Abstract
Rationale
Neuroactive steroids might be therapeutic alternatives for benzodiazepines because they have similar anxiolytic, sedative and anticonvulsant effects, and their actions at different modulatory sites on GABAA receptors might confer differences in adverse effects.
Objectives
This study used drug discrimination to compare discriminative stimuli produced by positive GABAA modulators that vary in their site of action on GABAA receptors.
Methods
Two groups of rats discriminated either 3.2 mg/kg of pregnanolone or 0.56 mg/kg of midazolam from vehicle while responding under a fixed ratio 10 schedule of food presentation.
Results
Pregnanolone, midazolam and flunitrazepam produced ≥80% drug-lever responding in both groups; each drug was more potent in rats discriminating pregnanolone. Pentobarbital produced ≥80% drug-lever responding in all rats discriminating pregnanolone and in 1/3 of the rats discriminating midazolam with larger doses decreasing response rates to <20% of control. Morphine and ketamine produced predominantly saline-lever responding in both groups. Flumazenil antagonized midazolam and flunitrazepam in both groups; slopes of Schild plots were not different from unity and pA2 values for flumazenil ranged from 5.86 to 6.09. Flumazenil did not attenuate the discriminative stimulus effects of pregnanolone.
Conclusions
The midazolam and pregnanolone discriminative stimuli were qualitatively similar, although the effects of pentobarbital were not identical in the two groups. Although acute effects of midazolam and pregnanolone are similar, suggesting that neuroactive steroids might retain the therapeutic effects of benzodiazepines, differences emerge during chronic treatment, indicating that neuroactive steroids might produce fewer adverse effects than benzodiazepines.
Keywords: regnanolone, midazolam, flumazenil, drug discrimination, Schild analyses, rats
γ-Aminobutyric acidA (GABAA) receptors are important therapeutic targets, and drugs that act at benzodiazepine binding sites on GABAA receptors are used clinically. Despite their effectiveness and large margin of safety, there are adverse effects associated with the clinical use of benzodiazepines, including the development of tolerance and dependence (Lader 2008; Cloos and Ferreira 2008). Treatment might be improved by developing drugs that retain the therapeutic effects of benzodiazepines while reducing their adverse effects. One strategy for identifying potential replacements for benzodiazepines is to target different binding sites on GABAA receptors, such as the neuroactive steroid site. Like benzodiazepines, neuroactive steroids can positively modulate GABAA receptors (Lan and Gee 1994), although several differences have been observed between benzodiazepines and neuroactive steroids. First, while benzodiazepines have one binding site on GABAA receptors, neuroactive steroids have two, and they can facilitate the actions of GABA through one site and directly activate the channel through the other site (Hosie et al. 2006). Second, binding sites for benzodiazepines are present only on a subset of GABAA receptors (Doble and Martin 1992); in contrast, binding sites for neuroactive steroids are likely present on most GABAA receptors (Hosie et al. 2006). Finally, effects of benzodiazepines appear to be mediated solely by GABAA receptors, whereas effects of neuroactive steroids might also involve other receptors, including N-methyl-D-aspartate (NMDA), 5-hydroxytryptamine (5-HT3) and sigma (σ1) receptors (Maurice et al. 2001; Rupprecht et al. 2001; Dubrovsky 2005).
These differences between benzodiazepines and neuroactive steroids appear to confer differences in their behavioral effects. For example, during chronic treatment, tolerance develops readily to many effects of benzodiazepines, including rate-decreasing (McMahon and France 2002b) and anticonvulsant effects (Gonsalves and Gallagher 1987; Löscher et al. 1996). In contrast, although tolerance develops to some effects of neuroactive steroids (e.g., decreases in locomotor activity, Marshall et al.1997; impairment in Morris Water Maze, Türkmen et al. 2006), it does not develop to their rate-decreasing (McMahon and France 2002a) or anticonvulsant effects (Kokate et al. 1998; Reddy and Rogawski 2000). Although tolerance to neuroactive steroids is not detected, other consequences of chronic administration are evident; specifically, increased potency of pentylenetetrazole suggests the development of dependence (McMahon and France 2002a) and decreased potency of diazepam suggests the development of cross tolerance to benzodiazepines (Reddy and Rogawski 2000). Together, these findings indicate that the absence of tolerance to neuroactive steroids is not due to inadequate dosing conditions. Another difference between benzodiazepines and neuroactive steroids is evident during chronic benzodiazepine treatment when tolerance develops to benzodiazepines and cross tolerance does not develop to neuroactive steroids (McMahon et al. 2007; Gerak 2009). Thus, under conditions where the potency of benzodiazepines is decreased, the potency of neuroactive steroids does not appear to change; this important difference among positive GABAA modulators could be exploited clinically.
