Abstract
Purpose
Treatment options are limited for advanced pancreatic cancer progressive after gemcitabine therapy. The vascular endothelial growth factor (VEGF) pathway is biologically important in pancreatic cancer, and docetaxel has modest anti-tumor activity. We evaluated the role of the anti-VEGF antibody bevacizumab as second-line treatment for patients with metastatic pancreatic cancer.
Design
Patients with metastatic adenocarcinoma of the pancreas who had progressive disease on a gemcitabine-containing regimen were randomized to receive bevacizumab alone or bevacizumab in combination with docetaxel.
Results
Thirty-two patients were enrolled; 16 to bevacizumab alone (Arm A) and 16 to bevacizumab plus docetaxel (Arm B). Toxicities were greater in Arm B with the most common grade 3/4 nonhematologic toxicities including fatigue, diarrhea, dehydration and anorexia. No confirmed objective responses were observed. At 4 months, 2/16 patients in Arm A and 3/16 in Arm B were free from progression. The study was stopped according to the early stopping rule for futility. Median PFS and OS were 43 days and 165 days in Arm A and 48 days and 125 days in Arm B. Elevated D-dimer levels and thrombin-antithrombin complexes were associated with decreased survival and increased toxicity.
Conclusion
Bevacizumab with or without docetaxel does not have antitumor activity in gemcitabine-refractory metastatic pancreatic cancer. Baseline and on-treatment D-dimer and thrombin-antithrombin complex levels are associated with increased toxicity and decreased survival.
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer death in the United States, with over 37,000 new cases in 2008, and a nearly equal number of deaths. The current standard initial therapy for metastatic pancreatic adenocarcinoma is gemcitabine-based [1, 2]. However, median survival remains approximately 6 months. Prospective studies of chemotherapy in patients who remained eligible for second-line therapy revealed low objective response rates and poor survival [3–6].
Vascular endothelial growth factor (VEGF) is produced by multiple cancers in an autocrine fashion to promote tumor neovascularization and directly stimulate tumor cells [7]. When compared to normal pancreas, human pancreatic cancers demonstrate increased expression of VEGF and its receptors [8]. Frequently, these angiogenic molecules are associated with increased microvessel density and upregulation of tissue factor [9,10], a 47-kDa transmembrane protein that is the principal trigger of the intrinsic coagulation cascade [10]. Activation of the coagulation system in pancreatic cancer through tissue factor may contribute not only to clinical thrombosis but also to tumor progression and chemotherapy resistance, mediated by activated thrombin leading to endothelial cell proliferation [11, 12].
Bone marrow-derived cells containing endothelial-specific antigens (circulating endothelial cells, or CEC) have been identified and characterized in the blood of patients with malignancy and may be a surrogate marker of angiogenesis [13]. Drugs targeting the VEGF pathway have been shown to work synergistically with paclitaxel to ameliorate a chemotherapy-induced surge of CEC [14]. We sought to determine if bevacizumab alone or in combination with docetaxel would effect the levels of CEC and proangiogenic growth factors such as VEGF, bFGF [15], tissue factor [10] and angiogenic coagulation factors [11].
In colorectal, lung and breast cancer, the combination of the anti-VEGF antibody bevacizumab with chemotherapy results in improved clinical outcome with non-overlapping toxicities [16–19]. Given the modest activity of docetaxel in pancreatic cancer [20] and the potential for exploiting angiogenic inhibition for therapeutic gain, we conducted this randomized phase II trial of bevacizumab with or without docetaxel for patients with gemcitabine-refractory metastatic pancreatic cancer. The primary objective was to assess progression-free survival, with evaluation of toxicity, coagulation, and angiogenic biomarkers as secondary objectives. Given the potential interaction of VEGF and coagulation markers on toxicities of bevacizumab therapy, we hypothesized that these markers would correlate with patient toxicity in this trial and evaluated this as an exploratory objective.
