Abstract
Pentalenic acid (1) has been isolated from many Streptomyces species as a co-metabolite of with the sesquiterpenoid antibiotic pentalenolactone and related natural products. We have previously reported the identification of a 13.4-kb gene cluster in the genome of Streptomyces avermitilis implicated in the biosynthesis of the pentalenolactone family of metabolites consisting of 13 ORFs. Detailed molecular genetic and biochemical studies have revealed that at least 7 genes are involved in the biosynthesis of the newly discovered metabolites, neopentalenoketolactone, but no gene specifically dedicated to the formation of pentalenic acid (1) was evident in the same gene cluster. The wild type strain of S. avermitilis as well as its derivatives mainly produce pentalenic acid (1) together with neopentalenoketolactone (9). Disruption of the sav7469 gene encoding a cytochrome P450 (CYP105D7), members of which class are associated with the hydroxylation of many structurally different compounds, abolished production of pentalenic acid (1). The sav7469-deletion mutant derived from SUKA11 carrying pKU462::ptl-clusterΔptlH accumulated 1-deoxypentalenic acid (5), but not pentalenic acid (1). Reintroduction of an extra-copy of the sav7469 gene to SUKA11 Δsav7469 carrying pKU462::ptl-clusterΔptlH restored the production of pentalenic acid (1). Recombinant CYP105D7 prepared from Escherichia coli catalyzed the oxidative conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1) in the presence of the electron-transport partners, ferredoxin and ferredoxin reductase, both in vivo and in vitro. These results unambiguously demonstrate that CYP105D7 is responsible for the conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1), a shunt product in the biosynthesis of the pentalenolactone family of metabolites.
Keywords: Sesquiterpene, Biosynthesis, Streptomyces avermitilis, pentalenolactone, cytochrome P450
Introduction
Pentalenolactone is a sesquiterpenoid antibiotic first discovered in 1957 in the culture extracts of Streptomyces roseogriseus [1] and subsequently isolated from numerous Streptomyces microorganisms [2–4]. Pentalenolactone is active against both Gram-positive and Gram-negative strains of bacteria as well as pathogenic and saprophytic fungi [5]. Pentalenolactone is also a potent and specific antiviral agent, inhibiting the replication of DNA viruses, such as the causal agent of herpes simplex HSV-1 and HSV-2 [6], and can inhibit vascular smooth muscle cell proliferation [7]. The first step in pentalenolactone biosynthesis is the cyclization of farnesyl diphosphate, the universal precursor of all sesquiterpenes, to pentalenene (2), which is the parent hydrocarbon of the pentalenolactone family of metabolites. From the results of feeding experiments for pentalenolactone biosynthesis, a variety of plausible intermediates in the conversion of pentalenene to pentalenolactone have been isolated, including 1-deoxypentalenic acid (5; as glucuronate ester) [4] as well as pentalenic acid (1) [8, 9], a demonstrated shunt metabolite of the main pentalenolactone biosynthetic pathway. The fact that tritium label from (1R)-[1-3H] pentalenene (2) was lost upon formation of pentalenic acid (1) but was retained in the derived pentalenolactone has definitively ruled out 1 as an intermediate in the formation of pentalenolactone [9]. A plausible biosynthetic pathway for pentalenolactone biosynthesis has been proposed based on the structures of these isolated pentalenolactone metabolites, but until recently, however, there had been no direct experimental evidence for biosynthetic sequence leading from pentalenene (2) to the pentalenolactone family of metabolites.
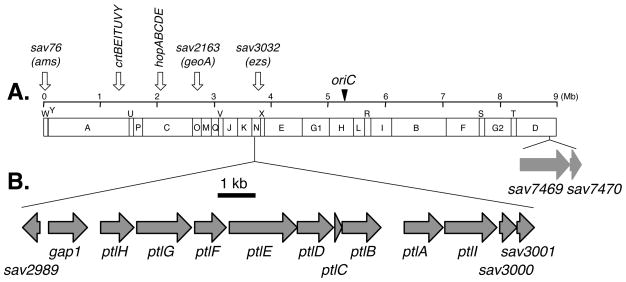
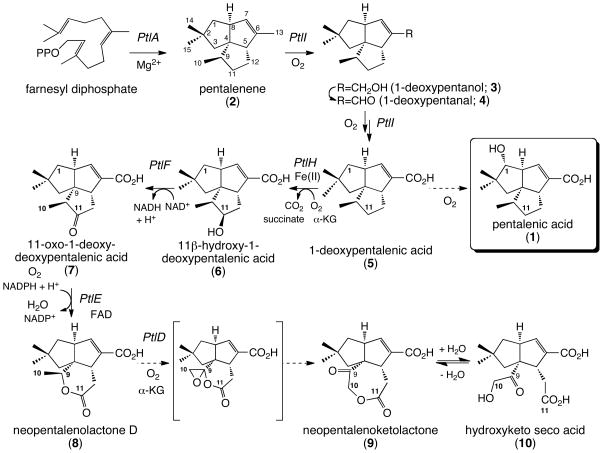
Streptomyces avermitilis is a Gram-positive soil bacterium responsible for the production of the anthelminthic macrocyclic lactone avermectins that are widely used in human and veterinary medicine. Sequencing of the complete 9.03-Mb linear genome of S. avermitilis revealed at least 37 presumed biosynthetic gene clusters related to secondary metabolism, with at least 6 of them encoding putative terpenoid biosynthetic pathways as shown in Fig. 1-A [10–13]. Among the latter, a 13.4-kb gene cluster centered at 3.75 Mb in the S. avermitilis genome and containing 13 unidirectionally transcribed open reading frames (ORFs) (sav2990 - sav3002) has been implicated in the biosynthesis of pentalenolactone-like metabolites (Fig. 1-B). Although pentalenolactone itself has not been detected in the organic extract of S. avermitilis, we have isolated the common pentalenolactone shunt metabolite pentalenic acid (1) from these cultures [14]. Deletion of the 13.4-kb operon from S. avermitilis abolished production of both neopentalenoketolactone (9) and pentalenic acid (1), while transfer of the entire gene cluster to S. lividans 1326, a strain that normally does not produce pentalenolactones, resulted in generation of pentalenic acid (1). In parallel with these molecular genetic investigations, we have been systematically expressing in E. coli the individual genes from the 13.4-kb biosynthetic cluster in order to identify the natural substrates and intrinsic biochemical reactions for each ORF (Fig. 2). Direct evidence for the function of the 13.4-kb cluster came from the expression of recombinant ptlA (sav2998) [14], ptlI (sav2999) [15], ptlH (sav2991) [16, 17], ptlF (sav2993) [18] and ptlE (sav2994) [19], respectively. The fact that the ΔptlD mutant of S. avermitilis accumulates neopentalenolactone D (8) suggests that 8 is likely to be the natural substrate for the ptlD gene product in the formation of neopentalenoketolactone (9) [20].