Given the differences between benzodiazepines and neuroactive steroids, their acute behavioral effects might also not be identical. One procedure in which differences in the acute effects of benzodiazepines and neuroactive steroids have been observed is drug discrimination. Drugs acting at benzodiazepine, barbiturate or neuroactive steroid binding sites have been established as discriminative stimuli, and generally, they share discriminative stimulus effects (e.g., de la Garza and Johanson 1987; Ator et al. 1993; McMahon et al. 2001; Engel et al. 2001), although differences among positive GABAA modulators have been reported. For example, in subjects discriminating the barbiturate pentobarbital, neuroactive steroids and the benzodiazepine lorazepam produce pentobarbital-lever responding, suggesting that these drugs have similar discriminative stimulus effects; however, in subjects discriminating lorazepam, neither neuroactive steroids nor pentobarbital produce lorazepam-lever responding (Ator and Griffiths 1983; Ator et al. 1993). In addition, barbiturates and neuroactive steroids produce drug-lever responding in some, but not all, subjects discriminating the benzodiazepine midazolam (Evans and Johanson 1989; Gerak et al. 2008a), whereas barbiturates and benzodiazepines produce drug-lever responding in all subjects discriminating the neuroactive steroid pregnanolone (Vanover 1997; 2000; Engel et al. 2001; Gerak et al. 2008b). Differences in actions at GABAA receptors that account for differences in discriminative stimulus effects have not been well established, although actions of neuroactive steroids at receptors other than GABAA receptors might contribute to some of the observed differences between neuroactive steroids and benzodiazepines (Engel et al. 2001).
The goal of this study was to compare the pregnanolone and midazolam discriminative stimuli in rats. With the exception of the training drug, identical experimental conditions were used in two groups of rats. The discriminative stimuli were compared using two types of studies. First, positive GABAA modulators acting at different modulatory sites and drugs with primary mechanisms of action that do not involve GABAA receptors were tested alone to determine whether they produced drug-lever responding. Second, midazolam, another benzodiazepine flunitrazepam, and pregnanolone were studied in combination with flumazenil, a neutral GABAA modulator acting on benzodiazepine sites. Given the high pharmacological selectivity of drug discrimination procedures, these studies compared the acute effects of pregnanolone and benzodiazepines under conditions that would be most likely to identify differences between these compounds.
Methods
Subjects
Male Sprague-Dawley rats were housed individually in a humidity- and temperature-controlled vivarium under a 12-h light/dark cycle with experiments conducted during the light cycle. Rats had free access to water and received grain-based food pellets (Bio Serv, Inc, Frenchtown, NJ) during experimental sessions and rodent chow (Harlan Teklad, Madison, WI) in the home cage; sufficient chow was provided to maintain weights between 320 to 330 g. Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee at The University of Texas Health Science Center at San Antonio, and the guidelines of the Committee on Care and Use of Laboratory Animal Resources, National Research Council [Department of Health, Education and Welfare, publication No. (NIH) 85-23, revised 1996].
Apparatus
During sessions, rats were placed in chambers enclosed within sound-attenuating cubicles that had fans for ventilation (MED Associates, Inc., St. Albans, VT). Chambers were equipped with houselights, pellet troughs, pellet dispensers, response levers and stimulus lights located above each lever. White noise was present in the room to mask extraneous noise. An interface connected the chambers to a computer that controlled experimental events and recorded data using MED-PC/MEDSTATE NOTATION software (MED Associates, Inc., St. Albans, VT).
Procedure
One group of rats (n=11) discriminated 3.2 mg/kg of pregnanolone and another group (n=8) discriminated 0.56 mg/kg of midazolam while responding under a fixed-ratio 10 schedule of food presentation; other than the training drug, experimental sessions were identical in the two groups. Sessions were divided into 2-8 discrete cycles. Each cycle was 15 min in duration and began with a 10-min timeout period during which the chamber was dark and responding had no programmed consequence. Illumination of the stimulus lights above both levers signaled the end of the timeout period and the beginning of the response period, during which 10 responses on the lever designated correct by the injection given during the first minute of the timeout resulted in the delivery of a food pellet. Responses on the incorrect lever reset the response requirement on the correct lever. The lever designated correct following an injection of the training drug was counterbalanced among rats. Lights were extinguished and response periods ended after 5 min or the delivery of 10 pellets, whichever occurred first. Any time remaining between the end of the response period and the end of the cycle was a timeout; once the 15-min cycle ended, the 10-min timeout at the beginning of the subsequent cycle started.
During some training sessions, rats received the training drug at the beginning of the first cycle with a sham injection (rats were handled but did not receive an injection) given at the beginning of the second cycle; for both of these cycles, responding on drug lever resulted in delivery of food. During other training sessions, the two drug cycles were preceded by 1-5 cycles during which vehicle or sham was administered. For still other training sessions, only vehicle or sham was administered for 2-8 cycles. The first test session was conducted when the following criteria were satisfied for 5 consecutive or 6 of 7 training sessions: ≥80% of the total responses were emitted on the correct lever and fewer than 10 responses were emitted on the incorrect lever prior to delivery of the first food pellet. Thereafter, test sessions were conducted twice a week as long as the above criteria were satisfied during intervening training sessions.
Test sessions were identical to training sessions except that 10 consecutive responses on either lever resulted in the delivery of food and test compounds were administered during sessions. Dose-effect curves were determined using a cumulative-dosing procedure. On the first cycle, rats received vehicle; on subsequent cycles, rats received increasing doses of drug with the cumulative dose increasing by 0.25 or 0.5 log units per cycle. Dosing continued until ≥80% responding occurred on the drug lever or until rates decreased to <20% of control. The pregnanolone and midazolam discriminative stimuli were compared in two ways. First, dose-effect curves were determined for positive GABAA modulators acting on different sites (pregnanolone [1-10 mg/kg], midazolam [0.032-1 mg/kg], flunitrazepam [0.032-1 mg/kg] and pentobarbital [1-17.8 mg/kg]) and for drugs with primary mechanisms of action that do not involve GABAA receptors (ketamine [0.32-5.6 mg/kg] and morphine [0.32-10 mg/kg]). Second, dose-effect curves for midazolam, flunitrazepam and pregnanolone were redetermined following administration of flumazenil, a neutral GABAA modulator acting at benzodiazepine sites; flumazenil (1-5.6 mg/kg) was given on the first cycle with increasing doses of positive GABAA modulators given on subsequent cycles.