PATIENTS AND METHODS
Patients
Eligible patients had measurable, metastatic pancreatic adenocarcinoma which had progressed on one prior gemcitabine-containing regimen completed at least 4 weeks prior to enrollment. Patients who received gemcitabine as a component of adjuvant therapy and recurred within 6 months were eligible in the absence of other treatment for metastatic disease. Other eligibility criteria included: ECOG performance status of 0–1, adequate bone marrow (granulocytes ≥1,500/uL, leukocytes ≥3,000/uL, platelets ≥100,000/uL, hemoglobin ≥9 g/dL), renal (creatinine ≤2 mg/dL), and hepatic function (normal total bilirubin and transaminases ≤1.5x the upper limit of normal), and urine protein:creatinine (UPC) ratio <1.0.
Exclusion criteria included pregnancy or lactation, clinically significant cardiovascular disease (e.g., blood pressure of >160/110 mmHg on medication, previous myocardial infarction within 6 months, unstable angina), New York Heart Association (NYHA) Grade II or greater congestive heart failure, ventricular dysrhythmia requiring medication, clinically significant peripheral vascular disease, history of transient ischemic attack or cerebrovascular accident (stroke) within 6 months, or any brain metastases. Patients with prior history of a bleeding diathesis or coagulopathy were ineligible, but those on therapeutic anticoagulation were eligible. While the protocol initially excluded patients on therapeutic anticoagulation, expanding experience with bevacizumab demonstrated safety with therapeutic anticoagulation and this became standard in bevacizumab trials during this study’s conduct. All patients provided written informed consent according to federal and institutional guidelines and the study was approved by the Institutional Review Board at Fox Chase Cancer Center.
Drug Administration and Study Design
The study was conducted at Fox Chase Cancer Center between October, 2004 and December, 2006. Patients were randomly assigned in a 1:1 fashion to two concurrent cohorts. Those assigned to bevacizumab alone (Arm A) received bevacizumab at a dose of 10 mg/kg by intravenous infusion over 30–90 minutes once every 2 weeks. Patients assigned to receive bevacizumab plus docetaxel (Arm B) received bevacizumab as in Arm A with weekly docetaxel at a dose of 35 mg/m2 given intravenously over 1 hour on days 1, 8, and 15 of each 28 day cycle. Patients receiving docetaxel were given 8 mg of dexamethasone the night before treatment, within one hour of therapy, and 12 hours after each weekly docetaxel dose.
Cycle length was 28 days. CT scans of the chest, abdomen, and pelvis were performed at baseline and after every 2 cycles of therapy and response evaluated by RECIST criteria [21]. Study treatment continued until evidence of disease progression, unacceptable toxicity, or patient preference.
Dose Adjustments
Toxicity was graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE) v.3.0 (http://ctep.cancer.gov/reporting/ctc.html). The dose of docetaxel was held for ANC <1,000/mm3 or platelet count <75,000/mm3 until recovery. For febrile neutropenia or grade 4 thrombocytopenia, subsequent doses of docetaxel were reduced by 25%. For grade 3/4 non-hematologic toxicity attributable to docetaxel, the dose of docetaxel was held until recovery to ≤ grade 2 and restarted with a 25% dose-reduction. Bevacizumab was initially held for >500 mg protein/24 hour when the study began. With expanding safety experience with bevacizumab, this was amended during the study to hold bevacizumab for UPC ratio ≥3.5 or grade 2/3 hypertension and restart with a decrease to 5 mg/kg upon resolution. Bevacizumab was also held for development of DVT/PE and restarted at a dose of 5 mg/kg upon establishment of stable anticoagulation. Bevacizumab was permanently discontinued for development of bowel perforation or arterial thromboembolic event.
Correlative Studies
Coagulation Markers
Ten ml of peripheral blood were obtained on days 1, 15, 29, and 43 for evaluation of coagulation markers. D-dimer plasma concentrations were measured with the STA Liatest D-Di on the STAR coagulation analyzer. Serum thrombin-antithrombin levels, prothrombin fragment 1+ 2, tissue factor antigen and fibrinogen determination followed standard commercial procedures in the clinical laboratory.