Figure 1.
AseI-physical map of S. avermitilis and distribution of biosynthetic genes encoding terpenoid compounds (sav76; gene for avermitilol biosynthesis, crt; genes for isoneriatene biosynthesis, hop; genes for squalene and hopanoid biosynthesis, sav2163; gene for germacradienol and geosmin biosynthesis, sav3032; gene for epi-isozizaene biosynthesis) (A), and gene cluster for neopentalenoketolactone biosynthesis (sav2989; gene encoding MarR-family transcriptional regulator, gap1; gene for pentalenolactone-insensitive glyceraldehyde-3-phosphate dehydrogenase, ptlH; gene for 1-deoxypentalenic acid 11-β hydroxylase, ptlG; gene for transmembrane efflux protein, ptlF; 1-deoxy-11β-hydroxypentalenic acid dehydrogenase; ptlE; gene for Baeyer-Villiger monooxygenase, ptlD; gene for dioxygenase, ptlC; gene for hypothetical protein, ptlB; gene for farnesyl diphosphate synthase, ptlA; gene for pentalenene synthase, ptlI; gene for pentalenene C13 hydroxylase CYP183A1, sav3000; gene for AraC-family transcriptional regulator, sav3001; gene for lyase, sav3002; gene for hypothetical protein) (B). Two genes, sav7469 and sav7470 encode cytochrome P450 (CYP105D7) and ferredoxin (FdxH), respectively.
Figure 2.
Proposed neopentalenoketolactone biosynthetic pathway in S. avermitilis. α-KG indicates α-ketoglutarate.
We have thus elucidated all but the final step in the biosynthetic pathway for the pentalenolactone variant, neopentalenoketolactone (9), while the genetic and biochemical basis for the accumulation of pentalenic acid (1) in S. avermitilis remained to be clarified. The shunt product pentalenic acid (1) is thought to be formed by C-1 hydroxylation of 1-deoxypentalenic acid (5), an intermediate in the biosynthesis of the pentalenolactone and neopentalenolactone family of metabolites, presumably under the control of a suitable hydroxylase or CYP. Significantly, however, no candidate gene encoding such a hydroxylase is evident in the 13.4-kb ptl cluster. In fact, several S. avermitilis CYP genes are located within the biosynthetic clusters for avermectin, oligomycin, filipin, albaflavenone, and neopentalenolactone biosynthesis. Other CYP genes are distributed independently throughout the linear chromosome of S. avermitilis [20]. It is therefore of considerable interest to identify which S. avermitilis CYP gene is responsible for the conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1). We now provide evidence that CYP105D7 encoded by sav7469 is responsible for hydroxylation at the C-1 position of 1-deoxypentalenic acid (5), resulting in accumulation of pentalenic acid (1) as a shunt metabolite of pentalenolactone or neopentalenolactone biosynthesis.
Materials and methods
Bacterial strains
The large-deletion mutants of Streptomyces avermitilis, SUKA5, SUKA7 (SUKA5 Δsav7469), SUKA11 (SUKA5 Δptl-cluster::ermE) and SUKA13 (SUKA11 Δsav7469) [21], were used for production of neopentalenolactone and related compounds. Culture conditions for the sporulation and regeneration of protoplasts after PEG-mediated protoplast transformation have been previously described [22, 23].
Construction of ΔptlH deletion mutant
The ΔptlH deletion mutants were prepared using the entire 14.9-kb S. avermitlis ptl-cluster clone (pKU462::ptl-cluster), in which ptlA had been placed under the control of the ermE promoter, as previously described [14, 19], using as host strains S. avermitilis SUKA11 and SUKA13. In-frame ΔptlH-deletion mutant: forward primer, 5’-ACCACGGCCACTGAGGTCTCACAAGGAGATGAATCTGTGGCCAGTGAGTTCGAG CGACT-3’ (bold characters indicate the upstream region of ptlH on the chromosome and underlined characters correspond to the start codon of ptlH) and the reverse primer 5’-GCCCGCCGGGGAAAATCTCCGGGTGGGGGTTGGTCGTCACCCCGGGTACCGAGC GAAC-3’ (bold characters indicate the downstream region of ptlH on the chromosome and underlined characters correspond to the stop codon of ptlD). This primer pair was used for the amplification of the loxP-aad(3'')-loxP segment using pKU473 (pULwL::aad(3'')) as template [19]. The initial denaturation step (95 °C, 5 min) was followed by 5 cycles of amplification (95 °C, 0.5 min; 50 °C, 0.5 min; 72 °C, 1.0 min) followed by 25 cycles using an annealing temperature of 65 °C, and then a final incubation at 72 °C (10 min). The desired in-frame deletion plasmid, pKU462::ptl-clusterΔptlH, was constructed using the λ recombination system and Cre/loxP-based site-specific excision, as previously described [19, 21].
Isolation of products from S. avermitilis and its deletion mutants
The cultivation of in-frame deletion mutants was performed as previously described [14, 19]. After cultivation, an equal volume of methanol was added and mixed for 10 min. The mycelia were sedimented by centrifugation, the supernatant was evaporated under reduced pressure to remove methanol. The aqueous extract was diluted with a half volume of water, adjusted to pH 2 with 2 N HCl and extracted twice with a half volume of EtOAc. The resulting organic extracts were combined, dried under Na2SO4 and evaporated to dryness. The brownish oily residue was dissolved in 0.1 mL of benzene-methanol (9:1) and the products were methylated by adding 0.02 mL of trimethylsilyldiazomethane (TMS-CHN2; 10% in n-hexane) for 15 min at ambient temperature. After removal of solvent by evaporation, the methylated products were re-dissolved in a small amount of methanol and a portion of the extract was subjected to GC-MS analysis [Shimadzu GC-17A, 70 eV, electron impact, positive ion mode; 30 m x 0.25 mm φ Neutra Bond-5 capillary column (5% phenylmethylsilicon) using a temperature program of 50-280 °C and temperature gradient of 20 °C/min, inlet temperature 280 °C; run time 20 min].
