Abstract
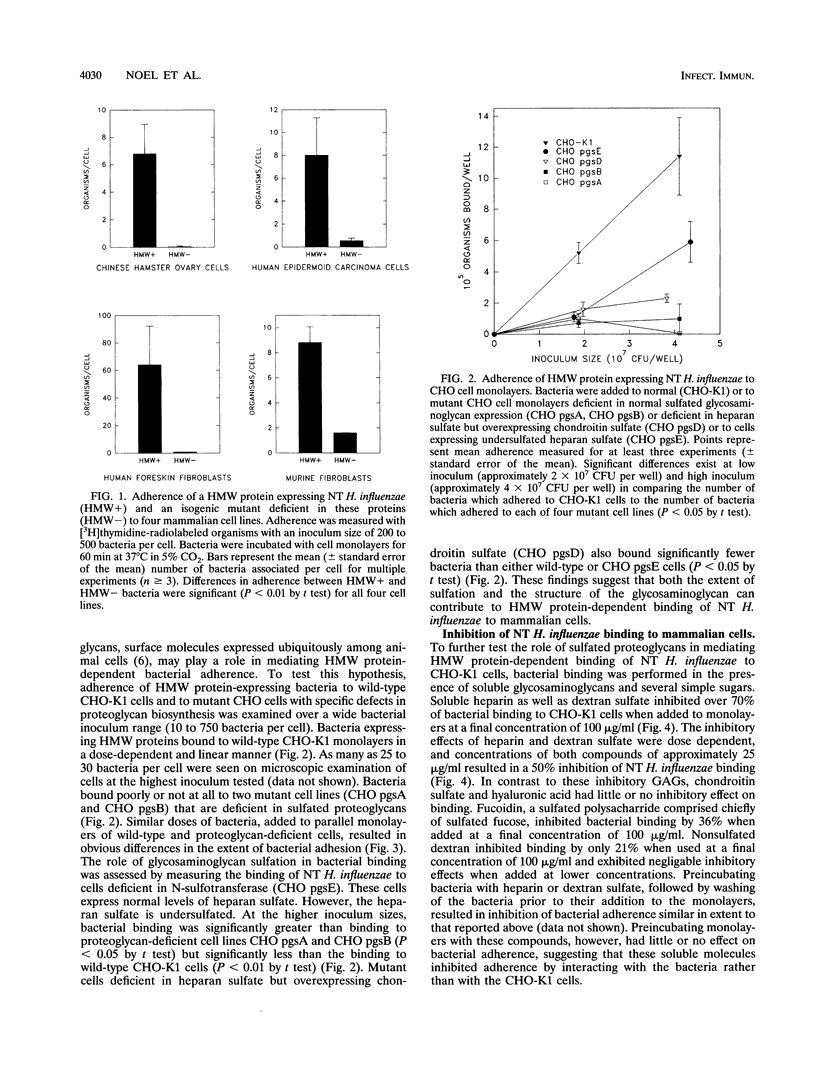
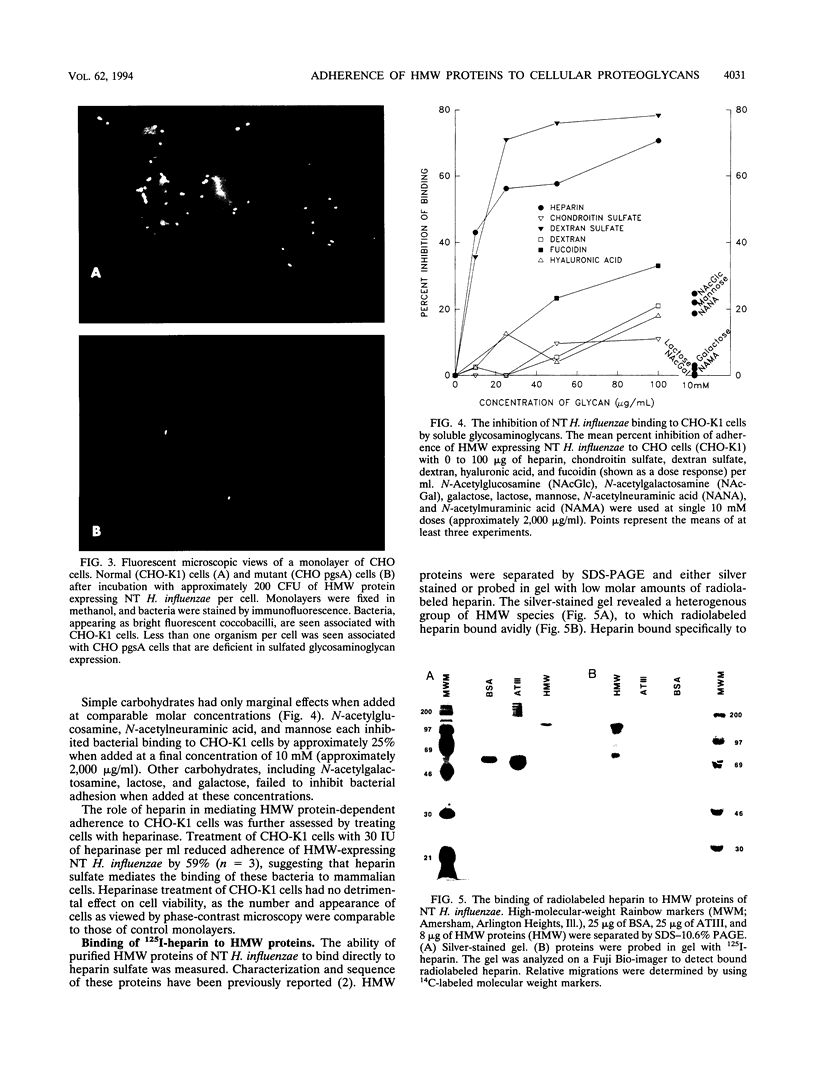
A family of high-molecular-weight (HMW) surface-exposed proteins of nontypeable Haemophilus influenzae (NT H. influenzae) mediated adherence of these organisms to human epithelium. To better understand the molecular basis for this adherence, the role of glycosaminoglycans (GAGs), substances commonly expressed on cell surfaces, was examined. Bacterial adherence to cells with specific deficiencies in GAG biosynthesis was measured. HMW protein-dependent bacterial adherence to normal cells was significantly greater than adherence to cells deficient in sulfated GAGs or to cells deficient in heparan sulfate but overexpressing chondroitin sulfate. Cells expressing undersulfated heparan sulfate exhibited intermediate levels of bacterial adherence. The addition of exogenous dextran sulfate or heparin inhibited over 70% of the adherence of NT H. influenzae to normal cells, whereas hyaluronic acid and chondroitin sulfate tested at the same concentration (100 micrograms/ml) inhibited bacterial adherence by less than 11%. Treatment of cells with heparinase significantly reduced bacterial adherence. Following electrophoretic separation, HMW proteins were shown to bind directly to radiolabeled heparin. These results indicate that HMW protein-dependent adherence of NT H. influenzae is mediated by cellular sulfated GAGs and that heparan sulfate may be the predominant GAG involved in this process. However, the decreased adherence of bacteria to cells expressing undersulfated heparan sulfate and the inhibition of bacterial adherence by the addition of exogenous dextran sulfate suggest that bacterial adhesion to mammalian cells is likely to be influenced by a variety of factors, including the degree of sulfation and the specificity of the carbohydrate moieties contained in the cellular proteoglycans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Bodor F. F. Development of serum bactericidal activity following nontypable Haemophilus influenzae acute otitis media. Pediatr Infect Dis J. 1990 May;9(5):333–339. doi: 10.1097/00006454-199005000-00006. