Abstract
The present study examines the effects of Cd81-null mutation on the development of the retinal pigment epithelium (RPE), specifically cell size and number of cells with multiple nuclei. The outlines of RPE in retinal flat mounts were stained with rhodamine-labeled phalloidin and RPE nuclei with Hoechst stain. The RPE layer was sampled to define the number of cells, the size of the RPE cells and the number of nuclei within the cells. The Cd81-null mutation caused an increase in the number of cells within the RPE layer. The cells were smaller than those in the wild type mice. Furthermore there was an increase in the number of mono-nucleated cells. In the posterior portion of the eye there was a significant increase in the number of multi-nucleated cells. The data indicate that CD81 plays a significant role in the final stages of RPE development, controlling cell number and overall developmental pattern.
Keywords: TAPA, Growth regulation, Retina, Tetraspanin
Introduction
The retinal pigment epithelium (RPE) is a single layer of cells between the outer segments of photoreceptors in neural retina and vascular choroids of the eye. These cells sit on a specialized basement membrane, Bruch's membrane. On the basal surface of the cells a series of specialized endfoldings are involved in the regulation of ion transport. On the lateral surface of the cells zonula occludins junctions help to isolate the internal environment of the retina. The apical surface of the cells consists of a specialized series of microvilli that are exquisitely involved in the phagocytosis of shed outer segments of photoreceptors. The cells of the RPE differentiate early in development as the outer cells of the optic cup undergo extensive proliferation forming a single layer interacting with the development of the choroid at their basal surface and the developing retina at their apical surface [1-3]. After birth the RPE cells continue to proliferate into the second postnatal week [4-6]. Unlike most cells in the body, some of the RPE cells go through the cell cycle but fail to undergo cytokinesis near the end of the proliferative phase. This results in the presence of many binucleated cells in the central portion of the eye [4-6].
We have found that tetraspanin CD81 is highly expressed by rat retina [7] and RPE [8]. CD81 is localized to the microvilli on the apical surface of RPE cells [8] and plays an important role in the phagocytosis of outer segments [9]. CD81 also appears to play a role in the interaction of the RPE with Bruch's membrane and lateral interactions between RPE cells [8]. [These interactions may be associated with the regulation of cell number in mice with a null mutation in Cd81, there is an increase number of RPE nuclei relative to wild-type littermates [10].] In this study we used a Cd81-null mouse to investigate the role of CD81 in the development of the RPE cell layer. CD81 is a member of the tetraspanin family of proteins and this small membrane protein is involved in the regulation of cell growth [11, 12]. In the rodent the highest levels of CD81 are found in the CNS and retina [8, 11]. Antibody directed against CD81 dramatically decreases the proliferation of cultured glial cells [11], including RPE cells [12]. A previous study demonstrated an increased number of RPE cell nuclei in the eyes of mice with Cd81-null mutation [10]. The present study examines the effects of this null mutation on the number and structure of the RPE, defining: the size of the RPE cells, the number of RPE cells, and the number of nuclei within each cell.
Experimental Procedures
Animals
All animal protocols used in this study were approved by the Animal Care and Use Committee of the University of Tennessee, Health Science Center. Adult mice with a Cd81-null mutation [13] were backcrossed (minimum of 8) onto the BALB/cJ and the 129X1/SvJ backgrounds. These backcross progeny are more than 99% homozygous for alleles derived from BALB/cJ and 129X1/SvJ. Cd81-null mice from both inbred backgrounds have difficulty breeding and are propagated as CD81 heterozygotes. Two groups of mice were used in the present study. The first group was mice on the 129/SvJ background and included 6 wild-type mice and 6 Cd81-null mice. In the second group, we used the progeny from F1 crosses between mice with the Cd81-null genotype on the BALB/cJ and 129X1/SvJ background. All of the mice in this latter group were identical, with one allele from the BALB/cJ strain and one from the 129X1/SvJ strain. We studied 4 Cd81-null mice and 4 wild-type (Cd81+/+) mice. All of the mice used in this study were over the age of 60 days. The results from both sets of mice were similar and we have pooled the data. The fact that there were fewer Cd81-null mice than expected indicates that we analyzed a subpopulation of the total number of Cd81-null mice conceived (see Rubinstein et al) [14]. We genotyped mice using polymerase chain reaction (PCR). Genomic DNA isolated from a 1 mm tail clip was used for each PCR reaction. All PCR reagents come from Promega (Taq DNA Polymerase, #163466) and performed in an MJ Research PTC-200 DNA engine. The primers identifying the NEO insert, 5′ GCCTTCTTGAC GAGTTCTTCTGAG-3′ and 5′-CATTGAAGGCATAA GAGGGCTTAC-3′, result in a 950 bp product. The primers identifying the normal Cd81 gene, 5′-CTCAACT GTTGTGGCTCCAAC-3′ and 5′-CCAATGAGGTACA GCTTCCC-3′, result in a 500 bp product. The reactions were run for 30 cycles at 94°C for 30 s, 58°C for 15 s, and 72°C for 1 min. We ran out the PCR products on a 1% agarose gel stained with ethidium bromide and viewed them on an ultraviolet light box. The normal CD81 band runs at approximately 500 bases; the Neo band runs at 950 bases.
