominent source of oxidative stress. MAO are a class of enzymes located in the outer mitochondrial membrane, deputed to the oxidative breakdown of key neurotransmitters such as norepinephrine, epinephrine and dopamine, and in the process generate H2O2. All these monoamines are endowed with potent modulatory effects on myocardial function. Thus, when the heart is subjected to chronic neuro-hormonal and/or peripheral hemodynamic stress, the abundance of circulating/tissue monoamines can make MAO-derived H2O2 production particularly prominent. This is the case of acute cardiac damage due to ischemia/reperfusion injury or, on a more chronic stand, of the transition from compensated hypertrophy to overt ventricular dilation/pump failure. Here, we will first briefly discuss mitochondrial status and contribution to acute and chronic cardiac disorders. We will illustrate possible mechanisms by which MAO activity affects cardiac biology and function, along with a discussion as to their role as a prominent source of reactive oxygen species. Finally, we will speculate on why MAO inhibition might have therapeutic value for treating cardiac affections of ischemic and non-ischemic origin.
Keywords: monoamine oxidase, reactive oxygen species, myocardial injury, heart failure, mitochondria
1. Introduction
Given its “highly-aerobic” mode of operation, the cardiac muscle is tightly dependent on oxidative energy supplied by mitochondria via fatty acid (FA) β-oxidation, electron transport chain and oxidative phosphorylation. Mitochondrial energy production depends on genetic factors that modulate normal mitochondrial function including enzyme activity and cofactor availability, and by environmental variables, such as the availability of fuels (i.e. sugars, fats and proteins) and oxygen. In the postnatal and adult heart, FAs are the primary energy substrate for ATP generation via oxidative phosphorylation and the mitochondrial respiratory chain, the most important source of cardiac energy [1-3]. Over 75% of the ATP produced via mitochondrial oxidative phosphorylation is used to support myocyte contractile activity. To this purpose, mitochondria appear to be clustered at sites of high ATP demand and are organized into highly ordered elongated bundles, regularly spaced between rows of myofilaments and in contact with the sarcoplasmic reticulum.
Thus, preserving mitochondrial function, and protecting it from noxae, is essential for myocardial cells to cope with increased workload and stresses of diverse origin. Moreover, mitochondria participate in intracardiac Ca2+ handling and homeostasis. Therefore, targeting mitochondrial dysfunction is increasingly appreciated as a new attractive avenue to correct redox and/or metabolic aspects of human cardiac disorders.
1.1. Mitochondrial functional disarray and cardiac disorders
Given its dependence on mitochondrial bioenergetics and metabolism, the cardiac muscle is especially vulnerable to mitochondrial derangements [4,5]. Insufficient cellular oxygenation leads to the inhibition of electron flow through the respiratory chain and consequently, an impairment of energy conservation and oxidative metabolism. The impairment of respiratory chain function prevents ADP phosphorylation to ATP at the level of F1F0ATP synthase for the loss in protonmotive force composed of membrane potential and proton gradient. If the latter is not generated, F1F0ATP synthase may function in a “reverse mode” thereby coupling ATP hydrolysis with proton pumping to maintain the mitochondrial membrane potential. The net result will be that the mitochondria will cease to represent the main source of intracellular ATP and become very powerful in hydrolyzing glycolytically produced ATP [6]. Further to this, mitochondrial Ca2+ homeostasis is also hampered. This typically occurs in ischemia/reperfusion (I/R) injury. Indeed, upon reperfusion the active accumulation of Ca2+ within the mitochondrial matrix due to the rise in cytosolic Ca2+ can lead to an overload resulting in permeability transition pore (PTP) opening, mitochondrial depolarization and cell death [7,8].
In addition to I/R injury, mitochondrial dysfunction is also chiefly involved in the onset and progression of congestive heart failure (CHF). In this syndrome, cardiac mitochondria display structural abnormalities and are usually decreased in number [9]. The process known as mitochondrial biogenesis is responsible for controlling mitochondrial number and assembly. For instance, a clear functional correlation has been found between physiological stimuli such as endurance training and mitochondrial biogenesis accompanying the increase in cardiac mass [10]. Conversely, the expression of peroxisome-proliferator-activated receptor gamma coactivator-1α (PGC-1α), the chief transcriptional coactivator involved in the regulation of mitochondrial metabolism and biogenesis, is downregulated in several models of heart failure [11-16] and in human failing heart [17]. PGC-1α is also implicated in the regulation of genes involved in the cellular uptake and mitochondrial oxidation of FAs via direct co-activation of peroxisome proliferator activated receptors (PPARs) [18,19]. This phenomenon likely accounts for the “fuel shift” in substrate preference from FA to glucose, steadily reported in the failing heart [20-23]. Yet, despite evidence showing that reduced FA oxidation is a deleterious event associated with heart failure appearance, early switch from FA to carbohydrate metabolism may improve cardiac efficiency, at least in the short-term [24,25]. Some animal studies support the view that FA uptake is reduced in failing hearts, although the few studies done in humans seem to convey more conflicting conclusions [21,26,27]. Thus, whether a shift towards glucose utilization is a compensatory or maladaptive event remains to be fully elucidated.
Consistent with the idea of an overall down-regulation of mitochondrial function in CHF, there is additional evidence showing that the activity of both respiratory chain complexes and ATP synthase is lower in this syndrome [28-30]. Accordingly, the regulation of oxidative phosphorylation by the phosphate acceptors AMP, ADP and creatine is impaired [31], whereas the levels of uncoupling proteins (directing the mitochondria to produce heat rather than ATP) are increased [32]. Hearts from dogs in which CHF is induced by tachypacing, closely resembling the adverse features of human end-stage CHF display markedly reduced activities of respiratory complex III and ATP synthase [33]. A recent study in the same model implies that complexes I and II might also be involved [34]. The reduction in ATP synthase activity has been categorized both as an early and persistent event in CHF development. Another proposed mechanism for mitochondrial alterations is the decrease in functional respirasomes in heart failure [35], which in turn should cause a decrease in oxidative phosphorylation and an increase in electron leakage. Taken together, these data are of crucial importance considering that mitochondrial bioenergetics is impaired also in patients with heart failure [36]. Both ATP and phosphocreatine, the prime cardiac energy reserve, are reduced in human myocardial infarction [37], mitochondrial creatine kinase activity is impaired [36] and the failing human heart has a 25–30% decline in ATP levels as determined in human biopsy specimens [38].
Genetic approach has provided many additional insights regarding the pathogenetic role of mitochondria in heart failure. Gene ablation in mice targeting a wide spectrum of genes encoding specific mitochondrial proteins results in severe cardiomyopathy. Adenine nucleotide translocator knockout mice and mice lacking cytochrome c oxidase subunit VIa-H both display dilated cardiomyopathy and severe cardiac ATP deficiency [39,40], which is thought to underlie the resulting cardiac phenotype.
Mitochondria are also involved in intracardiac Ca2+ handling, a key ion which plays a pivotal role in coordinating changes in cytosolic workload with mitochondrial energy metabolism in cardiomyocytes. When the heart has to match an increased demand for blood supply, Ca2+ is accumulated in mitochondria where it stimulates ATP production to cover the rise in energetic expenditures. The modalities/kinetics of Ca2+ uptake by mitochondria, the extra- and intracellular factors implicated in its regulation in normal hearts and the possible changes and functional consequences occurring in CHF have been recently thoroughly reviewed [41].
