Abstract
The retinoblastoma-susceptibility gene product (pRB) is a classical tumor suppressor. pRB regulates a number of cellular processes including proliferation, differentiation, and apoptosis. One of the essential mechanisms by which pRB, and the related p107 and p130 family members, act is through its interactions with the E2F class of transcription factors. E2F-1 transcription is necessary for entry into S-phase during the cell-cycle. pRB binds E2F-1 and represses transcription via recruitment of a histone deacetylase complex and by preventing co-activator complexes from binding E2F-1. Current dogma suggests that phosphorylation of pRB during mid- to late-G1 leads to release of E2F-1 and E2F-1 dependent transcriptional activation of essential S-phase genes. Here we show that pRB, and the related p107 protein, are modified by O-linked β-N-acetylglucosamine (O-GlcNAc) in an in vitro transcription/translation system. Furthermore, we show in vivo that pRB is more heavily glycosylated in G1 of the cell-cycle when pRB is known to be in an active, hypophosphorylated state. Finally, we demonstrate that E2F-1 associated pRB is modified by O-GlcNAc. These studies suggest that regulation of pRB function(s) may be controlled by dynamic O-GlcNAc modification, as well as phosphorylation.
Keywords: O-GlcNAc, pRB, Cell cycle, E2F
Introduction
Knudson proposed the existence of the retinoblastoma-susceptibility gene in 1971 (Knudson 1971). In the past 15 years, this tumor suppressor and related family members, p107 and p130, have been cloned and characterized [reviewed in (Chau and Wang 2003; Paggi et al. 1996; Stevaux and Dyson 2002; Zhu et al. 1994)]. All members of the retinoblastoma-susceptibility gene product (pRB) family have a characteristic “pocket domain” that is the site of many protein–protein interactions (Morris and Dyson 2001). While a variety of cellular processes appear to be regulated by the pRB family, a key mechanism of pRB action appears to be transcription factor binding and repression (Helin 1998). Consistent with the retinoblastoma-susceptibility gene product (pRB) being a tumor suppressor, inactivation of pRB is essential for progression through the G1/S transition of the cell-cycle and, thus, proliferation (Ewen 1994). In fact, many viral oncoproteins bind to pRB and sequester it from its endogenous binding partners (Helin 1998). One of the most well-characterized interacting proteins for pRB is E2F-1, a transcription factor for many essential S-phase genes (Helin 1998). In G1 of the cell-cycle pRB is bound to E2F-1 and this interaction prevents co-activator binding (Stevaux and Dyson 2002). Also, pRB recruits histone deacetylase chromatin remodeling complexes, including the mSin3-histone deacetylase complex (Lai et al. 2001), to further repress transcription. pRB becomes hyperphosphorylated through a cascade of cyclin-dependent kinase phosphorylation events in late G1, and E2F-1 is released to promote transcription of essential S-phase genes (Bartek et al. 1997). Thus, a key paradigm in cell-cycle progression and proliferation is that hypophosphorylated pRB is the “active” transcriptional repressor of E2F-1.
Post-translational modification on serine and threonine residues of nuclear and cytosolic proteins with O-linked β-N-acetylglucosamine (O-GlcNAc) is dynamic and inducible (reviewed in (Hart et al. 2007; Wells et al. 2001). In several cases, including the transcription factors c-myc and the estrogen receptor, there appears to be a reciprocal relationship between O-GlcNAc and serine/threonine phosphorylation (Cheng and Hart 2001; Chou et al. 1995; Comer and Hart 2000). Many transcription factors as well as the basal transcriptional machinery are modified by O-GlcNAc (Comer and Hart 1999). O-GlcNAc modification has been shown to modulate transcriptional activity in several cases [reviewed in (Comer and Hart 1999)]. O-GlcNAc addition and removal is a dynamic enzyme-catalyzed event similar to phosphorylation (Iyer and Hart 2003). The O-GlcNAc transferase (OGT), which adds the modification, associates with specific regions of the cell. For example, OGT is recruited to promoters by a mSin3A-histone deacetylase co-repressor complex and necessary for maximal gene silencing (Yang et al. 2002). At M phase, OGT is found at the mitotic spindle and the midbody (Slawson et al. 2005), and increased OGT expression alters aurora Kinase B and cyclin-dependent kinase 1 signaling in these areas (Slawson et al. 2008; Wang et al. 2010b). These data suggest that a subset of OGT is localized to discrete areas of the cell at different points of the cell cycle, and potentially O-GlcNAc modifying substrates involved in transcription and signaling in a spatial and temporal manner.