Tissue Processing
Mice were anesthetized with a mixture of xylazine (13 mg/kg AnaSed®) and ketamine (87 mg/kg Ketaset®) administered by intraperitoneal injection. The mice were per-fused through the heart with a solution of 0.01 M phosphate-buffered saline (PBS, pH 7.5) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH7.5). The perfusions were performed at the same time (between 1:00 and 2:00 PM) under similar conditions and light levels. The eyes were placed in 4% paraformaldehyde for 24 h, and then the cornea, iris, and lens were removed. The retina from each eye was removed in physiological saline. The actin ring within the RPE cell was stained with rhodamine-labeled phalloidin by placing the eyecup in TRITC-phalloidin (Sigma) at 5 Mg/ml in PBS and was allowed to remain in this solution for approximately 1 h. The nuclei of RPE cells were counterstained with Hoechst stain. The retinas were placed in Hoechst solution (final concentration of 0.12 μg/ml) and incubated for 15 min at room temperature. The tissue was rinsed and flat mounted with the RPE facing the coverslip. The RPE layer was photographed with an Optronics digital camera mounted on an Olympus SZX12 dissecting microscope. The resulting images were analyzed with the NIH Image Program to determine the total area of the retina (not including any attached ciliary body) from each mouse.
Quantification
The light microscopic measurements were made using a Leitz Orthoplan microscope connected to an Optronics digital camera with a final magnification of 250 X. Fields were selected and the location of each field was located on a low magnification picture of each retina. We attempted to select fields along a radius extending from the most posterior aspect of the eye to the periphery, such that the eye was divided into three concentric zones (the posterior 1/3, the middle 1/3 and the peripheral 1/3). The retina was photographed and the actin ring and the nuclei were merged. The double-labeled photomicrographs were then transferred to disk and MetaMorph version 6.2r2 (Molecular Devices, West Chester, PA) was used to measure the area of each cell, count the number of cells and log the number of nuclei within each cell. All the measurements were made blind to the genotype (Cd81-null or wild type) of the mouse. The genotype of each sample was revealed after the analysis. We entered all data into Microsoft Excel, and analyzed the differences in measurements using means and standard error of the mean (SE). The data was then analyzed using the unpaired student t-test, and the significance level was set at P < 0.05.
Results
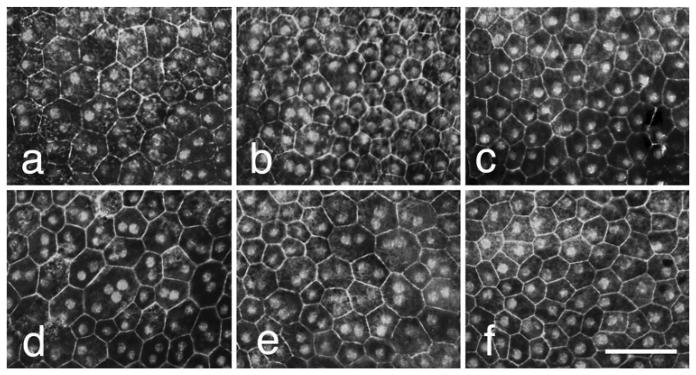
In a previous study of the effects of the Cd81-null mutation on the RPE in the mouse [10], we found that there was an 18% increase in the number of nuclei in the RPE layer. These data were counts of nuclei in transverse sections through the retina and as such, we were unable to define cells containing more than one nucleus. In most tissues the number of cells and the number of nuclei are equivalent; however, this is not the case for RPE cells in the mouse retina. Many of the RPE in the mouse retina are multi-nucleated [4-6]. The most direct approach to define the effects of the Cd81-null mutation on the number of RPE cells and nuclei is to examine stained whole mounts of the retina. By staining the actin rings of the RPE with rhoda-mine-labeled phalloidin and the nuclei with the Hoechst stain, we were able to define the individual RPE cells and the nuclei within them (Fig. 1).
Fig. 1.