2. Reactive oxygen species and myocardial response to stress
A growing body of evidence suggests that reactive oxygen species (ROS) and oxidative stress may contribute to I/R injury and the pathogenesis of myocardial remodeling and failure [42-45]. The pathological consequences of ROS are of major interest, but also not always fully clarified both in terms of source and modalities of damage. It is often difficult to dissect between numerous (and overlapping) signaling pathways mediated by ROS that, in turn, determine the cell fate. For instance, a number of cascades that mediate hypertrophic response, matrix remodeling and cellular dysfunction can be modulated by ROS, which have been demonstrated to activate a broad variety of hypertrophy signaling kinases and transcription factors.
Conversely, ROS and reactive nitrogen species can also contribute to several physiological processes and frequently act as signaling molecules. In cardiomyocytes, relatively low levels of H2O2 are known to activate extracellular signal regulated kinase (ERK) 1/2, p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) signaling, protein kinase C (PKC) and PI3 kinases as well as to stimulate protein synthesis [46]. This combined effect should favor myocardial survival and compensatory hypertrophy, at least initially. However, it is known that excess ROS production and/or limited buffering ROS capacity by resident tissues can fuel myocardial maladaptive hypertrophy, matrix remodeling and myocyte dysfunction. For instance, studies have shown that adverse remodeling induced by angiotensin II (ATII) can be explained by the generation of H2O2 that in turn activates Ras/Raf/ERK MAPK pathway and the transcription factor NFκB [46,47]. However, chronic infusion of ATII, used to induce left ventricular concentric hypertrophy, triggers the activation of NADPH oxidase (Nox) resulting in a substantial rise in cardiac superoxide levels [48]. Norepinephrine (NE) interacts with α- and β-adrenergic receptors and both receptor subtypes have been implicated in NE-induced ROS generation and hypertrophy [49]. In neonatal and/or adult rat myocytes, NE increased the level of intracellular ROS, and this effect could be inhibited by α-adrenergic receptor blockade, antioxidants or monoamine oxidase (MAO) A inhibition [49-51]. High levels of H2O2 also stimulate JNK and p-38 to induce apoptosis [52], thereby likely contributing to ventricular remodeling via this mechanism. Another MAPK family member linking ROS, hypertrophy and apoptosis in failing hearts is apoptosis signal-regulating kinase 1 (ASK-1), a redox-sensitive kinase upstream of JNK and p-38. ASK-1 activation is a good example illustrating how an excess of ROS production per se may not be sufficient to trigger an adverse signaling cascade in the heart. In fact, ASK-1 activation is tightly controlled by the redox status of thioredoxin 1. Only when thioredoxin is almost entirely oxidized, thus unveiling a substantial failure of the cellular anti-oxidant system, does ASK-1 trigger its cascade of adverse signaling events [53].
ROS also have potent effects on the extracellular matrix, stimulating cardiac fibroblast proliferation and activating metalloproteinases (MMPs) [54-56], effects central to fibrosis and cardiac remodeling. MMPs are generally secreted in an inactive form and are activated post-translationally by ROS from targeted interactions with critical cysteins in the propeptide autoinhibitory domain. In addition to changes in extracellular matrix composition, bursts of ROS and/or perturbations of the cellular antioxidant defenses are also responsible for the alterations of the cardiac contractile machinery, either at the myofilament or excitation-contraction coupling level [57,58]. This includes the modification of critical thiol groups on the channels, pumps and transporters implicated in the recruitment and intracellular Ca2+ handling. For instance, transient and reversible redox-modifications may increase the open probability of ryanodine receptor. However, persistent oxidative modifications may sustain diastolic Ca2+ leakage, contributing to cardiac arrhythmias and dampened contractility and/or relaxation [59].
In the setting of I/R injury, cytosolic and mitochondrial Ca2+ overload and ROS accumulation result in the opening of the PTP, recognized as a major determinant of myocyte injury following I/R [60,61]. Although PTP opening is particularly sensitive to altered redox balance [62], its opening and ROS formation are also linked in a vicious cycle since the latter is exacerbated after PTP opening in cardiac myoctes [63]. This amplification of oxidative stress targets myofibril proteins resulting in their oxidation and contractile dysfunction on the one hand [57], and determines the evolution of cell injury towards necrosis or apoptosis, on the other [64-67].
3. Sources of oxidative stress
A number of intracellular ROS sources have been identified in animal models of cardiac diseases. These include Nox, xanthine oxidase (XO) and nitric oxide synthase that, when uncoupled, can become a powerful ROS generator [42,43,68,69]. Concerning XO, it must be said upfront that in some species, including humans, the heart appears not to contain any [70,71]. Therefore, an explanation for the protective effects exerted by the XO inhibitor allopurinol in these species should be sought elsewhere. For example it is possible that allopurinol prevents the depletion of purine nucleotides which can be used as substrates for the resynthesis of ATP on reoxygenation [72].
It is generally accepted that, in cardiac myocytes, the largest amount of ROS in cardiac myocytes is formed within the mitochondria [73-76]. The majority of oxygen delivered to mitochondria is fully reduced to water at the level of Complex IV of the respiratory chain. Nevertheless, electrons flowing through the respiratory chain can be donated to oxygen at other sites resulting in the formation of partially reduced oxygen forms, such as superoxide anion. Indeed, superoxide is formed at the level of Complex I and III and rapidly dismutated into H2O2 by Mn-SOD. The finding that Mn-SOD deficient mice develop ROS toxicity and dilated cardiomyopathy [77], underlines the importance of ROS in this pathology and of mitochondria as the source and target of this altered redox equilibrium.
The mitochondrial respiratory chain is certainly the best-characterized site for ROS generation. Yet, other mitochondrial proteins, such as p66Shc, MAO and Nox4, are also emerging as major ROS producers and have potential pathophysiological relevance. p66Shc localizes partially to mitochondria where it catalyzes electron transfer from cytochrome c to oxygen [78], a process that can result in the formation of ROS. Indeed, ROS generation is reduced in cells lacking p66Shc and in p66Shc-/- mice, whose lifespan is increased by 30% [78-81]. Furthermore, a lack of p66Shc was shown to protect against diabetic cardiomyopathy and I/R injury in mice [82]. A significant role for MAO activity in the formation of H2O2 has been demonstrated in the brain, as MAO have been shown to produce more H2O2 than the mitochondrial respiratory chain [83]. Indeed, MAO are involved in numerous neuronal and psychiatric disorders, as demonstrated by the beneficial effects elicited by MAO inhibitiors. MAO-B seems to be involved in the loss of dopaminergic neurons that occurs in Parkinson's disease, most likely due to an increased dopamine catabolism that in turn would result in elevated ROS production responsible for the oxidative damage of nigrostriatal neurons [84]. An increase of MAO-B activity in the brain has been also associated with Alzheimer's and Huntington's disease. Recently, also Nox4 has been demonstrated to localize primarily in the mitochondria and to be a major source of oxidative stress in the failing heart [85,86].
4. MAO as a major source of ROS
4.1. Basic biochemical aspects
MAOs are flavoenzymes located within the outer mitochondrial membrane, responsible for the oxidative deamination of neurotransmitters and dietary amines. They exist in two isoforms, MAO-A and B, distinguished by different substrate specificity and inhibitor sensitivity. These two enzymes present 70% homology in their primary sequence [87,88] and both contain the pentapeptide Ser-Gly-Gly-Cys-Tyr, where the obligatory cofactor FAD is covalently bound through a thioether linkage to the cysteine residue, namely Cys406 in MAO-A and Cys397 in MAO-B [89,90]. This flavin moiety is the only redox-dependent factor necessary for their activity deployment. The 3D structures of human and rat MAO-A (h/rMAO-A) and human MAO-B (hMAO-B) have been resolved. Despite their high level of sequence identity (92%), there are several functional properties that differentiate hMAO-A from rMAO-A. hMAO-A exhibits a 10-fold lower affinity than rMAO-A for the specific inhibitor clorgyline [91], and a Phe208 to Ile mutation on MAO-A from human and rat [92,93] shows differential effects on activities and sensitivities to irreversible inhibition. The largest differences among the three known structures of MAO are the corresponding natures of their oligomeric states: hMAO-B and rMAO-A are dimers, whereas hMAO-A is a monomer [94]. However, pulsed electron paramagnetic resonance data showed that this is the case only in the detergent-solubilized, purified preparations while all rat and human MAOs are dimeric in their membrane-bound forms.
