Abstract
Background
Delirium is associated with a host of negative outcomes, including increased risk of mortality, longer hospital stay, and poor long-term cognitive function. The pathophysiology of delirium is not well understood. Cancer patients undergoing a bone marrow transplant (BMT) are at high risk for developing delirium and Proton Magnetic Resonance Spectroscopy (1H-MRS) could lead to better understanding of the delirium process.
Methods
Fourteen BMT patients and 10 controls completed 1H-MRS, positioned above the corpus callosum, shortly after delirium onset or at study end if no delirium occurred.
Results
In the BMT-delirium group, statistically significantly elevated tCho/tCr was found in contrast to the BMT-no delirium group (p<0.05). The BMT–delirium group also showed statistically significantly lesser NAA/tCho compared to both controls (p=0.01) and the BMT–no delirium group (p=0.04).
Conclusions
Elevated choline and reduced NAA indicate inflammatory processes and white matter damage as well as neuronal metabolic impairment. Further research is needed to separate the choline peaks, as well as more detailed collection of medication regimens to determine whether a higher choline concentration is a function of the delirium process or cancer treatment effects.
Keywords: delirium, spectroscopy, choline, cancer, magnetic resonance imaging, cognition
1. Introduction
Delirium is a disturbance of consciousness caused by a medical condition and characterized by rapid onset, fluctuating course and change in cognition. Delirium is assessed by a clinician using criteria from the DSM-IV (APA, 2000) or a validated screening instrument, both of which involve in-person interviewing of the patient. Delirium is associated with a host of negative outcomes, including increased risk of mortality, longer hospital stay, and poor long-term cognitive function (Beglinger et al., 2006; Fong et al., 2009; van Hemert et al., 1994). Additionally, patients with delirium may not accurately report physical symptoms, which may impact treatment decisions, such as appropriate medical management of pain. Delirium is common in cancer patients, but it is under-recognized and under-treated, particularly early in its course when it may be mistaken for anxiety about treatment or side effects. Certain risk factors have been identified, such as age, elevated blood urea nitrogen level, cognitive impairment, and use of opioids, but the underlying mechanism of delirium is unknown.
Proton Magnetic Resonance Spectroscopy (1H-MRS) has become a valuable tool in the study of both cancer and psychiatric disorders. 1H-MRS can measure levels of metabolites such as N-acetyl aspartate (NAA), trimethylamines, such as phosphocholine, glycerophosphocholine and choline (tCho), total creatine (tCre=phosphocreatine + creatine), myo-inositol (mI), and glutamate (Glu, containing both neurotransmitter glutamate and metabolic glutamate). These metabolites are essential for various cell processes and we are just beginning to understand their functions. TCre reflects high energy phosphate metabolism, and has been used as a reference value in many studies. NAA is selectively concentrated in neurons and numerous studies have shown NAA/Cr decrease in neurodegenerative diseases suggesting neuronal loss (Birken and Oldendorf, 1989). The choline peak in 1H-MRS consists of contributions from mainly glycerophosphocholine (GPC) phosphocholine (PC), and typically a small amount of free choline and is regulated by activity of phospholipase A2 (Boulanger et al., 2000), phosphodiesterphosphodiesterase and phosphodiesterphosphomonoesterase. Therefore, in 1H-MRS in 3 Tesla fields, the peak is referred to as total choline (tCho). Total choline is thought to represent an increase in membrane turnover, with increased GPC indicating membrane degradation and increased PC being a precursor to membrane synthesis (e.g., found in replicating cancers). Only 31P-MRS can separate these signals to determine their relative contributions to tCho abnormalities as might be seen with disparate pathological conditions (e.g., tumors, demyelination, inflammatory processes)(Brenner et al., 1993). Myo-inositol has been used as a glial marker and may be involved with inflammation. Glutamate (Glu) is the highest concentrated amino acid in the brain, but it is not readily detected by in vivo proton MRS because it is often contaminated by the glutamine (Gln) signal. Posse et al. have shown that Glu can be resolved with Gln at 3T by using the short TE PEPSI sequence. (Posse et al., 2007) Although glutamate is usually thought of as the major excitatory neurotransmitter, it also has many other fates in the brain, including oxidation for energy, incorporation into proteins, and formation of glutamine, gamma-aminobutyric acid (GABA) and glutathione (McKenna, 2007). Measurement of brain metabolites in patients with active delirium could lead to a better understanding of the pathophysiology of delirium. There are few published reports using 1H-MRS in patients with delirium, in part due to the difficulties of scanning acutely ill and often frail patients with altered consciousness. Patients with delirium have cognitive impairments or other symptoms (e.g., agitation, delusions) that make following directions challenging. The small number of spectroscopy studies conducted in patients with delirium suggests metabolic abnormalities in the brain are present. For example, neurospsychiatric systemic lupus erythematosus patients suffering short-term cognitive dysfunction showed changes in NAA and tCho (Axford et al., 2001; Sibbitt et al., 1997) where tCho was elevated in patients with less severe clinical symptoms and NAA was reduced in patients with more severe disease. Cancer patients undergoing a bone marrow transplant or peripheral blood hematopoietic stem cell transplant (BMT) are at high risk for developing post-transplant delirium, with rates as high as 50%(Fann et al., 2002), the vast majority of which occur in the first two weeks (Beglinger et al., 2006). By imaging BMT patients who developed delirium as well as BMT patients that did not, and a group of healthy comparison subjects, differences in brain metabolite levels among these populations might point to a biomarker for delirium.