Since O-GlcNAc has a yin-yang relationship with phosphorylation, is involved in transcriptional repression (Zachara and Hart 2002), and cell cycle control (Slawson et al. 2005), we decided to investigate pRB for O-GlcNAc modification. After determining that pRB and p107 were O-GlcNAc modified in vitro, we examined the in vivo glycosylation state of early G1-isolated pRB, the form of pRB known to be hypophosphorylated and actively binding E2F-1. Here, we show that E2F-1 associated hypophosphorylated pRB is indeed modified by O-GlcNAc.
Experimental procedures
In vitro transcription/translation
The plasmids for pRB (pLitmusRB), p107 (pBSIIKSp107), and the nucleoporin p62 (pGEMc62) transcription/translation have been previously described (2,19). In vitro transcription/translation (ITT) in rabbit reticulocyte lysate with radiolabeled methionine was performed according to the manufacturers protocol (TNT system, Promega) with time of reaction varied between 1 and 2.5 h. Where indicated, the kinase inhibitor staurosporine was added at 60 nM. The generation of each protein was confirmed by SDS–PAGE (see inset of Fig. 1 for example).
Fig. 1.
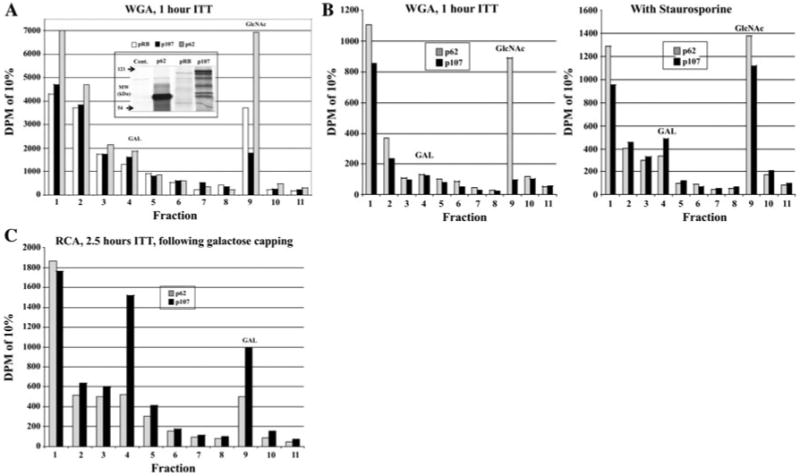
pRB and p107 are O-GlcNAc modified in vitro. pRB, p107, and nucleoporin p62 (positive control for O-GlcNAc modification) were in vitro transcribed/translated (ITT) with radiolabeled methionine. a Following 1 h of ITT, lysates containing pRB, p107, or p62 were separated by WGA chromatography and O-GlcNAc modified proteins specifically eluted with free GlcNAc and counted by liquid scintillation. Inset is an autoradiogram of 5% of ITT reaction following SDS–PAGE (Cont. is no plasmid DNA added). pRb full-length product is marked with a gray arrow while p107 and p62 are marked in black. b Following 2.5 h of ITT in the presence or absence of the broad-based kinase inhibitor staurosporine (60nM final concentration), WGA chromatography and liquid scintillation counting were performed on p107 and p62. c Following 2.5 h of ITT, p107- or p62-containing lysates had a galactose added to terminal GlcNAc residues with β-1,4-galactosyltransferase. Extracts were then passed over the galactose binding lectin RCA and modified proteins eluted with free galactose and counted by liquid scintillation
Wheat germ agglutinin chromatography
In vitro transcription/translation reactions were subjected to wheat germ agglutinin (WGA) chromatography as previously described (Roquemore et al. 1994). Radioactivity in 10% of resulting fractions was quantified by a liquid scintillation counter. The first two fractions are omitted from the graphs for scale since the counts are extremely high due to the presence of free radiolabeled methionine. A representative experiment is shown in each case (all experiments were performed in at least triplicate).
Galactosyltransferase labeling
Proteins had their terminal GlcNAc residues capped with unlabeled or radiolabeled galactose by β-1,4-galactosyltransferase as previously described (Roquemore et al. 1994). Proteins were then subjected to RCA chromatography as previously described (Roquemore et al. 1994) or separated by SDS–PAGE and visualized by autoradiography.