MAO-A preferentially catalyzes the oxidative deamination of NE and serotonin (5-HT) and is inhibited by low concentrations of clorgyline. In contrast, MAO-B has a major affinity for phenylethylamine and benzylamine, and is inhibited by selegiline [84]. Both isoforms catalyze the deamination of dopamine, tyramine, octopamine and tryptamine and are inhibited by pargyline.
The prototypic reaction catalyzed by MAO is the following:
Kinetic studies have shown that the binding of the amine group to the enzyme precedes the binding of oxygen [95]. In the first moment, the reduction of the cofactor FAD yields an aldehyde intermediate and ammonia, while in the second moment the oxidized form of the prosthetic group is restored with the concomitant production of H2O2. The aldehyde intermediate is rapidly metabolized to corresponding acid by the action of aldehyde dehydrogenase.
The distribution of MAO in various tissues of different species has been investigated through use of specific inhibitors, immunohistochemistry, enzyme autoradiography and in situ hybridization [96-98]. MAO location has been intensively studied particularly in the brain, where MAO-A has been prevalently found in noradrenergic neurons, whereas MAO-B has been detected in serotoninergic and histaminergic neurons and in glial cells [99-102]. In peripheral tissues, MAO-A has been found in the placenta, liver, intestine and thyroid gland, while platelets, liver and kidney contain mainly MAO-B. Human cardiomyocytes contain both enzymes, although MAO-A appears to be the predominant isoform [103,104].
4.2. The physiological role of MAOs
The main physiological role of MAO is the degradation of endogenous monoamine neurotransmitters and dietary amines, such as tyramine. In peripheral tissues, MAO are involved in the oxidative catabolism of amines from the blood and in preventing the entry of dietary amines into the circulation. In the central and peripheral nervous system, intraneuronal MAO-A and -B protect neurons from exogenous amines, terminate the actions of amine neurotransmitters and regulate the contents of intracellular amine stores.
From a different angle, studies employing selective pharmacological MAO inhibition and its genetic ablation have further delineated their physiological roles. Deletion of MAO-A and MAO-B genes has proven their important roles in neurotransmitter metabolism and behavior. MAO-A knockout mice have elevated brain levels of 5-HT, NE and, to a lesser extent, dopamine [105]. Conversely, only 2-phenylethylamine is increased in MAO-B knockout mice [84]. Both MAO-A and -B knockout mice show increased reactivity to stress, similar to that observed after administration of non-selective MAO inhibitors. In humans, MAO-A deficiency results in borderline mental retardation and impaired impulse control, whereas a lack of MAO-B doesn't lead to intellectual impairment or behavioral disturbances [106]. Deletion of both genes in a male patient has been shown to cause a profound mental retardation, severe developmental delay, intermittent hypotonia and stereotypical hand movements [107]. Although these studies indicate that MAO is not essential for survival, gene deletion approaches has proven that MAO-A activity is important during neuronal development. A compulsive-aggressive behavior resulting from lack of MAO-A function in humans and mice [108,109] might reflect the importance of 5-HT during development and can be mimicked by the administration of the MAO-A inhibitor clorgyline during the early postnatal period. Studies of MAO-A knockout mice have also shown the maintenance of 5-HT levels to be important for the normal development of thalamocortical axons and the aggregation of neurons to form barrels [110].
The roles of MAO in terminating the actions of neurotransmitters/dietary amines in central and peripheral nervous system and in the extraneuronal tissue have been extensively studied. In contrast, much less attention has been dedicated to the products of their activity. The monoamine catabolism byproducts are aldehydes, ammonia and H2O2, a reactive oxygen species that could be toxic per se at high concentrations or it could generate hydroxyl radical when in the presence of free Fe2+. Aldehyde intermediates are also toxic for the biological systems and a decrease in aldehyde dehydrogenase activity, which is associated with oxidative stress [111], might further contribute to the exacerbation of damage. These are aspects of MAO biochemical and pharmacological profile that certainly deserve further attention.
5. MAO in cardiac pathophysiology
5.1. MAO in hypertrophy and heart failure
Despite their wide distribution in animal and human organs/tissues and their association with mitochondria, the role of MAOs has been primarily investigated in central and peripheral nervous system or in the gastro-intestinal tract. It is only recently that the cardiovascular field has focused on the role of MAO activity in the heart, with much of the pioneering work performed by Parini's group. This research team was the first to demonstrate the role of MAO-A as an important myocardial source of ROS. Depending on the concentrations of substrate available, ROS produced by MAO-A are able to trigger separate signaling pathways leading respectively to cell proliferation and hypertrophy or apoptosis [112] (Fig. 1). In one study, Bianchi and coworkers demonstrated that low concentrations of 5-HT are able to induce cardiomyocyte hypertrophy in a MAO-A dependent manner, involving activation of ERK1/2, an essential signaling molecule for cell growth [113]. The use of pharmacological inhibitors demonstrated that hypertrophy induced by 5-HT involves a dual action by both receptors and MAO-A. Indeed, in vascular smooth muscle cells 5-HT1B/D receptor stimulation induced ERK phosphorylation, whereas H2O2 generated by MAO was responsible for ERK translocation into the nucleus [112]. In addition to 5-HT, in vitro studies from our group have also recently unraveled a major role for MAO-A in NE-induced hypertrophy [51]. Using neonatal rat and adult mouse cardiomyocytes, we have found that NE acts in part independently from α- or β-adrenergic receptor binding. Employing a pharmacological and genetic approach, we showed that NE induced hypertrophy is also partially mediated by MAO-A generated ROS through a mechanism likely involving nuclear factors of activated T cells 3 and 4, transcription factors involved in maladaptive hypertrophic signaling. Furthermore, we have demonstrated that the stimulation of MAO-A activity is sufficient to trigger myocyte hypertrophy, independently of receptor activation.
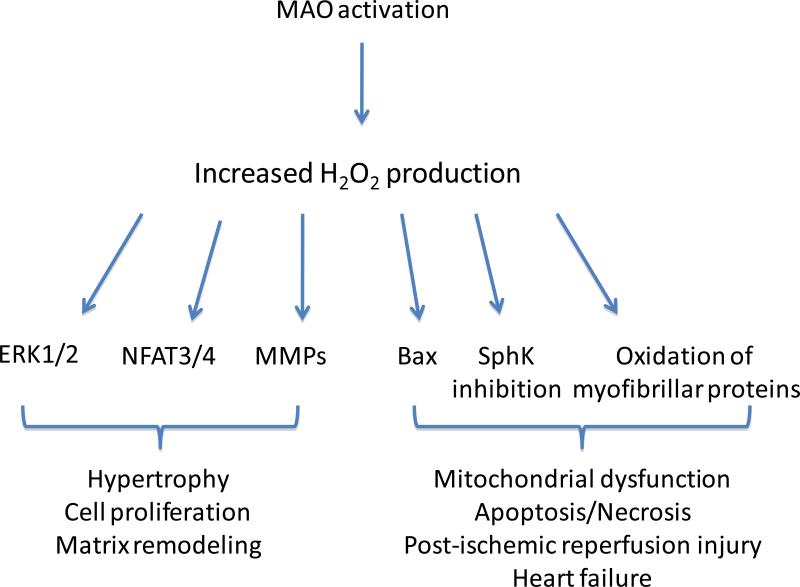
Figure 1. Signaling pathways triggered by MAO-induced H2O2 formation.