Chemical Shift Imaging (CSI) is a 1H-MRS non-invasive technique with rapid acquisition of spectral data from a large spatial area. This study examined the neurochemistry of delirium by measuring brain metabolites using CSI in older cancer patients who underwent a BMT. Those BMT patients who developed delirium were compared to BMT patients who did not show delirium and normal comparison subjects who did not have cancer or BMT. Although this is an exploratory study, based on the limited literature we hypothesized that BMT patients with delirium would have abnormal NAA and tCho compared to those without delirium and controls.
2. Methods
2.1 Participants and Procedures
The protocol and all study procedures were approved by the University of Iowa Institutional Review Board. All participants provided written informed consent and received financial compensation for their participation. Twenty-four patients were recruited from the University of Iowa Blood and Marrow Transplantation Program (BMTP) between 2006 to 2008, when they were being treated with an allogeneic or autologous BMT. Additionally, 10 healthy controls were recruited and matched generally for the age and gender of the participants with delirium. Most controls were family members or friends of the BMT patients and thus were well-matched to the stressful conditions the patients experienced. Participants were assessed at a pretransplantation visit with a 90-minute screening battery that assessed cognitive and psychiatric functioning and delirium status. Additional demographic and medical information was obtained from computerized records. During their inpatient stay, participants completed testing twice weekly for two weeks post-transplant or until the delirium resolved with a briefer battery to monitor for delirium and cognitive functioning. Additionally, if staff noticed symptoms of delirium during a non-assessment day, the research team was notified and a visit was scheduled as soon as possible. Control participants were tested on the same schedule as the BMT participants to simulate a “pre-transplant” and “inpatient” visits. All assessments were conducted by a trained research assistant or neuropsychologist.
2.2 Measures
The Delirium Rating Scale-Revised (Trzepacz et al., 1988; Trzepacz et al., 2001) is a scale of delirium severity based on all available information from patient interview, family, and nurses’ reports, cognitive tests, and medical reports, measured over a 24 hour period (DRS-R cutoff =15 for severity or=18 total score). The Memorial Delirium Assessment Scale (MDAS) (Breitbart et al., 1997) measures delirium presence and severity and can be administered multiple times in one day (cutoff ≥ 8). Patients were classified as delirious if they met either cutoff score. The following tests were also given twice weekly during the inpatient stay to measure neuropsychological status and assist with delirium assessment: Trail Making Test, Modified Mini Mental Status Exam, and the Repeatable Battery for the Assessment of Neuropsychological Status List Learning, Coding, Fluency and Digit Span subtests. Medical comorbidity was assessed with the Hematopoeitic Cell Transplant-Comorbidity Index.