Ricinus communis agglutinin chromatography
Fractions previously subjected to galactosyltransferase labeling were separated by ricinus communis agglutinin (RCA) chromatography as previously described (Roquemore et al. 1994). Radioactivity in 10% of resulting fractions was quantified by a liquid scintillation counter.
Cell culture and extracts
HeLa S3 cells and 3T3-L1 preadipocytes were grown in 10% FBS/DMEM and 10% Calf Serum/DMEM, respectively. HeLa S3 cells were arrested in early G1 with 66 μM lovastatin for 27 h (Reed et al. 1994). Two days postconfluent 3T3-L1 preadipocytes were allowed to reenter the cell cycle synchronously into mitotic clonal expansion using a differentiation cocktail previously described (Student et al. 1980) and harvested at reported times. Extracts of cells were made in 1% NP-40 in TBS with protease inhibitors and 10 M PUGNAc [to prevent O-GlcNAc removal, (Haltiwanger et al. 1998)].
Immunopurification, western blotting, and lectin blotting
Extracts were pre-cleared for 30 min at 4°C with normal rabbit and mouse IgG (Santa Cruz) and Protein A/G conjugated agarose. Commercially available agarose-conjugated antibodies to pRB (Santa Cruz) and E2F-1 (Santa Cruz) with Protein A/G agarose were added to extracts overnight with gentle rotation at 4°C. For co-purification purposes, pelleted proteins were washed four times with 1% NP-40, 0.25% deoxylcholate in TBS and then boiled in Laemmli buffer. For stringent washing, 0.1% SDS was added to the wash buffer. Immunopurified proteins were separated by SDS–PAGE, transferred to PVDF, and Western blotted with antibodies or lectin blotted by succinylated WGA (sWGA) coupled to horseradish peroxidase [as described, (Roquemore et al. 1994)] and visualized by enhanced chemiluminescents (ECL).
Results
In vitro transcribed/translated pRB and p107 are O-GlcNAc modified
As a first step towards investigating the post-translational status of pRB and p107, radiolabeled proteins were generated by in vitro transcription/translation (ITT) in rabbit reticulocyte lysate for 1 h. The known O-GlcNAc modified protein nucleoporin p62 (p62) was generated as a positive control(Lubas et al. 1995). All three proteins were bound and were specifically eluted with free N-acetylglucosamine (GlcNAc) from the terminal GlcNAc binding lectin WGA-agarose conjugate (Fig. 1a). If ITT was allowed to continue for 2.5 h, very little p107, compared to p62, bound the WGA-agarose column (there was no appreciable effect on pRB; data not shown). However, inhibition of kinases, using the broad-based kinase inhibitor staurosporine (Juan et al. 1998) during the ITT significantly increased the amount of O-GlcNAc modified p107 (Fig. 1b). p62 binding was only slightly influenced by the presence of staurosporine, suggesting that a reciprocal relationship between glycosylation and staurosporine-sensitive phosphorylation was occurring for p107, but not for p62. To further confirm that p107 was modified by O-GlcNAc, ITT reactions of p107 and p62 were subjected to galactosyltransferase capping of terminal GlcNAc residues with unlabeled galactose, separated via the terminal galactose binding lectin RCA-agarose, and specifically eluted with galactose (Fig. 1c). Thus both pRB and p107 are O-GlcNAc modified in vitro and there appears to be a yin-yang relationship between O-GlcNAc modification and staurosporine-sensitive phosphorylation of p107.
pRB isolated from HeLa S3 cells is modified with O-GlcNAc
Having established that pRB was O-GlcNAc modified in vitro, we next wanted to establish whether pRB was modified in vivo. pRB immunopurified from pre-cleared lysates of asynchronous HeLa S3 cells was separated via SDS–PAGE. Following transfer, gels were either Western blotted with an antibody to pRB or lectin blotted with succinylated WGA (sWGA) coupled to horseradish peroxidase to detect the O-GlcNAc modification (Fig. 2). Ovalbumin was used as a positive control, and sWGA binding to ovalbumin, but not to pRB, was lost upon PNGaseF treatment indicating that pRB is not N-linked glycosylated (data not shown).
Fig. 2.