VEGF and bFGF plasma levels
Eight ml of peripheral blood were obtained on days 1, 15, 29, 43, and upon removal from study for evaluation of VEGF and bFGF plasma levels. The 8 ml blood sample was collected into yellow top vacutainer tubes containing acid citrate dextrose, centrifuged and plasma aliquoted and stored at −70°C. Enzyme-linked immunosorbent assay (ELISA) was used to determine plasma VEGF and bFGF levels using a quantitative sandwich enzyme immunoassay technique (Quantikine® Human VEGF and bFGF Immunoassay, R&D Systems, Minneapolis, MN) according to the manufacturer protocol.
Circulating endothelial cells (CEC)
CEC were identified by flow cytometry. 6.5 ml of whole blood was diluted with PBS and then layered onto Ficoll-Paque for isolation of peripheral blood mononuclear cells layer by gradient centrifugation. The cells were incubated with fluorochrome-conjugated antibodies for 30 minutes at 4°C in the dark, washed in FACS buffer and fixed with 1% paraformaldehyde. Propidium iodide was added to cells 24 hours later to identify nucleated cells. CEC were characterized as CD45 negative and CD146 positive. Additional phenotypic evaluation was performed using a panel of monoclonal antibodies which included anti-CD34, annexin V (apoptosis), CD133, and VEGFR2 (anti-KDR).
Statistical Considerations
The primary endpoint for this trial was progression-free survival (PFS), defined as the time from randomization to progression or death. At the time our study began, the most favorable reports from second-line pancreatic cancer trials reported median PFS of 3 months. As 2 months would represent our initial disease evaluation, we projected that a proportion of patients progression-free at 4 months of less than 25% would be of no interest. The study treatment would be of interest if the proportion of patients progression-free at 4 months was at least 50%. Twenty-three patients per arm would be needed to test the null hypothesis: p≤0.25 against the alternative hypothesis: p≥0.5 at the 19.3% level of significance and with 95.1% power. The trial employed an early stopping rule for futility. If ≤3 of the first 16 patients enrolled to each treatment arm were progression-free at 4 months, that treatment arm would be terminated. Overall (OS) and progression-free survival (PFS) were estimated by Kaplan-Meier methodology.
Secondary clinical objectives included evaluation of toxicity, response rate, and overall survival. Secondary laboratory correlative objectives included assessing the relationship of coagulation markers, circulating endothelial cells, VEGF, and bFGF to clinical outcome. We investigated the relationship between the correlative biomarkers and clinical outcomes using regression analysis to find significant covariates. The analysis was conducted in SAS and Minitab software. A covariate was selected as a significant predictor if its p value from the mixed model analysis was less than 0.05.
Toxicity Index calculations
Toxicity data for each patient at each cycle were summarized into a Toxicity Index (TI, range 0–5), computed according to previously published methodology by our group [22]. We chose to calculate the TI for this study as an exploratory analysis of the toxicity for a given patient. The TI can be generalized to accommodate the differential impact of various toxicities by applying relative weights or appropriate transformations to the CTCAE-graded toxicities before the application of the algorithm to compute the TI. For example, we considered grade 3 neutropenia, leukopenia, and anemia non-dose-limiting, and we re-coded these as the observed CTCAE grade minus 1 (to a minimum of 0 if no toxicity was present). By design, a TI score greater than or equal to 3 corresponds to the toxicity grade definition given by CTCAE and the maximum toxicity grade is the integer part of the final score. For example, a TI of 3.0 indicates a single grade 3 toxicity, whereas a score of 3.5 indicates that the patient experienced at least one grade 3 toxicity plus additional toxicity. Thus, the TI preserves the highest toxicity grade. All toxicity grades are taken into account, although lower grades will tend to contribute less to the final score. Nonetheless, a large number of toxicities of the same grade will generate a TI score just slightly less than that generated by a single toxicity of the next higher grade.
The Generalized Estimating Equations (GEEs) approach was used to analyze the correlated TI data. The GEE method employs the quasi-likelihood approach in the model which avoids the need of a distributional assumption about the response. Autoregressive correlation matrix structure was used for GEE method. Since most covariates were the measurement of biomarkers at different time points and highly correlated, univariate analysis was implemented to find significant covariates in predicting the correlated TI data. The procedure of GENMOD in SAS was used. The analysis was conducted in SAS and Minitab software. A covariate was selected as a significant predictor if its p value from the mixed model analysis was less than 0.05.