2.3 Neuroimaging
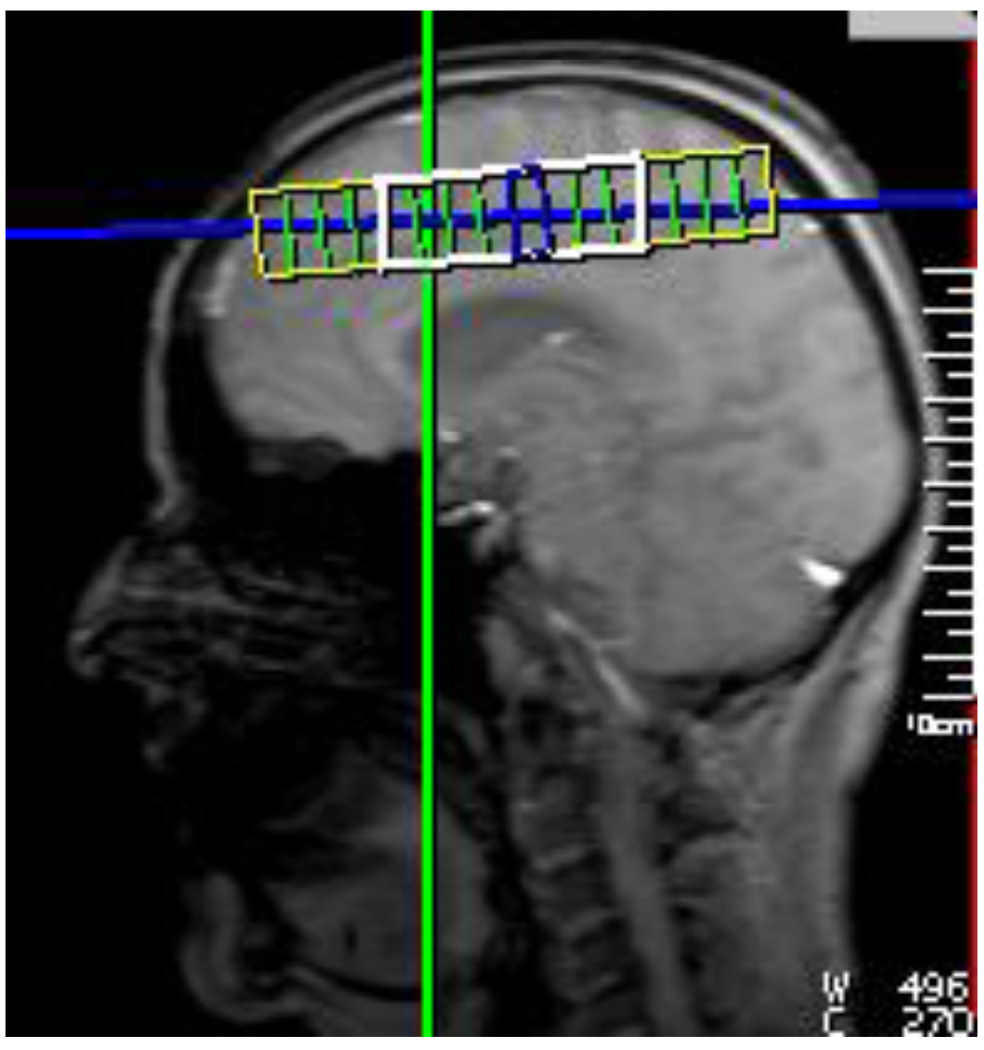
Patients were imaged as soon as possible after the first identification of delirium (mean=15.6 days from transplant to scan). BMT patients who never developed delirium over the two weeks were scanned at the end of that period (mean=14.7 days from transplant to scan). Healthy comparison participants were scanned at the end of the two week study period as well. MR imaging and spectroscopy were obtained from all subjects using a Siemens TIM Trio 3T MR scanner using a 12 channel head coil. Localizer images were obtained first, followed by a coronal T1 MPRAGE, a coronal T2 Turbo Spin Echo, and an axial FLAIR image that were used for anatomical localization. A 2D H-MRSI CSI scan using the PEPSI sequence (Posse et al., 2007) (TR = 2500 ms, TE = 30 ms, NEX = 6, FOV = 120×120 mm, VOI = 60×60 mm, 8×8 matrix size interpolated to 16×16, water saturation = 35 Hz) was then collected above the corpus callosum. The location of the matrix is shown in Figure 1. This location was selected because it is a brain slice that produces a good shim for CSI measurements and contains white matter from frontal, temporal and parietal lobes. A water reference scan was obtained by repeating the CSI scan with no water suppression using only one average.
Figure 1.
Location of the 2D CSI grid above the corpus callosum. The yellow indicates the FOV, and the white indicates the VOI.