Construction of the sav7469 expression cassette
Since the sav7470 gene (8,897,117-8,894,401 nt in the S. avermitilis genome) encoding a ferredoxin (FdxH) is located 14-bp downstream of the sav7469 gene, the two genes appear to belong to a single transcriptional unit. An operon containing both sav7469 and sav7470 was amplified by PCR from template DNA from cosmid CL_228_E11 using the primer pair, forward: 5′-GCCCATATGACAGAGCCCGGTACGTCCGT-3′ (underlined characters indicate the NdeI site and bold characters correspond to the start codon of sav7469) and reverse: 5′-GCACTAGTTCAGCTTGCCGACCGCGGGAC-3′ (underlined characters indicate the SpeI site and bold characters correspond to the stop codon of sav7470). The amplified DNA segment was digested with NdeI and SpeI and the digested segment was ligated with the NdeI/XbaI fragment of pKU460::xylAp-sav3129-sav5675 [24]. The resultant recombinant plasmid pKU460::xylAp-sav7469-sav7470-sav3129-sav5675 was digested with NdeI/HindIII and the resultant NdeI/HindIII segment sav7469-sav7470-sav3129-sav5675 was ligated with the large segmant of NdeI/HindIII pKU460::rpsJp [21]. The plasmid isolated from the resulting transformants was digested with NheI/HindIII and the NheI/HindIII segment, rpsJp-sav7469-sav7470-sav3129-sav5675, was ligated with the large segment of NheI/HindIII pKU490::hph and the ligation product was used to transform E. coli DH5α. The desired plasmid, pKU490::rpsJp-sav7469-sav7470-sav3129-sav5675, obtained by selection for hygromycin B resistance, was transferred into S. avermitilis SUKA13 carrying pKU462::ptl-clusterΔptlH by conjugation, as previously described [25].
Heterologous expression and purification of CYP105D7 (SAV_7469)
The sav7469 gene (8,895,895-8,897,103 nt in S. avermitilis) was amplified by PCR with template DNA from cosmid CL_228_E11 using the primer pair, forward: 5′-CGGAATTCCATATGACAGAGCCCGGTACGTCCGTGTC-3′ (underlined characters indicate the NdeI site) and reverse: 5′-GGACTAGTTCAGTGGTGGTGGTGCCAGGTCACGGGGAGTTCCAGCATC-3′ (underlined characters indicate the SpeI site and bold characters represent His4 tag). The PCR program employed was as follows: initial denaturation step (96 °C, 2 min), followed by 30 cycles of amplification (96 °C, 30 s; 65 °C, 30 s; and 72 °C, 60 s), and then a final incubation at 72 °C (5 min). The resultant amplicon and pET17b vector were each then double-digested with NdeI and SpeI. The digested PCR product was ligated with the large NdeI/SpeI fragment of pET17b by T4 DNA ligase. The ligation mixture was used to transform competent cells of E. coli JM109. The resultant plasmid, pET17b::sav7469, isolated from cultures grown on LA medium containing 50 μg/mL of ampicillin, was used to transform E. coli BL21 CodonPlus (DE3). Expression of the sav7469 gene and purification of C-terminal His4-tagged CYP105D7 was performed by procedures similar to those described previously [26]. The purified CYP105D7 (10 mg/mL) was flash-frozen in small aliquots in liquid N2 and stored at −80 °C. The measurement of UV-visible absorption spectrum under oxidized and reduced conditions, and CO-difference spectrum was performed by procedures described previously [27].
Preparation of 1-deoxypentalenic acid (5)
The culture conditions for the production of 1-deoxypentalenic acid by S. avermtilis SUKA13 carrying pKU462::ptl-clusterΔptlH were described previously [19]. Two liters of the culture supernatant were acidified to pH 2.5 with 2 N HCl and the organic products were extracted twice with 1 L of EtOAc. The organic extracts were combined, dried under Na2SO4 and evaporated to dryness. The brownish oily material was dissolved in 2 mL of benzene-methanol (9:1) and treated with 0.1 mL of TMS-CHN2 for 15 min at room temperature. After removal of solvent, methylated products were subjected to silica gel column chromatography using CHCl3 as eluent with collection of the (5) methyl ester-rich fraction. Pure (5)-methyl ester was obtained by preparative HPLC developing with 90% acetonitrile in water. The resulting (5)-methyl ester (27 mg) was hydrolyzed with 5 mM K2CO3 in 30% (v/v) aq. methanol (reflux, 24 h). After removal of the methanol under reduced pressure, the resultant aqueous solution was adjusted to pH 2.5 with 2 N HCl and extracted with equal volume of EtOAc to give 17 mg of 1-deoxypentalenic acid (5) as a colorless oil. The structure of 5 was confirmed by the 1H and 13C NMR spectra.