RESULTS
Patient Characteristics
Thirty-two patients were enrolled in this phase II trial; 16 assigned to Arm A (bevacizumab alone) and 16 to Arm B (bevacizumab plus docetaxel). Patient characteristics are listed in Table 1. Twenty two patients had liver metastases. The majority had an ECOG PS of 1. A total of 98 cycles of therapy were delivered: 16 patients in Arm A received 44 cycles of bevacizumab (median 2, average 2.75 cycles per patient) and 16 patients in arm B received 54 cycles of therapy (median 3, average 3.33 cycles per patient).
Table 1.
Patient characteristics.
| Characteristic | Arm A | Arm B |
|---|---|---|
| Total | 16 | 16 |
| Age median, years | 67 | 56.5 |
| Average cycles | 2.75 | 3.33 |
| Total cycles | 44 | 50 |
| Male | 6 | 8 |
| Female | 10 | 8 |
| ECOG PS0 | 7 | 5 |
| PS1 | 9 | 11 |
| Metastatic disease | All 16 | All 16 |
| Sites of disease | ||
| Liver | 10 | 12 |
| Lymph nodes | 2 | 2 |
| Lung | 5 | 4 |
| Peritoneum | 4 | 2 |
| Previous surgery | 5 | 8 |
| Previous chemoradiation | 5 | 8 |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status
Toxicity
Table 2 lists all toxicities by treatment arm and grade regardless of attribution. As expected, both hematologic and non-hematologic toxicities were more common in Arm B compared to Arm A. In Arm B, 4/16 (25%) developed grade 3/4 neutropenia necessitating docetaxel dose adjustment. The most common grade 3/4 non-hematologic toxicities in Arm B included nausea/vomiting, hypoalbuminemia, and fatigue. Bevacizumab dose adjustment or discontinuation was performed in 4 patients in each arm [proteinuria (2), hypertension (2), thrombosis (3), and perforation (1)]. Seven patients in Arm A and eight in Arm B had SAEs, including 3 episodes of DVT and 2 of PE, one grade 4 gastrointestinal bleeding, and one bowel perforation. Two patients treated with docetaxel and bevacizumab had cholangitis and liver abscesses judged as probably related to treatment. As anticipated, development of an SAE was highly associated with decreased survival, HR=4.14, p=0.001 (Table 3).
Table 2.
Toxicity data by grade (all attributions)
| Toxicity grade: | Number of patients | |||
|---|---|---|---|---|
| Arm A (n=16) | Arm B (n=16) | |||
| 1–2 | 3–4 | 1–2 | 3–4 | |
| Non-hematologic | ||||
| Anorexia | 10 | 0 | 9 | 2 |
| Cholangitis | - | 0 | - | 2 |
| Dehydration | 0 | 3 | 1 | 3 |
| Diarrhea | 2 | 0 | 8 | 2 |
| DVT/Pulmonary embolism | 0 | 2/1 | 0 | 0/2 |
| Dysgeusia | 0 | 0 | 3 | 0 |
| Fatigue | 12 | 1 | 11 | 4 |
| GI bleeding | - | 1 | - | 2 |
| Hyperkalemia | 2 | 0 | 2 | 2 |
| Hyperuricemia | 0 | 2 | 0 | 0 |
| Hypoalbuminemia | 9 | 0 | 6 | 4 |
| Hypertension | 2 | 1 | 1 | 0 |
| Injection site rxn | 0 | 0 | 1 | 1 |
| Nail changes | 0 | 0 | 3 | 1 |
| Nausea and vomiting | 6 | 1 | 5 | 4 |
| Paresthesias | 0 | 0 | 3 | 1 |
| Perforation | 0 | 0 | 0 | 1 |
| Peritonitis | - | 1 | - | 1 |
| Proteinuria | 6 | 0 | 7 | 1 |
| Watery eyes | 0 | 0 | 6 | 0 |
| Hematologic | ||||
| Anemia | 12 | 0 | 13 | 2 |
| Leukopenia | 5 | 0 | 5 | 6 |
| Neutropenia | 4 | 0 | 3 | 4 |
| Thrombocytopenia | 2 | 0 | 3 | 0 |
Table 3.