The pattern of labeling for the actin ring (labeled with Phalloidin) and nuclei within the cells (labeled with the Hoechst stain) is shown in 3 photomicrographs from wild-type mice (a–c) and from Cd81-null mice (d–f). The micrographs were taken from the posterior portion of the eye (a and d) the mid portion of the eye (b and e) and the peripheral region of the eye (c and f). Notice that in both mice the peripheral cells are mono-nucleated (c and f). In the middle portion of the retinas more bi-nucleated cells are present (b and e). In the posterior portion of the globe many bi-nuclear cells are observed and in the Cd81-null eye a cell is present that contains 4 nuclei. This type of cell was only observed in the Cd81−/− eye. The scale bar in F represents 50 μm
When examining the retinas from the wild-type mice at low magnification, the most obvious feature of the RPE cell layer is the relatively low density of RPE cells in the center of the retina and the increase in density of RPE cells in the periphery of the retina. In contrast to cell density, the density of RPE nuclei appeared to be approximately the same across the entire RPE layer. The difference in cell and nuclei density was previously described in the mouse by Bodenstein and Sidman [4-6]. The cells in the posterior pole are multi-nucleated and the cells in the periphery being mono-nucleated. When examining the eyes of the Cd81-null at low magnification, the general appearance was similar to that of the wild-type eye. A closer examination of the retinas revealed an apparent increase in the number of nuclei within the RPE cell layer of the Cd81−/− eyes (Fig. 1). There also appeared to be an increase in number of bi-nucleated in the Cd81-null eyes. In the Cd81-null samples we observed cells with four nuclei (Fig. 1d). The cells with four nuclei were only observed in the posterior pole of the eye and only in Cd81−/− eyes and were not seen in the wild-type mice.
To quantify the change in the RPE cells observed in the Cd81-null eye, three regions along an axis from the optic nerve head were selected and photographed (135 μm × 170 μm). The locations of the fields were identified on a photomicrograph of the entire retina. The linear distance from the optic nerve head was measured. Each photographed field was then identified as being within the posterior 1/3 of the globe, middle 1/3 of the globe or the periphery of the globe. Each field was analyzed to define: the number of cells; the number of nuclei; the area of each cell; the number of mono-nucleated cells; and the number of bi-nucleated cells. The results of this analysis are summarized in Table 1. There were a number of significant changes in the RPE layer within the Cd81-null mouse relative to the wild-type littermates. The density of cells across the retina increased by 16.8 % in the Cd81-null mice (P < 0.05) and there was a 15% increase in the density of nuclei (P < 0.05). This resulted in a 10% overall decrease in the average area of the cells within the Cd81-null RPE cell layer (P < 0.05). There were also significant increases in the density of mono-nucleated RPE cells. Thus, across the entire RPE layer there was an increase number of nuclei and cells with a decrease in the average size of the cells.
Table 1.
The mean and standard errors of the mean are shown for the entire eye, the posterior 1/3, the middle 1/3 and the peripheral 1/3
| Cell density (cells/mm2) |
Nuclei density (nuclei/mm2) |
Cell area (μm2) |
Mononucleated (cells/mm2) |
Binucleated (cells/mm2) |
|
|---|---|---|---|---|---|
| Cd81−/− eye | 2899 ± 93.8 | 3512 ± 86.2 | 372.5 ± 10.62 | 2297 ± 121.6 | 597 ± 47.4 |
| WT eye | 2481 ± 51.4* | 3053 ± 47.2* | 415.1 ± 8.07* | 1916 ± 73.7* | 561 ± 34.1 |
| Cd81−/− posterior | 2424 ± 136.8 | 3402 ± 119.0 | 443.3 ± 19.15 | 1463 ± 174.8 | 953 ± 59.4 |
| WT posterior | 2262 ± 56.7 | 3072 ± 59.0* | 454.9 ± 12.56 | 1465 ± 81.3 | 787 ± 42.1* |
| Cd81−/− middle | 3151 ± 190.0 | 3816 ± 183.3 | 337.3 ± 15.40 | 2499 ± 219.9 | 645 ± 68.6 |
| WT middle | 2431 ± 71.2* | 3036 ± 77.5* | 412.1 ± 11.63* | 1829 ± 94.2* | 599 ± 48.4 |
| Cd81−/− peripheral | 3189 ± 114.0 | 3309 ± 127.5 | 326.6 ± 11.09 | 3070 ± 112.7 | 119 ± 38.6 |
| WT peripheral | 2858 ± 124.7 | 3049 ± 118.5 | 364.4 ± 15.03* | 2666 ± 145.8* | 191 ± 45.6 |
For each of these regions we quantified the: Cell Density, Nuclear Density, Average Cell Area, the number of Mononucleated cells, and the number of Binucleated cells. The asterisk represents measures that were statistically different at the 0.05 level using the student t test
When we examine the cells within the different regions of the retina, it is clear that the effects of Cd81-null mutation were not constant across the entire surface of the RPE layer. Different regions of the eye were more severely affected by this mutation. The intermediate portion of the retina (the middle 1/3) was the most severely affected. There was a significant increase in the number of cells and the number of nuclei, along with the significant decrease in cell size (Table 1 and Fig. 2). The peripheral 1/3 of the retina and posterior regions (central 1/3) were not as affected by the null mutation (see Table 1 and Fig. 2). In the posterior region only the nuclear density and number of bi-nucleated cells increased. This can be explained simply by a relative increase in the number of bi-nucleated RPE cells. In the peripheral portion of the RPE layer there was a decrease in cell area of the cells and an increase in the number of mono-nucleated cells, which represents a generalized increase in overall cell number. These data suggest that within the layer of RPE cells, the cells that remain in cell cycle later in development are more affected by the Cd81-null mutation and this may relate to the developmental maturation of the RPE layer.