In vitro conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1) by CYP105D7
The catalytic reaction of CYP105D7 was performed using the following reaction mixture: 0.4 mM 1-deoxypentalenic acid (5) in DMSO, 4.4 μM spinach ferredoxin, 0.05 U of spinach ferredoxin-NADP+ reductase, 0.5 mM NADP+, 0.5 mM glucose-6-phosphate, 0.5 U of glucose-6-phosphate dehydrogenase, 50 mM Tris-HCl (pH 8.0), 10% (v/v) of glycerol and 1.1 μM purified C-terminal His4-tagged CYP105D7, in a total volume of 200 μL. The reaction mixture without CYP105D7 was pre-incubated for 15 s at 25 °C before addition of CYP105D7 and incubation for 2 h at 30 °C with shaking. The enzymatic reaction was terminated by adding 20 μL of 1 N HCl and the product was extracted using 2 x 100 μL of EtOAc. The combined organic extracts were dried over Na2SO4 and evaporated to dryness. The residue was dissolved in 100 μL of benzene-methanol (9:1) and 20 μL of TMS-CHN2 was added. After 15 min at room temperature, the reaction mixture was evaporated to dryness and the residue was dissolved in 20 μL methanol. A five microliters portion of the methylated product was analyzed to GC-MS. For kinetic measurements, assays were performed under the above conditions over a range of substrate concentrations. The steady-state kinetic parameters, Km, Vmax and kcat, were calculated by fitting the observed GC-MS TIC values to the Michaelis-Menten equation by non-linear, least-squares regression. Reported standard deviations correspond to the statistical errors limits of the data fit. The dissociation constant KD of CYP105D7 and 1-deoxypentalenic acid (5) was obtained as previously described [15]. 1-Deoxypentalenic acid (5) dissolved in DMSO was added at increasing concentrations to a stock solution of 1.1 μM of CYP105D7 in 50 mM Tris-HCl (pH 8.0) and 10% (v/v) glycerol. The concentration of DMSO was adjusted to 0.1% (v/v). UV difference spectra were recorded from 0 to 100 μM of 1-deoxypentalenic acid (5). The dissociation constant KD was calculated from the saturation curve of the measured delta value (ΔAU=A364 nm - A416 nm) versus substrate. All kinetic and dissociation data were obtained by the triplicate experiments.
Results
Production of pentalenic acid (1) in mutants of S. avermitilis
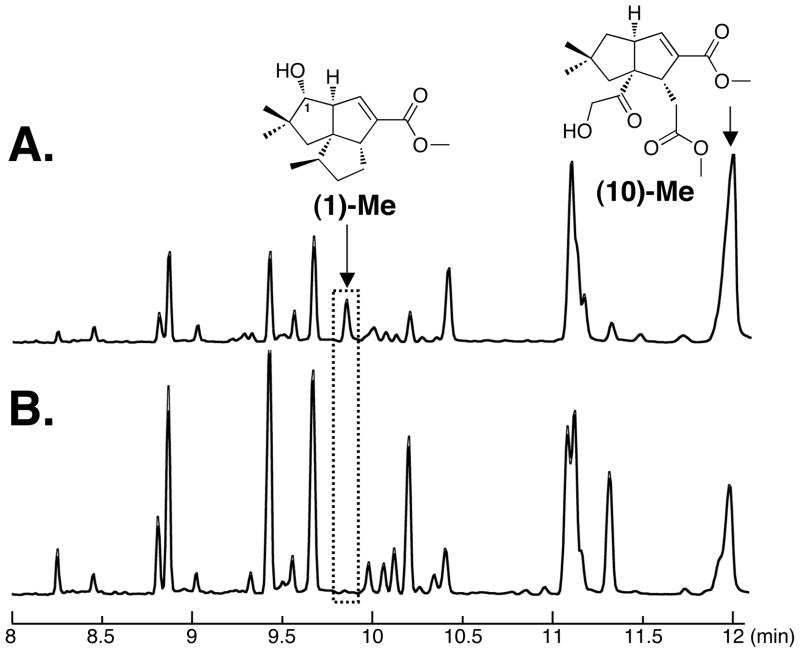
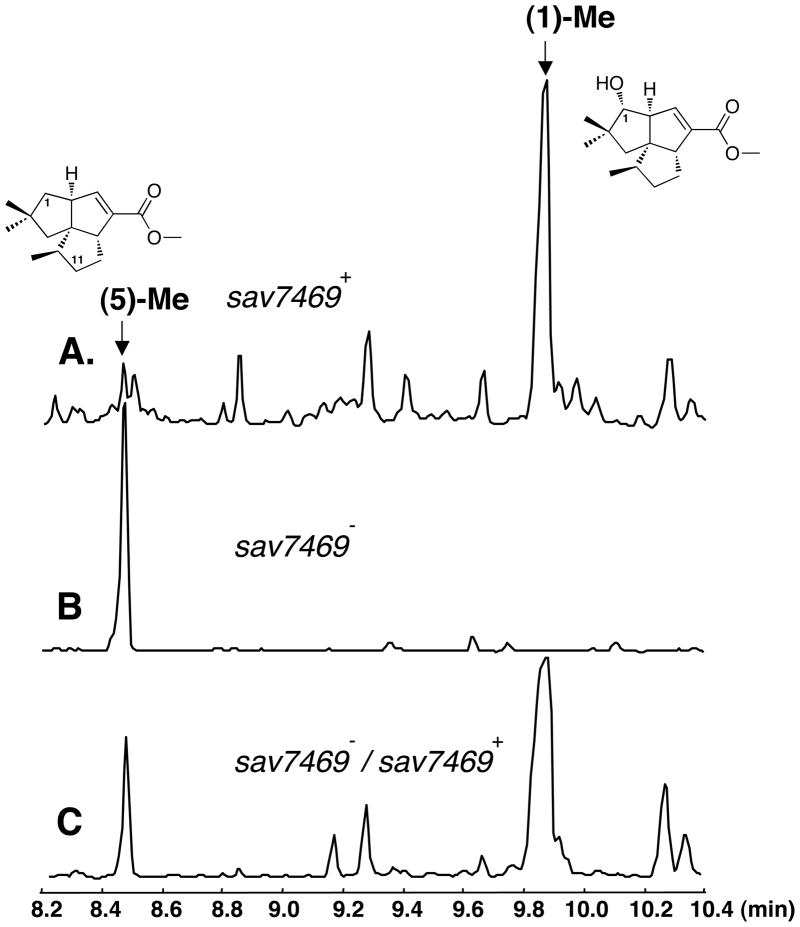
S. avermitilis harbors a biosynthetic gene cluster for a newly discovered branch of the classical pentalenolactone family tree, with the microorganism producing a new type of pentalenolactone derivative which we have termed neopentalenolactone [19]. One of the large-deletion mutants of S. avermitilis, SUKA5, produced pentalenic acid (1) and neopentalenoketolactone (9), which underwent facile conversion to the hydroxyketo seco acid form (10) (Fig. 3A). We had independently constructed sav7469-deletion mutants for the bioconversion experiments. The mutant SUKA7 which harbors a deletion of the gene encoding CYP105D7 (SAV_7469) still produced neopentalenoketolactone (9) and its hydrolyzed derivative (10) but did not produce pentalenic acid (1) (Fig. 3B). This result indicates that CYP105D7 is most likely involved in the formation of pentalenic acid (1) in S. avermitilis. To clarify the role of CYP105D7 in the hydroxylation of 1-deoxypentalenic acid (5) at C-1, a biosynthetically blocked mutant with a disrupted ptlH (sav2991) gene [16], encoding a non-heme iron, α-ketoglutarate-dependent hydroxylase that catalyzes the hydroxylation of 1-deoxypentalenic acid (5) at C-11 to generate 1-deoxy-11β-hydroxypentalenic acid (6), was constructed by in-frame deletion using the λ recombination and Cre/loxP excision systems [19]. Since the C-11 hydroxylation was not functional in SUKA11 carrying pKU462::ptl-clusterΔptlH, this deletion mutant produced 1-deoxypentalenic acid (5) accompanied by relatively large amounts of pentalenic acid (1) (Fig. 4A). Furthermore, when the sav7469-deletion was introduced into the SUKA11 carrying pKU462::ptl-clusterΔptlH mutant the resultant double deletion mutant, SUKA13 carrying pKU462::ptl-clusterΔptlH, accumulated only 1-deoxypentalenic acid (5) (Fig. 4B). Pentalenic acid (1) production could be restored by introduction of an extra copy of sav7469, which was co-expressed along with two genes encoding the S. avermitilis electron-transport partners, ferredoxin (SAV_3129; FdxD) and ferredoxin reductase (SAV_5675; FprD) into the double deletion mutant (Fig. 4C) because the production of oxidative product(s) of epi-isozizaene derivatives was enhanced by co-expression of these two genes with sav3032-sav3031 [24]. These results indicate that pentalenic acid (1) is generated in S. avermitilis by hydroxylation of 1-deoxypentalenic acid (5) at C-1 catalyzed by CYP105D7.
Figure 3.
GC-MS analysis of EtOAc extracts treated with TMS-diazomethane of S. avermitilis SUKA5 (A) and SUKA7 (SUKA5 Δsav7469::aadA). The two strains were cultured at 28 °C with shaking at 200 rpm for 96 h.
Figure 4.
GC-MS analysis of EtOAc extracts treated with TMS-diazomethane of SUKA11 carrying pKU462::ptl-clusterΔptlH (A), SUKA13 (SUKA11 Δsav7469::aadA) carrying pKU462::ptl-clusterΔptlH (B), and SUKA13 carrying pKU462::ptl-clusterΔptlH and pKU493::rpsJp-sav7469-sav7470-sav3129-sav5675 (C).
Characterictics of CYP105D7
From a BLAST homology search using the NCBI nr database, S. avermitilis CYP105D7 was found to be similar at the amino acid sequence level to ZP_05504847 of Streptomyces sp. C (395 aa, 77% identity and 86% positive matches), BAG55292 of Streptomyces sp. A-1544 (409 aa, 75% identity and 87% positive matches), ZP_05536090 of S. viridochromogenes DSM 40736 (398 aa, 75% identity and 86% positive matches), ZP_05021349 of S. sviceus ATCC 29083 (413 aa, 75% identity and 85% positive matches), ZP_04683686 of S. ghanaensis ATCC 14672 (401 aa, 73% identity and 84% positive matches), ZP_05528097 (CYP105D4) of S. lividans TK24 (406 aa, 74% identity and 85% positive matches), NP_625076 (CYP105D5) of S. coelicolor A3(2) (412 aa, 74% identity and 85% positive matches), P26911 (CYP105D1) of S. griseus (412 aa, 71% identity and 80% positive matches) and BAG50412 of S. griseolus (395 aa, 69% identity and 80% positive matches).
To confirm the predicted function of CYP105D7, the recombinant protein was expressed in the T7-RNA polymerase-based expression host E. coli BL21 CodonPlus (DE3). Since induction with IPTG (0.1 to 0.5 mM) was not effective for the expression of sav7469, the cultivation for the E. coli transformant carrying pET17b::sav7469 was performed without IPTG induction. The soluble C-terminal His4-tagged CYP105D7 was purified by sequential Ni2+-affiny chromatography, ion-exchange chromatography, and gel filtration chromatography. The recovered deep red recombinant CYP105D7 (15 mg from 2 L of culture) was judged to be more than 90% pure by SDS-PAGE.
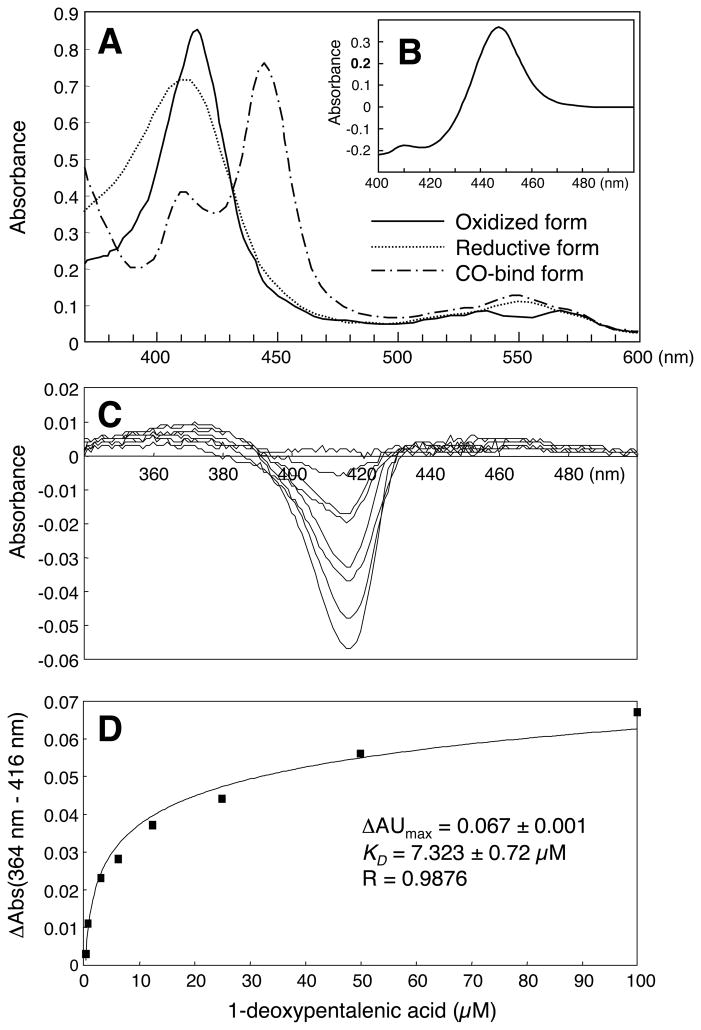
The UV-visible absorption spectrum of the oxidized-form of recombinant CYP105D7 showed a Soret peak plus β and α bands at 418, 534 and 568 nm, respectively, suggesting a typical low-spin state spectrum (Fig. 5A). The relatively sharp peak at 447 nm in the CO-difference spectrum of recombinant CYP105D7 confirmed that the protein was correctly folded (Fig. 5B). The difference spectra showed a typical type I spectral shift and positive peaks were observed in the difference binding spectra at 364 and 416 nm recorded at increasing concentrations of 5 (Fig. 5C). The plot of the measured delta value (ΔAU=A364 nm–A416 nm) versus substrate concentration is shown in Fig. 5D. The ΔAUmax and a KD values were calculated as 0.067±0.001 and 7.3±0.7 μM, respectively.