Univariate analysis of factors predicting overall survival (OS) using Cox regression model.
| Overall Survival | ||
|---|---|---|
| Variable | p-value | Hazard Ratio |
| SAE | 0.001 | 4.14 |
| Dd-C1D1 | <0.0001 | 1.32 |
| Dd-C2D1 | 0.013 | 1.45 |
| Dd-C2D15 | 0.006 | 1.40 |
| TAT-C1D15 | 0.038 | 1.10 |
| TAT-C2D15 | 0.002 | 1.33 |
Abbreviations: SAE, severe adverse event; Dd, D-dimer; TAT, thrombin/antithrombin complex; C1D1 denotes results obtained on day 1 of cycle 1, respectively.
To estimate the additive effect of multiple heterogeneous treatment side effects, we used the toxicity index (TI) which is a number between 0 and 5 taking into account interactions of multiple toxicities: any score greater than or equal to 3 corresponds to a DLT, and the maximum toxicity grade is the integer part of the final score. After adjusting for correlated outcome data and controlling for cycle, the TI for those in treatment Arm A (average 0.89, range 0–4.78) was lower by 50% (shown as a factor.−0.506, p=0.02, 95% CI: [−1.11, −0.10], Table 4) than that for patients in Arm B (average 1.55, range 0–4.95). This indicates that combination of docetaxel with bevacizumab is an overall more toxic regimen compared to bevacizumab alone.
Table 4.
Univariate analysis of factors predicting toxicity index (TI).
| Parameter | Parameter coefficient†† | Standard Error | Lower 95% CI* | Upper 95% CI | P value |
|---|---|---|---|---|---|
| ARM A† | −0.506 | 0.200 | −0.897 | −0.114 | 0.011 |
| SAE | 0.663 | 0.220 | 0.232 | 1.095 | 0.003 |
| Dd-C1D1 | 0.116 | 0.031 | 0.054 | 0.177 | <0.0001 |
| Dd-C3D1 | 0.464 | 0.133 | 0.203 | 0.724 | <0.0001 |
| Dd-C3D15 | 0.242 | 0.102 | 0.041 | 0.442 | 0.018 |
| TAT-C3D15 | 0.282 | 0.060 | 0.164 | 0.400 | <0.0001 |
| CEC-C2D1 | 0.002 | 0.001 | 0.000 | 0.004 | 0.025 |
| VEGF-C2D1 | −0.005 | 0.002 | −0.009 | 0.000 | 0.033 |
| VEGF-C2D15 | −0.005 | 0.002 | −0.009 | 0.000 | 0.030 |
Patients on Arm A (bevacizumab only) had lower TI.
Parameter coefficient describes amount of change in the TI if a parameter changes by one unit.
CI, confidence interval.
SAE, severe adverse event; Dd, D-dimer; TAT, thrombin/antithrombin complex; CEC, circulating endothelial cells; VEGF, vascular endothelial growth factor
Clinical Outcome
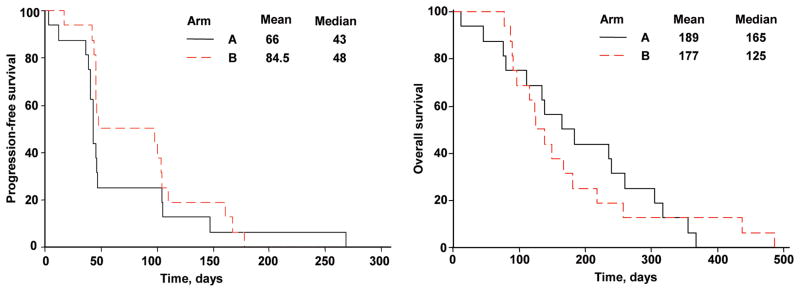
All patients who entered the study have died. The best response on the first evaluation at 2 months was stable disease in 4 patients in Arm A and in 8 patients treated in Arm B. There were no confirmed responses. At 4 months, only 2/16 patients in Arm A and 3/16 in Arm B were free from progression. Thus, the study was stopped according to the early stopping rule for futility. Median PFS and OS were 43 days and 165 days in Arm A and 48 days and 125 days in Arm B (Figures 1A and 1B).