2.4 Spectral Processing
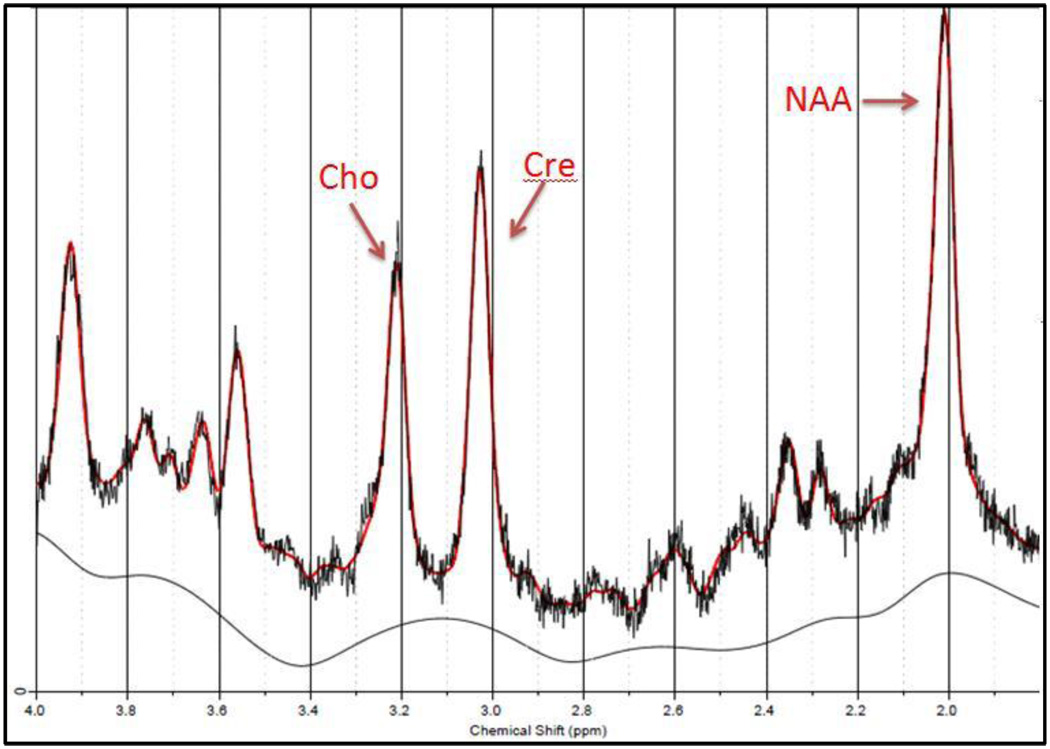
The software package LCModel was used to quantify the CSI data and the water reference scan was used to do water scaling and eddy current correction. The reliability of the metabolite peak fits were measured with Cramer-Rao Lower Bound, and any result below 20% was determined to be a reliable fit. Ratios with respect to the creatine and choline peaks were used to avoid partial volume effects. An example of the LCModel spectral quantification is shown in Figure 2.
Figure 2.
Example of a spectroscopy voxel spectrum of a BMT patient with deliruim. The NAA, Cho, and Cre metabolite peaks are labeled. The red line shows the LCModel metabolite quantification fit to the data and the black line shows the LCModel corrected baseline.
2.5 Data Analysis
The CSI VOI includes the inner 8 × 8 voxels. To avoid the inclusion of outlier metabolites, the median of each metabolite value for these 64 voxels was chosen for each subject. The three groups (BMT-no delirium, BMT-delirium, controls) were examined for baseline demographic differences. A priori planned paired-group comparisons were conducted examining age-adjusted group means for metabolite levels using a weighted least squares regression model to account for differences in group variances, a more conservative approach given the small sample size. Nonparametric tests were also run and showed similar results. The associations of metabolite levels with demographic and clinical variables were explored using age-adjusted Spearman correlations.
3. Results
Twenty-four patients who were admitted to the University of Iowa BMTP for BMT and 10 controls consented to participate. Ten patients and one control who completed the cognitive assessments were not able to tolerate or complete a usable MRI for a variety of reasons (e.g., metal in body, too ill, claustrophobic) and were excluded from the analyses. The final sample of 23 was comprised of 5 BMT patients with delirium, 9 BMT patients who did not experience delirium and 9 controls. The BMT patients had lymphomas (n=5), multiple myeloma (n=5) or leukemias (n=4), and all received myeloablative therapy (100%). Four out of 5 patients with delirium had lymphoma. A greater number of patients received an autologous transplant (N=9, 64%) than an allogeneic (N=5; 36%) transplant. Patients who underwent an autologous BMT received high dose, multi-agent chemotherapy for myeloablative therapy. Allogeneic BMT patients mostly received total body irradiation and high dose chemotherapy or Busulfan-based high-dose chemotherapy. The gender composition and average level of education between the three groups (control, BMT-delirium, and BMT-no delirium) did not differ, but patients who had delirium were older than patients without delirium (p=0.02, df=1) and more likely to have lymphoma (χ2 =5.06, p=0.02, df=1). See Table 1 for patient characteristics. There were no group differences on baseline global cognitive performance on the 3MS (F=1.67, p=0.22, df=2), but the RBANS Total Score revealed group differences (F=3.72, p=0.04, df=2) with controls performing better than both patient groups (Least Squares Mean Control=97.44 vs. BMT-no delirium=88.67 vs. BMT-delirum=87.80).
Table 1.
Subject Characteristics
| Demographics | Transplant | Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Total (N) |
M (N) |
F (N) |
Age (M) |
Education (M Yrs) |
Auto- (N) |
Allo- (N) |
Lymphoma (N) |
Leukemia (N) |
Multiple Myeloma (N) |
| Controls | 9 | 4 | 5 | 58.3 | 14.6 | 0 | 0 | 0 | 0 | 0 |
| BMT-Delirium | 5 | 4 | 1 | 63.8 | 12.6 | 4 | 1 | 4 | 0 | 1 |
| BMT-No Delirium | 9 | 4 | 5 | 55.4 | 13.4 | 5 | 4 | 1 | 4 | 4 |
| Total | 23 | 12 | 11 | 58.4 | 13.7 | 9 | 5 | 5 | 4 | 5 |
Demographics: M=male, F=female, Age and Education (both reported as mean years). Transplant type: Auto= autologous or Allo=allogeneic. Cancer diagnosis: lymphoma, leukemia, or multiple myeloma. BMT=bone marrow transplant.
