Abstract
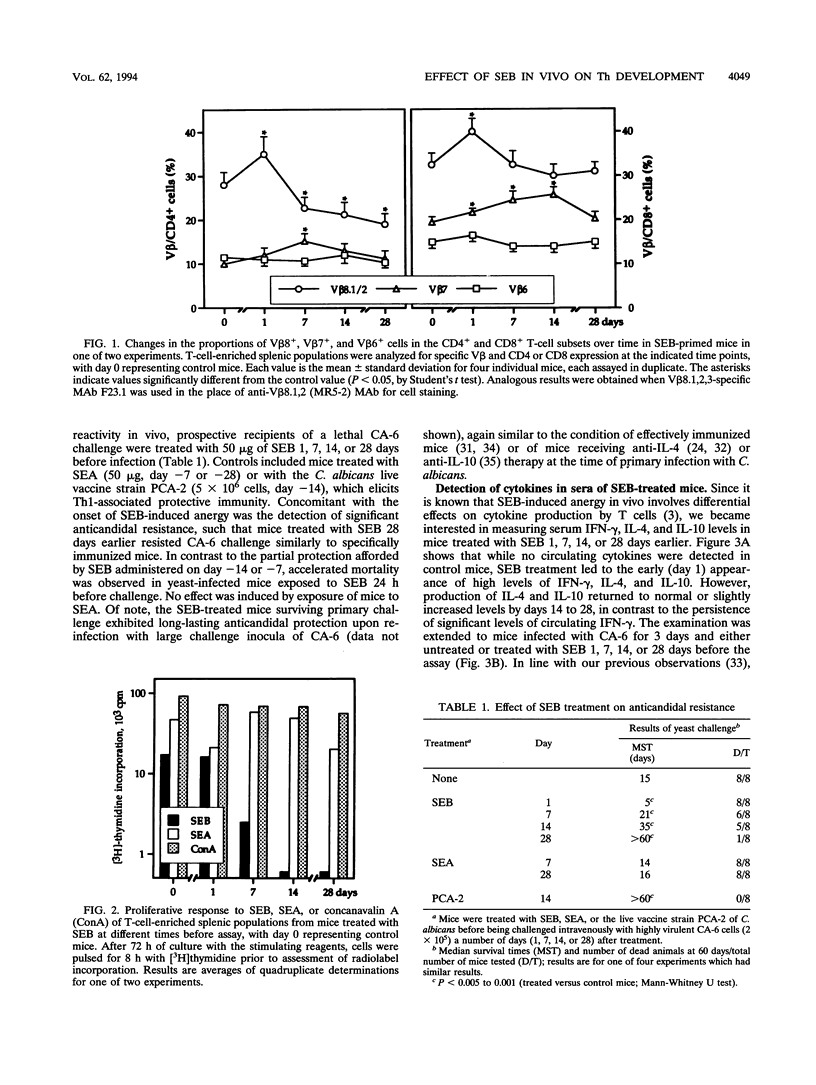
Staphylococcal enterotoxin B (SEB) is a bacterial superantigen that specifically activates T cells bearing V beta 8 T-cell receptor domains, which eventually leads to a long-lasting state of clonal anergy accompanied by selective cell death in the targeted CD4+ subset. Because the superantigen is known to promote Th1 cell differentiation in vitro, we have investigated the effect of SEB treatment on the course of Th2-associated progressive disease in mice infected systemically with Candida albicans. On the basis of the kinetics of SEB-induced changes in CD4+ cells and production in sera of interleukin 4 (IL-4), IL-10, and gamma interferon, we obtained evidence that V beta 8+ cell anergy concomitant with infection abolished the early IL-4/IL-10 response of the host to the yeast, ultimately leading to a state of resistance characterized by gamma interferon secretion in vitro by antigen-specific CD4+ cells. In contrast, SEB administered near the time of challenge resulted in accelerated mortality. Significant resistance to infection was also afforded by exposure of mice to a retrovirally encoded endogenous superantigen. These data suggest that CD4+ V beta 8+ T cells play an important role in vivo in the initiation of a Th2 response to C. albicans and that suppression of their activity may alter the qualitative development of the T-cell response and the outcome of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B., Papadimitriou J. M. What's new in the mechanisms of host resistance to Candida albicans infection? Pathol Res Pract. 1990 Aug;186(4):527–534. doi: 10.1016/S0344-0338(11)80477-2. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Coutinho A., Burlen-Defranoux O., Khazaal I., Coltey M., Jacquemart F., Le Douarin N., Salaün J. Thymic epithelium induces neither clonal deletion nor anergy to Mls 1a antigens. Eur J Immunol. 1992 Jun;22(6):1397–1404. doi: 10.1002/eji.1830220611. [DOI] [PubMed] [Google Scholar]
- Baschieri S., Lees R. K., Lussow A. R., MacDonald H. R. Clonal anergy to staphylococcal enterotoxin B in vivo: selective effects on T cell subsets and lymphokines. Eur J Immunol. 1993 Oct;23(10):2661–2666. doi: 10.1002/eji.1830231041. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Cenci E., Mencacci A., Schiaffella E., Mosci P., Puccetti P., Romani L. Mucosal and systemic T helper cell function after intragastric colonization of adult mice with Candida albicans. J Infect Dis. 1993 Dec;168(6):1449–1457. doi: 10.1093/infdis/168.6.1449. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Coker L. A., 3rd, Mercadal C. M., Rouse B. T., Moore R. N. Differential effects of CD4+ and CD8+ cells in acute, systemic murine candidosis. J Leukoc Biol. 1992 Mar;51(3):305–306. doi: 10.1002/jlb.51.3.305. [DOI] [PubMed] [Google Scholar]
- Gaur A., Fathman C. G., Steinman L., Brocke S. SEB induced anergy: modulation of immune response to T cell determinants of myoglobin and myelin basic protein. J Immunol. 1993 Apr 1;150(7):3062–3069. [PubMed] [Google Scholar]
- Gollob K. J., Nagelkerken L., Coffman R. L. Endogenous retroviral superantigen presentation by B cells induces the development of type 1 CD4+ T helper lymphocytes. Eur J Immunol. 1993 Oct;23(10):2565–2571. doi: 10.1002/eji.1830231028. [DOI] [PubMed] [Google Scholar]