Increased H2O2 production by MAO can trigger different signaling pathways in different cell types. Lower levels of H2O2 activate ERK1/2, NFAT3/4 and MMPs in cardiac myocytes and vascular smooth muscle cells resulting in hypertrophy, cell proliferation and matrix remodeling. Instead, higher levels of MAO activity are associated with Bax upregulation, SphK inhibition, myofibrillar oxidation and mitochondrial dysfunction leading to cell death in heart failure or following ischemia/reperfusion. MAO: monoamine oxidase, ERK: extracellular signal regulated kinase, NFAT: nuclear factor of activated T cells, MMPs: metalloproteinases, SphK: sphingosine kinase.
The important role of MAO in the transition from compensated hypertrophy to heart failure was demonstrated by in vivo studies. MAO-A expression and activity increase in rats with aging [114], a condition often associated with heart failure. Similarly, MAO-B expression is elevated in the myocardium of dogs in which CHF was induced by tachypacing [115]. However, these studies did not correlate changes in MAO activity with alterations in cardiac function. This possible causative relationship was examined in mice in which CHF was induced by pressure overload due to transverse aortic constriction (TAC). In these hearts NE degradation by MAO-A was augmented and accompanied by increased oxidative stress [51]. Pharmacological or genetic inhibition of MAO-A in this CHF model confirmed its central role as a major in vivo trigger of cardiac oxidative stress, left ventricle (LV) remodeling and apoptosis. Indeed, both approaches, while blunting excess ROS production, completely prevented left ventricular dilation and pump dysfunction in these chronically stressed hearts. It is also worth mentioning that the use of clorgyline normalized the protein abundance of the neuronal NE transporter, and likely ameliorated its function resulting in an improved NE bioavailability and cycling. Thus, clorgyline which has no major cardiac impact on sham-operated mice has been shown to benefit TAC hearts through at least two mechanisms. One is through the reduction in ROS burden, therefore countering the adverse structural and functional sequelae of increased oxidative stress. Indeed, triggering H2O2 formation from MAO-A results in the activation of MMPs and LV remodeling. Moreover, this excess in H2O2 production can also target mitochondria themselves, resulting in the amplification of the oxidative stress, mitochondrial dysfunction, PTP opening, release of cytochrome c, caspase activation and apoptosis (Fig. 2). Some of these deleterious events occurred in TAC hearts, but were almost completely abated by MAO-inhibition via clorgyline. The other mechanism concerns the rescued levels of available neuronal NE pool, denoting an improved catecholamine cycling.
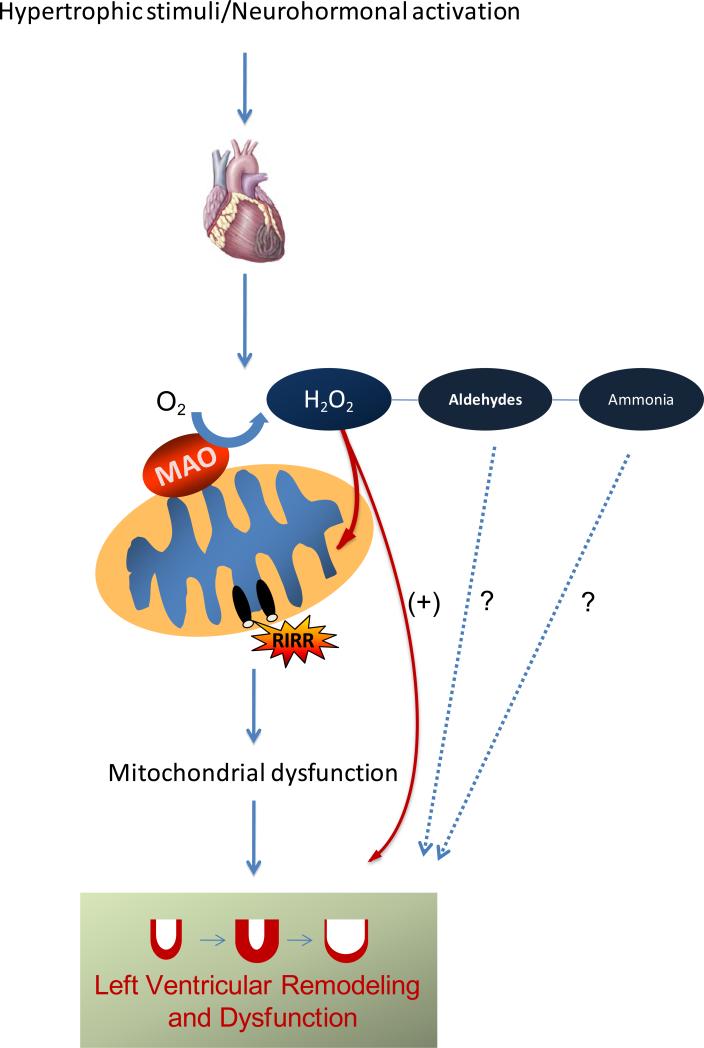
Figure 2. Mechanism for MAO activation and consequent mitochondrial dysfunction in heart failure.
Hyperadrenergic activation following a hypertrophic stimulus (such as pressure overload) results in increased availability of catecholamines for MAO-mediated degradation. This in turn leads to elevated H2O2 formation that can directly influence ventricular remodeling and myocardial function, but it may also target mitochondria resulting in permeability transition pore opening and ROS induced ROS release (RIRR), further amplifying the damage in the heart. It should not be excluded that other products of MAO activity, such as aldehydes and ammonia, may also participate to these processes.
In an apparent contrast to the studies mentioned above, Lairez and colleagues have observed exacerbated ventricular hypertrophy in MAO-A knockout mice subjected to aortic banding, and have attributed this effect to the hyperactivation of 5-HT2A receptors in MAO-A knockout mice [116]. This discrepancy might be explained by some adaptive mechanisms present in the knockout mice (such as overexpression of 5-HT2A receptors, increased catecholamine and 5-HT content) and by the fact that the experimental observations performed in this study were limited to the compensated hypertrophy time-frame and not extended to later stages in which left ventricular dilation and pump failure is evident. Regardless, there is no doubt that MAO-A expression and/or activity is a major contributing factor for the development of pathologic hypertrophy and heart failure, at least in rodents. Intriguing enough is the finding that MAO-A expression is elevated in pathological hypertrophy and heart failure in rats, but not in physiological hypertrophy induced by exercise [117]. It goes without saying that many additional, fundamental questions follow. First and foremost, what are the signaling cascades that activate MAO-A expression/activity in adverse cardiac remodeling? Second, are these same pathways silent in hearts subjected to physiological stress as such exercise? And if so, why? Another important question that begs future attention is whether the oxidative stress present in CHF models such as pressure overload is due to MAO activation alone or also due to other sources. A cross-talk between MAO, mitochondrial respiratory chain, Nox4 or p66Shc is likely and could result in the amplification of the oxidative stress. However, there is not enough evidence in the literature at the moment to link these processes together and/or to attribute relative importance to one or another source. Further studies addressing these basic unknowns will greatly help to consolidate our understanding on the contribution of each of these components to CHF development.