Figure 1.
Progression-free survival (A) and overall survival (B) for all patients by treatment arm.
Correlative studies
Coagulation Parameters
All 32 patients had a total of 150 peripheral blood specimens drawn for coagulation parameters during the first 2 cycles of therapy. A Cox regression model was used to conduct the univariate analysis to determine significant covariates associated with overall survival. Elevated D-dimer levels obtained at three timepoints (C1D1, C2D1, and C2D15) were associated with worse OS with respective hazard ratios of 1.32 (p<0.001), 1.45 (p=0.013) and 1.40 (p=0.006) (Table 3). Elevated thrombin-antithrombin complex levels on treatment (C1D15 and C2D15) were weakly associated with decreased survival (Table 3). Other coagulation parameters were not associated with survival.
Increased pre- and on-treatment D-dimer levels were associated with increased risk for SAE (not shown, p<0.001, Mann-Whitney test) and high TI (Table 4) (p<0.001, Mann-Whitney test). Elevated thrombin-antithrombin complex level at one time-point (C3D15) was associated with increased TI (Table 4).
VEGF and circulating endothelial cells
Pre- and on-treatment plasma VEGF levels were obtained in all 32 patients and ranged from 13.7 to 759 pg/mL (median, 67.7 pg/mL). There was no relationship between baseline or on-treatment VEGF levels and response to therapy, PFS, or OS (data not shown). Similarly, there was no clear relationship between baseline or on-treatment CEC level and clinical outcome. There was a weak relationship between elevated VEGF and CEC levels on treatment and increased TI (Table 4).
DISCUSSION
In this randomized Phase II clinical trial we found that bevacizumab with or without docetaxel had no significant activity as second-line therapy for patients with metastatic pancreatic adenocarcinoma. Only 3/16 patients in the docetaxel plus bevacizumab arm and 2/16 in the bevacizumab alone treatment arm were free of progression at 4 months and thus accrual to the trial was halted per the early stopping rule. Exploratory coagulation studies suggest that baseline and on-treatment D-dimer and thrombin-antithrombin complex levels are associated with increased toxicity and decreased survival.
Our findings demonstrating a lack of clinical benefit of bevacizumab in pancreatic cancer are disappointing, given recent benefit in other diseases including colorectal, lung, and breast cancer [17–19]. However, they are consistent with the results of Kindler et al. who reported the results of a large phase III trial of gemcitabine with or without bevacizumab utilizing the same dose (10 mg/kg) that we chose for this trial [23]. The authors reported no improvement in overall survival with the addition of bevacizumab to gemcitabine in the front-line setting. Why bevacizumab therapy improves outcome in other malignancies but not in pancreatic cancer is unclear. One proposed mechanism of bevacizumab therapy is a normalization of peritumoral blood vessels which allows improved chemotherapy delivery to the tumor [24]. Chemotherapy regimens result in much higher objective antitumor activity in colorectal, breast, and lung cancer compared to pancreatic cancer. In colorectal cancer, bevacizumab monotherapy does not have clear clinical activity but improves efficacy of active chemotherapy agents [16]. Thus, one limitation of bevacizumab-based therapy in pancreatic cancer may be a lack of an effective chemotherapy partner. Additionally, VEGF depletion in pancreatic adenocarcinoma through bevacizumab may be insufficient to interfere with tumor angiogenesis and VEGFR signaling due to redundancy in VEGF family ligands and receptors [25, 26]. Since our trial was developed, the combination of 5-FU and oxaliplatin has been reported to improve PFS and OS compared to 5-FU alone in the second-line treatment of advanced pancreatic cancer [27]. Whether bevacizumab may potentiate this chemotherapy combination in a similar manner to colorectal cancer requires future study.