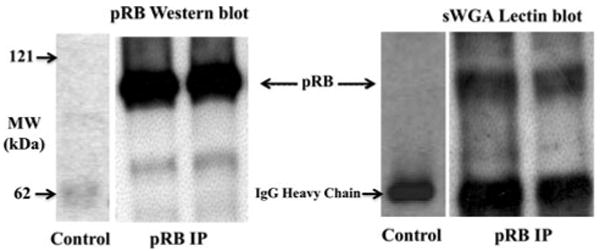
pRB is O-GlcNAc modified in vivo. In duplicate, pRB from pre-cleared lysates of HeLa S3 cells was immunopurified and separated by SDS–PAGE. Following transfer, pRB was detected by Western blotting and the presence of the O-GlcNAc modification by lectin blotting with O-GlcNAc specific sWGA coupled to HRP
pRB is more heavily glycosylated in G1 of the cell cycle
We then wanted to investigate whether a reciprocal relationship between phosphorylation and O-GlcNAc modification existed for pRB. Since it is well established that pRB is in a hypophosphorylated state in G1 of the cell-cycle (8), we compared the O-GlcNAc modification of pRB in G1 arrested versus asynchronous growing cells. Logarithmically growing HeLa cells were allowed to continue growing asynchronously or were arrested in G1 of the cell-cycle by treatment with lovastatin, a HMGCoA reductase inhibitor (Reed et al. 1994; Sinensky et al. 1990). pRB was immunopurified with a mouse monoclonal antibody coupled to agarose, and non-specific mouse IgG with protein A/G agarose was used as a negative control. Following stringent washing, the resulting proteins were subjected to galactose capping of terminal GlcNAc residues using galactosyltransferase and radiolabeled UDP-Galactose. Equal amounts of pRB, as judged by Western blotting, from each sample were separated by SDS–PAGE and visualized by autoradiography (Fig. 3). pRB was more heavily O-GlcNAc modified when isolated from G1 arrested cells compared to asynchronous cells.
Fig. 3.
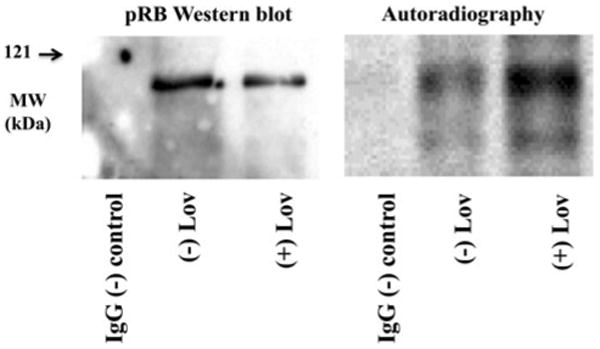
pRB is more heavily O-GlcNAc modified in G1 of the cell-cycle. pRB from pre-cleared lysates of HeLa S3 arrested in G1 with lovastatin or growing asynchronously was immunopurified. Equal amounts of pRB, as well as the antibody alone, were subjected to radioactive galactose capping of the O-GlcNAc modification via β-1,4-galactosyltransferase, separated by SDS–PAGE, and Western blotted with an anti-pRB antibody or visualized by autoradiography
E2F-1 associated pRB is modified by O-GlcNAc
During G1 of the cell cycle hypophosphorylated pRB binds and represses the transcription factor E2F-1 (7). Thus, based on our previous results, we wanted to determine if E2F-1 associated pRB is O-GlcNAc modified. 3T3-L1 preadipocytes were harvested in the early G1 phase of the cell cycle during the first round of mitotic clonal expansion. E2F-1 was immunopurified and proteins separated by SDS–PAGE. Co-purifying pRB was detected by Western blotting, and the presence of O-GlcNAc on E2F-1- associated pRB was detected by sWGA-HRP lectin blotting (Fig. 4). Thus, active, hypophosphorylated, hyperglycosylated pRB is bound to E2F-1 during early G1 of the cell cycle.
Fig. 4.