Drugs
Pregnanolone (5β-pregnan-3α-ol-20-one; Steraloids, Inc., Newport, RI) was dissolved in 45% (w/v) 2-hydroxypropyl-γ-cyclodextrin. Midazolam hydrochloride (Bedford Laboratories, Bedford, OH) and ketamine hydrochloride (racemate; Fort Dodge Laboratories, Fort Dodge, IA) were purchased as commercially prepared solutions and diluted with sterile 0.9% saline. Flunitrazepam (Sigma-Aldrich Co., St. Louis, MO) was dissolved in a vehicle containing 20% emulphor, 10% ethanol and 70% sterile 0.9% saline. Pentobarbital sodium (Sigma-Aldrich Co., St. Louis, MO) and morphine sulfate (Research Technology Branch, National Institute on Drug Abuse, Rockville, MD) were dissolved in sterile 0.9% saline. Flumazenil (Hoffman LaRoche, Nutley, N.J., USA) was dissolved in a vehicle comprising 40% propylene glycol, 50% sterile 0.9% saline and 10% ethanol. Drugs were administered i.p. typically in a volume of 1 ml/kg body weight. Doses are expressed in the form listed above in mg/kg body weight.
Data analyses
The number of sessions (mean ± 1 SEM) required for rats to satisfy the initial testing criteria were compared between the two groups using a t-test. Control (no drug) response rates were obtained for individual rats by first averaging rates across cycles within sessions during which vehicle or sham was administered and rats satisfied the testing criteria; these rates obtained for individual sessions were then averaged across 10 training sessions (mean ± 1 SEM). Control response rates were compared between the two groups using a t-test. Response rates obtained during test sessions were expressed as a percentage of the control rate for each subject and averaged across subjects. Discrimination data were not included in the analyses when response rates were less than 20% of control for an individual rat. The percentage of responses on the drug-appropriate lever and rates, expressed as a percentage of control, were plotted as a function of dose. For drugs that produced ≥80% drug-lever responding in both groups, dose-effect curves obtained in the two groups were compared by simultaneously fitting straight lines to the individual dose-effect curves using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA). Straight lines were fitted to the linear portion of the dose-effect curves which included one data point below 25%, one data point above 75% and all data points in between. To determine the simplest model that best fit the data, slopes of the linear portion of the dose-effect curves in rats discriminating pregnanolone were compared to those obtained in rats discriminating midazolam using an F-ratio test. Slopes that were significantly different required the more complex model to fit the data; when slopes were not different, a simpler model was used by selecting a common slope. Dose-effect curves could then be further compared by determining whether the data were best fit by a common intercept. Significance was set at P<0.05.
Schild plots were constructed to confirm simple, competitive interactions between flumazenil and the benzodiazepines in the two groups of rats. First, the dose of benzodiazepine needed to produce 50% drug-appropriate responding (ED50) was estimated for individual rats by fitting straight lines as described above and using linear regression or interpolation, depending on the number of available data points, to calculate ED50 values. Dose ratios were determined for each dose of flumazenil by dividing the ED50 of the benzodiazepine in the presence of flumazenil by the ED50 of the benzodiazepine alone; dose ratios were then averaged across rats and plotted as a function of flumazenil dose (–log [mol/kg]) using the method of Arunlakshana and Schild (1959). Straight lines were fitted to each Schild plot using GraphPad and the following equation: log (dose ratio-1) = -log(molar dose of flumazenil)*slope + intercept. Schild plots generated under each condition (i.e., two benzodiazepines in each of two groups) were compared using an F-ratio test (Kenakin 1993; Koek et al. 2000) with slope of each Schild plot constrained to unity in the simpler model and allowed to vary in the more complex model. If the calculated F value was not significant, then the simpler model (i.e., common slope) was used. When slopes of Schild plots were constrained to unity, differences in apparent pA2 values were determined by fitting each data set and comparing the results with an F-ratio test. When slopes were not constrained, apparent pA2 values (95% CI) were determined using linear regression of Schild plots.