We utilized the toxicity index (TI) to characterize toxicity for this trial. This methodology has been described previously and represents an effort to give a more global assessment of the toxicity that each patient is experiencing by accommodating their differential impact [22]. In a disease such as pancreatic cancer where patients frequently experience multiple toxicities and disease-related morbidities, the TI may be of higher value in providing a complete impression of toxicity. As expected, the TI was higher for the combination compared to the single agent bevacizumab treatment arm. In terms of bevacizumab-related toxicities, we observed 5 episodes of DVT/PE and one bowel perforation. While our bowel perforation rate is higher than that reported by Kindler et al. [23, 28] and in colorectal cancer trials [29], this likely reflects our smaller sample size. Our DVT/PE rate was typical for advanced pancreatic cancer patients and for cancer patients in general receiving bevacizumab [30].
Deep venous thrombosis is common in pancreatic cancer and has been associated with decreased response to chemotherapy and shorter PFS and OS [31]. Some evidence suggests that anticoagulation can improve survival in selected subgroups of patients with advanced malignancy [32]. We thus hypothesized that plasma coagulation factors would similarly be associated with worse outcome and increased toxicity. The relationship was strongest for D-dimer, with elevated levels both at baseline and on treatment associated with inferior survival and increased toxicity. Our findings with D-dimer parallel several reports on the prognostic significance of D-dimer on OS in lung cancer [33–35], ovarian cancer [36] and breast cancer [37]. While the sample size of the current trial is modest. Thrombin/antithrombin complexes are also a marker of coagulation activation and significantly elevated in the blood of cancer patients compared to healthy controls [38, 39]. The relationship of elevated TAT levels to increased toxicity and decreased survival was more modest in our study and confined to specific time points.
The development of a plasma biomarker of bevacizumab effect would be of high clinical value. We hypothesized at the conception of this trial that plasma CEC and VEGF levels might predict for clinical outcome from bevacizumab therapy. Nucleated cells containing endothelial-specific antigens (circulating endothelial cells, or CEC) have been identified and characterized in the blood of patients with a number of pathologic conditions, including malignancy [40, 41]. However, we found no clear relationship of either marker to clinical outcome in this cohort of pancreatic cancer patients receiving bevacizumab. Kindler et al. also found no relationship of circulating VEGF levels to clinical benefit from bevacizumab in pancreatic cancer [28]. While CEC remain an attractive potential marker of anti-angiogenic therapy, their use remains investigational.
In conclusion, we observed no evidence of clinical activity of bevacizumab alone or with docetaxel in pancreatic cancer patients previously treated with gemcitabine. However, baseline and on-treatment D-dimer and thrombin-antithrombin complex levels are associated with increased toxicity and decreased survival.
Acknowledgments
Support: This publication was supported by Genentech Inc. and by the FCCC Core Grant P30 CA006927 from the NIH.
Footnotes
Author Disclosures: Barbara Burtness: research funding from Genentech and consultancy to Genentech and Sanofi.