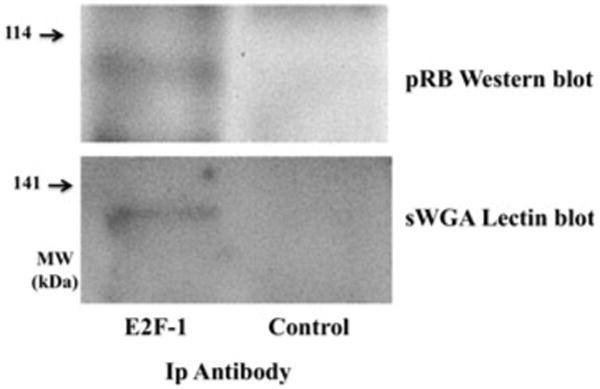
E2F-1 associated pRB is O-GlcNAc modified. E2F-1 was immunopurified from 3T3-L1 preadipocytes with an antibody to E2F-1, as well as normal rabbit IgG as a negative control, in early G1 (4 h) of the cell-cycle, and the resulting proteins separated by SDS–PAGE. Following transfer, the presence of co-purifying pRB was detected by Western blotting and O-GlcNAc modified pRB was detected by lectin blotting with sWGA coupled to HRP
Discussion
We chose to investigate the possibility that the retinoblastoma-susceptibility gene product (pRB) was O-GlcNAc modified for several reasons. First, it had been demonstrated by others that p53, another tumor suppressor, is O-GlcNAc modified (Shaw et al. 1996). Second, O-GlcNAc modification has been shown to be involved in repressing transcription in some systems (Comer and Hart 1999) and pRB represses E2F-1 mediated transcription (Stevaux and Dyson 2002). Third, alterations in O-GlcNAc levels alter cell cycle progression (Slawson et al. 2005). Next, pRB is known to be active when it is hypophosphorylated (Kaelin 1999), and several proteins have been shown to have a reciprocal relationship between their modification with O-GlcNAc and phosphate (Comer and Hart 2000; Wang et al. 2008, 2010b). Thus, we hypothesized that if pRB was modified by O-GlcNAc then it might be O-GlcNAc modified in a cell-cycle dependent manner. Further, we hypothesized that E2F-1 associated pRB might in fact be modified with O-GlcNAc. Finally, we hypothesized that O-GlcNAc might modulate the cleavage of pRB by Caspase-3 (Chau et al. 2002) since there is compelling evidence for O-GlcNAc playing a role in apoptosis [reviewed in (Wells et al. 2003)].
As a first step towards testing our hypotheses, we expressed pRB and the related p107 protein in an in vitro transcription/translation rabbit reticulocyte lysate system. This system contains O-GlcNAc transferase activity and has been used previously to identify O-GlcNAc modified proteins (Roquemore et al. 1994). In the absence of microsomes, the ITT system is incapable of complex glycosylation. Thus, the binding of the translated proteins to WGA-agarose (Fig. 1a) is strong evidence for O-GlcNAc modification. Interestingly, initial experiments were conducted for 2.5 h of ITT, and only a very small fraction of p107 appeared to be O-GlcNAc modified (Fig. 1b in the absence of staurosporine). Several experiments were conducted to address this result. First, ITT incubation times were adjusted showing that 1 h gave optimal O-GlcNAc modification (Fig. 1a and data not shown). Second, ITT was performed in the presence of staurosporine (Fig. 1b), a broad-based kinase inhibitor that blocks pRB phosphorylation (Juan et al. 1998), since O-GlcNAc and phosphorylation can compete for the same serine/threonine residues on certain proteins (Hanover 2001). Inhibition of staurosporine-sensitive kinases enhanced the O-GlcNAc modification of p107 but had little effect on the positive control for glycosylation, nucleoporin p62 (Fig. 1b). This suggests a reciprocal relationship between phosphorylation and glycosylation on p107, but the molecular mechanism of this relationship remains to be elucidated. Finally, since WGA prefers to bind proteins with multiple terminal GlcNAc residues, we galactose-capped the O-GlcNAc post-translational modification on p107 and p62 and performed RCA chromatography to demonstrate the presence of O-GlcNAc on p107 (Fig. 1c).
Having demonstrated that pRB (and p107) is glycosylated in vitro, we sought to determine if pRB was O-GlcNAc modified in vivo. First, we immunopurified pRB from HeLa S3 cells and performed sWGA lectin chromatography to demonstrate that pRB was O-GlcNAc modified (Fig. 2). Furthermore, PNGaseF treatment, which removes complex N-glycosylation, had no effect on WGA binding (data not shown). Interestingly, we have previously developed an O-GlcNAc specific IgM antibody (Comer et al. 2001) but were unable to detect an immunoreactive pRB band with this antibody or with a recently developed O-GlcNAc specific IgG antibody (Teo et al. 2010; data not shown). This suggests to us that there may be some peptide specificity in the developed O-GlcNAc antibodies.
pRB is known to be phosphorylated in a cell-cycle dependent manner (Ewen 1994); thus, we examined whether pRB might be O-GlcNAc modified in a reciprocal manner. We demonstrated that pRB is hyperglycosylated in G1 cell-cycle arrested HeLa S3 cells compared with pRB from asynchronous growing cells as analyzed by radioactive galactose capping of the O-GlcNAc sugar (Fig. 3). Galactose-labeling of pRB also serves as further proof that pRB is indeed O-GlcNAc modified. Since pRB is in a hypophosphorylated state in G1 (Ewen 1994), we conclude that there is a reciprocal cell-cycle dependent relationship between phosphorylation and O-GlcNAc modification of pRB.