Figure 5.
UV spectra of oxidized, reduced and CO-bound forms of C-terminal His4-tagged CYP105D7 (A) and its CO-difference spectrum (B). UV difference binding spectra of CYP105D7 and 1-deoxypentalenic acid (5) binding (C), saturation curve for CYP105D7 and 1-deoxypentalenic acid (5) (D).
In vitro hydroxylation of 1-deoxypentalenic acid (5) to pentalenic acid (1) catalyzed by recombinant CYP105D7
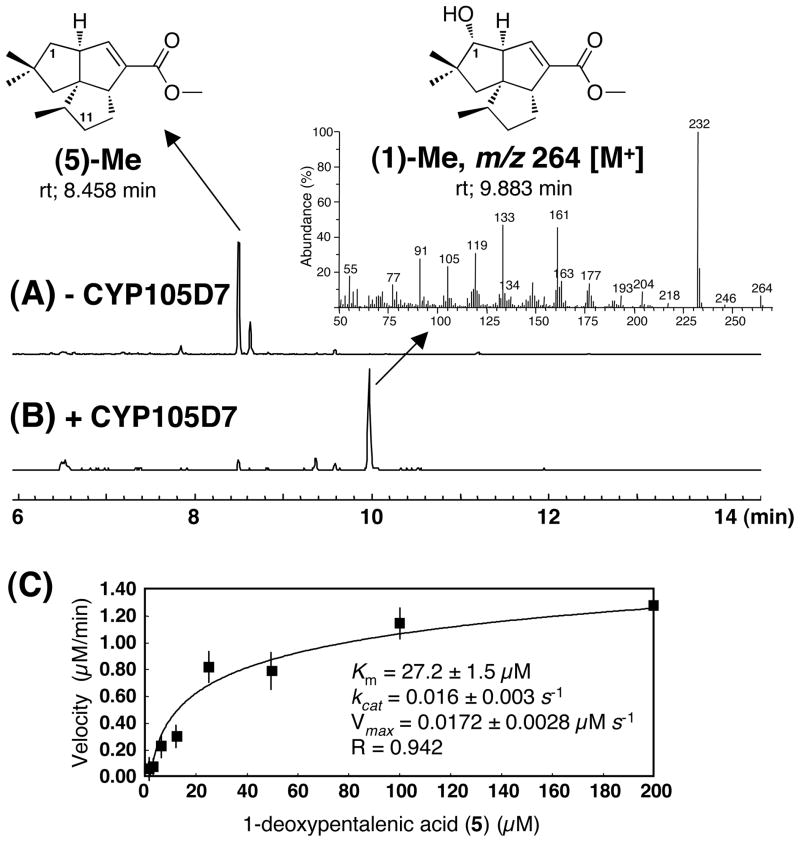
The reaction mixture was incubated in the presence of 2 μM recombinant CYP105D7 for 2 h at 30 °C with shaking. The substrate 1-deoxypentalenic acid (5) was almost completely consumed, based on the disappearance of the GC-MS peak (ret. time 8.458 min) corresponding to 5-methyl ester, while the formation of the product pentalenic acid (1) was evident from the appearance of a methyl ester peak (1-methyl ester), ret. time 9.883 min. In a parallel control incubation carried out in the absence of CYP105D7, there was no observable hydroxylation of 5 to 1. (Fig. 6A). The EI-MS fragmentation pattern and the retention time of the peak corresponding to pentalenic acid (1) methyl ester were identical to those of an authentic sample (Fig. 6B).
Figure 6.
GC-MS analysis of EtOAc extracts treated with TMS-diazomethane from incubation of 1-deoxypentalenic acid (5) with (B) or without (A) CYP105D7 protein in the presence of a NADPH-generating system, electron-transport partners (spinach ferredoxin and ferredoxin reductase) and Michaelis-Menten plot of initial velocity of formation of pentalenic acid (1) (C).
The pH-dependence of the CYP105D7-catalyzed reaction was measured at pH 6.0, 6.5, 7.0, 7.5 and 8.0. The maximum rate of formation of pentalenic acid (1) was observed at pH 8.0 (data not shown). The steady-state kinetic parameters of the CYP105D7-catalyzed hydroxylation reaction were Km(5) of 27.2±2.5 μM, Vmax 0.0172±0.003 μM s−1 and kcat 0.116±0.003 s−1 (Fig. 6C). To examine the substrate specificity of CYP105D7, several intermediates of pentalenolactone biosynthesis were incubated with CYP105D7. The recombinant CYP105D7 protein could not catalyze the C-1 hydroxylation of pentalenene (2), 1-deoxy-11β-hydroxypentalenic acid (6), 1-deoxy-11-oxopentalenic acid (7), or the corresponding methyl esters of 6, 7 and 5, respectively. CYP105D7 is therefore specific for the C-1 hydroxylation of 1-deoxypentalenic acid (1).