References
- 1.Burris HA, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 3.Reni M, et al. A multi-centre retrospective review of second-line therapy in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2008 doi: 10.1007/s00280-007-0653-y. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich-Pur H, et al. Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer. 2003;88(8):1180–4. doi: 10.1038/sj.bjc.6600883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebbia V, et al. Second-line chemotherapy in advanced pancreatic carcinoma: a multicenter survey of the Gruppo Oncologico Italia Meridionale on the activity and safety of the FOLFOX4 regimen in clinical practice. Ann Oncol. 2007;18(Suppl 6):vi124–7. doi: 10.1093/annonc/mdm240. [DOI] [PubMed] [Google Scholar]
- 6.Tsavaris N, et al. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs. 2005;23(4):369–75. doi: 10.1007/s10637-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 7.Shojaei F, Ferrara N. Antiangiogenesis to treat cancer and intraocular neovascular disorders. Lab Invest. 2007;87(3):227–30. doi: 10.1038/labinvest.3700526. [DOI] [PubMed] [Google Scholar]
- 8.Chung GG, et al. Vascular endothelial growth factor, FLT-1, and FLK-1 analysis in a pancreatic cancer tissue microarray. Cancer. 2006;106(8):1677–84. doi: 10.1002/cncr.21783. [DOI] [PubMed] [Google Scholar]
- 9.Riess H. Antiangiogenic strategies in pancreatic cancer. Recent Results Cancer Res. 2008;177:123–9. doi: 10.1007/978-3-540-71279-4_14. [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13(10):2870–5. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 11.Tsopanoglou NE, Maragoudakis ME. Role of thrombin in angiogenesis and tumor progression. Semin Thromb Hemost. 2004;30(1):63–9. doi: 10.1055/s-2004-822971. [DOI] [PubMed] [Google Scholar]
- 12.Trikha M, Nakada MT. Platelets and cancer: implications for antiangiogenic therapy. Semin Thromb Hemost. 2002;28(1):39–44. doi: 10.1055/s-2002-20563. [DOI] [PubMed] [Google Scholar]
- 13.Gora-Tybor J, et al. Evaluation of circulating endothelial cells as noninvasive marker of angiogenesis in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(1):62–7. doi: 10.1080/10428190802549883. [DOI] [PubMed] [Google Scholar]
- 14.Shaked Y, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14(3):263–73. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51(2):143–58. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Giantonio BJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Rougier P, et al. A phase II study: docetaxel as first-line chemotherapy for advanced pancreatic adenocarcinoma. Eur J Cancer. 2000;36(8):1016–25. doi: 10.1016/s0959-8049(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Rogatko A, et al. Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin Cancer Res. 2004;10(14):4645–51. doi: 10.1158/1078-0432.CCR-03-0535. [DOI] [PubMed] [Google Scholar]
- 23.Kindler HL, et al. A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB. ASCO Meeting Abstracts. 2007;25(18 suppl):4508. [Google Scholar]
- 24.Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 27.Pelzer U, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol (Meeting Abstracts) 2008;26(15_suppl):4508. [Google Scholar]
- 28.Kindler HL, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23(31):8033–40. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 29.Heinzerling JH, Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006;63(5):334–7. doi: 10.1016/j.cursur.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Nalluri SR, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300(19):2277–85. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 31.Tetzlaff ED, et al. The impact on survival of thromboembolic phenomena occurring before and during protocol chemotherapy in patients with advanced gastroesophageal adenocarcinoma. Cancer. 2007;109(10):1989–95. doi: 10.1002/cncr.22626. [DOI] [PubMed] [Google Scholar]
- 32.Kuderer NM, et al. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110(5):1149–61. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 33.Altiay G, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol) 2007;19(7):494–8. doi: 10.1016/j.clon.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Antoniou D, et al. Predictive value of D-dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer. 2006;53(2):205–10. doi: 10.1016/j.lungcan.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Buccheri G, Torchio P, Ferrigno D. Plasma levels of D-dimer in lung carcinoma: clinical and prognostic significance. Cancer. 2003;97(12):3044–52. doi: 10.1002/cncr.11432. [DOI] [PubMed] [Google Scholar]
- 36.Gadducci A, et al. Preoperative D-dimer plasma assay is not a predictor of clinical outcome for patients with advanced ovarian cancer. Gynecol Oncol. 1997;66(1):85–8. doi: 10.1006/gyno.1997.4704. [DOI] [PubMed] [Google Scholar]
- 37.Dirix LY, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86(3):389–95. doi: 10.1038/sj.bjc.6600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Avello A, et al. Coagulative system activation and fibrinolytic system inhibition activities arise from tumoral draining vein in colon carcinoma. Thromb Res. 2001;104(6):421–5. doi: 10.1016/s0049-3848(01)00383-8. [DOI] [PubMed] [Google Scholar]
- 39.Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol. 2001;106(1–2):33–42. doi: 10.1159/000046587. [DOI] [PubMed] [Google Scholar]
- 40.Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313(5794):1785–7. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 41.Beaudry P, et al. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;11(9):3514–22. doi: 10.1158/1078-0432.CCR-04-2271. [DOI] [PubMed] [Google Scholar]