Results
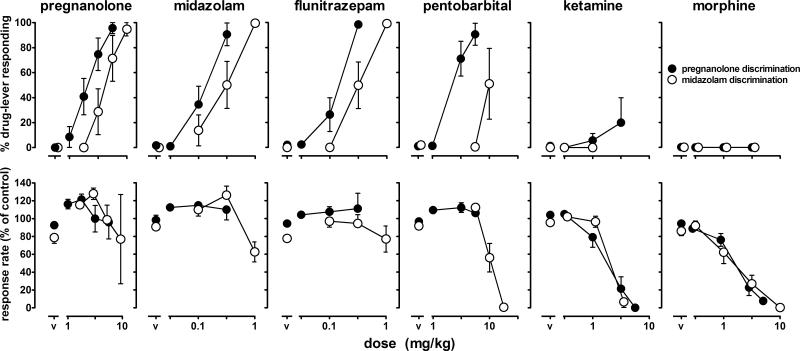
The number of training sessions required to satisfy the testing criteria was 26.0 ± 4.1 in rats discriminating pregnanolone and 20.1 ± 1.8 in rats discriminating midazolam; there was no difference between groups. When vehicle or sham were administered, response rates obtained in rats discriminating pregnanolone (0.86 ± 0.06 responses/sec) were not significantly different from those obtained in rats discriminating midazolam (0.73 ± 0.04 responses/sec). Pregnanolone dose dependently increased pregnanolone-lever responding with ≥80% of total responses occurring on the drug lever at a cumulative dose of 5.6 mg/kg (solid symbols, upper left panel, Figure 1); midazolam dose dependently increased midazolam-lever responding with ≥80% of total responses occurring on the drug lever at a cumulative dose of 1 mg/kg (open symbols, upper panel, 2nd from left, Figure 1). This dose of pregnanolone did not decrease response rates, although 1 mg/kg of midazolam decreased rates to 63% of control (lower panels, Figures 1).
Figure 1.
Discriminative stimulus and rate-decreasing effects of pregnanolone, midazolam, flunitrazepam, pentobarbital, ketamine and morphine in rats discriminating either pregnanolone (solid symbols) or midazolam (open symbols). For rats discriminating pregnanolone, data from 12 rats are shown for pregnanolone, ketamine and morphine and data from 11 rats are shown for midazolam, flunitrazepam and pentobarbital. For rats discriminating midazolam, data from 8 rats are shown for midazolam, flunitrazepam, ketamine and morphine; data from 7 rats are shown for pregnanolone and data from 6 rats are shown for pentobarbital. Ordinates: top panel, percentage of total responses emitted on the drug (i.e., pregnanolone or midazolam) lever; bottom panel, average rate expressed as a percentage of control response rate. Abscissa: dose in mg/kg. Points above V represent the effects of vehicle.
Pregnanolone, midazolam and flunitrazepam produced ≥80% drug-lever responding in both groups (upper panel, Figure 1). Straight lines fitted to the dose-effect curves indicated that slopes were not significantly different between groups. The simplest model that could be used to fit the data was one with a common slope and different intercepts (pregnanolone: F (1,73)=15.98, P=0.0002; midazolam: F(1,54)=8.84, P=0.0044; flunitrazepam: F(1,54)=21.14, P<0.0001), demonstrating that these positive GABAA modulators were more potent in rats discriminating pregnanolone. Despite similarities between groups for these three positive modulators, a fourth drug acting at another binding site had different effects in the two groups. In rats discriminating pregnanolone, a cumulative dose of 5.6 mg/kg of pentobarbital occasioned ≥80% drug-lever responding in all 11 rats (solid circles, upper panel, 4th from left, Figure 1) and produced modest rate-decreasing effects (solid circles, lower panel, Figure 1). In contrast, pentobarbital produced midazolam-lever responding in only 2 of the 6 rats in which it was studied with a cumulative dose of 10 mg/kg resulting in 51% drug-lever responding and decreasing rates to 56% of control; a larger dose of pentobarbital (17.8 mg/kg) decreased rates to 0.6% of control (open circles, lower panel, 4th from left, Figure 1). Morphine and ketamine occasioned <20% drug-lever responding in both groups up to doses that decreased rates to <20% of control (right 4 panels, Figure 1).
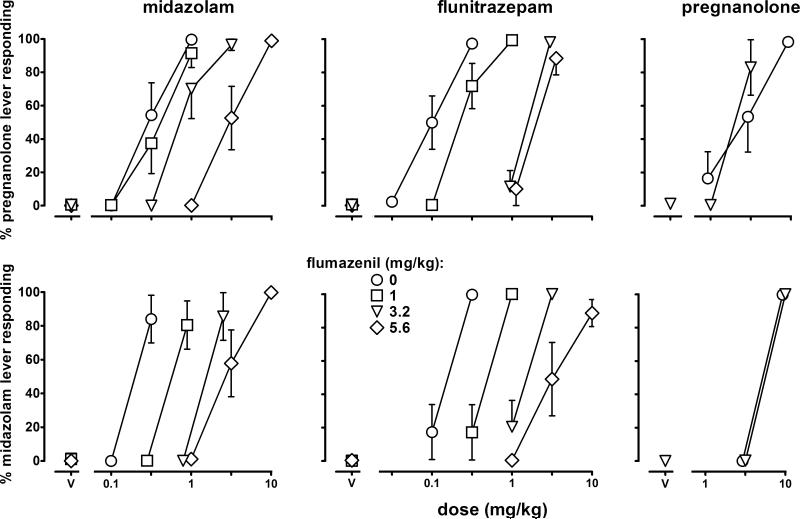
To further compare the pregnanolone and midazolam discriminative stimuli, flumazenil was studied in combination with the three drugs that produced ≥80% drug-lever responding in both groups of rats. Flumazenil dose dependently antagonized midazolam and flunitrazepam, shifting their dose-effect curves to the right in rats discriminating pregnanolone (upper panels, Figure 2) or midazolam (lower panels, Figure 2). Comparison of fits indicated no significant difference between Schild plots generated with unconstrained slopes and those generated with slopes constrained to unity; consequently, further analyses of these data were conducted using the simpler model and data were plotted with slopes constrained to unity (Figure 3). In addition to slopes, apparent pA2 values were also not significantly different across benzodiazepines or groups with pA2 values ranging from 5.86 to 6.09 (Table 1). In contrast to the antagonism obtained when flumazenil was studied in combination with benzodiazepines, flumazenil did not attenuate the discriminative stimulus effects of pregnanolone (right panels, Figure 2).