Data remain very scant on the possible involvement of MAO-B in this setting. Despite the fact that the role of MAO-A in myocardial failure is better described, the potential contribution of MAO-B should not be disregarded as it appears to be up-regulated in some experimental models of CHF. The relative expression of each MAO isoform varies enormously across different tissues and species. For instance, MAO-A is the predominant isoform in the rat heart, with little or no MAO-B activity, whereas exactly the opposite is true in the case of the mouse myocardium [118]. The majority of the studies were performed in rats, therefore highlighting the deleterious effects of MAO-A derived ROS. Interestingly though, specific MAO-A inhibition in mouse hearts subjected to pressure overload is able to completely prevent oxidative stress, apoptosis, LV dysfunction and remodeling [51]. The involvement of specific MAO substrates might count as a partial explanation for this observation. However, it is also tempting to speculate that each isoform might be differently expressed in distinct cell types where the consequences of increased oxidative stress can determine separate outcomes. Further studies are necessary to explore this possibility.
5.2. MAO in I/R injury
Although lower 5-HT concentrations promote cell proliferation, survival and hypertrophy, higher 5-HT concentrations lead to cardiomyocyte apoptosis. This effect can be entirely attributed to MAO-A activation as apoptosis was completely blocked by the MAO inhibitor pargyline with the 5-HT2B antagonist failing to inhibit this response [119]. ROS generated by MAO in the presence of elevated 5-HT concentrations induced release of cytochrome c, upregulation of proapoptotic Bax and downregulation of antiapoptotic Bcl-2 proteins [119]. These in vitro findings pertain to in vivo pathophysiological situations because I/R injury is associated with increased interstitial 5-HT released by activated platelets [120]. Therefore, a role for MAO-A is plausible. Indeed, in an in vivo rat model of I/R injury, infarct size was dramatically reduced in animals treated with pargyline or clorgyline prior to I/R as compared to untreated rats [119]. However, another study performed in isolated Langendorff perfused mouse hearts showed that MAO inhibition by pargyline is equally effective in preventing I/R induced injury despite the fact that 5-HT was absent in that model [121]. Although the role of MAO remains central in mediating I/R injury, the importance of 5-HT as a major MAO substrate warrants further investigation.
MAO-A generated ROS in I/R induce sphingosine kinase inhibition, ceramide accumulation and sphingosine-1-phosphate (S1P) degradation in cardiac myocytes thereby leading to mitochondria-mediated apoptosis [122]. This is yet another interesting example of the dual role of ROS in the regulation of cell proliferation, survival and cell death; the control of the sphingolipid rheostat (sphingostat), the dynamic balance between the S1P and ceramide, by MAO-A and the consequent regulation of opposing signaling pathways that ultimately determine the cell fate. In the smooth muscle cells, oxidation of low concentrations of 5-HT or tyramine by MAO-A triggers MMP2, neutral sphingomyelinase-1 and sphingosine kinase resulting in cell proliferation [123]. Considering that MAO-A controls the activity of sphingosine kinase that, in turn, controls the levels of both proapoptotic ceramide and antiapoptotic S1P, it may be an important drug target for regulating I/R injury in the heart and vascular wall remodeling.
6. The therapeutic potential of MAO inhibitors
Despite the fact that experimental studies strongly support a key role of oxidative stress in the pathophysiology of I/R injury and heart failure, the outcome of clinical trials using different antioxidant approaches remains disappointing. There is no doubt that some drugs already in use for CHF treatment may act indirectly to ameliorate excessive oxidative stress. This is the case of angiotensin-converting enzyme inhibitors and AT receptor blockers that may reduce G protein-linked signaling through Nox. Likewise, β-blockers may provide some benefits as β-adrenergic receptor activation is coupled to the generation of ROS [124]. In addition, drugs such as carvedilol or nebivolol may have additional antioxidant properties independent of the β-blocking effect [125,126], whereas drugs such as statins and hydralazine have indirect inhibitory effects on ROS production [127]. As hinted before, the beneficial effects observed in animal CHF models with XO inhibitors such as allo-or oxy-purinol were not reproduced in humans [128]. Finally, large clinical trials of the antioxidant vitamins A and E and their precursors have been disappointing [129,130]. Conversely, ubiquinone, an antioxidant, caused a small but significant increase in ejection fraction in a meta-analysis of 11 randomized clinical trials [131]. Considering these results, it is possible that more specific targeting of the sources of oxidative stress may ultimately provide more effective approaches to reversing cardiac remodeling.
6.1. MAO inhibitors
The investigation of the therapeutic potential of MAO inhibitors started in the early „50s as anti-tuberculosis treatment with iproniazid was shown to improve the mood and inhibit MAO activity. Nowadays, there is a wide range of MAO inhibitors at our disposal, and this class of drugs has been proven effective against many diseased states including affective disorders, neurodegenerative diseases, stroke and eugeric aging. Just to provide a brief census of them, they can be classified in three major groups based on their specificity for each isoform and by the nature of their binding to the enzyme [84]. There are:
-
-
Irreversible and non selective inhibitors, such as phenelzine and tranylcypromine;
-
-
Irreversible and selective inhibitors, such as selegiline for MAO-B and clorgyline for MAO-A;
-
-
Reversible and selective MAO-A inhibitors (RIMAs), such as moclobemide.
All the irreversible MAO inhibitors form N(5) flavin adducts, with the exception of tranylcypromine that forms the flavin C(4a) adduct with MAO-B [94]. Differences in inhibitor specificity are due to distinct architecture of the active sites between the two isoenzymes. The active site cavity of MAO-B is bipartite and determined by the conformation of Ile199 that functions as a gating residue and may result in a closed or open conformation. The corresponding residue in human and rat MAO-A is Phe208, but the bulky side Phe chain impedes such conformational flexibility, reduces the space of the entrance cavity and therefore interferes with the binding of specific MAO-B inhibitors [94]. hMAO-B can bind compounds of different sizes; depending on the conformation of gating residue Ile199, the cavity can host either small inhibitors or cavity-filling ligands. Therefore, small compounds such as isatin and tranylcypromine show similar binding affinities for both isoforms, whereas cavity-filling ligands, such as safinamide, are highly specific for MAO-B [94].
6.2. Current use for MAO inhibitors
MAO inhibitors have been used for decades for the treatment of depression [84]. The antidepressant properties result from selective MAO-A inhibition in the central nervous system, which leads to increased brain levels of dopamine, NE and 5-HT. Some of the non-selective irreversible inhibitors, such as isocarboxazide, phenelzine and tranylcypromine, are still in clinical use for the treatment of mood disorders, along with the reversible MAO inhibitors moclobemide and toloxatone. As levels of MAO-B are increased in patients with Parkinson's disease, the MAO-B inhibitor selegiline is used as a dopamine sparing agent or as adjunct to L-DOPA and has been shown to be effective both as an adjuvant to L-DOPA and as monotherapy [84]. Recently, a new MAO-B inhibitor rasagiline has been commercialized for the treatment of the symptoms of Parkinson's disease. Combination of MAO-B inhibitor with cholinesterase inhibitor is beneficial for the treatment of Alzheimer's disease [132]. Selegiline has also been shown to reduce the peripheral tissue damage that results from cardiac failure [133], and that arising in the brain after cerebral ischemia [134] in animal models. This protective effect has been attributed to a decrease in hydrogen peroxide production generated by MAO upon reperfusion. Selegiline has also been shown to increase Bcl2 to Bax ratio and activate the translocation of anti-apoptotic protein kinases, PKC and PKC [135], although the mechanisms leading to these effects are as yet not completely elucidated. A protective outcome following selegiline treatment have also been observed at concentrations lower than those required for MAO-B inhibition, suggesting that they may be independent of MAO-B inhibition.