Discussion
The wild type strain of S. avermitilis and its large-deletion derivative SUKA5, as well as S. lividans 1326 carrying the entire ptl cluster of S. avermitilis, each produce pentalenic acid (1), a known shunt metabolite in the biosynthesis of the pentalenolactone family of antibiotics [9]. In principle, pentalenic acid (1) might be formed by the hydroxylation of 1-deoxypentalenic acid (5) at C-1 or the oxidation of pentalenene (2) at both C-1 and C-13. Pentalenic acid (1) is normally isolated as a common co-metabolite of pentalenolactone and its derivatives, including the biosynthetic intermediate, 1-deoxypentalenic acid (5) [28]. Although at least four genes in the S. avermitilis ptl cluster have been shown to encode oxygenases implicated in the biosynthesis of neopentalenoketolactone biosynthesis, the gene responsible for the formation of pentalenic acid (1) by hydroxylation of 1-deoxypentalenic acid (5) is not present in the ptl cluster. Therefore, we sought to determine which of the 33 cytochrome P450 (CYP) genes that have been identified in the genome of S. avermitilis would be responsible for the generation of pentalenic acid (1). The altered product profile in the SUKA7 large deletion mutant that lacks the sav7469 gene encoding CYP105D7 indicates that production of pentalenic acid (1) depends on the presence of sav7469 gene. This relationship was further examined and supported using biosynthetically blocked mutants of the large-deletion mutants SUKA5 and SUKA7.
It is well-known that CYP105 family monooxygenases have a remarkable capacity for broad-based xenobiotic metabolism, utilizing compounds of diverse structure and chemistry [20, 29]. In the ptl cluster for the biosynthesis of neopentalenoketolactone, the only CYP gene involved in the formation of an intermediate for neopentalenolactones, is ptlI (sav2999) whose gene product (CYP183A1) has been shown to oxidize the C-13 methyl of pentalenene (2) to give 1-deoxypentalenic acid (5) [15]. Since no further metabolites of pentalenic acid (1) have been isolated from S. avermitilis, pentalenic acid (1) was thought to be formed by direct hydroxylation of 1-deoxypentalenic acid (5) catalyzed by CYP105D7. Notably, this C-1 oxidation involves removal of a different diastereotopic proton from that which is lost in the final oxidative rearrangement that results in the formation of pentalenolactone [9].
The in vitro enzymatic reaction unambiguously established that CYP105D7 is responsible for the conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1). Although CYP105D7 has been shown to have a relatively broad substrate range, being able to catalyze the hydroxylation of milbemycin A5, compactin, diclofenac, lauric acid and narigenin (unpublished data), no pentalenolactone-related compounds other than 1-deoxypentalenic acid (5) could be hydroxylated by CYP105D7. Since pentalenene (2) was not oxidized by CYP105D7, the formation of the shunt metabolite pentalenic acid (1) in S. avermitilis is conclusively shown to involve sequence conversion of farnesyl diphosphate to pentalenene (2) by pentalenene synthase (PtlA), PtlI-catalyzed oxidation of 2 to 1-deoxypentalenic acid (5), and finally CYP105D7-catalyzed hydroxylation of 5 is to pentalenic acid (1). We have previously demonstrated that exoconjugants of S. lividans 1326 carrying the S. avermitilis ptl cluster produced only pentalenic acid (1) [14]. Since S. lividans also possesses a gene encoding a member of the CYP105D subfamily of monooxygenases, CYP105D4 [20], the S. lividans exoconjugant carrying the ptl cluster most likely generated 1-deoxypentalenic acid (5) which presumably served as the substrate for hydroxylation by CYP105D4 to pentalenic acid (1). Genes encoding CYP105D subfamily monooxygenases have been found in all genome-sequenced Streptomyces, including draft genome sequenced microorganisms. It is therefore likely that other pentalenolactone-producing Streptomyces strains might possess the similar genes encoding members of the CYP105D subfamily of monooxygenases. Accumulation of the shunt product pentalenic acid (1) in pentalenolactone-producing microorganisms would therefore result from the abortive hydroxylation of the normal 1-deoxypentalenic acid (5) intermediate by CYP105D subfamily monooxygenase in the producing microorganism. Streptomyces exfoliates UC5319 also accumulates pentalenolactone H as a minor component, derived by C-1 hydroxylation of pentalenolactone F [9]. It is likely that a specific CYP105D subfamily monooxygenase in this microorganism might be able to catalyze the requisite oxidtion of pentalenolactone F.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan, a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) 20310122 and a research grant of the Institute for Fermentation, Osaka, Japan (H.I.), and by NIH Grant GM30301 (D.E.C.).