Figure 2.
Effects of flumazenil in combination with midazolam, flunitrazepam and pregnanolone in rats discriminating pregnanolone (n=8, 11 and 6, respectively) or midazolam (n=7, 6 and 4, respectively). Ordinates: percentage of total responses emitted on the drug (i.e., pregnanolone or midazolam) lever. Abscissa: dose in mg/kg. Points above V represent the effects of flumazenil alone.
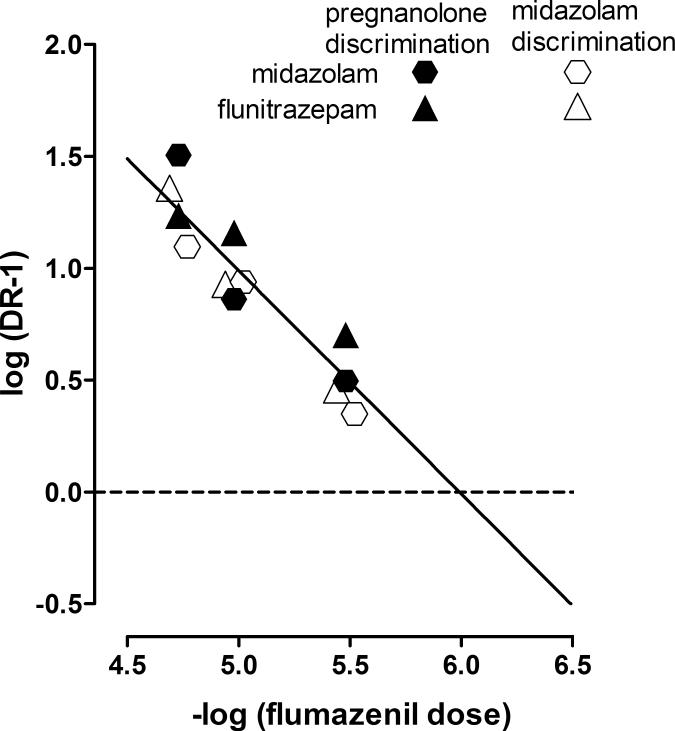
Figure 3.
Schild plots constructed from data shown in Figure 2. Closed symbols: pregnanolone discrimination. Open symbols: midazolam discrimination. Triangles: flunitrazepam. Hexagons: midazolam. Ordinate: logarithm of the dose ratio-1. Abscissa: negative logarithm of the molar dose of flumazenil.
Table 1.
Slopes of Schild plots and pA2 values of flumazenil determined with constrained and unconstrained slopes.
| Slope (unconstrained) | pA2 (unconstrained) | pA2 (constrained) | ||
|---|---|---|---|---|
| Pregnanolone discrimination | Flunitrazepam | -0.73 (-1.25, -0.21) | 6.20 (5.50, 6.48) | 6.09 (5.94, 6.24) |
| Midazolam | -1.26 (-1.75, -0.77) | 5.97 (5.62, 6.91) | 6.02 (5.87, 6.17) | |
| Midazolam discrimination | Flunitrazepam | -1.17 (-1.85, -0.50) | 5.71 (5.38, 6.04) | 5.98 (5.78, 6.18) |
| Midazolam | -1.02 (-1.48, -0.57) | 5.74 (5.49, 5.99) | 5.86 (5.72, 6.00) | |
Discussion
The goal of the current study was to compare the neuroactive steroid pregnanolone with the benzodiazepine midazolam using drug discrimination procedures; differences in the acute behavioral effects of these drugs might be most evident when they are compared using procedures that have high pharmacological selectivity. Two groups of rats discriminated either pregnanolone or midazolam from vehicle under identical experimental conditions; the only difference between groups was the training drug. Pregnanolone and the benzodiazepines produced similar effects in rats discriminating pregnanolone, as compared to those discriminating midazolam, with all three drugs producing ≥80% drug-lever responding in both groups. Each of these positive GABAA modulators was 2- to 3-fold more potent in rats discriminating pregnanolone. Flumazenil antagonized the discriminative stimulus effects of midazolam and flunitrazepam, but not pregnanolone. In addition, drugs that do not act at GABAA receptors produced predominantly saline-lever responding in both groups. Thus, the pregnanolone and midazolam discriminative stimuli appear to be qualitatively similar, although differences in the potencies of pregnanolone, midazolam and flunitrazepam suggest that a training dose of 3.2 mg/kg of pregnanolone is quantitatively different from a training dose of 0.56 mg/kg of midazolam.
This quantitative difference between the two discriminative stimuli might account for differences in the discriminative stimulus effects of pentobarbital. Rate-decreasing effects of pentobarbital were not evident at doses that produced ≥80% pregnanolone-lever responding; however, doses of pentobarbital that would be expected to produce midazolam-lever responding, based on differences between groups in potencies of the other 3 positive GABAA modulators, had rate-decreasing effects. Thus, one possibility is that differences in the discriminative stimulus effects of pentobarbital in the two groups are due to the quantitative difference between the two training conditions.