- Held W., Shakhov A. N., Izui S., Waanders G. A., Scarpellino L., MacDonald H. R., Acha-Orbea H. Superantigen-reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J Exp Med. 1993 Feb 1;177(2):359–366. doi: 10.1084/jem.177.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Nagata M., Utsugi T., Yoon J. W. Prevention of autoimmune type I diabetes by CD4+ suppressor T cells in superantigen-treated non-obese diabetic mice. J Immunol. 1993 Oct 15;151(8):4362–4370. [PubMed] [Google Scholar]
- Kim C., Siminovitch K. A., Ochi A. Reduction of lupus nephritis in MRL/lpr mice by a bacterial superantigen treatment. J Exp Med. 1991 Dec 1;174(6):1431–1437. doi: 10.1084/jem.174.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. T., Vitetta E. S. Memory T cells are anergic to the superantigen staphylococcal enterotoxin B. J Exp Med. 1992 Aug 1;176(2):575–579. doi: 10.1084/jem.176.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Baschieri S., Lees R. K. Clonal expansion precedes anergy and death of V beta 8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991 Aug;21(8):1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- Matthews R. C. The 14th C. L. Oakley Lecture. Candida albicans HSP 90: link between protective and auto immunity. J Med Microbiol. 1992 Jun;36(6):367–370. doi: 10.1099/00222615-36-6-367. [DOI] [PubMed] [Google Scholar]
- Migita K., Ochi A. The fate of anergic T cells in vivo. J Immunol. 1993 Feb 1;150(3):763–770. [PubMed] [Google Scholar]
- Nagelkerken L., Gollob K. J., Tielemans M., Coffman R. L. Role of transforming growth factor-beta in the preferential induction of T helper cells of type 1 by staphylococcal enterotoxin B. Eur J Immunol. 1993 Sep;23(9):2306–2310. doi: 10.1002/eji.1830230938. [DOI] [PubMed] [Google Scholar]
- Nagelkerken L., Gollob K. J., Tielemans M., Coffman R. L. Role of transforming growth factor-beta in the preferential induction of T helper cells of type 1 by staphylococcal enterotoxin B. Eur J Immunol. 1993 Sep;23(9):2306–2310. doi: 10.1002/eji.1830230938. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Shibata N., Podzorski R. P., Herron M. J. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev. 1991 Jan;4(1):1–19. doi: 10.1128/cmr.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K. A., Ellenhorn J. D., Bruce D. S., Bluestone J. A. In vivo T-cell activation by staphylococcal enterotoxin B prevents outgrowth of a malignant tumor. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1074–1078. doi: 10.1073/pnas.88.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Yuh K., Migita K., Kawabe Y. Effects of staphylococcal toxins on T-cell activity in vivo. Chem Immunol. 1992;55:115–136. [PubMed] [Google Scholar]
- Puccetti P., Mencacci A., Cenci E., Spaccapelo R., Mosci P., Enssle K. H., Romani L., Bistoni F. Cure of murine candidiasis by recombinant soluble interleukin-4 receptor. J Infect Dis. 1994 Jun;169(6):1325–1331. doi: 10.1093/infdis/169.6.1325. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Locksley R. M. Lessons from Leishmania: a model for investigations of CD4+ subset differentiation. Infect Agents Dis. 1992 Feb;1(1):33–42. [PubMed] [Google Scholar]