References
- 1.Koe BK, Sobin BA, Celmer WD. PA 132, a New Antibiotic. I. Isolation and Chemical Properties. Antibiot Annu. 1957:672–675. [PubMed] [Google Scholar]
- 2.Martin DG, Slomp G, Mizsak S, Duchamp DJ, Chidester CG. The Structure and Absolute Configuration of Pentalenolactone (PA 132) Tetrahedron Lett. 1970;11:4901–4904. doi: 10.1016/s0040-4039(00)99739-9. [DOI] [PubMed] [Google Scholar]
- 3.Keller-Schierlein W, Lemke J, Nyfeler R, Zähner H. Stoffwechelprodukte von Mikroorganismen. 105. Arenaemycin E, D, und C. Arch Mikrobiol. 1972;84:301–316. [PubMed] [Google Scholar]
- 4.Takahashi S, Takeuchi M, Arai M, Seto H, Otake N. Studies on the Biosynthesis of Pentalenolactone. V. Isolation of Deoxypentalenylglucuron. J Antibiot. 1983;36:226–228. doi: 10.7164/antibiotics.36.226. [DOI] [PubMed] [Google Scholar]
- 5.English AR, McBride TJ, Lynch JE. PA 132, A New Antibiotic. II. In Vitro and in Vivo Studies. Antibiot Annu. 1957:682–687. [PubMed] [Google Scholar]
- 6.Nakagawa A, Tomoda H, Hao MV, Okano K, Iwai Y, Omura S. Antiviral Activities of Pentalenolactones. J Antibiot. 1985;38:1114–1115. doi: 10.7164/antibiotics.38.1114. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda M, Fukuda A, Takagi M, Morita M, Shimada Y. Inhibitory effect of pentalenolactone on vascular smooth muscle cell proliferation. Eur J Pharm. 2001;411:45–53. doi: 10.1016/s0014-2999(00)00894-3. [DOI] [PubMed] [Google Scholar]
- 8.Seto H, Sasaki T, Uzawa J, Takeuchi S, Yonehara H. Studies on the Biosynthesis of Pentalenolactone. Part II. Isolation of Pentalenic Acid and Pentalenolactone H. Tetrahedron Lett. 1978;19 :4411–4412. [Google Scholar]
- 9.Cane DE, Oliver JS, Harrison PHM, Abell C, Hubbard BR, Kane CT, Lattman R. The Biosynthesis of Pentalenene and Pentalenolactone. J Am Chem Soc. 1990;112:4513–4524. [Google Scholar]
- 10.Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol. 2003;21:526–531. doi: 10.1038/nbt820. [DOI] [PubMed] [Google Scholar]
- 11.Nett M. Ikeda H. Moore BS. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat Prod Rep. 2009;26:1362–1384. doi: 10.1039/b817069j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cane DE, He X, Kobayashi S, Omura S, Ikeda H. Geosmin biosynthesis in Streptomyces avermitilis. Molecular cloning, expression and mechanistic study of the Germacradienol/Geosmin synthase. J Antibiot. 2006;59:471–479. doi: 10.1038/ja.2006.66. [DOI] [PubMed] [Google Scholar]
- 13.Wayne KW, Chou W, Fanizza I, Uchiyama T, Komatsu M, Ikeda H, Cane DE. Genome Mining in Streptomyces avermitilis: Cloning and characterization of SAV_76, the synthase for a New sesquiterpene. Avermitilol. J Am Chem Soc. 2010;132:8850–8851. doi: 10.1021/ja103087w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tetzlaff CN, You Z, Cane DE, Takamatsu S, Omura S, Ikeda H. A gene cluster for biosynthesis of the sesquiterpenoid antibiotic pentalenolactone in Streptomyces avermitilis. Biochemistry. 2006;45:6179–6186. doi: 10.1021/bi060419n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quaderer R, Omura S, Ikeda H, Cane DE. Pentalenolactone biosynthesis. Molecular cloning and assignment of biochemical function to PtlI, a cytochrome P450 of Streptomyces avermitilis. J Am Chem Soc. 2006;128:13036–13037. doi: 10.1021/ja0639214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You Z, Omura S, Ikeda H, Cane DE. Pentalenolactone biosynthesis. Molecular cloning and assignment of biochemical function to PtlH, a non-heme iron dioxygenase of Streptomyces avermitilis. J Am Chem Soc. 2006;128:6566–6567. doi: 10.1021/ja061469i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You Z, Omura S, Ikeda H, Cane DE, Jogl G. Crystal structure of the non-heme iron dioxygenase PtlH in pentalenolactone biosynthesis. J Biol Chem. 2007;282:36552–36560. doi: 10.1074/jbc.M706358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You Z, Omura S, Ikeda H, Cane DE. Pentalenolactone biosynthesis: Molecular cloning and assignment of biochemical function to PtlF, a short-chain dehydrogenase from Streptomyces avermitilis, and identification of a new biosynthetic intermediate. Arch Biochem Biophys. 2007;459:233–240. doi: 10.1016/j.abb.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Tetzlaff CN, Takamatsu S, Iwatsuki M, Komatsu M, Ikeda H, Cane DE. Genome Mining in Streptomyces avermitilis: A Biochemical Baeyer-Villiger Reaction and Discovery of a New Branch of the Pentalenolactone Family Tree. Biochemistry. 2009;48:6431–6440. doi: 10.1021/bi900766w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamb DC, Ikeda H, Nelson DR, Ishikawa J, Skaug T, Jackson C, Omura S, Waterman MR, Kelly SL. Cytochrome p450 complement (CYPome) of the avermectin-producer Streptomyces avermitilis and comparison to that of Streptomyces coelicolor A3(2) Biochem Biophys Res Commun. 2003;307:610–619. doi: 10.1016/s0006-291x(03)01231-2. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu M, Uchiyama T, Omura S, Cane DE, Ikeda H. Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci USA. 2010;107:2646–2651. doi: 10.1073/pnas.0914833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda H, Kotaki H, Omura S. Genetic studies of avermectin biosynthesis in Streptomyces avermitilis. J Bacteriol. 1987;169:5615–5621. doi: 10.1128/jb.169.12.5615-5621.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda H, Takada Y, Pang CH, Tanaka H. Omura S. Transposon mutagenesis by Tn4560 and applications with avermectin-producing Streptomyces avermitilis. J Bacteriol. 1993;175:2077–2082. doi: 10.1128/jb.175.7.2077-2082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takamatsu S, Lin X, Nara A, Komatsu M, Cane DE, Ikeda H. Characterization of a silent sesquiterpenoid biosynthetic pathway in Streptomyces avermitilis controlling epi-isozizaene and albaflavenone biosynthesis and isolation of a new oxidized epi-isozizaene metabolite. Microb Biotechnol. doi: 10.1111/j.1751-7915.2010.00209.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu M, Tsuda M, Omura S, Oikawa H, Ikeda H. Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc Natl Acad Sci USA. 2008;105:7422–7427. doi: 10.1073/pnas.0802312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu LH, Fushinobu S, Ikeda H, Wakagi T, Shoun H. Crystal structures of cytochrome P450 105P1 from Streptomyces avermitilis: conformational flexibility and histidine ligation state. J Bacteriol. 2009;191:1211–1219. doi: 10.1128/JB.01276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 28.Cane DE, Sohng JK, Williard PG. Isolation and structure determination of pentalenolactones A, B, D, and F. J Am Chem Soc. 1992;57:844–851. [Google Scholar]
- 29.Lamb DC, Skaug T, Song SL, Jackson CJ, Podust LM, Waterman MR, Kell DB, Kelly DE, Kelly SL. The cytochrome P450 complement (CYPome) of Streptomyces coelicolor A3(2) J Biol Chem. 2002;277:24000–24005. doi: 10.1074/jbc.M111109200. [DOI] [PubMed] [Google Scholar]