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992 Apr;60(4):1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey E. J., Stinson M. W. Heparin-inhibitable basement membrane-binding protein of Streptococcus pyogenes. Infect Immun. 1988 Jul;56(7):1715–1721. doi: 10.1128/iai.56.7.1715-1721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin A. D., Witt K. R., Jackson R. L. Visualization of heparin-binding proteins by ligand blotting with 125I-heparin. Anal Biochem. 1984 Mar;137(2):368–373. doi: 10.1016/0003-2697(84)90099-x. [DOI] [PubMed] [Google Scholar]
- Esko J. D. Genetic analysis of proteoglycan structure, function and metabolism. Curr Opin Cell Biol. 1991 Oct;3(5):805–816. doi: 10.1016/0955-0674(91)90054-3. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Rostand K. S., Weinke J. L. Tumor formation dependent on proteoglycan biosynthesis. Science. 1988 Aug 26;241(4869):1092–1096. doi: 10.1126/science.3137658. [DOI] [PubMed] [Google Scholar]
- Farley M. M., Stephens D. S., Kaplan S. L., Mason E. O., Jr Pilus- and non-pilus-mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990 Feb;161(2):274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- Frevert U., Sinnis P., Cerami C., Shreffler W., Takacs B., Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993 May 1;177(5):1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., Ferrieri P. Adherence of Haemophilus influenzae to human epithelial cells. Scand J Infect Dis. 1984;16(3):271–278. doi: 10.3109/00365548409070400. [DOI] [PubMed] [Google Scholar]
- Lampe R. M., Mason E. O., Jr, Kaplan S. L., Umstead C. L., Yow M. D., Feigin R. D. Adherence of Haemophilus influenzae to buccal epithelial cells. Infect Immun. 1982 Jan;35(1):166–172. doi: 10.1128/iai.35.1.166-172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron R. G., Hök A., Esko J. D., Gay S., Hök M. Binding of heparan sulfate to type V collagen. A mechanism of cell-substrate adhesion. J Biol Chem. 1989 May 15;264(14):7950–7956. [PubMed] [Google Scholar]