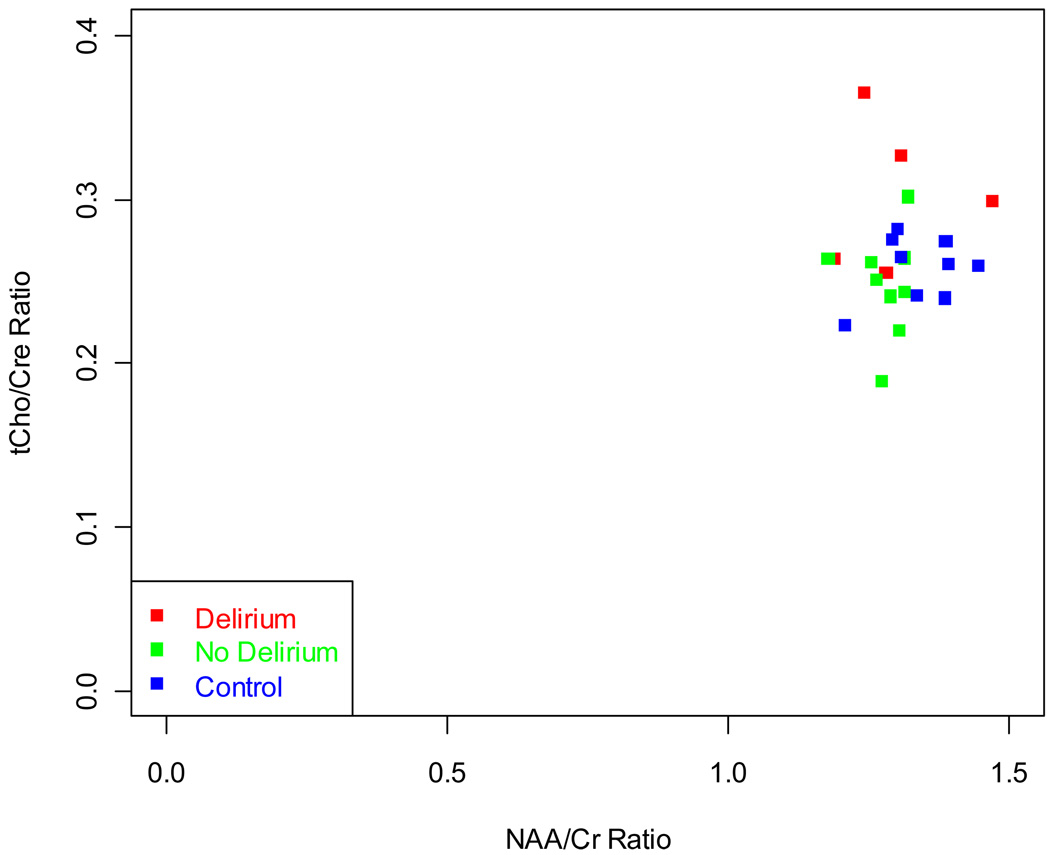
There were no significant differences in NAA/tCre, mI/tCre, Glu/tCre ratios among the three groups. In the BMT- delirium group, statistically significantly elevated tCho/tCre was found in contrast to the BMT-no delirium group (t=2.11, p=0.049, df=19). The tCho/tCre vs. NAA/tCre plot is shown in Figure 3. The BMT – delirium group also showed statistically significantly lesser NAA/tCho compared to both controls (t=−2.79, p=0.012, df=19) and the BMT – no delirium group (t=−2.25, p=0.037, df=19). Transplant type, disease severity, medical comorbidity burden at baseline and cognition were not associated with choline level. NAA/tCho was associated with lymphoma diagnosis (r=0.74, p=0.004, df=11) and memory score (r=0.63, p=0.002, df=11), but not transplant type, disease severity, or medical comorbidity burden.
Figure 3.
A plot of tCho/Cr ratio vs. NAA/Cr ratio for all subjects show outliers in the Delirium group that have elevated total Choline.