It should be pointed out that non-selective, irreversible MAO inhibitors might cause adverse reactions when taken together with drugs such as sympathomimetics, in case of hypertension or when taken in combination with cheese or other foods particularly rich in tyramine content. These combinations cause hypertensive crises (the so-called “cheese reaction”) that may result even in sudden death. For decades, this side-effect was the major drawback for the use of MAO inhibitors in clinic. However, non-reversible MAO-B inhibitors and RIMAs are devoid of these undesirable effects and can be used by patients on unrestricted diet [136]. Thus, considering the availability of these amenable inhibitors and the encouraging results obtained with MAO inhibitors in experimental CHF and I/R injury, MAO inhibition might represent a potential new class of compounds to employ for treatment of ischemic and non ischemic cardiac disorders. Along this line, it would be interesting to test the hypothesis that patients treated with MAO inhibitors experience a lower incidence of cardiac injury, especially myocardial infarction. In this respect, it is worth mentioning that at the moment there are no data available concerning the role of MAO-derived oxidative stress related to atherosclerosis. However, MAO could be implicated also in chronic vascular alterations considering that a tight link between mitochondrial ROS formation and vascular injury has been demonstrated [137].
Very often, “old mates” make surprises. Therefore, we hope that focusing our attention on these mitochondrial companions will lead us to learn increasingly more on the role of certain disturbances such as myocardial tissue redox imbalance and altered communication between the peripheral nervous system and the cardiac muscle in acute and chronic heart pathobiology. This in turn will allow us to enrich our therapeutic armamentarium against many chronic and severely invalidating cardiac conditions such as CHF.
Acknowledgments
This work was supported by American Heart Association (Postdoctoral fellowship to NK), NIH grants R01 HL075265 and R01 HL091923 (NP), University of Padova, Cariparo Foundation, MIUR (FDL).
Abbreviations
- 5-HT
serotonin
- ASK-1
apoptosis signal-regulating kinase 1
- AT
angiotensin
- CHF
congestive heart failure
- ERK
extracellular signal regulated kinase
- FA
fatty acid
- I/R
ischemia/reperfusion
- JNK
c-Jun N-terminal kinase
- LV
left ventricle
- MAPK
mitogen-activated protein kinase
- MAO
monoamine oxidase
- MMPs
metalloproteinases
- NE
norepinephrine
- Nox
NADPH oxidase
- PGC-1α
peroxisome-proliferator-activated receptor gamma coactivator-1α
- PKC
protein kinase C
- PPARs
peroxisome-proliferator-activated receptors
- PTP
permeability transition pore
- RIMAs
reversibile MAO-A inhibitors
- ROS
reactive oxygen species
- S1P
sphingosine-1-phosphate
- TAC
transverse aortic constriction
- XO
xantine oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr. Probl. Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 2.Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail. Rev. 2002;7:115–130. doi: 10.1023/a:1015320423577. [DOI] [PubMed] [Google Scholar]
- 3.BING RJ, SIEGEL A, UNGAR I, GILBERT M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am. J. Med. 1954;16:504–515. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- 4.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim. Biophys. Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 5.Jennings RB, Ganote CE. Mitochondrial structure and function in acute myocardial ischemic injury. Circ. Res. 1976;38:I80–I91. [PubMed] [Google Scholar]
- 6.Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail. Rev. 2007;12:249–260. doi: 10.1007/s10741-007-9028-z. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 9.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 10.Rimbaud S, Garnier A, Ventura-Clapier R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol. Rep. 2009;61:131–138. doi: 10.1016/s1734-1140(09)70015-5. [DOI] [PubMed] [Google Scholar]
- 11.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J. Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoll J, Monassier L, Garnier A, N'Guessan B, Mettauer B, Veksler V, Piquard F, Ventura-Clapier R, Geny B. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J. Appl. Physiol. 2006;101:385–391. doi: 10.1152/japplphysiol.01486.2005. [DOI] [PubMed] [Google Scholar]
- 13.Kemi OJ, Hoydal MA, Haram PM, Garnier A, Fortin D, Ventura-Clapier R, Ellingsen O. Exercise training restores aerobic capacity and energy transfer systems in heart failure treated with losartan. Cardiovasc. Res. 2007;76:91–99. doi: 10.1016/j.cardiores.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Sun CK, Chang LT, Sheu JJ, Wang CY, Youssef AA, Wu CJ, Chua S, Yip HK. Losartan preserves integrity of cardiac gap junctions and PGC-1 alpha gene expression and prevents cellular apoptosis in remote area of left ventricular myocardium following acute myocardial infarction. Int. Heart J. 2007;48:533–546. doi: 10.1536/ihj.48.533. [DOI] [PubMed] [Google Scholar]
- 15.Sano M, Wang SC, Shirai M, Scaglia F, Xie M, Sakai S, Tanaka T, Kulkarni PA, Barger PM, Youker KA, Taffet GE, Hamamori Y, Michael LH, Craigen WJ, Schneider MD. Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure. EMBO J. 2004;23:3559–3569. doi: 10.1038/sj.emboj.7600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastiani M, Giordano C, Nediani C, Travaglini C, Borchi E, Zani M, Feccia M, Mancini M, Petrozza V, Cossarizza A, Gallo P, Taylor RW, d'Amati G. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J. Am. Coll. Cardiol. 2007;50:1362–1369. doi: 10.1016/j.jacc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol. Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- 20.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 21.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 22.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 23.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 24.Taegtmeyer H. Metabolism--the lost child of cardiology. J. Am. Coll. Cardiol. 2000;36:1386–1388. doi: 10.1016/s0735-1097(00)00870-6. [DOI] [PubMed] [Google Scholar]
- 25.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J. Biol. Chem. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 26.Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 27.Funada J, Betts TR, Hodson L, Humphreys SM, Timperley J, Frayn KN, Karpe F. Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLoS. One. 2009;4:e7533. doi: 10.1371/journal.pone.0007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marin-Garcia J, Goldenthal MJ, Moe GW. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc. Res. 2001;52:103–110. doi: 10.1016/s0008-6363(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 29.Quigley AF, Kapsa RM, Esmore D, Hale G, Byrne E. Mitochondrial respiratory chain activity in idiopathic dilated cardiomyopathy. J. Card Fail. 2000;6:47–55. doi: 10.1016/s1071-9164(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 30.Casademont J, Miro O. Electron transport chain defects in heart failure. Heart Fail. Rev. 2002;7:131–139. doi: 10.1023/a:1015372407647. [DOI] [PubMed] [Google Scholar]
- 31.Lewandowski ED. Cardiac carbon 13 magnetic resonance spectroscopy: on the horizon or over the rainbow? J. Nucl. Cardiol. 2002;9:419–428. doi: 10.1067/mnc.2002.125811. [DOI] [PubMed] [Google Scholar]
- 32.Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364:1786–1788. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- 33.Marin-Garcia J, Goldenthal MJ, Moe GW. Mitochondrial pathology in cardiac failure. Cardiovasc. Res. 2001;49:17–26. doi: 10.1016/s0008-6363(00)00241-8. [DOI] [PubMed] [Google Scholar]
- 34.Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, Samantapudi D, Paolocci N, Tomaselli GF, Kass DA, Van Eyk JE. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ. Cardiovasc. Genet. 2010;3:78–87. doi: 10.1161/CIRCGENETICS.109.871236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosca MG, Hoppel CL. New aspects of impaired mitochondrial function in heart failure. J. Bioenerg. Biomembr. 2009;41:107–112. doi: 10.1007/s10863-009-9215-9. [DOI] [PubMed] [Google Scholar]