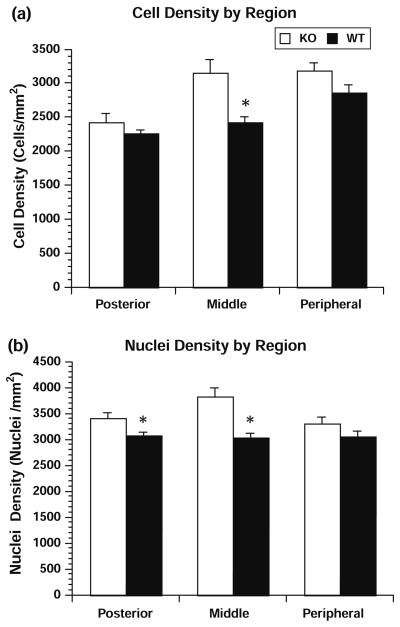
Fig. 2.
Two measures are shown in bar histograms with the means being represented by the bar and the standard error of the mean by the error bars. When looking at the cell density (a) there was no difference in the posterior or peripheral portion of the eye comparing the Cd81-null globes (KO) relative to the wild-type globes (WT). There was a significant increase at the P < 0.05 level for the cells in the middle portion of the globe. When we examined nuclear density (b), there was a significant increase in the density of the nuclei within the posterior and middle portion of the eye in the Cd81-null globes relative to the wild-type globes. If all of the data is combined there is a significant increase in total density of cells and total nuclear density in the Cd81-null globes relative to the wild-type globes (see Table 1)
Discussion
The data from the current analysis demonstrates an increase in the number of RPE cells and nuclei in the eyes of the Cd81−/− relative to the wild-type eyes. Song et al found that Cd81-null mice had an average of 43,400 ± 890 RPE nuclei (mean ± SE) per retina, whereas their wild-type littermates had an average of 36,900 ± 650 RPE nuclei per retina [10]. This represented an 18% increase RPE nuclei in the retinas of the Cd81-null mice, a difference that attained significance (P < 0.005). We have extended these observations by demonstrating an overall increase in the number of cells within the Cd81-null eye. Furthermore, we have shown that different regions of the RPE cell layer are differentially affected by the null mutation. The most peripheral portion of the RPE cell layer is the least affected by the Cd81-null mutation; while, the most dramatic changes occurred in the middle 1/3 of the RPE cell layer. In an extensive study of RPE in the mouse, Bodenstein and Sidman (1987) found that there were two phases of growth within the RPE layer [4-6]. The early pattern was characterized by a continuing shift of the mitotic activity from the center of the retina outward forming an edge-biased pattern of growth. After P5 this peripheral edge-biased pattern ceases and a second late pattern of growth was observed with scattered mitotic activity over the central 2/3 of the retina [4-6]. Based on the changes observed in the Cd81-null mice the mutation is affecting both the early peripheral phase and the later maturations phase, for there is an increase in the number of mono-nucleated cells in the periphery and an increase in multinucleated cells in the middle and posterior RPE cell layer. These effects of the Cd81-null mutation are consistent with other reported phenomenon associated with CD81. Originally, CD81 was termed a target of an anti-proliferative antibody, TAPA-1 [15]. As that name implies, antibodies directed against an extracellular epitope on CD81 block cell-cycle progression in cultured cells [11, 12, 15, 16]. Further proof of the role of CD81 in the regulation of cell growth comes from studies demonstrating that the brains of Cd81-null mice are larger than those of wild-type mice, and contain significantly more astrocytes and microglial cells, which are known to express CD81 [11, 12, 17]. In this study, there was a significant increase in RPE cells in Cd81-null mice compared to their wild-type littermates. The present study demonstrates that CD81 is important in regulating CD81 cell cycle within the RPE, resulting in an increase in the number of nuclei, mono-nucleated cells and bi-nucleated in the RPE cell layer of the Cd81-null mouse relative to the wild-type mouse.