4. Discussion
This study examined an important and difficult to assess question using 1H-MRS – what is the pathophysiology of delirium in cancer patients? Cancer patients receiving BMT were separated into two groups, BMT with delirium and BMT without delirium, and were compared to healthy controls. Several brain metabolite levels were analyzed and compared with the patient characteristics. The main findings of this study were that BMT patients who developed delirium had significantly lower NAA/tCho ratio, were older and were more likely to have lymphoma when compared to the BMT participants who did not develop delirium and controls. The BMT-delirium patients also had significantly elevated tCho/tCre ratio compared to BMT-no delirium patients.
Ratios are commonly used in 1H-MRS to avoid the partial volume effects of CSF in voxels. In the absence of technically more difficult absolute concentration calculations, a definitive conclusion as to which metabolite is changing is not possible which is a limitation of this study. As mentioned previously, reduced NAA has indicated neuronal damage, and higher tCho concentration may indicate demyelination. Therefore, NAA/tCho is a sensitive, though not very specific, marker for brain tissue damage. Assuming Cr is remaining constant, the higher tCho/tCre finding between BMT-delirium patients and BMT-no delirium patients indicates choline may be the metabolite associated with the delirium process. Choline levels have been examined in cancer patients using spectroscopy, and there is interest in its utility as a biomarker for staging and diagnosis (Gillies and Morse, 2005; Sardanelli et al., 2009), although these studies focus on solid tumor classification and residual disease, which is not relevant in our sample. Our results show an elevated choline correlation specifically with delirium while no significant change in choline was seen in the post transplant cancer patients without delirium or the control group.
Beyond neuroimaging in cancer patients, studies using spectroscopy have found choline abnormalities in neuropsychiatric populations. Given impaired cognition in delirium, other disorders with cognitive dysfunction may be a relevant comparison group. Elevated choline levels in gray matter have been found in Alzheimer disease patients (Pfefferbaum et al., 1999), possibly due to cellular membrane turnover, however, findings have been mixed. Higher choline levels have also been associated with cognitive dysfunction in white matter of patients with neuropsychiatric systemic lupus erythematosus (Axford et al., 2001). Because they saw elevated tCho in patients with minor clinical features and reduced NAA in patients with more severe disease that were stable longitudinally, Axford, et al. hypothesized that choline elevations are caused by an inflammatory process without cell membrane degradation or demyelination that precedes NAA loss and irreversible neuronal damage. Further evidence of elevated choline linked with inflammatory damage is found in patients with multiple sclerosis (Brenner et al., 1993). These in vivo metabolic changes were confirmed with a histological study on an animal model to confirm inflammatory processes with no neuronal loss (Brenner et al., 1993). Higher choline levels have been found in patients with schizophrenia (Martinez-Granados et al., 2008). Martinez-Granados and colleagues argue that cortical regions are regulated with cholinergic activation by basal ganglia-thalamo-cortical circuit, and failure in this circuit could lead to hallucinations. Elevated choline has also been associated with myelin breakdown. In a recent review of neuroimaging studies in delirium (Soiza et al., 2008), white matter lesions were one of the two major brain abnormalities identified in patients who developed delirium (along with general atrophy). Elevated choline was early recognized in white matter T2 hyperintensities in a comparable brain slice in Alzheimer’s disease and vascular dementia patients (Constans et al., 1995). Perturbations of cholinergic neurotransmission have been widely implicated in causes of delirium (Trzepacz et al., 1996). It has been documented that anticholinergic drugs can produce delirium states (Cancelli et al., 2009). Gibson et al. suggest that while there are many causes of impaired cholinergic neurotransmission, it represents the final common pathway for the development of delirium (Gibson et al., 1991). However, since acetylcholine is a minor contributor to the in vivo 1H MRS choline peak, detected choline changes in delirium most likely represent abnormal phospholipid turnover.
However, reduced NAA cannot be ruled out as a cause of the lower NAA/tCho ratio. Reduced NAA indicates neuronal metabolic impairment, which may be reversible (De Stefano et al., 1995), or neuronal loss which is irreversible. By combining the likelihood of increased choline and reduced NAA in the NAA/tCho ratio, we expected to increase the sensitivity for metabolic abnormality in delirium. Low NAA/Cho ratios have been found in different psychiatric and neurologic disorders, notably schizophrenia, where both reduced NAA and increased choline have been reported (Shirayama et al., 2009). One additional caveat bears mention; because lymphoma diagnosis was associated with delirium and lower NAA concentration, it is possible that treatment for lymphoma is driving the NAA finding. Many medications used for lymphoma and other cancers cross the blood-brain barrier and may affect spectroscopy results due to the neurotoxic effects of chemotherapy (Serkova et al., 2004). To explore this we examined the conditioning regimen of all BMT patients by chart review and found that lymphoma patients were more commonly taking lipid-soluble medications known to cross the blood brain barrier (i.e., carmustine, etoposide, cytarabine), however only 3 of the 5 patients with delirium were taking these medications and only one with a strong choline signal. Carmustine was not statistically related to either tCho/tCre (p=0.86, df=11) or NAA/tCho (p=0.08, df=11) in this sample. Nevertheless, carmustine has been associated with white matter changes by two months post-chemotherapy in a small sample of breast cancer patients who had BMT (Brown et al., 1998). Spectroscopy was also performed in that study and there were no changes in NAA/Cr or tCho/tCre over the same interval post-transplant as patients in the current study, but there was a trend of minor reductions in NAA/Cr over a longer follow-up period (6 to 10 months). Thus, further study is warranted in cancer patients with delirium to determine whether changes in NAA and tCho are associated with the delirium process, late effects of chemotherapy or some interaction of both.