- 36.Scheubel RJ, Tostlebe M, Simm A, Rohrbach S, Prondzinsky R, Gellerich FN, Silber RE, Holtz J. Dysfunction of mitochondrial respiratory chain complex I in human failing myocardium is not due to disturbed mitochondrial gene expression. J. Am. Coll. Cardiol. 2002;40:2174–2181. doi: 10.1016/s0735-1097(02)02600-1. [DOI] [PubMed] [Google Scholar]
- 37.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 38.Neubauer S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 39.Radford NB, Wan B, Richman A, Szczepaniak LS, Li JL, Li K, Pfeiffer K, Schagger H, Garry DJ, Moreadith RW. Cardiac dysfunction in mice lacking cytochrome-c oxidase subunit VIaH. Am. J. Physiol Heart Circ. Physiol. 2002;282:H726–H733. doi: 10.1152/ajpheart.00308.2001. [DOI] [PubMed] [Google Scholar]
- 40.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat. Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 41.Liu T, O'Rourke B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr. 2009;41:127–132. doi: 10.1007/s10863-009-9216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 44.Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid. Redox. Signal. 2006;8:2111–2124. doi: 10.1089/ars.2006.8.2111. [DOI] [PubMed] [Google Scholar]
- 45.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res. Cardiol. 2009;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 46.Sabri A, Hughie HH, Lucchesi PA. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid. Redox. Signal. 2003;5:731–740. doi: 10.1089/152308603770380034. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 48.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ. Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 49.Luo JD, Xie F, Zhang WW, Ma XD, Guan JX, Chen X. Simvastatin inhibits noradrenaline-induced hypertrophy of cultured neonatal rat cardiomyocytes. Br. J. Pharmacol. 2001;132:159–164. doi: 10.1038/sj.bjp.0703792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amin JK, Xiao L, Pimental DR, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J. Mol. Cell Cardiol. 2001;33:131–139. doi: 10.1006/jmcc.2000.1285. [DOI] [PubMed] [Google Scholar]
- 51.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ. Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J. Mol. Cell Cardiol. 2003;35:615–621. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 53.Kondo N, Nakamura H, Masutani H, Yodoi J. Redox regulation of human thioredoxin network. Antioxid. Redox. Signal. 2006;8:1881–1890. doi: 10.1089/ars.2006.8.1881. [DOI] [PubMed] [Google Scholar]
- 54.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 55.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail. Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 56.Kaludercic N, Lindsey ML, Tavazzi B, Lazzarino G, Paolocci N. Inhibiting metalloproteases with PD 166793 in heart failure: impact on cardiac remodeling and beyond. Cardiovasc. Ther. 2008;26:24–37. doi: 10.1111/j.1527-3466.2007.00034.x. [DOI] [PubMed] [Google Scholar]
- 57.Canton M, Skyschally A, Menabo R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur. Heart J. 2006;27:875–881. doi: 10.1093/eurheartj/ehi751. [DOI] [PubMed] [Google Scholar]
- 58.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 59.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res. Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Lisa F, Canton M, Menabo R, Dodoni G, Bernardi P. Mitochondria and reperfusion injury. The role of permeability transition. Basic Res. Cardiol. 2003;98:235–241. doi: 10.1007/s00395-003-0415-x. [DOI] [PubMed] [Google Scholar]
- 62.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 63.Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, Naik TJ, Prasad SV, Ardehali H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J. Biol. Chem. 2009;284:2080–2087. doi: 10.1074/jbc.M804570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernardi P, Petronilli V, Di Lisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem. Sci. 2001;26:112–117. doi: 10.1016/s0968-0004(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 65.Di Lisa F, Menabo R, Canton M, Petronilli V. The role of mitochondria in the salvage and the injury of the ischemic myocardium. Biochim. Biophys. Acta. 1998;1366:69–78. doi: 10.1016/s0005-2728(98)00121-2. [DOI] [PubMed] [Google Scholar]
- 66.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 67.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 68.Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc. Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell Cardiol. 2002;34:379–388. doi: 10.1006/jmcc.2002.1526. [DOI] [PubMed] [Google Scholar]
- 70.Downey JM, Miura T, Eddy LJ, Chambers DE, Mellert T, Hearse DJ, Yellon DM. Xanthine oxidase is not a source of free radicals in the ischemic rabbit heart. J Mol. Cell Cardiol. 1987;19:1053–1060. doi: 10.1016/s0022-2828(87)80350-4. [DOI] [PubMed] [Google Scholar]
- 71.Eddy LJ, Stewart JR, Jones HP, Engerson TD, McCord JM, Downey JM. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am. J Physiol. 1987;253:H709–H711. doi: 10.1152/ajpheart.1987.253.3.H709. [DOI] [PubMed] [Google Scholar]
- 72.Lasley RD, Ely SW, Berne RM, Mentzer RM., Jr. Allopurinol enhanced adenine nucleotide repletion after myocardial ischemia in the isolated rat heart. J Clin. Invest. 1988;81:16–20. doi: 10.1172/JCI113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 74.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 75.Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 78.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 80.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J. Biol. Chem. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 81.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P, Lanfrancone L, Pelicci PG. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 82.Rota M, LeCapitaine N, Hosoda T, Boni A, De AA, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ. Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 83.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 84.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 85.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, Seeburg PH, Shih JC. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. U. S. A. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edmondson DE, Mattevi A, Binda C, Li M, Hubalek F. Structure and mechanism of monoamine oxidase. Curr. Med. Chem. 2004;11:1983–1993. doi: 10.2174/0929867043364784. [DOI] [PubMed] [Google Scholar]
- 89.Edmondson DE, Binda C, Mattevi A. The FAD binding sites of human monoamine oxidases A and B. Neurotoxicology. 2004;25:63–72. doi: 10.1016/S0161-813X(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 90.Abell CW, Kwan SW. Molecular characterization of monoamine oxidases A and B. Prog. Nucleic Acid Res. Mol. Biol. 2001;65:129–156. doi: 10.1016/s0079-6603(00)65004-3. [DOI] [PubMed] [Google Scholar]
- 91.Tsugeno Y, Hirashiki I, Ogata F, Ito A. Regions of the molecule responsible for substrate specificity of monoamine oxidase A and B: a chimeric enzyme analysis. J. Biochem. 1995;118:974–980. doi: 10.1093/jb/118.5.974. [DOI] [PubMed] [Google Scholar]
- 92.Tsugeno Y, Ito A. A key amino acid responsible for substrate selectivity of monoamine oxidase A and B. J. Biol. Chem. 1997;272:14033–14036. doi: 10.1074/jbc.272.22.14033. [DOI] [PubMed] [Google Scholar]
- 93.Geha RM, Chen K, Shih JC. Phe(208) and Ile(199) in human monoamine oxidase A and B do not determine substrate and inhibitor specificities as in rat. J. Neurochem. 2000;75:1304–1309. doi: 10.1046/j.1471-4159.2000.751304.x. [DOI] [PubMed] [Google Scholar]
- 94.Edmondson DE, Binda C, Wang J, Upadhyay AK, Mattevi A. Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry. 2009;48:4220–4230. doi: 10.1021/bi900413g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tipton KF, Boyce S, O'Sullivan J, Davey GP, Healy J. Monoamine oxidases: certainties and uncertainties. Curr. Med. Chem. 2004;11:1965–1982. doi: 10.2174/0929867043364810. [DOI] [PubMed] [Google Scholar]