Drugs acting at neuroactive steroid or barbiturate sites can directly activate chloride channels whereas benzodiazepines can only facilitate the actions of GABA to increase chloride influx (Simmonds 1981; Hattori et al. 1986; Lambert et al. 2001; Hosie et al. 2006). In this regard, neuroactive steroids would appear to be more similar to barbiturates than to benzodiazepines; however, pregnanolone, along with midazolam and flunitrazepam, produced discriminative stimulus effects in all rats at doses smaller than those that decreased response rates, and pentobarbital did not, suggesting that direct activation of GABAA receptors does not entirely account for this difference between pentobarbital and other positive GABAA modulators. In other studies conducted in rats discriminating benzodiazepines, the discriminative stimulus effects of barbiturates are qualitatively similar to those of benzodiazepines (Shannon and Herling 1983; Garcha et al. 1985; Woudenberg and Slangen 1989), although two observations are consistent with differences observed between these positive modulators in the current study. First, drug-lever responding produced by pentobarbital is accompanied by rate-decreasing effects in rats discriminating 0.4 mg/kg of midazolam and drug-lever responding produced by benzodiazepines is not (Garcha et al. 1985). Second, when rats discriminate two doses of midazolam and a no-drug condition under a three-lever procedure, midazolam produces a dose-dependent switch in responding from the no-drug lever to the lever associated with the small training dose and finally to the lever associated with the large training dose; in contrast, pentobarbital produces a dose-dependent switch from the no-drug lever to the lever associated with the small training dose with larger doses decreasing response rates rather than producing a switch to the lever associated with the large training dose (Sannerud and Ator 1995). Thus, although there might not be marked qualitative differences between pentobarbital and other positive GABAA modulators, the prominence of the rate-decreasing effects of pentobarbital and not those of pregnanolone or the benzodiazepines suggest that the effects of these positive modulators are not identical.
Under some conditions, actions of neuroactive steroids at receptors other than GABAA receptors, including NMDA, 5-HT3 and σ1 receptors, have been implicated in the discriminative stimulus effects of pregnanolone (Engel et al. 2001; Shannon et al. 2005). In the current study, ketamine, a drug which acts at NMDA receptors, did not produce drug-lever responding in either group of rats, although it was studied up to a dose that markedly decreased responding. In contrast, MK-801, a drug with actions similar to those of ketamine, produced 73% drug-lever responding in rats discriminating 5 mg/kg of pregnanolone (Engel et al. 2001). Such differences among studies might be due to procedural differences. For example, in the current study, drug discrimination data were included in the analyses only when response rates were >20% of control whereas the earlier study reported discrimination data as long as rats completed one fixed ratio (Engel et al. 2001), although applying the criterion from the earlier study to the current data set would only modestly increase drug-lever responding in a few rats.
Drug interaction studies and quantitative pharmacological analyses can be exceptionally useful in understanding mechanisms of action of drugs and have been used extensively to characterize the effects of benzodiazepines in monkeys (Paronis and Bergman, 1999; Lelas et al. 2000; McMahon and France 2005). In the current study, flumazenil, a neutral modulator of GABAA receptors acting at benzodiazepine sites, antagonized the discriminative stimulus effects of benzodiazepines in both groups of rats, and Schild analyses confirmed similarities in the mechanism of action of benzodiazepines across training drugs. The slope of each Schild plot was not different from unity, indicating that interactions were competitive and reversible with one population of receptors involved in the discriminative stimulus effects of midazolam or flunitrazepam, regardless of training drug. Similar pA2 values for flumazenil in rats discriminating pregnanolone or midazolam indicated that effects of midazolam and flunitrazepam are mediated by the same population of receptors in the two groups. The pA2 values for flumazenil obtained in the current study are similar to those reported for flumazenil in another study in which it antagonized the discriminative stimulus effects of β-CCE, a negative GABAA modulator acting at benzodiazepine sites (Rowlett et al. 1999). Although the discriminative stimulus effects of β-CCE are very different from those of benzodiazepines (current study; Rowlett et al. 1999; Lelas et al. 2000), similar pA2 values for flumazenil indicate that the same population of receptors mediates their effects.
Because flumazenil and pregnanolone act at different modulatory sites on GABAA receptors, flumazenil would not be expected to attenuate the discriminative stimulus effects of pregnanolone. In fact, flumazenil enhances the discriminative stimulus effects of neuroactive steroids in rhesus monkeys, suggesting that flumazenil is a low-efficacy positive modulator rather than a neutral modulator in monkeys (McMahon and France 2006). In the current study, flumazenil did not alter the discriminative stimulus effects of pregnanolone regardless of the training drug. Not surprisingly, flumazenil does not attenuate other behavioral effects of neuroactive steroids in rats, including anxiolytic effects (Brot et al. 1997; Bitran et al. 1999) and suppression of ultrasonic vocalizations in pups (Vivian et al. 1997). Collectively, these results indicate that the discriminative stimulus effects of pregnanolone are similar to those of the benzodiazepines with modest differences between pentobarbital and other positive GABAA modulators.