- Love D. C., Esko J. D., Mosser D. M. A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. J Cell Biol. 1993 Nov;123(3):759–766. doi: 10.1083/jcb.123.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlman T., Burgess W. H. Detection and characterization of heparin-binding proteins with a gel overlay procedure. Anal Biochem. 1990 Jul;188(1):159–163. doi: 10.1016/0003-2697(90)90545-k. [DOI] [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991 Feb;62(1):59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- Neyts J., Snoeck R., Schols D., Balzarini J., Esko J. D., Van Schepdael A., De Clercq E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992 Jul;189(1):48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- Noel G. J., Barenkamp S. J., St Geme J. W., 3rd, Haining W. N., Mosser D. M. High-molecular-weight surface-exposed proteins of Haemophilus influenzae mediate binding to macrophages. J Infect Dis. 1994 Feb;169(2):425–429. doi: 10.1093/infdis/169.2.425. [DOI] [PubMed] [Google Scholar]
- Ortega-Barria E., Pereira M. E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991 Oct 18;67(2):411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- Pancake S. J., Holt G. D., Mellouk S., Hoffman S. L. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J Cell Biol. 1992 Jun;117(6):1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C. C., Patrick G. S., Kaplan S. L., Barrish J., Mason E. O., Jr Adherence kinetics of Haemophilus influenzae type b to eucaryotic cells. Pediatr Res. 1989 Nov;26(5):500–503. doi: 10.1203/00006450-198911000-00028. [DOI] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Falkow S., Barenkamp S. J. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham S. M., van Alphen L., Mooi F. R. Fimbria-mediated adherence and hemagglutination of Haemophilus influenzae. J Infect Dis. 1992 Jun;165 (Suppl 1):S97–S99. doi: 10.1093/infdis/165-supplement_1-s97. [DOI] [PubMed] [Google Scholar]