Acknowledgments
The authors wish to thank Grace Geisert, O.D. for the mice used in this study. This study was supported by PHS grants R01EY017841 (EEG) and 3P30 EY13080 (Dianna Johnson, PI), funds provided by unrestricted grant from Research to Prevent Blindness and funds provided by the Community Foundation of Greater Memphis.
References
- 1.Zhao S, Overbeek PA. Regulation of choroid development by the retinal pigment epithelium. Mol Vis. 2001;7:277–282. [PubMed] [Google Scholar]
- 2.Coulombre JL, Coulombre AJ. Influence of mouse neural retina on regeneration of chick neural retina from chick embryonic pigmented epithelium. Nature. 1970;228:559–560. doi: 10.1038/228559a0. [DOI] [PubMed] [Google Scholar]
- 3.Rapaport DH, Rakic P, Yasamura D, et al. Genesis of the retinal pigment epithelium in the macaque monkey. J Comp Neurol. 1995;363:359–376. doi: 10.1002/cne.903630303. [DOI] [PubMed] [Google Scholar]
- 4.Bodenstein L, Sidman RL. Growth and development of the mouse retinal pigment epithelium. I. Cell and tissue morpho-metrics and topography of mitotic activity. Dev Biol. 1987;121:192–204. doi: 10.1016/0012-1606(87)90152-7. [DOI] [PubMed] [Google Scholar]
- 5.Bodenstein L, Sidman RL. Growth and development of the mouse retinal pigment epithelium. II. Cell patterning in experimental chimaeras and mosaics. Dev Biol. 1987;121:205–219. doi: 10.1016/0012-1606(87)90153-9. [DOI] [PubMed] [Google Scholar]
- 6.Bodenstein L, Sidman RL. Cell patterning in vertebrate development: models and model systems. Curr Top Dev Biol. 1987;21:1–29. doi: 10.1016/s0070-2153(08)60131-3. [DOI] [PubMed] [Google Scholar]
- 7.Clarke K, Geisert EE., Jr The target of the antiproliferative antibody (TAPA) in the normal and injured rat retina. Mol Vis. 1998;4:3. [PubMed] [Google Scholar]
- 8.Geisert EE, Jr, Abel HJ, Fan L, et al. Retinal pigment epithelium of the rat express CD81, the target of the anti-proliferative antibody (TAPA) Invest Ophthalmol Vis Sci. 2002;43:274–280. [PubMed] [Google Scholar]
- 9.Chang Y, Finnemann SC. Tetraspanin CD81 is required for the alpha v beta5-integrin-dependent particle-binding step of RPE phagocytosis. J Cell Sci. 2007;120:3053–3063. doi: 10.1242/jcs.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song BK, Levy S, Geisert EE., Jr Increased density of retinal pigment epithelium in cd81−/− mice. J Cell Biochem. 2004;92:1160–1170. doi: 10.1002/jcb.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisert EE, Jr, Yang L, Irwin MH. Astrocyte growth, reactivity, and the target of the antiproliferative antibody, TAPA. J Neurosci. 1996;16:5478–5487. doi: 10.1523/JNEUROSCI.16-17-05478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisert EE, Jr, Williams RW, Geisert GR, et al. Increased brain size and glial cell number in CD81-null mice. J Comp Neurol. 2002;453:22–32. doi: 10.1002/cne.10364. [DOI] [PubMed] [Google Scholar]
- 13.Maecker HT, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med. 1997;185:1505–1510. doi: 10.1084/jem.185.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubinstein E, Ziyyat A, Prenant M, et al. Reduced fertility of female mice lacking CD81. Dev Biol. 2006;290:351–358. doi: 10.1016/j.ydbio.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Oren R, Takahashi S, Doss C, et al. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelic S, Levy S, Suarez C, et al. CD81 regulates neuron-induced astrocyte cell-cycle exit. Mol Cell Neurosci. 2001;17:551–560. doi: 10.1006/mcne.2000.0955. [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra S, Geisert EE, Jr, Dijkstra CD, et al. CD81 and microglial activation in vitro: proliferation, phagocytosis and nitric oxide production. J Neuroimmunol. 2001;114:151–159. doi: 10.1016/s0165-5728(01)00240-5. [DOI] [PubMed] [Google Scholar]