Like benzodiazepines, positive modulators acting at neuroactive steroid sites produce anxiolytic (Wieland et al. 1997), sedative (Lancel 1999; Vanover et al. 1999) and anticonvulsant effects (Gasior et al. 2000; Kokate et al. 1994; Reddy and Rogawski 2001) and can reverse ethanol withdrawal (Finn et al. 2000). Although neuroactive steroids are not yet available for clinical use, similarities between their acute behavioral effects and those of benzodiazepines, which are largely supported by the current study, suggest that they might have therapeutic utility. Moreover, differences between these drug classes emerge during chronic treatment. In particular, during chronic treatment with neuroactive steroids, tolerance does not develop under conditions that result in the development of dependence or cross tolerance to benzodiazepines (Reddy and Rogawski 2000; McMahon and France 2002a). To the extent that similarities in the acute behavioral effects predict similarities in their therapeutic effects, the clinical effectiveness of neuroactive steroids might be similar to that of benzodiazepines while differences in the development of tolerance and dependence could provide a distinct advantage over benzodiazepines. Neuroactive steroids could give clinicians another option for the treatment of conditions like anxiety, insomnia or ethanol withdrawal.
Acknowledgements
The author thanks R. Lopez and J. Kite for their excellent technical assistance.
These studies were supported by U.S. Public Health Service Grant DA017240. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Lorazepam and pentobarbital drug discrimination in baboons: cross-drug generalization and interaction with Ro 15-1788. J Pharmacol Exp Ther. 1983;226:776–782. [PubMed] [Google Scholar]
- Ator NA, Grant KA, Purdy RH, Paul SM, Griffiths RR. Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol. 1993;241:237–243. doi: 10.1016/0014-2999(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABAA receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Cloos J-M, Ferriera V. Current use of benzodiazepines in anxiety disorders. Curr Opin Psychiatry. 2008;22:90–95. doi: 10.1097/YCO.0b013e32831a473d. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J Pharmacol Exp Ther. 1987;243:955–962. [PubMed] [Google Scholar]
- Doble A, Martin IL. Multiple benzodiazepine receptors: no reason for anxiety. Trends Pharmacol Sci. 1992;13:76–81. doi: 10.1016/0165-6147(92)90027-4. [DOI] [PubMed] [Google Scholar]
- Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog NeuroPsychopharmacol Biol Psych. 2005;29:169–192. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;297:489–495. [PubMed] [Google Scholar]
- Evans SM, Johanson CE. Discriminative stimulus properties of midazolam in the pigeon. J Pharmacol Exp Ther. 1989;248:29–38. [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, Crabbe JC. Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther. 2000;292:394–405. [PubMed] [Google Scholar]
- Garcha HS, Rose IC, Stolerman IP. Midazolam cue in rats: generalization tests with anxiolytic and other drugs. Psychopharmacology. 1985;87:233–237. doi: 10.1007/BF00431814. [DOI] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–1196. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Gerak LR. Selective changes in sensitivity to benzodiazepines, and not other positive GABAA modulators, in rats receiving flunitrazepam chronically. Psychopharmacology. 2009;204:667–677. doi: 10.1007/s00213-009-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, McMahon LR, France CP. Acute cross tolerance to midazolam, and not pentobarbital and pregnanolone, after a single dose of chlordiazepoxide in monkeys discriminating midazolam. Behav Pharmacol. 2008a;19:796–804. doi: 10.1097/FBP.0b013e32831c3b40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Winsauer PJ, Moerschbaecher JM. Overlapping, but not identical, discriminative stimulus effects of the neuroactive steroid pregnanolone and ethanol. Pharmacol Biochem Behav. 2008b;89:473–479. doi: 10.1016/j.pbb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves SF, Gallagher DW. Time course for development of anticonvulsant tolerance and GABAergic subsensitivity after chronic diazepam. Brain Res. 1987;405:94–99. doi: 10.1016/0006-8993(87)90993-0. [DOI] [PubMed] [Google Scholar]
- Hattori K, Oomura Y, Akaike N. Diazepam action on gamma-aminobutyric acid-activated chloride currents in internally perfused frog sensory neurons. Cell Mol Neurobiol. 1986;6:307–323. doi: 10.1007/BF00711116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Synaptic receptor function. Trends Pharmacol Sci. 1993;14:431–432. doi: 10.1016/0165-6147(93)90178-m. [DOI] [PubMed] [Google Scholar]
- Koek W, Assié MB, Zernig G, France CP. In vivo estimates of efficacy at 5-HT1A receptors: effects of EEDQ on the ability of agonists to produce lower-lip retraction in rats. Psychopharmacology. 2000;149:377–387. doi: 10.1007/s002130000374. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- Lader M. Effectiveness of benzodiazepines: do they work or not? Expert Rev Neurother. 2008;8:1189–1191. doi: 10.1586/14737175.8.8.1189. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev. 2001;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- Lan NC, Gee KW. Neuroactive steroid actions at the GABAA receptor. Hor Behav. 1994;28:537–544. doi: 10.1006/hbeh.1994.1052. [DOI] [PubMed] [Google Scholar]
- Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. Antagonism of the discriminative stimulus effects of positive gamma-aminobutyric acidA modulators in rhesus monkeys discriminating midazolam. J Pharmacol Exp Ther. 2000;294:902–908. [PubMed] [Google Scholar]