A strength of this study is the novel use of spectroscopy imaging in a frail and difficult to assess sample to examine brain abnormalities that may be shed light on the pathophysiology of delirium. However, there are a number of limitations to be acknowledged, most notably the small sample size. Nearly half (10/24) of the patients were unable to tolerate the scanning session or were too ill to attempt a scan. Thus the power to detect group differences was limited and analyses should be considered a preliminary effort to identify targets for future research. Despite the fact that power was low and participants who completed the scans were likely less ill (and possibly less metabolically abnormal) than those who did not complete scans, our results indicated some group differences that merit future examination. Age was also different between the groups, and although we used age-adjusted means in our analyses, age is a potential confound and future larger studies should attempt to recruit more age-matched samples. An additional limitation of this study is that while using 1H-MRS, the total choline peak cannot reveal the compounds responsible for the higher tCho/tCre. 31P-MRS in conjunction with 1H-MRS has been used to resolve the phosphomonoester and phosphodiester components of the proton choline peak (Potwarka et al., 1999) and future studies may benefit from the use of 31P-MRS to study the choline metabolite contributions. Additionally, longitudinal studies are needed to determine whether pre-transplant spectroscopy can be used as a measure of post-transplant risk for developing delirium, which could have important clinical implications. It is also important to conduct follow-up scans to determine whether the MRS abnormalities resolve after the delirium is no longer present. Future studies should also collect detailed information about the types of medication regimens used before transplant, including dosages and timing of treatment, to investigate whether systematic differences in treatment may be a risk factor for delirium and the neurochemical changes identified in our sample using MRS. Finally, the analysis strategy selected of taking the median metabolic values precluded separate white and gray matter contributions to be differentiated.
Although there is limited literature on brain metabolic activity during acute delirium, studies on related neurological diseases and disorders point to possible causes for the elevated choline levels seen in this study, including inflammatory processes and white matter damage. Additionally, NAA/tCho was lower in the delirium group compared to controls suggesting neuronal metabolic impairment. While we have interpreted the MRS changes as possible markers of the delirium process, it is possible that these changes preceded delirium and served as a risk factor for the later development of delirium.
Acknowledgements
The authors wish to acknowledge the patients and families who volunteered their time to participate in this study, the nurses and physicians of our participants and Sara Van Der Heiden, Jana Hanson and Karen Parrott for their assistance. Support for this research was provided by the University of Iowa Cancer and Aging Program in the Holden Comprehensive Cancer Center (NCI/NIA P20 CA 103672, PI: Lubaroff).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None of the authors have any financial conflicts of interest to report with regard to the content of this mauscript.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition - TR ed. Washington DC: APA; 2000. [Google Scholar]
- Axford JS, Howe FA, Heron C, Griffiths JR. Sensitivity of quantitative (1)H magnetic resonance spectroscopy of the brain in detecting early neuronal damage in systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2001;60(2):106–111. doi: 10.1136/ard.60.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger LJ, Duff K, Van Der Heiden S, Parrott K, Langbehn D, Gingrich R. Incidence of delirium and associated mortality in hematopoietic stem cell transplantation patients. Biology of Blood Marrow Transplant. 2006;12(9):928–935. doi: 10.1016/j.bbmt.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience & Biobehavior Reviews. 1989;13(1):23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Boulanger Y, Labelle M, Khiat A. Role of phospholipase A(2) on the variations of the choline signal intensity observed by 1H magnetic resonance spectroscopy in brain diseases. Brain Research Brain Research Review. 2000;33(2–3):380–389. doi: 10.1016/s0165-0173(00)00037-0. [DOI] [PubMed] [Google Scholar]
- Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. Journal of Pain and Symptom Management. 1997;13(3):128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- Brenner RE, Munro PM, Williams SC, Bell JD, Barker GJ, Hawkins CP, Landon DN, McDonald WI. The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magnetic Resonance in Medicine. 1993;29(6):737–745. doi: 10.1002/mrm.1910290605. [DOI] [PubMed] [Google Scholar]