- Romani L., Cenci E., Mencacci A., Spaccapelo R., Grohmann U., Puccetti P., Bistoni F. Gamma interferon modifies CD4+ subset expression in murine candidiasis. Infect Immun. 1992 Nov;60(11):4950–4952. doi: 10.1128/iai.60.11.4950-4952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Mosci P., Vitellozzi G., Grohmann U., Puccetti P., Bistoni F. Course of primary candidiasis in T cell-depleted mice infected with attenuated variant cells. J Infect Dis. 1992 Dec;166(6):1384–1392. doi: 10.1093/infdis/166.6.1384. [DOI] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Spaccapelo R., Mosci P., Puccetti P., Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993 Feb 1;150(3):925–931. [PubMed] [Google Scholar]
- Romani L., Mencacci A., Grohmann U., Mocci S., Mosci P., Puccetti P., Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992 Jul 1;176(1):19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Tonnetti L., Spaccapelo R., Cenci E., Wolf S., Puccetti P., Bistoni F. Interleukin-12 but not interferon-gamma production correlates with induction of T helper type-1 phenotype in murine candidiasis. Eur J Immunol. 1994 Apr;24(4):909–915. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- Romani L., Mocci S., Bietta C., Lanfaloni L., Puccetti P., Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991 Dec;59(12):4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Puccetti P., Mencacci A., Cenci E., Spaccapelo R., Tonnetti L., Grohmann U., Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994 Apr 1;152(7):3514–3521. [PubMed] [Google Scholar]
- Rott O., Wekerle H., Fleischer B. Protection from experimental allergic encephalomyelitis by application of a bacterial superantigen. Int Immunol. 1992 Mar;4(3):347–353. doi: 10.1093/intimm/4.3.347. [DOI] [PubMed] [Google Scholar]
- Röcken M., Müller K. M., Saurat J. H., Müller I., Louis J. A., Cerottini J. C., Hauser C. Central role for TCR/CD3 ligation in the differentiation of CD4+ T cells toward A Th1 or Th2 functional phenotype. J Immunol. 1992 Jan 1;148(1):47–54. [PubMed] [Google Scholar]
- Röcken M., Urban J. F., Shevach E. M. Infection breaks T-cell tolerance. Nature. 1992 Sep 3;359(6390):79–82. doi: 10.1038/359079a0. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Assenmacher M., Radbruch A. Regulation of T helper cell cytokine expression: functional dichotomy of antigen-presenting cells. Eur J Immunol. 1993 Jan;23(1):191–199. doi: 10.1002/eji.1830230130. [DOI] [PubMed] [Google Scholar]
- Scott P. Selective differentiation of CD4+ T helper cell subsets. Curr Opin Immunol. 1993 Jun;5(3):391–397. doi: 10.1016/0952-7915(93)90058-z. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R. L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Sinha A. A., Lopez M. T., McDevitt H. O. Autoimmune diseases: the failure of self tolerance. Science. 1990 Jun 15;248(4961):1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Vanier L. E., Prud'homme G. J. Cyclosporin A markedly enhances superantigen-induced peripheral T cell deletion and inhibits anergy induction. J Exp Med. 1992 Jul 1;176(1):37–46. doi: 10.1084/jem.176.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. R., Hutchinson J., Sprent J. Mls antigens: immunity and tolerance. Chem Immunol. 1992;55:87–114. [PubMed] [Google Scholar]