- Löscher W, Rundfeldt C, Hönack D, Ebert U. Long-term studies on anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. I. Comparison of diazepam, clonazepam, clobazam and abecarnil. J Pharmacol Exp Ther. 1996;279:561–572. [PubMed] [Google Scholar]
- Marshall FH, Stratton SC, Mullings J, Ford E, Worton SP, Oakley NR, Hagan RM. Development of tolerance in mice to the sedative effects of the neuroactive steroid minaxolone following chronic exposure. Pharmacol Biochem Behav. 1997;58:1–8. doi: 10.1016/s0091-3057(96)00132-3. [DOI] [PubMed] [Google Scholar]
- Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res Brain Res Rev. 2001;37:116–132. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Gerak LR, France CP. Potency of positive γ-aminobutyric acidA modulators to substitute for a midazolam discriminative stimulus in untreated monkeys does not predict potency to attenuate a flumazenil discriminative stimulus in diazepam-treated monkeys. J Pharmacol Exp Ther. 2001;298:1227–1235. [PubMed] [Google Scholar]
- McMahon LR, France CP. Acute and chronic effects of the neuroactive steroid pregnanolone on schedule-controlled responding in rhesus monkeys. Behav Pharmacol. 2002a;13:545–555. doi: 10.1097/00008877-200211000-00004. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Daily treatment with diazepam differentially modifies sensitivity to the effects of gamma-aminobutyric acidA modulators on schedule-controlled responding in rhesus monkeys. J Pharmacol Exp Ther. 2002b;300:1017–1025. doi: 10.1124/jpet.300.3.1017. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Negative GABAA modulators attenuate the discriminative stimulus effects of benzodiazepines and the neuroactive steroid pregnanolone in rhesus monkeys. Psychopharmacology. 2005;181:697–705. doi: 10.1007/s00213-005-0028-1. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Differential behavioral effects of low efficacy positive GABAA modulators in combination with benzodiazepines and a neuroactive steroid in rhesus monkeys. Br J Pharmacol. 2006;147:260–268. doi: 10.1038/sj.bjp.0706550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Javors MA, France CP. Changes in relative potency among positive GABAA receptor modulators upon discontinuation of chronic benzodiazepine treatment in rhesus monkeys. Psychopharmacology. 2007;192:135–145. doi: 10.1007/s00213-006-0692-9. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. Apparent pA2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther. 1999;290:1222–1229. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Discriminative stimulus effects of ethyl-β-carboline-3-carboxylate at two training doses in rats. Psychopharmacology. 1999;145:324–332. doi: 10.1007/s002130051065. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, di Michele F, Hermann B, Strohle A, Lancel M, Romeo E, Holsboer F. Neuroactive steroids: molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res. 2001;37:59–67. doi: 10.1016/s0165-0173(01)00123-0. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Ator NA. Drug discrimination analysis of midazolam under a three-lever procedure: I. Dose-dependent differences in generalization and antagonism. J Pharmacol Exp Ther. 1995;272:100–111. [PubMed] [Google Scholar]
- Shannon EE, Porcu P, Purdy RH, Grant KA. Characterization of the discriminative stimulus effects of the neuroactive steroid pregnanolone in DBA/2J and C57BL/6J inbred mice. J Pharmacol Exp Ther. 2005;314:675–685. doi: 10.1124/jpet.104.082644. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Herling S. Discriminative stimulus effects of diazepam in rats: evidence for a maximal effect. J Pharmacol Exp Ther. 1983;227:160–166. [PubMed] [Google Scholar]
- Simmonds MA. Distinction between the effects of barbiturates, benzodiazepines and phenytoin on responses to gamma-aminobutyric acid receptor activation and antagonism by bicuculline and picrotoxin. Br J Pharmacol. 1981;73:739–747. doi: 10.1111/j.1476-5381.1981.tb16810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkmen S, Löfgren M, Birzniece V, Bäckström T, Johansson IM. Tolerance development to Morris water maze test impairments induced by acute allopregnanolone. Neuroscience. 2006;139:651–659. doi: 10.1016/j.neuroscience.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Vanover KE. Discriminative stimulus effects of the endogenous neuroactive steroid pregnanolone. Eur J Pharmacol. 1997;327:97–101. doi: 10.1016/s0014-2999(97)89647-1. [DOI] [PubMed] [Google Scholar]
- Vanover KE. Effects of benzodiazepine receptor ligands and ethanol in rats trained to discriminate pregnanolone. Pharmacol Biochem Behav. 2000;67:483–487. doi: 10.1016/s0091-3057(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Suruki M, Robledo S, Huber M, Wieland S, Lan NC, Gee KW, Wood PL, Carter RB. Positive allosteric modulators of the GABAA receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology. 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Barros HMT, Manitiu A, Miczek KA. Ultrasonic vocalizations in rat pups: modulation at the γ-aminobutryic acidA receptor complex and the neurosteroid recognition site. J Pharmacol Exp Ther. 1997;282:318–325. [PubMed] [Google Scholar]
- Wieland S, Belluzzi J, Hawkinson JE, Hogenkamp D, Upasani R, Stein L, Wood PL, Gee KW, Lan NC. Anxiolytic and anticonvulsant activity of a synthetic neuroactive steroid Co 3-0593. Psychopharmacology. 1997;134:46–54. doi: 10.1007/s002130050424. [DOI] [PubMed] [Google Scholar]
- Woudenberg F, Slangen JL. Discriminative stimulus properties of midazolam: comparison with other benzodiazepines. Psychopharmacology. 1989;97:466–470. doi: 10.1007/BF00439549. [DOI] [PubMed] [Google Scholar]