- Brown MS, Stemmer SM, Simon JH, Stears JC, Jones RB, Cagnoni PJ, Sheeder JL. White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. American Journal of Neuroradiology. 1998;19(2):217–221. [PMC free article] [PubMed] [Google Scholar]
- Cancelli I, Beltrame M, Gigli GL, Valente M. Drugs with anticholinergic properties: cognitive and neuropsychiatric side-effects in elderly patients. Journal of the Neurological Sciences. 2009;30(2):87–92. doi: 10.1007/s10072-009-0033-y. [DOI] [PubMed] [Google Scholar]
- Constans JM, Meyerhoff DJ, Gerson J, MacKay S, Norman D, Fein G, Weiner MW. H-1 MR spectroscopic imaging of white matter signal hyperintensities: Alzheimer disease and ischemic vascular dementia. Radiology. 1995;197(2):517–523. doi: 10.1148/radiology.197.2.7480705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magnetic Resonance Medicine. 1995;34(5):721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- Fann JR, Roth-Roemer S, Burington BE, Katon WJ, Syrjala KL. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer. 2002;95(9):1971–1981. doi: 10.1002/cncr.10889. [DOI] [PubMed] [Google Scholar]
- Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Blass JP, Huang HM, Freeman GB. The cellular basis of delirium and its relevance to age-related disorders including Alzheimer's disease. International Psychogeriatric. 1991;3(2):373–395. doi: 10.1017/s1041610291000820. [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annual Review Biomedical Engineering. 2005;7:287–326. doi: 10.1146/annurev.bioeng.7.060804.100411. [DOI] [PubMed] [Google Scholar]
- Martinez-Granados B, Brotons O, Martinez-Bisbal MC, Celda B, Marti-Bonmati L, Aguilar EJ, Gonzalez JC, Sanjuan J. Spectroscopic metabolomic abnormalities in the thalamus related to auditory hallucinations in patients with schizophrenia. Schizophrenia Research. 2008;104(1–3):13–22. doi: 10.1016/j.schres.2008.05.025. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. Journal of Neuroscience Research. 2007;85(15):3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo brain concentrations of N-acetyl compounds, creatine, and choline in Alzheimer disease. Archives of General Psychiatry. 1999;56(2):185–192. doi: 10.1001/archpsyc.56.2.185. [DOI] [PubMed] [Google Scholar]
- Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins B, Mullins P, Renshaw P, Ugurbil K, Lim KO, Alger JR. Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magnetic Resonance in Medicine. 2007;58(2):236–244. doi: 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- Potwarka JJ, Drost DJ, Williamson PC, Carr T, Canaran G, Rylett WJ, Neufeld RW. A 1H-decoupled 31P chemical shift imaging study of medicated schizophrenic patients and healthy controls. Biological Psychiatry. 1999;45(6):687–693. doi: 10.1016/s0006-3223(98)00136-x. [DOI] [PubMed] [Google Scholar]
- Sardanelli F, Fausto A, Di Leo G, de Nijs R, Vorbuchner M, Podo F. In vivo proton MR spectroscopy of the breast using the total choline peak integral as a marker of malignancy. American Journal of Roentgenology. 2009;192(6):1608–1617. doi: 10.2214/AJR.07.3521. [DOI] [PubMed] [Google Scholar]
- Serkova NJ, Christians U, Benet LZ. Biochemical mechanisms of cyclosporine neurotoxicity. Molecular Interventions. 2004;4(2):97–107. doi: 10.1124/mi.4.2.7. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E, Ikehira H, Hashimoto K, Iyo M. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: A preliminary study. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Sibbitt WL, Jr, Haseler LJ, Griffey RR, Friedman SD, Brooks WM. Neurometabolism of active neuropsychiatric lupus determined with proton MR spectroscopy. American Journal of Neuroradiology. 1997;18(7):1271–1277. [PMC free article] [PubMed] [Google Scholar]
- Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, Maclullich AM. Neuroimaging studies of delirium: a systematic review. Journal of Psychosomatic Research. 2008;65(3):239–248. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Research. 1988;23(1):89–97. doi: 10.1016/0165-1781(88)90037-6. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT, Ho V, Mallavarapu H. Cholinergic delirium and neurotoxicity associated with tacrine for Alzheimer's dementia. Psychosomatics. 1996;37(3):299–301. doi: 10.1016/S0033-3182(96)71569-4. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(2):229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- van Hemert AM, van der Mast RC, Hengeveld MW, Vorstenbosch M. Excess mortality in general hospital patients with delirium: a 5-year follow-up of 519 patients seen in psychiatric consultation. Journal of Psychosomatic Research. 1994;38(4):339–346. doi: 10.1016/0022-3999(94)90038-8. [DOI] [PubMed] [Google Scholar]