Since it is the active, hypophosphorylated form of pRB that binds and represses E2F-1 during G1 of the cell-cycle (Slansky and Farnham 1996), we investigated whether E2F-1 associated pRB is O-GlcNAc modified. In order to synchronize cells at G0 and then release them into the cell-cycle we chose to use the well-established 3T3-L1 preadipocyte cell line (Student et al. 1980; Tang et al. 2003). Upon reaching confluence, the preadipocytes exit the cell cycle but reenter in a synchronous manner upon treatment with a differentiation cocktail of methylisobutylxanthine, dexamethasone, and insulin for two rounds of mitotic clonal expansion before differentiation into adipocytes (Tang et al. 2003). E2F-1 was immunopurified from the preadipocytes 4 h (early G1 of the cell-cycle, (Tang et al. 2003)) following reentry into the cell cycle and the presence of co-purifying O-GlcNAc modified pRB was detected by sWGA lectin chromatography (Fig. 4). Thus, O-GlcNAc modified pRB is co-purifying with E2F-1.
These data set the groundwork for future investigations into the role of O-GlcNAc on pRB and other family members. Determining the sites of modification should allow for mutagenesis studies to determine if the O-GlcNAc modification is necessary for the pRB-E2F-1 association. Further, pRB is clearly involved in multiple cellular processes beyond G1/S cell-cycle control including apoptosis and differentiation (Chau and Wang 2003; De Luca et al. 1996; Herwig and Strauss 1997; Stevaux and Dyson 2002). In an attempt to address the role of O-GlcNAc modification of pRB in apoptosis, we examined the previously documented cleavage of pRB by caspase-3 in vitro (Dou and An 1998). Following ITT, pRB was separated into two pools: that which did not bind WGA (hypoglycosylated) and that which did and was specifically eluted by free GlcNAc (hyperglycosylated). Both pools were compared for the efficiency by which Caspase-3, an executioner protease in apoptosis (Dou and An 1998), could cleave the protein and no differences were found in catalytic efficiency (data not shown, analysis carried out with O-GlcNAcase as positive control as previously described by Butkinaree et al. 2008). This experiment was especially relevant since we have previously demonstrated that O-GlcNAcase, the enzyme that removes O-GlcNAc from nucleocytoplasmic proteins, is an efficient substrate for Caspase-3 (Butkinaree et al. 2008; Wells et al. 2002a) and there is existing indirect evidence for O-GlcNAc playing a role in apoptosis [reviewed in (Wells et al. 2003)]. It remains to be determined if there is an in vivo consequence of O-GlcNAc modification of pRB in regard to apoptosis.
Given that the hypophosphorylated, hyperglycosylated form of pRB occurs when the protein is active in repressing E2F-1 mediated transcription, it seems likely that this enigmatic post-translational modification is modulating the function(s) of pRB. Also, since p107 O-GlcNAc modification and staurosporine-sensitive phosphorylation appear to be reciprocal in vitro, investigation of the role of O-GlcNAc in modulating the entire family of pRB-related proteins appears warranted. Determining the role that O-GlcNAc plays in modulating the activities of pRB and other family members is an area of current and future research and should be facilitated by the site-mapping strategies that have been recently developed by our group and others (Chalkley and Burlingame 2003; Chalkley et al. 2009; Greis et al. 1996; Haynes and Aebersold 2000; Khidekel et al. 2003, 2007; Wang et al. 2010a; Wells et al. 2002b).
Acknowledgments
We would like to thank Liang Zhu for supplying us the pRB and p107 plasmids and Karen Wells and members of the Hart laboratory, past and present, for helpful discussions. This work was supported by a National Research Service Award Fellowship to LW (CA83261) and by National Institute of Health Grants (CA42486 to GWH and DK075069 to LW).
Abbreviations
- pRB
Retinoblastoma-susceptibility gene product
- O-GlcNAc
O-linked β-N-acetylglucosamine
- ITT
In vitro transcription/translation
- ECL
Enhanced chemiluminescents
- WGA
Wheat germ agglutinin
- RCA
Ricinus communis agglutinin
- sWGA
Succinylated WGA
- GlcNAc
N-acetylglucosamine
- HRP
Horseradish peroxidase
- p62
Nucleoporin p62
- Gal
Galactose
Contributor Information
Lance Wells, Complex Carbohydrate Research Center, University of Georgia, 315 Riverbend Road, Athens, GA 30602, USA.