- 96.Berry MD, Juorio AV, Paterson IA. The functional role of monoamine oxidases A and B in the mammalian central nervous system. Prog. Neurobiol. 1994;42:375–391. doi: 10.1016/0301-0082(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 97.Kitahama K, Maeda T, Denney RM, Jouvet M. Monoamine oxidase: distribution in the cat brain studied by enzyme- and immunohistochemistry: recent progress. Prog. Neurobiol. 1994;42:53–78. doi: 10.1016/0301-0082(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 98.Luque JM, Biou V, Nicholls JG. Three-dimensional visualization of the distribution, growth, and regeneration of monoaminergic neurons in whole mounts of immature mammalian CNS. J. Comp Neurol. 1998;390:427–438. doi: 10.1002/(sici)1096-9861(19980119)390:3<427::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 99.Arai R, Kimura H, Nagatsu I, Maeda T. Preferential localization of monoamine oxidase type A activity in neurons of the locus coeruleus and type B activity in neurons of the dorsal raphe nucleus of the rat: a detailed enzyme histochemical study. Brain Res. 1997;745:352–356. doi: 10.1016/s0006-8993(96)01239-5. [DOI] [PubMed] [Google Scholar]
- 100.Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience. 1988;25:439–456. doi: 10.1016/0306-4522(88)90250-3. [DOI] [PubMed] [Google Scholar]
- 102.Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985;230:181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- 103.Sivasubramaniam SD, Finch CC, Rodriguez MJ, Mahy N, Billett EE. A comparative study of the expression of monoamine oxidase-A and -B mRNA and protein in non-CNS human tissues. Cell Tissue Res. 2003;313:291–300. doi: 10.1007/s00441-003-0765-6. [DOI] [PubMed] [Google Scholar]
- 104.Saura J, Kettler R, Da PM, Richards JG. Quantitative enzyme radioautography with 3HRo 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAOB in rat CNS, peripheral organs, and human brain. J. Neurosci. 1992;12:1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lenders JW, Eisenhofer G, Abeling NG, Berger W, Murphy DL, Konings CH, Wagemakers LM, Kopin IJ, Karoum F, van Gennip AH, Brunner HG. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J. Clin. Invest. 1996;97:1010–1019. doi: 10.1172/JCI118492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whibley A, Urquhart J, Dore J, Willatt L, Parkin G, Gaunt L, Black G, Donnai D, Raymond FL. Deletion of MAOA and MAOB in a male patient causes severe developmental delay, intermittent hypotonia and stereotypical hand movements. Eur. J. Hum. Genet. 2010 doi: 10.1038/ejhg.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 109.Shih JC. Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B. Neurotoxicology. 2004;25:21–30. doi: 10.1016/S0161-813X(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 110.Rebsam A, Seif I, Gaspar P. Dissociating barrel development and lesion-induced plasticity in the mouse somatosensory cortex. J. Neurosci. 2005;25:706–710. doi: 10.1523/JNEUROSCI.4191-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mialet-Perez J, Bianchi P, Kunduzova O, Parini A. New insights on receptor-dependent and monoamine oxidase-dependent effects of serotonin in the heart. J. Neural Transm. 2007;114:823–827. doi: 10.1007/s00702-007-0695-7. [DOI] [PubMed] [Google Scholar]
- 113.Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J. 2005;19:641–643. doi: 10.1096/fj.04-2518fje. [DOI] [PubMed] [Google Scholar]
- 114.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Frances B. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am. J. Physiol Heart Circ. Physiol. 2003;284:H1460–H1467. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- 115.Ojaimi C, Qanud K, Hintze TH, Recchia FA. Altered expression of a limited number of genes contributes to cardiac decompensation during chronic ventricular tachypacing in dogs. Physiol Genomics. 2007;29:76–83. doi: 10.1152/physiolgenomics.00159.2006. [DOI] [PubMed] [Google Scholar]
- 116.Lairez O, Calise D, Bianchi P, Ordener C, Spreux-Varoquaux O, Guilbeau-Frugier C, Escourrou G, Seif I, Roncalli J, Pizzinat N, Galinier M, Parini A, Mialet-Perez J. Genetic deletion of MAO-A promotes serotonin-dependent ventricular hypertrophy by pressure overload. J. Mol. Cell Cardiol. 2009;46:587–595. doi: 10.1016/j.yjmcc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 117.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, Kang PM. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- 118.Dorris RL. A simple method for screening monoamine oxidase (MAO) inhibitory drugs for type preference. J Pharmacol Methods. 1982;7:133–137. doi: 10.1016/0160-5402(82)90025-0. [DOI] [PubMed] [Google Scholar]
- 119.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 120.Shimizu Y, Minatoguchi S, Hashimoto K, Uno Y, Arai M, Wang N, Chen X, Lu C, Takemura G, Shimomura M, Fujiwara T, Fujiwara H. The role of serotonin in ischemic cellular damage and the infarct size-reducing effect of sarpogrelate, a 5-hydroxytryptamine-2 receptor blocker, in rabbit hearts. J. Am. Coll. Cardiol. 2002;40:1347–1355. doi: 10.1016/s0735-1097(02)02158-7. [DOI] [PubMed] [Google Scholar]
- 121.Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim. Biophys. Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ. Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 123.Coatrieux C, Sanson M, Negre-Salvayre A, Parini A, Hannun Y, Itohara S, Salvayre R, Auge N. MAO-A-induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radic. Biol. Med. 2007;43:80–89. doi: 10.1016/j.freeradbiomed.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 124.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ. Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 125.Dandona P, Ghanim H, Brooks DP. Antioxidant activity of carvedilol in cardiovascular disease. J. Hypertens. 2007;25:731–741. doi: 10.1097/HJH.0b013e3280127948. [DOI] [PubMed] [Google Scholar]
- 126.Agabiti RE, Rizzoni D. Metabolic profile of nebivolol, a beta-adrenoceptor antagonist with unique characteristics. Drugs. 2007;67:1097–1107. doi: 10.2165/00003495-200767080-00001. [DOI] [PubMed] [Google Scholar]
- 127.Daiber A, Oelze M, Coldewey M, Kaiser K, Huth C, Schildknecht S, Bachschmid M, Nazirisadeh Y, Ullrich V, Mulsch A, Munzel T, Tsilimingas N. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem. Biophys. Res. Commun. 2005;338:1865–1874. doi: 10.1016/j.bbrc.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 128.Hare JM, Mangal B, Brown J, Fisher C, Jr., Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 2008;51:2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 129.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 130.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 131.Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J. Card Fail. 2006;12:464–472. doi: 10.1016/j.cardfail.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 132.Marin DB, Bierer LM, Lawlor BA, Ryan TM, Jacobson R, Schmeidler J, Mohs RC, Davis KL. L-deprenyl and physostigmine for the treatment of Alzheimer's disease. Psychiatry Res. 1995;58:181–189. doi: 10.1016/0165-1781(95)02714-8. [DOI] [PubMed] [Google Scholar]
- 133.Qin F, Shite J, Mao W, Liang CS. Selegiline attenuates cardiac oxidative stress and apoptosis in heart failure: association with improvement of cardiac function. Eur. J. Pharmacol. 2003;461:149–158. doi: 10.1016/s0014-2999(03)01306-2. [DOI] [PubMed] [Google Scholar]
- 134.Simon L, Szilagyi G, Bori Z, Orbay P, Nagy Z. (-)-D-Deprenyl attenuates apoptosis in experimental brain ischaemia. Eur. J. Pharmacol. 2001;430:235–241. doi: 10.1016/s0014-2999(01)01375-9. [DOI] [PubMed] [Google Scholar]
- 135.Weinreb O, Bar-Am O, Amit T, Chillag-Talmor O, Youdim MB. Neuroprotection via pro-survival protein kinase C isoforms associated with Bcl-2 family members. FASEB J. 2004;18:1471–1473. doi: 10.1096/fj.04-1916fje. [DOI] [PubMed] [Google Scholar]
- 136.White WB, Salzman P, Schwid SR. Transtelephonic home blood pressure to assess the monoamine oxidase-B inhibitor rasagiline in Parkinson disease. Hypertension. 2008;52:587–593. doi: 10.1161/HYPERTENSIONAHA.108.115873. [DOI] [PubMed] [Google Scholar]
- 137.Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondria and vascular pathology. Pharmacol. Rep. 2009;61:123–130. doi: 10.1016/s1734-1140(09)70014-3. [DOI] [PubMed] [Google Scholar]