Chad Slawson, Department of Biological Chemistry, Johns Hopkins University School of Medicine, 725 N. Wolfe St., Baltimore, MD 21205, USA.
Gerald W. Hart, Email: gwhart@jhmi.edu, Department of Biological Chemistry, Johns Hopkins University School of Medicine, 725 N. Wolfe St., Baltimore, MD 21205, USA.
References
- Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway in cell cycle control and cancer. Exp Cell Res. 1997;237:1–6. doi: 10.1006/excr.1997.3776. [DOI] [PubMed] [Google Scholar]
- Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW. Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem. 2008;283:23557–23566. doi: 10.1074/jbc.M804116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley RJ, Burlingame AL. Identification of novel sites of O-N-Acetylglucosamine modification of serum response factor using quadrupole time-of-flight mass spectrometry. Mol Cell Proteomics. 2003;2:182–190. doi: 10.1074/mcp.M300027-MCP200. [DOI] [PubMed] [Google Scholar]
- Chalkley RJ, Thalhammer A, Schoepfer R, Burlingame AL. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc Natl Acad Sci USA. 2009;106:8894–8899. doi: 10.1073/pnas.0900288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BN, Wang JY. Coordinated regulation of life and death by RB. Nat Rev Cancer. 2003;3:130–138. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- Chau BN, Borges HL, Chen TT, Masselli A, Hunton IC, Wang JY. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat Cell Biol. 2002;4:757–765. doi: 10.1038/ncb853. [DOI] [PubMed] [Google Scholar]
- Cheng X, Hart GW. Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: post-translational regulation of turnover and transactivation activity. J Biol Chem. 2001;276:10570–10575. doi: 10.1074/jbc.M010411200. [DOI] [PubMed] [Google Scholar]
- Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270:18961–18965. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- Comer FI, Hart GW. O-GlcNAc and the control of gene expression. Biochim Biophys Acta. 1999;1473:161–171. doi: 10.1016/s0304-4165(99)00176-2. [DOI] [PubMed] [Google Scholar]
- Comer FI, Hart GW. O-Glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J Biol Chem. 2000;275:29179–29182. doi: 10.1074/jbc.R000010200. [DOI] [PubMed] [Google Scholar]
- Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem. 2001;293:169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- De Luca A, Esposito V, Baldi A, Giordano A. The retinoblastoma gene family and its role in proliferation, differentiation and development. Histol Histopathol. 1996;11:1029–1034. [PubMed] [Google Scholar]
- Dou QP, An B. RB and apoptotic cell death. Front Biosci. 1998;3:d419–d430. doi: 10.2741/a288. [DOI] [PubMed] [Google Scholar]
- Ewen ME. The cell cycle and the retinoblastoma protein family. Cancer Metastasis Rev. 1994;13:45–66. doi: 10.1007/BF00690418. [DOI] [PubMed] [Google Scholar]
- Greis KD, Hayes BK, Comer FI, Kirk M, Barnes S, Lowary TL, Hart GW. Selective detection and site-analysis of O-GlcNAc-modified glycopeptides by beta-elimination and tandem electrospray mass spectrometry. Anal Biochem. 1996;234:38–49. doi: 10.1006/abio.1996.0047. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- Hanover JA. Glycan-dependent signaling: O-linked N-acetylglucosamine. Faseb J. 2001;15:1865–1876. doi: 10.1096/fj.01-0094rev. [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Haynes PA, Aebersold R. Simultaneous detection and identification of O-GlcNAc-modified glycoproteins using liquid chromatography-tandem mass spectrometry. Anal Chem. 2000;72:5402–5410. doi: 10.1021/ac000512w. [DOI] [PubMed] [Google Scholar]
- Helin K. Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet Dev. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- Herwig S, Strauss M. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur J Biochem. 1997;246:581–601. doi: 10.1111/j.1432-1033.1997.t01-2-00581.x. [DOI] [PubMed] [Google Scholar]
- Iyer SP, Hart GW. Dynamic nuclear and cytoplasmic glycosylation: enzymes of O-GlcNAc cycling. Biochemistry. 2003;42:2493–2499. doi: 10.1021/bi020685a. [DOI] [PubMed] [Google Scholar]
- Juan G, Gruenwald S, Darzynkiewicz Z. Phosphorylation of retinoblastoma susceptibility gene protein assayed in individual lymphocytes during their mitogenic stimulation. Exp Cell Res. 1998;239:104–110. doi: 10.1006/excr.1997.3885. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr Functions of the retinoblastoma protein. Bioessays. 1999;21:950–958. doi: 10.1002/(SICI)1521-1878(199911)21:11<950::AID-BIES7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, Ramakrishnan B, Qasba PK, Hsieh-Wilson LC. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J Am Chem Soc. 2003;125:16162–16163. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, Sun YE, Coon JJ, Peters EC, Hsieh-Wilson LC. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol. 2007;3:339–348. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Kennedy BK, Barbie DA, Bertos NR, Yang XJ, Theberge MC, Tsai SC, Seto E, Zhang Y, Kuzmichev A, et al. RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol Cell Biol. 2001;21:2918–2932. doi: 10.1128/MCB.21.8.2918-2932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubas WA, Smith M, Starr CM, Hanover JA. Analysis of nuclear pore protein p62 glycosylation. Biochemistry. 1995;34:1686–1694. doi: 10.1021/bi00005a025. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Dyson NJ. Retinoblastoma protein partners. Adv Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- Paggi MG, Baldi A, Bonetto F, Giordano A. Retinoblastoma protein family in cell cycle and cancer: a review. J Cell Biochem. 1996;62:418–430. doi: 10.1002/(SICI)1097-4644(199609)62:3%3C418::AID-JCB12%3E3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Reed SI, Bailly E, Dulic V, Hengst L, Resnitzky D, Slingerland J. G1 control in mammalian cells. J Cell Sci Suppl. 1994;18:69–73. doi: 10.1242/jcs.1994.supplement_18.10. [DOI] [PubMed] [Google Scholar]
- Roquemore EP, Chou TY, Hart GW. Detection of O-linked N-acetylglucosamine (O-GlcNAc) on cytoplasmic and nuclear proteins. Methods Enzymol. 1994;230:443–460. doi: 10.1016/0076-6879(94)30028-3. [DOI] [PubMed] [Google Scholar]
- Shaw P, Freeman J, Bovey R, Iggo R. Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene. 1996;12:921–930. [PubMed] [Google Scholar]
- Sinensky M, Beck LA, Leonard S, Evans R. Differential inhibitory effects of lovastatin on protein isoprenylation and sterol synthesis. J Biol Chem. 1990;265:19937–19941. [PubMed] [Google Scholar]
- Slansky JE, Farnham PJ. Introduction to the E2F family: protein structure and gene regulation. Curr Top Microbiol Immunol. 1996;208:1–30. doi: 10.1007/978-3-642-79910-5_1. [DOI] [PubMed] [Google Scholar]
- Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–32956. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell. 2008;19:4130–4140. doi: 10.1091/mbc.E07-11-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevaux O, Dyson NJ. A revised picture of the E2F transcriptional network and RB function. Curr Opin Cell Biol. 2002;14:684–691. doi: 10.1016/s0955-0674(02)00388-5. [DOI] [PubMed] [Google Scholar]
- Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3–L1 preadipocytes. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo CF, Ingale S, Wolfert MA, Elsayed GA, Not LG, Chatham JC, Wells L, Boons GJ. Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat Chem Biol. 2010;6:338–343. doi: 10.1038/nchembio.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci USA. 2008;105:13793–13798. doi: 10.1073/pnas.0806216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Udeshi ND, O'Malley M, Shabanowitz J, Hunt DF, Hart GW. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2010a;9:153–160. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal. 2010b;3:ra2. doi: 10.1126/scisignal.2000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002a;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. Mapping Sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics. 2002b;1:791–804. doi: 10.1074/mcp.m200048-mcp200. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci. 2003;60:222–228. doi: 10.1007/s000180300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. The emerging significance of O-GlcNAc in cellular regulation. Chem Rev. 2002;102:431–438. doi: 10.1021/cr000406u. [DOI] [PubMed] [Google Scholar]
- Zhu L, Enders GH, Wu CL, Starz MA, Moberg KH, Lees JA, Dyson N, Harlow E. Growth suppression by members of the retinoblastoma protein family. Cold Spring Harb Symp Quant Biol. 1994;59:75–84. doi: 10.1101/sqb.1994.059.01.011. [DOI] [PubMed] [Google Scholar]


